Abstract
The skin commensal bacillus Propionibacterium acnes is known to play a major role in the development of acne vulgaris and it is established that this bacteria is involved both in the induction and maintenance of the inflammatory phase of acne. The corticotropin releasing hormone (CRH), a neuropeptide originally isolated from the hypothalamus, is also produced by the skin. CRH has been reported to play a role in the inflammation, the production of sebum and finally the differentiation of keratinocytes. At the therapeutic level, zinc is known to act specifically on inflammatory lesions with still partially known mechanisms and thus could play an important role in the development of inflammatory acne lesions. Our objective was to study the modulation of CRH expression by keratinocytes induced by P. acnes extracts. CRH expression was examined using immunohistochemistry technique on deep-frozen sections of normal human skin explants incubated with two different extracts of P. acnes and with or without zinc salts. We observed that the membrane fraction (FM) of P. acnes increased the CRH expression in the epidermis. This result indicates that P. acnes, by stimulating the production of CRH, can both modulate the differentiation of keratinocytes and increase the local inflammation, arguing that this bacterium plays a role not only in the development of inflammatory acne lesions but also in the formation of the microcomedo in the early stages of acne.
Key words: acne, Propionibacterium acnes, stress, corticotropin releasing hormone, zinc
Introduction
Acne vulgaris, the most common pathology of the skin, is a chronic inflammatory disease of the pilosebaceous unit (PSU). Several pathogenic factors such as ductal hypercornification, increased sebum production, abnormality of the microbial flora within the PSU and secretion of mediators of inflammation contribute to the aetiology of this multifactorial disease.1 Although P. acnes has been associated with acne, the precise mechanisms governing the development and progression of acne remain unclear.
In response to systemic stress, the hypothalamic-pituitary-adrenal (HPA) axis is activated.2 The process begins with the hypothalamic release of corticotropin releasing hormone (CRH) which stimulates the production of adrenocorticotropic hormone (ACTH) and other proopiomelanocortin (POMC) peptides via the activation of CRH receptor type 1 (CRH-R1). It was reported that ACTH is also produced outside the pituitary tissue, including the skin.3–6 Interestingly it has been proposed that skin has an equivalent of the central HPA axis.5,7–10 There is increasing evidence that the cutaneous nervous system modulates physiologic and pathophysiologic effects in the skin.11 Although similar to its systemic equivalent, the cutaneous HPA axis is responsive to local stressors (solar, thermal, chemical, biological, etc.,) and resultantly activates the neuronal, endocrine and immune systems in the skin.
As acne is obviously exacerbated under acute or chronic psychological stress11–13 the role of CRH in this pathology appears as an important point in the development of lesions. In humans, CRH is synthesized among others by keratinocytes,14 immune cells15 and human mast cells16,17 under the influence of a stress. Interestingly, CRH is also reported to play a role in the regulation of keratinocytes proliferation and differentiation representing an important step in the early stages of the development of acne lesions. Moreover CRH is known to act on inflammation by inducing the degranulation of mast cells, the release of inflammatory cytokines and the modulation of immune cells.18–20 And finally CRH acts also on sebum production by promoting lipogenesis in human sebocytes and thus by increasing the seborrhoea.21,22 Consequently, CRH could be strongly implicated in the development of acne.
Furthermore, several studies have underlined an important role of P. acnes in the pathophysiology of inflammatory lesions.23–29
In this context, we hypothesized that P. acnes is able to increase CRH expression by keratinocytes, representing a local stress in acne. We thus evaluated the modulation of CRH expression in the epidermis induced by the microbial stressor P. acnes.
Results
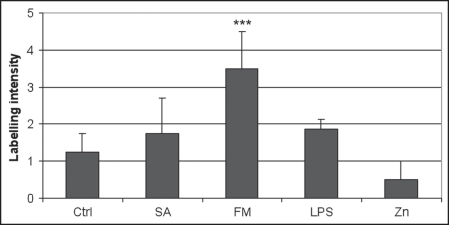
In all cases, we observed that the mean expression of CRH was moderate (1.25 ± 0.50) in control medium and was significantly increased (p < 0.05) in presence of P. acnes FM (3.50 ± 1.00). However the slight overexpression detected in the presence of SA (1.75 ± 0.96) was not significant (p > 0.05). This increased expression of CRH was observed after an incubation of three hours with P. acnes extracts.
Concerning LPS which is a proinflammatory substance, a slight increase (1.88 ± 0.25) in the expression of CRH was noted while zinc gluconate decreased this expression (0.50 ± 0.50) in comparison with the control medium, for all donors. However the modulations observed were not significant (Figs. 1 and 2).
Figure 1.
Evaluation of the expression of corticotrophin-releasing hormone in the epidermis of 4 donors after an incubation of 3 hours in the presence of Medium (Ctrl), SA (P. acnes supernatant A), FM (P. acnes membrane fraction), LPS (E. coli Lipopolysaccharide), Zn (Zinc Gluconate) (scale: null labelling (0), very weak labelling (1), weak labelling (2), moderate labelling (3), strong labelling (4) and very strong labelling (5). (***p value < 0.05).
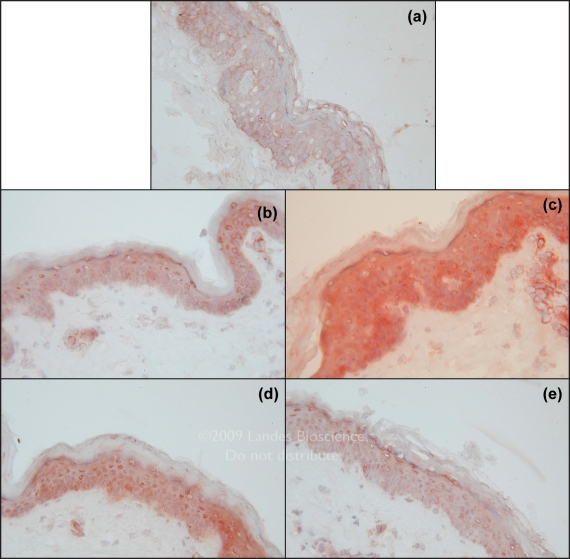
Figure 2.
Detection of the expression of corticotrophin-releasing hormone in the skin after an incubation of three hours in the presence of Medium (Ctrl) (A), SA (P. acnes supernatant A) (B), FM (P. acnes membrane fraction) (C), LPS (E. coli Lipopolysaccharide) (D), Zn (Zinc Gluconate) (E). (Magnification ×40).
Discussion
In the present work, we demonstrate that P. acnes stimulates the production of CRH by keratinocytes. In addition we observe that zinc gluconate used in inflammatory acne decreases the epidermal CRH production.
Concerning P. acnes extracts, the stimulating effect is specifically obtained with FM and not with SA. While FM contains P. acnes membrane components, in particular peptidoglycan and lipoteichoic acid, Supernatant A (SA) contains only cytosolic proteins. Our result thus confirms that the CRH overexpression is mediated by at least one component of FM, suggesting that P. acnes acts on the CRH production through a direct contact with keratinocytes membranes. Notably, the induction of CRH in the presence of FM is more important than this obtained in the presence of LPS which is considered as a reference among proinflammatory substances.
It is widely accepted that inflammatory acne may be mainly mediated by different factors secreted by P. acnes, such as lipases which induce the liberation of proinflammatory free fatty acids from sebum.30,31 Recently, it has also been described that P. acnes triggers anti-microbial peptide and cytokine secretion of keratinocytes in vitro.23–25 P. acnes, itself, is able to induce cytokines-like such as TNFα, IL-1α and IL-8.26,27 Moreover the genome sequence of P. acnes is known to encode many factors that may have inflammatory potential.28 But, recently it has also been shown that P. acnes was able to induce keratinocytes proliferation and filaggrin expression by keratinocytes,29 indicating that this bacterium could play a role not only in the development of inflammation in acne lesions but also in the formation of the comedo.
The role of zinc salts has already been reported in several studies and beneficial effects were described on inflammatory lesions in mild to moderate acne. It has been shown until now that its anti-inflammatory activity has different targets.32 Indeed zinc acts via an inhibition of polynuclear cells chemotaxis.33 The anti-inflammatory effects of zinc could be attributed to its ability to decrease TNFα and IL-6 production34,35 or to inhibit TLR-2 expression by keratinocytes.36 The modulation of integrins expression by zinc is also described.37,38 At the same time, zinc is known to have a direct influence on P. acnes development by its antimicrobial activities39 and its ability to reduce the resistance of P. acnes strains to erythromycin.40 Finally, zinc is also able to modulate the 5α-reductase, a key enzyme implicated in the transformation of testosterone leading to an exacerbated seborrhoea.41 By these many actions, zinc is able to improve inflammatory acne. In this study, we suggest another mechanism by which zinc salts could be able to improve acne by decreasing the epidermal CRH expression. The fact that the modulation observed with zinc salts was not significant can be mainly due to the low level of basal CRH production in skin control but interestingly the decreased expression of CRH was noted for all the four donors studied. Moreover, the model used was very close to in vivo. In this context, zinc salts could represent a potential new way to counteract acne by targeting CRH.
The potential interest of CRH in acne was recently suggested by the discovery of an overexpression of CRH in the PSU.21 Indeed, CRH has been reported to play several roles potentially strongly implicated in the development of acne lesions. First, as CRH was reported to promote lipogenesis in human sebocytes and thus to increase the seborrhoea, it could represent a key factor in the development of acne.21,22 Moreover, CRH is also known to act as a growth factor in the skin by activating the CRH-R1. CRH is thus described as an activator of keratinocytes differentiation.42 It is also an inhibitor of the early and late apoptosis of many skin cell types such as keratinocytes, dermal fibroblasts and melanocytes.43 Finally CRH has also a pro inflammatory activity.18 Interestingly, it was suggested that CRH could act as a local endocrine mediator that enhances inflammatory responses to bacterial antigens.44
In this study, we demonstrate for the first time that P. acnes acts as a strong local stressor at the origin of CRH overexpression by keratinocytes, in the epidermis. Thus, as CRH modifies the differentiation of keratinocytes, we confirm that this bacterium has a spectrum of action, not limited to inflammation but also in the early stages of the formation of acne lesions.29 This confirms the fundamental role played by this bacterium which can be detected as a local stressor by the skin and could potentially lead to the development of acne lesions.
In conclusion, CRH represents a new target for P. acnes in the formation of both retentional and inflammatory lesions. Zinc gluconate seems to be able to decrease the production of CRH by keratinocytes, thus explaining its role in the improvement of acne. But this point has to be confirmed by further studies.
Materials and Methods
Bacterial extracts.
Two extracts of P. acnes IP53113T (Pierre Fabre, Toulouse, France) were made available to us. The strain was first described in 1968. The membrane fraction (FM) contained peptidoglycan and lipoteichoic acid. Supernatant A (SA) contained cytosolic proteins. The membrane fractions of the bacteria were resuspended in DMEM (Dulbecco’s Modified Eagle Medium) (Sigma-Aldrich, Saint-Quentin Fallavier, France).
LPS extracted from E. coli 0111:B4 (Sigma, St. Louis, USA), was reconstituted in PBS (Phosphate-Bufferd Saline). As LPS is a pro inflammatory substance, it was used as positive control of the inflammation. LPS was diluted in DMEM (Dulbecco’s Modified Eagle Medium) (Sigma-Aldrich) medium with a final concentration of 1 µg/mL and incubated with cutaneous explants.
Trace element.
As zinc salts are an anti inflammatory treatment of acne lesions it was used as negative control. Zinc gluconate (Labcatal, Montrouge, France), was diluted in DMEM with a final zinc concentration of 1 µg/mL and incubated with cutaneous explants.
Skin explants technique.
Punches (4 mm in diameter) from abdominal skin of 6 healthy donors, considered as an healthy skin model, were incubated at 37°C in a moist atmosphere in the presence of 5% CO2 for 3, 6 or 24 hours in DMEM. The medium contained P. acnes extracts at the following concentrations: FM ½ or SA 1/5 or Zn 1 µg/mL. Medium alone was used as a control. After incubation of 3, 6 or 24 h, explants were removed from the culture medium and frozen at −80°C.
Immunoperoxidase.
Sections (5 µm thick) were then cut with a cryostat, fixed in acetone at 4°C for 10 min and frozen at −20°C. The non-specific sites were saturated for 30 mn with TBS (Tris-Buffered Saline) 0.05% Tween20 w/v (Sigma-Aldrich), 0.1% BSA w/v (Bovine Serum Albumin) (Sigma-Aldrich). CRH was detected with an immunoperoxidase technique, using PBS for dilution and TBS for washes. The rabbit anti-CRH Serum (1/50) (Phoenix Pharmaceuticals, Strasbourg, France) was deposited upon the slides for one hour in a humid environment at room temperature. Rinses of 10 mn were made between each stage using TBS, 0.1% BSA, 0.1%. The slides were incubated successively with a secondary biotinylated antibody (DAKO ChemTek detection kit peroxidase/AEC, Rabbit/Mouse, Trappes, France) (30 mn) and streptavidin coupled with peroxidase (30 mn). Reaction was stopped with distilled water (10 min) and counter-staining was done with Mayer haemalun (VWR International, Fontenay-sous-Bois, France) for about two minutes. Slides were rinsed with distilled water, and mounted in an aqueous medium. The control section included omission of primary antibody. Two different examiners read the slides. Labelling intensity was scored on a five-point scale: null labelling (0), very weak labelling (1), weak labelling (2), moderate labelling (3), strong labelling (4) and very strong labelling (5).
Statistical analysis.
CRH protein levels were expressed as the mean ± SD. To determine the differences among all experimental groups (Ctrl, SA, FM, LPS, Zn), a Kruskal-Wallis test was performed, followed by Dunnett’s test to isolate the group(s) that differed from the control group. Significance for stimulating effects of SA, FM, LPS or Zn compared with controls, was assessed at the p-value <0.05.
Acknowledgements
We thank Pierre Fabre Dermatologie laboratories for their financial support. Zinc Gluconate was kindly provided by Labcatal laboratories.
Abbreviations
- P. acnes
Propionibacterium acnes
- E. coli
Escherichia coli
- CRH
corticotropin releasing hormone
- FM
membrane fraction
- SA
supernatant A
- Zn
zinc
- LPS
lipopolysaccharide
- Ctrl
control
- HPA
hypothalamic-pituitary-adrenal
- ACTH
adrenocorticotropic hormone
- POMC
proopiomelanocortin
- CRH-R1
corticotropin receptor type 1
- PSU
pilosebaceous unit
- PBS
phosphate-buffered saline
- TBS
tris-buffered saline
- DMEM
dulbecco’s modified eagle medium
- TNFα
tumor necrosis factor alpha
- IL-1α
interleukin-1 alpha
- IL-8
interleukin-8
- TLR-2
toll-like receptor-2
Footnotes
Previously published online as a Dermato-Endocrinology E-publication: http://www.landesbioscience.com/journals/dermatoendocrinology/article/8102
References
- 1.Zouboulis CC, Eady A, Philpott M, Goldsmith LA, Orfanos C, Cunliffe WC, Rosenfield R. What is the pathogenesis of acne? Exp Dermatol. 2005;14:143–152. doi: 10.1111/j.0906-6705.2005.0285a.x. [DOI] [PubMed] [Google Scholar]
- 2.Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. 1995;332:1351–1362. doi: 10.1056/NEJM199505183322008. [DOI] [PubMed] [Google Scholar]
- 3.Chakraborty AK, Funasaka Y, Slominski A, Ermak G, Hwang J, Pawelek JM, Ichihashi M. Production and release of proopiomelanocortin (POMC) derived peptides by human melanocytes and keratinocytes in culture: Regulation by ultraviolet B. Biochim Biophys Acta. 1996;1313:130–138. doi: 10.1016/0167-4889(96)00063-8. [DOI] [PubMed] [Google Scholar]
- 4.Wakamatsu K, Graham A, Cook D, Thody AJ. Characterisation of ACTH peptides in human skin and their activation of the melanocortin-1 receptor. Pigment Cell Res. 1997;10:288–297. doi: 10.1111/j.1600-0749.1997.tb00688.x. [DOI] [PubMed] [Google Scholar]
- 5.Slominski A, Wortsman J, Luger T, Paus R, Solomon S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev. 2000;80:979–1020. doi: 10.1152/physrev.2000.80.3.979. [DOI] [PubMed] [Google Scholar]
- 6.Ito N, Ito T, Kromminga A, Bettermann A, Takigawa M, Kees F, et al. Human hair follicles display a functional equivalent of the hypothalamic-pituitary-adrenal axis and synthesize cortisol. Faseb J. 2005;19:1332–1334. doi: 10.1096/fj.04-1968fje. [DOI] [PubMed] [Google Scholar]
- 7.Slominski A, Mihm MC. Potential mechanism of skin response to stress. Int J Dermatol. 1996;35:849–851. doi: 10.1111/j.1365-4362.1996.tb05049.x. [DOI] [PubMed] [Google Scholar]
- 8.Slominski A, Wortsman J, Pisarchik A, Zbytek B, Linton EA, Mazurkiewicz JE, Wei ET. Cutaneous expression of corticotropin-releasing hormone (CRH), urocortin and CRH receptors. Faseb J. 2001;15:1678–1693. doi: 10.1096/fj.00-0850rev. [DOI] [PubMed] [Google Scholar]
- 9.Slominski A, Pisarchik A, Tobin DJ, Mazurkiewicz JE, Wortsman J. Differential expression of a cutaneous corticotropin-releasing hormone system. Endocrinology. 2004;145:941–950. doi: 10.1210/en.2003-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slominski A, Wortsman J, Tuckey RC, Paus R. Differential expression of HPA axis homolog in the skin. Mol Cell Endocrinol. 2007;266:143–149. doi: 10.1016/j.mce.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zouboulis CC, Bohm M. Neuroendocrine regulation of sebocytes—A pathogenetic link between stress and acne. Exp Dermatol. 2004;13:31–35. doi: 10.1111/j.1600-0625.2004.00254.x. [DOI] [PubMed] [Google Scholar]
- 12.Kraus SJ. Stress, acne and skin surface free fatty acids. Psychosom Med. 1970;32:503–508. doi: 10.1097/00006842-197009000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Koo JY, Smith LL. Psychologic aspects of acne. Pediatr Dermatol. 1991;8:185–188. doi: 10.1111/j.1525-1470.1991.tb00856.x. [DOI] [PubMed] [Google Scholar]
- 14.Slominski A, Ermak G, Mazurkiewicz JE, Baker J, Wortsman J. Characterization of corticotropin-releasing hormone (CRH) in human skin. J Clin Endocrinol Metab. 1998;83:1020–1024. doi: 10.1210/jcem.83.3.4650. [DOI] [PubMed] [Google Scholar]
- 15.Karalis K, Muglia LJ, Bae D, Hilderbrand H, Majzoub JA. CRH and the immune system. J Neuroimmunol. 1997;72:131–136. doi: 10.1016/s0165-5728(96)00178-6. [DOI] [PubMed] [Google Scholar]
- 16.Slominski AT. Proopiomelanocortin signaling system is operating in mast cells. J Invest Dermatol. 2006;126:1934–1936. doi: 10.1038/sj.jid.5700342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalantaridou S, Makrigiannakis A, Zoumakis E, Chrousos GP. Peripheral corticotropin-releasing hormone is produced in the immune and reproductive systems: Actions, potential roles and clinical implications. Front Biosci. 2007;12:572–580. doi: 10.2741/2083. [DOI] [PubMed] [Google Scholar]
- 18.O’Kane M, Murphy EP, Kirby B. The role of corticotropin-releasing hormone in immune-mediated cutaneous inflammatory disease. Exp Dermatol. 2006;15:143–153. doi: 10.1111/j.1600-0625.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 19.Theoharides TC, Donelan JM, Papadopoulou N, Cao J, Kempuraj D, Conti P. Mast cells as targets of corticotropin-releasing factor and related peptides. Trends Pharmacol Sci. 2004;25:563–568. doi: 10.1016/j.tips.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 20.Theoharides TC, Kalogeromitros D. The critical role of mast cells in allergy and inflammation. Ann N Y Acad Sci. 2006;1088:78–99. doi: 10.1196/annals.1366.025. [DOI] [PubMed] [Google Scholar]
- 21.Ganceviciene R, Graziene V, Fimmel S, Zouboulis CC. Involvement of the corticotropin-releasing hormone system in the pathogenesis of acne vulgaris. Br J Dermatol. 2008 doi: 10.1111/j.1365-2133.2008.08959.x. [DOI] [PubMed] [Google Scholar]
- 22.Zouboulis CC, Seltmann H, Hiroi N, Chen W, Young M, Oeff M, et al. Corticotropin-releasing hormone: An autocrine hormone that promotes lipogenesis in human sebocytes. Proc Natl Acad Sci USA. 2002;99:7148–7153. doi: 10.1073/pnas.102180999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagy I, Pivarcsi A, Koreck A, Szell M, Urban E, Kemeny L. Distinct strains of Propionibacterium acnes induce selective human β-defensin-2 and interleukin-8 expression in human keratinocytes through toll-like receptors. J Invest Dermatol. 2005;124:931–938. doi: 10.1111/j.0022-202X.2005.23705.x. [DOI] [PubMed] [Google Scholar]
- 24.Schaller M, Loewenstein M, Borelli C, Jacob K, Vogeser M, Burgdorf WH, Plewig G. Induction of a chemoattractive proinflammatory cytokine response after stimulation of keratinocytes with Propionibacterium acnes and coproporphyrin III. Br J Dermatol. 2005;153:66–71. doi: 10.1111/j.1365-2133.2005.06530.x. [DOI] [PubMed] [Google Scholar]
- 25.Jugeau S, Tenaud I, Knol AC, Jarrousse V, Quereux G, Khammari A, Dreno B. Induction of toll-like receptors by Propionibacterium acnes. Br J Dermatol. 2005;53:1105–1113. doi: 10.1111/j.1365-2133.2005.06933.x. [DOI] [PubMed] [Google Scholar]
- 26.Vowels BR, Yang S, Leyden JJ. Induction of proinflammatory cytokines by a soluble factor of Propionibacterium acnes: Implications for chronic inflammatory acne. Infect Immun. 1995;63:3158–3165. doi: 10.1128/iai.63.8.3158-3165.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graham GM, Farrar MD, Cruse-Sawyer JE, Holland KT, Ingham E. Proinflammatory cytokine production by human keratinocytes stimulated with Propionibacterium acnes and P. acnes GroEL. Br J Dermatol. 2004;150:421–428. doi: 10.1046/j.1365-2133.2004.05762.x. [DOI] [PubMed] [Google Scholar]
- 28.Bruggemann H, Henne A, Hoster F, Liesegang H, Wiezer A, Strittmatter A, et al. The complete genome sequence of Propionibacterium acnes, a commensal of human skin. Science. 2004;305:671–673. doi: 10.1126/science.1100330. [DOI] [PubMed] [Google Scholar]
- 29.Jarrousse V, Castex-Rizzi N, Khammari A, Charveron M, Dreno B. Modulation of integrins and filaggrin expression by Propionibacterium acnes extracts on keratinocytes. Arch Dermatol Res. 2007;299:441–447. doi: 10.1007/s00403-007-0774-5. [DOI] [PubMed] [Google Scholar]
- 30.Zouboulis CC. Exploration of retinoid activity and the role of inflammation in acne: Issues affecting future directions for acne therapy. J Eur Acad Dermatol Venereol. 2001;15:63–67. doi: 10.1046/j.0926-9959.2001.00015.x. [DOI] [PubMed] [Google Scholar]
- 31.Farrar MD, Ingham E. Acne: Inflammation. Clin Dermatol. 2004;22:380–384. doi: 10.1016/j.clindermatol.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 32.Dreno B, Moyse D, Alirezai M, Amblard P, Auffret N, Beylot C, et al. Multicenter randomized comparative double-blind controlled clinical trial of the safety and efficacy of zinc gluconate versus minocycline hydrochloride in the treatment of inflammatory acne vulgaris. Dermatology. 2001;203:135–140. doi: 10.1159/000051728. [DOI] [PubMed] [Google Scholar]
- 33.Dreno B, Trossaert M, Boiteau HL, Litoux P. Zinc salts effects on granulocyte zinc concentration and chemotaxis in acne patients. Acta Derm Venereol. 1992;72:250–252. [PubMed] [Google Scholar]
- 34.Gueniche A, Viac J, Lizard G, Charveron M, Schmitt D. Protective effect of zinc on keratinocyte activation markers induced by interferon or nickel. Acta Derm Venereol. 1995;75:19–23. doi: 10.2340/00015555751923. [DOI] [PubMed] [Google Scholar]
- 35.Sainte-Marie I, Jumbou O, Tenaud I, Dreno B. Comparative study of the in vitro inflammatory activity of three nickel salts on keratinocytes. Acta Derm Venereol. 1998;78:169–172. doi: 10.1080/000155598441459. [DOI] [PubMed] [Google Scholar]
- 36.Jarrousse V, Castex-Rizzi N, Khammari A, Charveron M, Dreno B. Zinc salts inhibit in vitro Toll-like receptor 2 surface expression by keratinocytes. Eur J Dermatol. 2007;17:492–496. doi: 10.1684/ejd.2007.0263. [DOI] [PubMed] [Google Scholar]
- 37.Tenaud I, Sainte-Marie I, Jumbou O, Litoux P, Dreno B. In vitro modulation of keratinocyte wound healing integrins by zinc, copper and manganese. Br J Dermatol. 1999;140:26–34. doi: 10.1046/j.1365-2133.1999.02603.x. [DOI] [PubMed] [Google Scholar]
- 38.Tenaud I, Leroy S, Chebassier N, Dreno B. Zinc, copper and manganese enhanced keratinocyte migration through a functional modulation of keratinocyte integrins. Exp Dermatol. 2000;9:407–416. doi: 10.1034/j.1600-0625.2000.009006407.x. [DOI] [PubMed] [Google Scholar]
- 39.Strauss JS, Stranieri AM. Acne treatment with topical erythromycin and zinc: effect of Propionibacterium acnes and free fatty acid composition. J Am Acad Dermatol. 1984;11:86–89. doi: 10.1016/s0190-9622(84)70139-3. [DOI] [PubMed] [Google Scholar]
- 40.Dreno B, Foulc P, Reynaud A, Moyse D, Habert H, Richet H. Effect of zinc gluconate on propionibacterium acnes resistance to erythromycin in patients with inflammatory acne: in vitro and in vivo study. Eur J Dermatol. 2005;15:152–155. [PubMed] [Google Scholar]
- 41.Sugimoto Y, Lopez-Solache I, Labrie F, Luu-The V. Cations inhibit specifically type I 5 alpha-reductase found in human skin. J Invest Dermatol. 1995;104:775–778. doi: 10.1111/1523-1747.ep12606985. [DOI] [PubMed] [Google Scholar]
- 42.Zbytek B, Pikula M, Slominski RM, Mysliwski A, Wei E, Wortsman J, Slominski AT. Corticotropin-releasing hormone triggers differentiation in HaCaT keratinocytes. Br J Dermatol. 2005;152:474–480. doi: 10.1111/J.1365-2133.2005.06217.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Slominski A, Zbytek B, Pisarchik A, Slominski RM, Zmijewski MA, Wortsman J. CRH functions as a growth factor/cytokine in the skin. J Cell Physiol. 2006;206:780–791. doi: 10.1002/jcp.20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zbytek B, Slominski AT. CRH mediates inflammation induced by lipopolysaccharide in human adult epidermal keratinocytes. J Invest Dermatol. 2007;127:730–732. doi: 10.1038/sj.jid.5700607. [DOI] [PMC free article] [PubMed] [Google Scholar]