Abstract
Background:
Pigment epithelium-derived factor (PEDF) was first isolated from the medium conditioned by human fetal retinal pigment epithelial cells and has been detected in a broad range of human fetal and adult tissues. Recent studies have indicated that PEDF activity is inhibitory to angiogenesis.
Objective:
To study the expression and distribution of pigment epithelium-derived factor (PEDF) in human melanocytes, malignant melanoma cells and tissues.
Results:
PEDF was expressed in human melanocytes. The expression of PEDF protein diminished in the following orders healthy skin, pigmented nevus and human malignant melanoma (p < 0.001). Both the expression of PEDF mRNA and protein was much lower or almost absent in the malignant melanoma cell line A375 than that in human melanocytes (p < 0.001).
Methods:
The expression and distribution of PEDF in human healthy skin, pigmented nevus and malignant melanoma were studied. The expression of PEDF mRNA in human melanocytes and malignant melanoma cell line A375 was measured by reverse transcription polymerase chain reaction (RT-PCR) and PEDF protein was detected by immunohistochemical method and Western blotting analysis.
Conclusion:
The lack of PEDF expression may contribute to the pathogenesis of malignant melanoma.
Key words: malignant melanoma, pigmented nevus, pigment epithelium derived factor, melanocytes, angiogenesis
Introduction
Malignant melanoma (MM) is characterized by rapid growth, intense angiogenesis, vascular malformation and poor survival rate. The incidence rate of MM is 2–16.4 per 100,000, accounting for about 5% of all skin cancers increasing each year. The mortality rate is extremely high, contributing to about 75% of all skin cancers causing death.
Pigment epithelium-derived factor (PEDF), initially isolated from the retinal pigment epithelial cells, is a 50 kDa glycoprotein recognized for its neurotrophic activity on the neural crest-derived cells.1,2 It shares high sequence homology with other serine proteinase inhibitors (serpins) and behaves like a non-inhibitory serpin.3 PEDF has been detected in many tissues and recent studies have demonstrated that the PEDF gene might be a candidate gene for tumor. It can induce differentiation in retinoblastoma tumor cells in vitro4 and protect retinal neurons from apoptotic death. The present study focuses on on the potential role of PEDF in the pathogenesis of MM. The expression and distribution of PEDF were also detected in other tissues and evaluated.
Results
Immunohistochemical staining for PEDF.
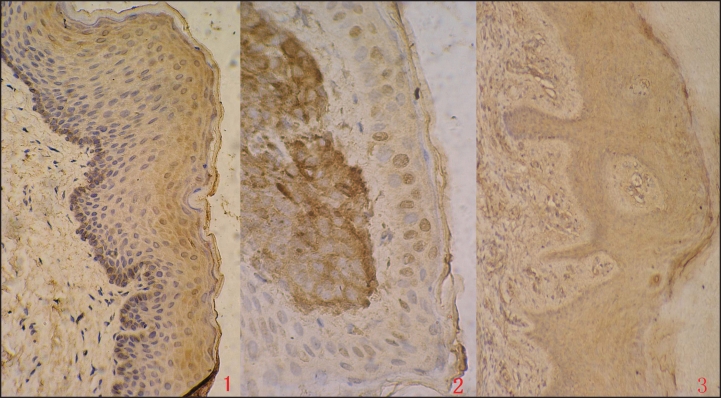
Immunostaining for PEDF was performed on healthy skin, pigmented nevus and MM specimens (Fig. 1). The expression of PEDF could be observed in cytoplasm under the microscope. For specimens stained, positive cells in 10 random fields (x40) under microscope were counted and the average of the percentages was taken. The scoring was as follows: 0 indicating that positive cells ≤20% of the total, (1): 21%–50%, (2): 51%–80%; (3): ≥81%. Moreover, the scoring was also given based on the levels of the color stained for the majority of positive cells: 1 for yellow, 2 for yellow-brown and 3 for deep brown. Then, the positivity of each specimen was evaluated by the summation of the both scores: 0 was regarded as negative (−), 1–2 (+) as weak, 3–4 as positive (++), 5–6 (+++) as strongly positive.
Figure 1.
Immunohistochemical staining for PEDF expression in healthy skin, pigmented nevus and MM (×40) (1) healthy skin; (2) pigmented nevus; (3) MM.
The expression of PEDF varied in different types of specimens (Table 1). PEDF was strongly positive in healthy skin, followed by pigmented nevus, with the lowest expression in MM. The difference was statistically significant among the three groups (Rank sum test, χ2 = 14.478, p < 0.001). The positive signal was most intense in healthy skin cells. The frequency and the intensity of PEDF immunostaining decreased greatly from healthy skin cells to malignant melanoma cells. Furthermore, the comparison of each two specimens as healthy skin either to pigmented nevus or to MM showed significant difference (Fisher’s Exact test, p < 0.01, Table 2), but not for the pigmented nevus and MM (p > 0.05).
Table 1.
Expression of PEDF in healthy skin, pigmented nevus and MM
| Group | No. | − | + | ++ | +++ |
| healthy skin | 20 | 3 | 0 | 11 | 6 |
| pigmented nevus | 12 | 7 | 2 | 3 | 0 |
| MM | 6 | 4 | 2 | 0 | 0 |
Table 2.
Comparison of PEDF expression between each two groups
| 99% confidence limit | |||
| p value | Low limit | Upper limit | |
| healthy skin/pigmented nevus | 0.0032 | 0.001745 | 0.004655 |
| healthy skin/MM | 0.0005 | 0 | 0.001076 |
| pigmented nevus/MM | 0.6218 | 0.609309 | 0.634291 |
Western blotting.
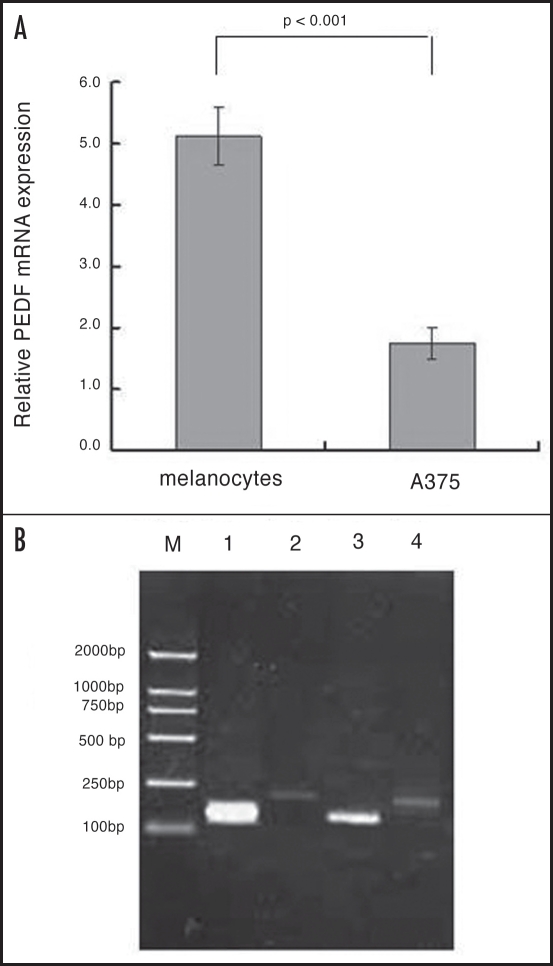
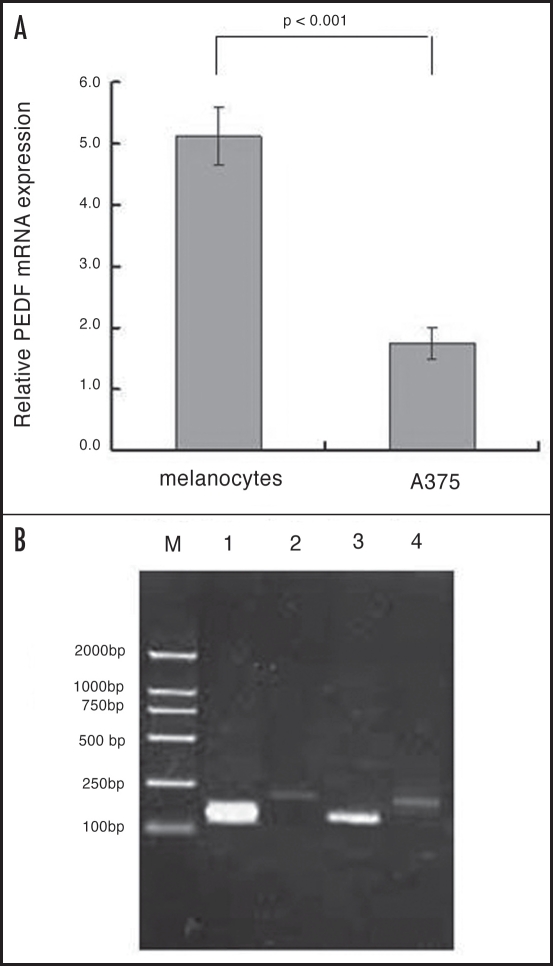
The expression of the PEDF protein in the normal human melanocytes and human malignant A375 melanoma cells was analyzed with anti-PEDF monoclonal antibody. The antibody recognized a clear band in human melanocytes, while the band was undetectable in A375. Consistent with the observations, optical density (OD) values of PEDF bands relative to GAPDH in two cells were 1.44 ± 0.13 and 0.10 ± 0.03. Significant difference (unpaired Student’s t test, p < 0.001, Fig. 2) was shown between the two groups. The expression of PEDF in normal human melanocytes was much higher than that of A375 cells.
Figure 2.
Western blot analysis of PEDF in normal human melanocytes and human malignant melanoma cell line A375 (N = 3).
PEDF mRNA expression in normal human melanocytes and melanoma cell line.
Semi-quantitative evaluation of PEDF normalized to that of GAPDH revealed that PEDF mRNA was abundant in human melanocytes, but was markedly reduced in A375 cells (Fig. 3). The relative amount of PEDF mRNA expression in normal human melanocytes and A375 was 5.12 ± 0.47 and 1.75 ± 0.26 respectively. The amount of PEDF mRNA found in normal human melanocytes was much greater than that found in melanoma cell line.
Figure 3.
The PEDF RT-PCR products in normal human melanocytes relative to the melanoma cell lines M: Markers for bp; (1) melanocytes/PEDF; (2) melanocytes/GAPDH; (3) A375/PEDF; (4) A375/GAPDH.
Discussion
Malignant melanoma cells arise from malignant devolution of melanocytes. MM are highly malignant and belong to 1–2% of all systemic malignant tumors. The majority of patients with metastasized MM die within 10 years. Melanomas are characterized by rapid cell proliferation and are able to invade and damage surrounding tissues. So, an additional prognostic indicator for those patients who should be treated aggressively or less aggressively would be extremely useful.
Pigment epithelium-derived factor (PEDF) was first purified from the conditioned medium of human retinal pigment epithelial cells as a factor with potent differentiating activity in human retinoblastoma cells in 1989.1 The 50 kD protein is encoded by a single gene that locates at the terminal portion of the short arm of chromosome 17. Amino acid sequence shows PEDF is a noninhibitory member of the serine protease inhibitor (serpin) family.5 In 1994, Tombran-Tink et al.6 sublocalized PEDF to 17p13.1-pter, shared the loci with p53, which is one of the most powerful tumor suppressor genes. PEDF thus maps to a region containing a number of cancer-related loci and is considered a candidate gene for MM.
PEDF is widely expressed throughout the human tissues, and exhibits multiple biological activities. It has been established as a potent anti-angiogenic molecule,7 and downregulation of PEDF is prevalently seen in a range of tumors. Cheung LW et al.8 found that the expression of PEDF was greatly reduced in ovarian tumors and in ovarian cancer cell lines. In addition, exogenous PEDF inhibited the growth of cultured human ovarian surface epithelial cells as well as ovarian cancer cell lines. Abramson LP et al.9,10 found that reduced level of PEDF in Wilms’ tumor removed a critical endogenous renal barrier to angiogenesis facilitating tumor cell survival. Zhang L et al.11 found that PEDF was reduced at both protein and mRNA level in non-small cell lung cancer (NSCLC) compared with normal lung tissues, and the reduction was associated with an increase in microvessel density in tumors and significantly associated with TNM (Tumor, Node, Metastasis) stage, tumor size and the overall survival. In particular, Yang H et al.12 found that angiostatin decreases cell migration and vascular endothelium growth factor (VEGF) to PEDF RNA ratio in vitro and in a murine ocular melanoma model. These studies indicated that PEDF might be a potential anti-tumor agent, causing both direct and indirect tumor suppression.13,14 However, the expression of PEDF, and the role of PEDF in MM and their relationship has not yet been determined.
In this report, we studied the expression and distribution of PEDF in human melanocytes, MM cells and tissues. Our results showed that PEDF was strongly positive in healthy skin, followed by pigmented nevus, with the lowest expression in MM by immunochemical staining. Moreover, we used anti-PEDF monoclonal antibody to detect the expression of the PEDF protein in normal human melanocytes and human malignant A375 melanoma cells, and found PEDF was barely expressed in A375 cells. Lastly, we extracted the RNA for RT-PCR. Semi-quantitative evaluation showed that the transcription of PEDF was barely detectable in A375 cells. Taken together, these results indicate that PEDF might be involved in the process of tumor development.
Angiogenesis is under the exquisite control of a network of angiogenic factors and anti-angiogenic factors. Previous studies have shown that PEDF plays an important role in negating the angiogenic process in pathological conditions. PEDF itself, or its biologically active fragment, is currently considered an agent of great interest in the prospects for future MM therapy. Li et al.15 found that PEDF offered greater protection to the retina and the method of delivering therapeutically active drugs have potentially clinical advantages for longer-term treatments of retinal diseases. Yamagishi et al.16 reported that PEDF inhibits the Ang II-induced T cell proliferation by blocking autocrine production of IL-2 via suppression of NADPH oxidase-mediated reactive oxygen species (ROS) generation. Blockade by PEDF of T cell activation may become a novel therapeutic target for atherosclerosis. Garcia et al.17 found that PEDF overexpression by melanoma cells greatly inhibited subcutaneous tumor formation and completely prevented lung and liver metastasis. Abe et al.18 found that overexpression of PEDF decreased angiogenesis and inhibited the growth of human malignant melanoma cells in vivo. Furthermore, Takenaka et al.19 found that PEDF could induce apoptosis and inhibition of vascular endothelial growth factor (VEGF) expression in MG63 human osteosarcoma cells. In vitro, PEDF dose-dependently inhibited growth and induced apoptosis in melanoma cells, which was completely blocked by treatments with a neutralizing antibody against FasL, highlighting the two beneficial effects of PEDF in melanoma growth, the suppression of tumor angiogenesis, and the induction of FasL-dependent apoptosis in tumor cells. Therefore, PEDF could be a promising novel therapeutic agent for the treatment of patients with certain types of cancer including melanoma.20
In conclusion, we have demonstrated a significant difference in the expression and distribution of the PEDF protein among healthy skin, pigmented nevus and MM, indicating that the lack of PEDF expression might be a potent factor for enhancement of angiogenesis in MM. Its “gliastatic” features, its effects on differentiation, and its anti-angiogenesis effects make PEDF an appealing potential agent for the control of tumor growth. We are currently in the process of studying the regulation of PEDF expression in MM with the aim to provide more information to help establish new and exciting strategies in inhibiting tumor growth.
Materials and Methods
Cell culture.
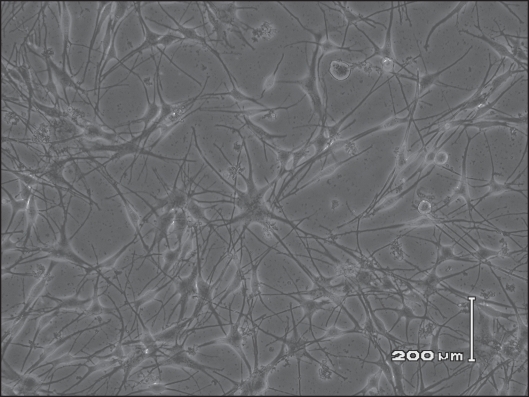
Human malignant melanoma cell line A375 (obtained from the cell bank of the Chinese Academy of Science) were cultured in DMEM medium and supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 100 IU/ml penicillin G and 100 mg/ml streptomycin at 37°C in a humidified 5% CO2 atmosphere (Fig. 4). Normal human melanocytes (surgically taken from healthy men’s foreskin) were routinely cultured in 254 medium with 0.5% fetal bovine serum, hydrocortisone (0.18 µg/ml), insulin (5 µg/ml), basic fibroblast growth factor (bFGF, 3 ng/ml), at 37°C in a humidified 5% CO2 atmosphere (Fig. 5).
Figure 4.
Cultured malignant A375 melanoma cells in vitro (×300).
Figure 5.
Primary cultured human melanocytes in vitro (×200).
Tissue specimens.
Healthy skin specimens were surgically taken from 20 patients aged less than 50 who underwent circumcision. Histological specimens of pigmented nevus were collected from 12 patients at Hua Shan Hospital, Fudan University; paraffin wax embedded tissues of six patients with MM were kindly provided by Tumor Hospital, Fudan University. All specimens were diagnosed clinically and pathologically.
Immunohistochemical staining.
The following primary antibodies were used: a monoclonal antibody specific to human PEDF (1/200 dilution; R&D, USA). Immunoperoxidase staining of formalin fixed, paraffin wax embedded tissue sections were performed using an ordinary biotin-streptavidin method. Briefly, sections were dewaxed, rehydrated in a descending alcohol series, heated in an 800 W microwave oven at maximal power for five minutes in 10 mM citric buffer (pH 6.0), and washed with phosphate buffered saline (PBS; pH 7.3). The sections were then immersed in 0.3% hydrogen peroxide in methanol for 20 minutes at room temperature to block endogenous peroxidase activity. After non-specific sites were blocked with 5% normal goat serum in PBS for one hour, the sections were incubated with anti-PEDF monoclonal antibodies overnight at 4°C. In subsequent steps, we used the Vectastain ABC kit using DAB as a chromogen (Changdao Biotech, Shanghai, China). The sections were then counterstained with haematoxylin. In every immunohistochemical staining, we performed additional staining without primary antibody in parallel as a negative control.
Western blotting.
Subconfluent A375 and melanocytes cells were washed three times with ice cold PBS, harvested by scraping, and homogenised in an appropriate amount of homo-genizing buffer (20 mM Hepes buffer containing 50 mmol/L Tris-cl (Ph 8.0), 0.02% NaN3, 100 mg/L PMSF, 150 mmol/L NaCl, 1 mg/L Aprotinin and 1% Triton X-100). The homogenates were centrifuged at 12,000 revolutions per minute (rpm) for two minutes at 4°C, and the supernatants were obtained. After determining the protein concentration by means of the BCA protein assay, an equal amount of protein (50 mg) from each sample was subjected to 10% sodium dodecyl sulfate poly-acrylamide gel electrophoresis and electrophoretically transferred to a nitrocellulose membrane. After blocking with 5% non-fat dry milk/PBS with 0.1% Tween 20 for one hour at room temperature, the membrane was incubated with anti-PEDF antibody (1/400 dilution) at 4°C overnight. After incubation with the secondary antibody (rabbit antimouse IgG absorbed horseradish peroxidase labelled (1/1,000 dilution, Zhongshan Biotech, Beijing, China) for one hour at room temperature, immuno-reactive bands were detected using the enhanced chemiluminescence western blotting analysis system. We also used glyceraldehyde-3-phosphatedehydrogenase (GAPDH) as the endogenous reference control. The manipulation above was repeated three times.
Reverse transcription-polymerase chain reaction.
Total RNA was extracted from cells by routine methods of Phenol-Chloroform. The quality of the RNA samples was qualified by electrophoresis through agarose gels and staining with ethidium bromide. The 18S and 28S RNA bands were visualized under ultraviolet light.
Total RNA (1 ug) was processed directly to cDNA by reverse transcription. The RT-PCR conditions were as follows. The RNA products were first placed in 70°C for 10 min, then moved to ice water immediately. All the reagents were put into an epperndorf tube in a total volume of 10 ul (2 ul 25 mM MgCl2, buffer, 0.5 ul 10 mM dNTPs, 0.5 ul RNAsin, 1 ul AMV reverse transcriptase, 1 ul Oligo dT, as well as the 1 ug RNA products as described above). All the reagents were incubated in 37°C for 10 min, 42°C for 60 min, 99°C for 5 min, 0–5°C (in ice water) for 3 min. The cDNA was then produced and reserved in −20°C.
Primers for PEDF and GAPDH were designed according to the references21 (Table 3) and synthesized by Ouyi Biotechnology Corporation, Shanghai, China, with the 5¢FAMreporter dye and the 3¢TAMRA quencher dye.
Table 3.
Primer sequence of PEDF and GAPDH
| Gene | Sequence | PCR product size |
| PEDF | upper primer 5′-AGGCCCAGAGTCCTGACGGG-3′ lower primer 5′-CCTTGAAGTGCGCCACACCG-3′ | 151 bp |
| GAPDH | upper primer 5′-GAAGGTGAAGGTCGGAGTCA-3′ lower primer 5′-GAAGATGGTGGTGATGGGATTTC-3′ | 226 bp |
With the production of cDNA, PCR was performed in the traditional manner. Each reaction contained 2 ul of each primer, 15 ul Taq Mix Buffer, as well as 5 ul RT products prepared above, in the total volume of 30 ul. Amplification was carried out for 36 cycles as follows: 94°C 30 s, 62°C 50 s (PEDF), 55°C (GAPDH), 72°C 1 min, after an initial denaturation step at 94°C for 5 min. An extension at 72°C for 10 min was used.
The PCR products were loaded onto an agarose gel for electrophoresis. The samples were run through the EB gels at 4 V/cm power for approximately one hour. The gel was dried and exposed to ultraviolet light for examination and photographed.
The manipulation above was also repeated for three times.
Statistical analysis.
Rank sum test was applied for comparison of PEDF expression among healthy skin group, pigmented nevus group and MM group. The relative expression of PEDF mRNA and protein between two groups was compared using the unpaired Student’s t-test. p values less than 0.05 were considered significant.
Acknowledgements
Funded by Shanghai Municipal Natural Science Foundation.
Footnotes
Previously published online as a Dermato-Endocrinology E-publication: http://www.landesbioscience.com/journals/dermatoendocrinology/article/7668
References
- 1.Tombran-Tink J, Chader GG, Johnson LV. PEDF: A pigment epithelium-derived factor with potent neuronal differentiative activity. Exp Eye Res. 1991;53:411–414. doi: 10.1016/0014-4835(91)90248-d. [DOI] [PubMed] [Google Scholar]
- 2.Becerra SP, Palmer I, Kumar A, Steele F, Shiloach J, Notario V, et al. Overexpression of fetal human pigment epithelium-derived factor in Escherichia coli. A functionally active neurotrophic factor. J Biol Chem. 1993;268:23148–23156. [PubMed] [Google Scholar]
- 3.Steele FR, Chader GJ, Johnson LV, Tombran-Tink J. Pigment epithelium-derived factor: Neurotrophic activity and identification as a member of the serine protease inhibitor gene family. Proc Natl Acad Sci USA. 1993;90:1526–1530. doi: 10.1073/pnas.90.4.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seigel GM, Tombran-Tink J, Becerra SP, Chader GJ, Diloreto DA, Jr, del Cerro C, et al. Differentiation of Y79 retinoblastoma cells with pigment epithelial-derived factor and interphotoreceptor matrix wash: Effects on tumorigenicity. Growth Factors. 1994;10:289–297. doi: 10.3109/08977199409010995. [DOI] [PubMed] [Google Scholar]
- 5.Steele FR, Chader GJ, Johnson LV, Tombran-Tink J. Pigment epithelium-derived factor: Neurotrophic activity and identification as a member of the serine protease inhibitor gene family. Proc Natl Acad Sci USA. 1993;90:1526–1530. doi: 10.1073/pnas.90.4.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tombran-Tink J, Pawar H, Swaroop A, Rodriguez I, Chader GJ. Localization of the gene for pigment epithelium-derived factor (PEDF) to chromosome 17p13.1 and expression in cultured human retinoblastoma cells. Genomics. 1994;19:266–272. doi: 10.1006/geno.1994.1057. [DOI] [PubMed] [Google Scholar]
- 7.Dawson DW, Volpert OV, Gillis P, Crawford SE, Xu H, Benedict W, et al. Pigment epithelium-derived factor: A potent inhibitor of angiogenesis. Science. 1999;285:245–248. doi: 10.1126/science.285.5425.245. [DOI] [PubMed] [Google Scholar]
- 8.Cheung LW, Au SC, Cheung AN, Ngan HY, Tombran-Tink J, Auersperg N, et al. Pigment epithelium-derived factor is estrogen sensitive and inhibits the growth of human ovarian cancer and ovarian surface epithelial cells. Endocrinology. 2006;147:4179–4191. doi: 10.1210/en.2006-0168. [DOI] [PubMed] [Google Scholar]
- 9.Abramson LP, Stellmach V, Doll JA, Cornwell M, Arensman RM, Crawford SE, et al. Wilms’ tumor growth is suppressed by anti-angiogenic pigment epithelium-derived factor in a xenograft model. J Pediatric Surg. 2003;38:336–342. doi: 10.1053/jpsu.2003.50104. [DOI] [PubMed] [Google Scholar]
- 10.Abramson LP, Browne M, Stellmach V, Doll J, Cornwell M, Reynolds M, et al. Pigment epithelium-derived factor targets endothelial and epithelial cells in Wilms’ tumor. J Pediatr Surg. 2006;41:1351–1356. doi: 10.1016/j.jpedsurg.2006.04.038. [DOI] [PubMed] [Google Scholar]
- 11.Zhang L, Chen J, Ke Y, Mansel RE, Jiang WG. Expression of pigment epithelial derived factor is reduced in non-small cell lung cancer and is linked to clinical outcome. Int J Mol Med. 2006;17:937–944. [PubMed] [Google Scholar]
- 12.Yang H, Xu Z, Iuvone PM, Grossniklaus HE. Angiostatin decreases cell migration and vascular endothelium growth factor (VEGF) to pigment epithelium derived factor (PEDF) RNA ratio in vitro and in a murine ocular melanoma model. Mol Vis. 2006;12:511–517. [PubMed] [Google Scholar]
- 13.Crawford SE, Stellmach V, Ranalli M, Huang X, Huang L, Volpert O, et al. Pigment epithelium- derived factor (PEDF) in neuroblastoma: A multifunctional mediator of Schwann cell antitumor activity. J Cell Sci. 2001;41:1351–1356. doi: 10.1242/jcs.114.24.4421. [DOI] [PubMed] [Google Scholar]
- 14.Ek ET, Dass CR, Choong PF. PEDF: A potential molecular therapeutic target with multiple anti-cancer activities. Trends in Molecular Medicine. 2006;12:497–502. doi: 10.1016/j.molmed.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 15.Li H, Tran VV, Hu Y, Mark Saltzman W, Barnstable CJ, Tombran-Tink J. A PEDF N-terminal peptide protects the retina from ischemic injury when delivered in PLGA nanospheres. Exp Eye Res. 2006;83:824–833. doi: 10.1016/j.exer.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 16.Yamagishi S, Matsui T, Nakamura K, Yoshida T, Shimizu K, Takegami Y, et al. Pigmentepithelium-derived factor (PEDF) inhibits angiotensin-II-induced vascular endothelial growth factor (VEGF) expression in MOLT-3 T cells through anti-oxidative properties. Microvasc Res. 2006;71:222–226. doi: 10.1016/j.mvr.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Garcia M, Fernandez-Garcia NI, Rivas V, Carretero M, Escamez MJ, Gonzalez-Martin A, et al. Inhibition of xenografted human melanoma growth and prevention of metastasis development by dual antiangiogenic/antitumor activities of pigment epithelium-derived factor. Cancer Res. 2004;64:5632–5642. doi: 10.1158/0008-5472.CAN-04-0230. [DOI] [PubMed] [Google Scholar]
- 18.Abe R, Shimizu T, Yamagishi S, Shibaki A, Amano S, Inagaki Y, et al. Overexpression of pigment epithelium-derived factor decreases angiogenesis and inhibits the growth of human malignant melanoma cells in vivo. Am J Pathol. 2004;164:1225–1232. doi: 10.1016/s0002-9440(10)63210-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takenaka K, Yamagishi S, Jinnouchi Y, Nakamura K, Matsui T, Imaizumi T. Pigment epithelium-derived factor (PEDF)-induced apoptosis and inhibition of vascular endothelial growth factor (VEGF) expression in MG63 human osteosarcoma cells. Life Sci. 2005;77:3231–3241. doi: 10.1016/j.lfs.2005.05.048. [DOI] [PubMed] [Google Scholar]
- 20.Abe R, Fujita Y, Yamagishi S. Angiogenesis and metastasis inhibitors for the treatment of malignant melanoma. Mini Rev Med Chem. 2007;7:649–661. doi: 10.2174/138955707780859440. [DOI] [PubMed] [Google Scholar]
- 21.Guan M, Yam HF, Su B, Chan KP, Pang CP, Liu WW, et al. Loss of pigment epithelium derived factor expression in glioma progression. J Clin Path. 2003;56:277–282. doi: 10.1136/jcp.56.4.277. [DOI] [PMC free article] [PubMed] [Google Scholar]