Abstract
Environmental pollutants can result in a variant of acne called ‘chloracne’. Chloracne is caused by systemic exposure to certain halogenated aromatic hydrocarbons ‘chloracnegens’, and is considered to be one of the most sensitive indicators of systemic poisoning by these compounds. Dioxin is the most potent environmental chloracnegen. Most cases of chloracne have resulted from occupational and non-occupational exposures, non-occupational chloracne mainly resulted from contaminated industrial wastes and contaminated food products. Non-inflammatory comedones and straw-colored cysts are the primary clinical manifestation of chloracne. Increasing of cysts in number is a signal of aggravation of chloracne. Generalized lesions can appear on the face, neck, trunk, exterimities, genitalia, axillary and other areas. Course of chloracne is chronic. Severity of chloracne is related to dosage of exposed chloracnegens, chloracnegenic potency and individual susceptibility. Histopathology of chloracne is characterized mainly by hyperplasia of epidermal cell, while follicular and sebaceous gland are taken placed by keratinized epidermal cell. The pathogenesis of chloracne maybe related to the imbalance of epidermal stem cell. Chloracne appears to be resistant to all tested forms of treatment. The only way to control chloracne is to prevent exposure to chloracnegens.
Key words: enviromental pollutant, chloracne, dioxin, acne vulgaris
Introduction
Althought technological progress has created great benefits for human being, at the same time a lot of industrial by-products and chemical wastes have been produced. Health of human being has been affected by these enviromental pollutants greatly.1 The skin is the largest organ in the human body and one of its main functions is to protect the body from noxious substances. Environmental pollutants are related to skin diseases such as contact dermatitis, chemical depigmentation and also chloracne.2 Acne vulgaris is generally considered to be a disorder of adolescence, but environmental pollutants can result in a variant of acne called chloracne, a typical enviromental skin disease, which is characterized by acnelike eruption of comedones (blackheads and whiteheads), cysts and pustules that occurs following systemic absorption of chemical ‘chloracnegens’.
Etiology of Chloracne
Chloracne results from environmental exposure to certain halogenated aromatic hydrocarbons and is considered to be one of the most sensitive indicators of systemic poisoning by these compounds. Chloracne was first described in 1887 by Von Bettman3 and by Herxheimer in 1889,4 who suggested that it was caused by chlorine exposure and hence called ‘chloracne’ based on the similarity of its clinical features with acne vulgaris. From then on, a number of chloracnegenic chemicals have been identified, such as chlorinated phenols, chloronaphthalenes, polychlorinated biphenyls (PCBs), and other polychlorinated compounds, which include polyhalogenated dibenzofurans, polychlorinated dibenzo-p-dioxins, and chlorinated azo- and azoxybenzenes.5 All chloracnegenic compounds are known to share certain structural features including molecular planarity and two benzene rings with halogen atoms occupying at least three of the lateral ring positions. The position of the halogen substitutions appears to be critical, as it is known that substitutions leading to molecular nonplanarity dramatically diminish chloracnegenic activity.2
Dioxin is a great family of halogenated aromatic hydrocarbons, which consists of tricyclic aromatic compounds, and hydrogen atoms can be substituted by up to eight chlorines, thus permitting about 75 isomers. Dioxins is ragarded to be class 1 carcinogen and endocrine disruptors, and also is the most potent environmental chloracnegen among chloracnegens. 2,3,7,8-Tetrachlorodibenzop-dioxin (TCDD) with four chlorine atoms in lateral positions is the most biologically active isomer, and is also the most toxic substances known6 (Fig. 1). Chloracne is the most consistent manifestation of dioxin intoxication, and considered to be its reliable “hallmarker.”7
Figure 1.
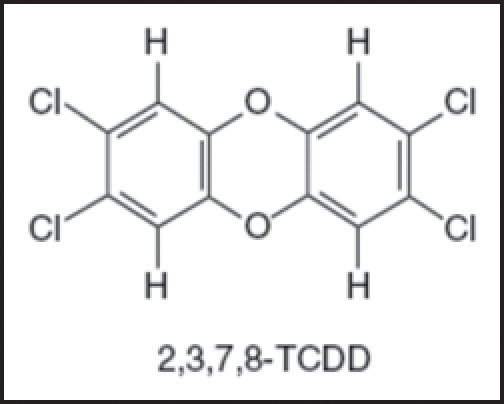
Chemical structure of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD).
Dioxins or other chloracnegens are absorbed into human body by direct contact, inhalation or ingestion. Generally the average concentration of TCDD in normal people is less than 10 ppt,8 while it is over several hundreds of ppt in patient with chloracne.9 Dioxin is stable and highly lipophilic, and its half-life in the human body is about 7∼11 years.10
Etiology of Chloracne
Most cases of chloracne have resulted from occupational and non-occupational exposures (environmental exposures). Non-occupational chloracne mainly resulted from contaminated industrial wastes, and contaminated food products.
In a study of 109 workers who had been engaged in the production of pentachlorophenol, chloracne among them had been noted since 1974. The prevalence of chloracne was 73.4% (80/109) in total and 95.2% (20/21) in a trichlorobenzene (TCB) tank area where dioxin and dibenzofurans levels were thousands of ppm.11 Another survey in 3,538 workers of a factory exposure to TCDD, 11% were found to have chloracne.12
In addition, a widely publicized example was the accident occurred at a chemical plant near Seveso of Italy in 1976. Two kilograms of TCDD were discharged into the atmosphere during an explosion and and 135 cases of chloracne were diagnosed among polluted 2,000 inhabitants (6.7%), most of them (88%) are children under 14-years-old, while healthy control is 0.1%.9 Another famous accident is the widespread ingestion of tainted rice cooking oil contaminated with PCBs in Yucheng in Taiwan, 17.5% have chloracne and healthy control is 1.3%;13 Examination of the skin in a group of 288 Vietnamese veterans with a remote (17–22 years) history of exposure to the herbicide known as Agent Orange revealed persistent chloracne lesions in 11.5% of these subjects.14
Clinical Features of Chloracne
Usually after exposure to chloracnegens, the first onset of chloracne is erythema or oedema on the face, then develop into non-inflammatory comedones and straw-colored cysts in a few days, occasionally pustules, non-infectious abscesses and scar can be seen.15
The distribution of the lesions of chloracne is characteristic. In the earlier time skin lesions appear on the face and neck, and later extend to trunk, exterimities, genitalia or other areas. Generally comedone appear more often on face and neck, especially the below and to the outer side of the eye (the so-called malar crescent) and in the postauricular triangles. The ear lobes, suboccipital hairline and groin are often involved. The cysts often appear on neck, shoulders, chest-back, penis, scrotum and axillary. The nose, perioral skin and supraorbital regions are usually spared. Increasing of cysts in number is a signal of aggravation of chloracne. Other skin lesions include decreased sebum secretion with skin xerosis, pigmentation, porphyrinopathy, hirsutism, skin thickening, palmoplantar hidrosis and palmoplantar hyperkeratotic. 15–17
Since chloracne is not only a skin disease but a systemic intoxical disease, so sometimes accompanied by other systemic symptoms such as fatigue, anorexia, neuropathy, impotence, dysfunction of liver function, hyperlipemia, anaemia, arthritis, thyromegaly and ophthalmitis.1
Chloracne is characterized by chronicity, which is because of that the chloracnegens are highly lipophilic and can remain in body fat for longer period. Normally from exposure to chloracnegens to the onset of clinical symptoms about 2–4 weeks, but after cessation of exposure it needs at least 2–3 yeas to recover, sometimes over 15–30 years.13,18 The severity of chloracne is related to intensity and duration of exposure, relative ‘chloracnegenic potency’ of the specific compound and individual susceptibility.7 Study showed that children (<8-years-old) and people with light hair color maybe more susceptible to the chloracnegens.8
Histopathology of Chloracne
Hambrick19 studied the histopathologic features of lesions of chloracne in different stages of evolution in human volunteers, and described that in early lesions of chloracne the epithelial cells of the outer root sheath forming the wall of the proximal portion of the infundibulum and the sebaceous duct appeared increased in number, resulting in dilatation of the proximal infundibulum. The walls of the sebaceous glands were thickened and merged with the infundibular wall. Hyperplasia of the epidermal cells, with incomplete keratinization, was manifested by a thick parakeratotic cell layer surrounding a mass of keratinized epidermal cells, which began the proximal filling of the infundibulum. In later stage lesions, almost all pilosebaceous units were involved. There was a definite diminution in the sebaceous glands, with reduced numbers of cells and reduced size of sebaceous lobules, which otherwise had a normal appearance. Still later stage lesions consisted of small comedones, with almost all vellus hair follicles showing thickening of the outer root sheath, dilated infundibula filled with sebum, and absence of sebaceous lobules. The maximum infundibular dilatation occurred proximally, resulting in either bottle-shaped formations, with the neck near the surface, or columnar funnels along the entire length of the infundibular structure.19–21
Chloracne is easy to be diagnosed by history of exposure to chloracnegens, characteristic clinical manifestations and histopathology, and high serum concentration of chloracnegens. Chloracne has acneiform comedo and cyst, but different from acne vulgaris in etiology, pathogenesis, clinical features, histopathology and treatment (Table 1).
Table 1.
Differential diagnosis of acne vulgaris and chloracne22
| Acne vulgaris | Chloracne | |
| Clinical features | ||
| Age group predilection site | Adolescence and early adulthood localized, including face, back chest | Any age group, predilected in children generalized, including retroauricular and malar, axillae, groin, extremities |
| Major lesions | Limited comedones, papules, pustules, cysts | Myriad comedones |
| Pathogenic factors | ||
| Inflammation lesions | Common | Very rare |
| Sebum production | Increased | Decreased |
| Microflora | Propionibacterium acnes and Propionibacterium granulosum | No bacteria |
| Androgen | Dependent | The role of androgens in chloracne is unknown |
| Histopathology | ||
| Sebaceous gland | Hypertrophic Atrophic; | Gradual replacement with keratinocytes |
| Sweat gland | Uninvoled | Palmoplantar hyperkeratotic lesions; acrosyringial plugging |
| Hair follicle | Thinning of the infundibular epithelial wall sebaceous gland duct; | General hyperplasia of the infundibulum and significant thickening of the upper follicle |
| Therapy | Effective under treatment of antibiotics, Resistant to therapy retinoids and other treatment |
Pathogenesis of Chloracne
The exact cellular and molecular pathogenesis of chloracne is still unclear. Since the major effect induced by chloracnegens in different skin structures is alteration of cellularity that results in prominent hyperplastic or hypoplastic responses. This indicates different epithelial structures in the skin respond to chloracnegens differentially: epidermis and infundibulum, which is characterized by epidermal-like type of differentiation undergo prominent hyperplasia, while sebaceous and sweat glands lose their secretory activity and are replaced by keratinizing cells.22
Earlier views ever thought that SGs are replaced by keratinized epithelial cells because SGs undergo squamous metaplasia and keratinization in patients with chloracne.23 Recently Panteleyev and Bickers22 produced a hypothesis to explain the pathogenesis of chloracne based on epidermal stem cell theory that chloracnegeninduced transformation of the pilosebaceous unit is driven by activation and accelerated exit of cells from the stem cell compartment coupled with a shift from a pilosebaceous differentiation pattern to an epidermal one. This may result in imbalance in early multipotent cells commitment and their preferential differentiation along an epidermal lineage with consequent diminution of SG and lower HF portion along with prominent epidermal/infundibular hyperplasia and hyperkeratinization. This process maybe controlled by a serious signal pathway of aryl hydrocarbon receptor(AhR), β-catenin, c-Myc, Indian Hedgehog (IHH) expressed in the cells.24,25
Treatment
Although some individual reports showed that retinoids, corticosteroid, dermabrasion and light electrodesiccation maybe useful in the treatment of chloracne, but actually chloracne appears to be resistant to all tested forms of treatment. So the only way to manage chloracne is to prevent exposure to chloracnegens.26 Once exposure happens, affected individuals should be removed from exposure sites and try to eliminate accumulated chloracnegens from the body. Recently a study found that a synthetic dietary fat substitute known as olestra can combine with chloracnegens and accelerate their fecal excretion,27 further study also showed that combined dietary olestra and caloric restriction causes a 30-fold increase in the rate of excretion of labelled compound,28 which maybe a perspective theray method in the future.
Footnotes
Previously published online as a Dermato-Endocrinology E-publication: http://www.landesbioscience.com/journals/dermatoendocrinology/article/7862
References
- 1.Pelclova D, Urban P, Preiss J, et al. Adverse health effects in humans exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) Environ Health. 2006;21:15–17. doi: 10.1515/reveh.2006.21.2.119. [DOI] [PubMed] [Google Scholar]
- 2.English JS, Dawe RS, Ferguson J. Environmental effects and skin disease. Br Med Bull. 2003;68:129–142. doi: 10.1093/bmb/ldg026. [DOI] [PubMed] [Google Scholar]
- 3.Bornemann W. Ueber die histologie der chloracne. Arch Derm Res. 1902;62:75–90. (Ger). [Google Scholar]
- 4.Herxheimer K. Uber chloracne. Munch Med Wochenschr. 1899;46:278. [Google Scholar]
- 5.Tindall JP. Chloracne and chloracnegens. J Am Acad Dermatol. 1985;13:539–558. doi: 10.1016/s0190-9622(85)70196-x. [DOI] [PubMed] [Google Scholar]
- 6.Schwetz BA, Norris JM, Sparschu GL, et al. Toxicology of chlorinated dibenzo-pdioxins. Environ Health Perspect. 1973;5:87–99. doi: 10.1289/ehp.730587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suskind RR. Chloracne, “the hallmark of dioxin intoxication”. Scand J Work Environ Health. 1985;11:165–171. doi: 10.5271/sjweh.2240. [DOI] [PubMed] [Google Scholar]
- 8.Link B, Gabrio T, Zoellner I, et al. Biomonitoring of persistent organochlorine pesticides, PCDD/PCDFs and dioxin-like PCBs in blood of children from South West Germany (Daded-Wuerttembergs) from 1993 to 2003. Chemosphere. 2005;58:1185–1201. doi: 10.1016/j.chemosphere.2004.09.061. [DOI] [PubMed] [Google Scholar]
- 9.Baccarelli A, Pesatori AC, Consonni , et al. Health status and plasma dioxin levels in chloracne cases 20 years after the Seveso, Italy accident. Br J Dermatol. 2005;152:459–465. doi: 10.1111/j.1365-2133.2005.06444.x. [DOI] [PubMed] [Google Scholar]
- 10.Wolfe WH, Michalek JE, Miner JC, et al. Determinants of TCDD half-life in veterans of operation ranch hand. J Toxicol Environ Health. 1994;41:481–488. doi: 10.1080/15287399409531858. [DOI] [PubMed] [Google Scholar]
- 11.Cheng WN, Coenraads PJ, Hao ZH, et al. A health survey of workers in the pentachlorophenol section of a chemical manufacturing plant. Am J Ind Med. 1993;24:81–92. doi: 10.1002/ajim.4700240108. [DOI] [PubMed] [Google Scholar]
- 12.Piacitelli L, Marlow D, Fingerhut M, et al. A retrospective job exposure matrix for estimating exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Am J Ind Med. 2000;38:28–39. doi: 10.1002/1097-0274(200007)38:1<28::aid-ajim4>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 13.Guo YL, Yu ML, Hsu CC, Rogan WJ. Chloracne, goiter, arthritis and anemia after polychlorinated biphenyl poisoning: 14-year follow-Up of the Taiwan Yucheng cohort. Environ Health Perspect. 1999;107:715–719. doi: 10.1289/ehp.99107715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Panteleyev AA, Roumak VS, Stepanova LV, Poznyakov SP, Bocharov BV. Clinical and ultrastructural characterization of human skin after exposure to dioxin-contaminated defoliants. Proceedings of Joint Russian-Vietnam Tropical Centre; Moscow. 1991. pp. 300–304. [Google Scholar]
- 15.Zugerman C. Chloracne. Clinical manifestations and etiology. Dermatol Clin. 1990;8:209–213. [PubMed] [Google Scholar]
- 16.Jensen NE. Chloracne: three cases. Proc R Soc Med. 1972;65:687–688. doi: 10.1177/003591577206500810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geusau A, Jurecka W, Nahavandi H, et al. Punctate keratoderma-like lesions on the palms and soles in a patient with chloracne: a new clinical manifestation of dioxin intoxication? Br J Dermatol. 2000;143:1067–1071. doi: 10.1046/j.1365-2133.2000.03846.x. [DOI] [PubMed] [Google Scholar]
- 18.Kerger BD, Leung HW, Scott P, et al. Age- and concentration-dependent elimination half-life of 2,3,7,8-tetrachlorodibenzo-p-dioxin in Seveso children. Environ Health Perspect. 2006;114:1596–1602. doi: 10.1289/ehp.8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hambrick GW. The effect of substituted naphthalenes on the pilosebaceous apparatus of rabbit and man. J Invest Dermatol. 1957;28:89–103. doi: 10.1038/jid.1957.11. [DOI] [PubMed] [Google Scholar]
- 20.Panteleyev AA, Thiel T, Rosenbach T, et al. Christiano Acne chlorina and acne vulgaris—casual likeness or causal homology? Arch Dermatol Res. 2000;292:577–581. doi: 10.1007/s004030000169. [DOI] [PubMed] [Google Scholar]
- 21.Pastor MA, Carrasco L, Izquierdo MJ, et al. Chloracne: histopathologic findings in one case. J Cutan Pathol. 2002;29:193–199. doi: 10.1034/j.1600-0560.2002.290401.x. [DOI] [PubMed] [Google Scholar]
- 22.Panteleyev AA, Bickers DR. Dioxin-induced chloracne—reconstructing the cellular and molecular mechanisms of a classic environmental disease. Exp Dermatol. 2006;15:705–730. doi: 10.1111/j.1600-0625.2006.00476.x. [DOI] [PubMed] [Google Scholar]
- 23.McConnell EE, Moore JA. Toxicopathology characteristics of the halogenated aromatics. Ann N Y Acad Sci. 1979;320:138–150. [PubMed] [Google Scholar]
- 24.Loertscher JA, Sattler CA, Allen-Hoffmann BL. 2,3,7,8-Tetrachlorodibenzo-p-dioxin alters the differentiation pattern of human keratinocytes in organotypic culture. Toxicol Appl Pharmacol. 2001;175:121–129. doi: 10.1006/taap.2001.9202. [DOI] [PubMed] [Google Scholar]
- 25.Huelsken J, Vogel R, Erdmann B, et al. beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105:533–545. doi: 10.1016/s0092-8674(01)00336-1. [DOI] [PubMed] [Google Scholar]
- 26.Gawkrodger DJ. Chloracne: causation, diagnosis and treatment. J Dermatol Treat. 1991;2:73–76. [Google Scholar]
- 27.Geusau A, Tschachler E, Meixner M, et al. Olestra increases faecal excretion of 2,3,7,8-tetrachlorodibenzo-p-dioxin. Lancet. 1999;354:1266–1267. doi: 10.1016/S0140-6736(99)04271-3. [DOI] [PubMed] [Google Scholar]
- 28.Redgrave TG, Wallace P, Jandacek RJ, Tso P. Treatment with a dietary fat substitute decreased Arochlor 1254 contamination in an obese diabetic male. J Nutr Biochem. 2005;16:383–384. doi: 10.1016/j.jnutbio.2004.12.014. [DOI] [PubMed] [Google Scholar]


