Abstract
A pilot study was conducted to compare lipid components of sebum from unaffected and acne-affected individuals. Nine males, 15–20 years old, with no acne, or with moderate to severe acne, were recruited. Facial images were taken with regular, polarized and fluorescent lights for each subject. Skin surface lipids were analyzed following collection of sebum using sebutapes. As expected, the subjects with acne had more (59%) sebum than the control subjects. Free fatty acids were the only lipid group that was reduced in the sebum of acne subjects. The specific lipid that differed the most between the two groups was squalene, which was upregulated in acne subjects by 2.2-fold on a quantitative basis. Squalene also represented a significantly greater proportion of the total sebaceous lipids in acne patients compared to controls (20% vs. 15%). The increase in the amount of squalene could represent a lipid marker for acne prone skin.
Key words: sebum, lipid, acne, fatty acid, squalene could represent a lipid marker for acne prone skin
Introduction
Acne is a skin disease affecting millions of young adults worldwide.1 Although its cause is multifactorial, changes in sebaceous gland functions including increased production of sebum is thought to be involved in the development of the disease.2 The sebaceous gland is considered nowadays to be an important endocrine organ embedded in the skin.3 Due to its close association with the hair follicle it forms the pilosebaceous unit of the skin. Sebum, a product of the sebaceous gland, is a mixture of lipids composed mainly of triglycerides (TG), wax esters (WE), squalene, free fatty acids (FFA) and smaller amounts of cholesterol, cholesterol esters (ChoE) and diglycerides.4–7 Sebum coats the hair and skin surface following a holocrine secretion into the infundibulum of the hair follicle.
Elevated sebum excretion is involved in the pathophysiology of acne,2,8,9 as body parts rich in sebaceous glands are the areas where acne lesions are manifested. While sebum is associated with acne in adolescence, it may also be of a benefit in lubricating the skin and contributing to a better skin barrier. Since acne is unique to humans it suggests that the unique sebaceous lipids might be associated with this human specific disease. The accumulation of squalene and the presence of unique fatty acids and lipids are unique manifestations of sebum.10,11 Our study aimed to identify possible lipid components within sebum, that could correlate with acne-prone skin their possible association with the molecular and cellular events involved in the generation of the acne lesion.
Several studies focused on dissecting the relationship between sebum output and the pathophysiology of acne. One of the very first studies focused on the measurements of the sebum secretion rate after an extensive depletion of previously secreted sebum with a bentonite clay and the subsequent use of specially designed disks. This study indicated that a high sebum secretion rate is the decisive factor in inflammatory acne.12 Moreover a recent study showed a correlation between higher sebum output and acne development.13
Most of the published studies assessed the total sebum output by instrumental analysis (sebumeter) or self-evaluation or by a sebum absorbent tape (Sebutape™). Sebutape™ is a specially designed tape that was proven to be reproducible and convenient to estimate sebaceous gland output.14 In addition Sebutape™ could also be used for collecting sebum for further quantitation of its components.14 Only few studies undertook the task to dissect the individual sebaceous lipids in search for specific lipids that could associate with the prevalence of acne. This is undoubtedly due to the cumbersome and laborious task that the individual extraction, separation and quantitation of lipids consists of. So far two studies have been reported and of course with a much smaller group size (n = 5 or 6).15,16 The only association found was lower levels of linoleic acid in acne patients and its displacement from sebaceous fatty acids in acylceramides of surface epidermal lipids.
In the current study we investigated differences in sebum composition between males, 15–20 years old, with no acne, or with severe acne. All of the healthy controls (no acne) subjects declared that they had acne 1–3 years ago. The scope of the study was to identify lipid markers between people with and without acne.
Results
Lipids, extracted from each sebutape, were subjected to thin layer chromatography (TLC), which separates the three major lipid classes: free fatty acids, triglycerides and wax and cholesterol esters. Each lipid class was later extracted separately from the silica and subsequently was subjected to saponification and derivatization in preparation for individual fatty acid analysis. This analysis was performed by gas chromatography and fluorescence ionization detection (GC-FID), resulting in estimates of the individual fatty acid population of each lipid class.
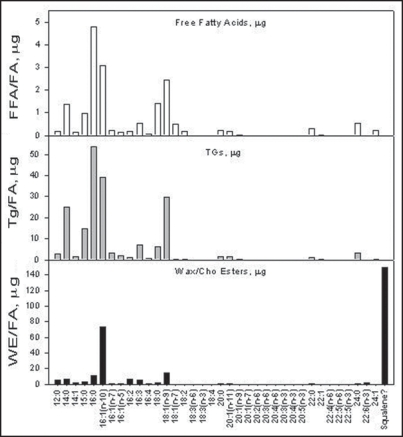
Figure 1 demonstrates a representative fatty acid profiles obtained from the three major lipid classes: FFA, TG and WE/ChoE. Interestingly, a predominant peak was identified in the WE/ChoE fraction of the sebum, that could not match the retention time of a known fatty acid standard. MS-MS analysis (data not shown) identified this peak as squalene. Apparently squalene runs on the top of the TLC profile, Figure 2, and it is co-extracted with the wax/cholesterol ester fraction. Subsequently it partitions with the derivatized mixture of the fatty acid esters that are injected into the GC-FID. This was further confirmed by spiking another fraction of the sample with a known concentration of squalene.
Figure 1.
Fatty acid profiles from lipid fractions. Micrograms of fatty acids obtained from GC-FID analysis. Squalene is co extracted with the WE/ChoE fraction.
Figure 2.
Fractionation of lipid classes and fatty acid analysis (FAA). Sebutapes were lipid extracted and the lipid extract was spotted on TLC where fractionation of the major lipid classes took place. The three major lipid fractions (FFA, TG, WE/ChoE) were subsequently prepared for GC-FID analysis.
Comparative analysis of fatty acids in sebum of subjects with and without acne did not yield statistical differences between the two groups. We therefore examined the total population of the lipids that constitute the major fraction of sebum.
Figure 3, summarizes the quantitative results in micrograms of lipid. It is apparent that only the total amount of lipid (micrograms, p = 0.002) and the amount of squalene (micrograms, p = 0.038) are increased in the sebum of acne subjects with a statistical significant difference. The subjects with acne had more sebum (59%) than the control subjects. The lipid that differed the most between the two groups was Squalene, which was upregulated in acne sebum by a 2.2-fold. The only class of lipids that was reduced in the sebum of acne subjects was the free fatty acids, which were suppressed more than 20%. However, this result did not demonstrate statistical significance. Note that in addition to the sum of the total lipid classes (FFA, TG, WE, ChoE, Squalene), the total amount of Sapienic acid was also included in Figure 3 (since it is the major fatty acid in human sebum). Squalene was subtracted from the total amount of the WE/ChoE fraction and was also graphed separately.
Figure 3.
Quantitative lipid analysis.
As seen in Figure 3, sebum from acne subjects contains 2.2-fold more squalene, 1.84-fold more triglycerides, 1.59-fold more sebum, 1.49-fold more sapienic acid, 1.33-fold more wax esters and reduced free fatty acid levels (0.79-fold) than the sebum of control subjects.
Qualitative analysis of sebum, Figure 4, also performed and demonstrated an increase in Squalene in subjects with acne (on a percent basis). In contrast, the free fatty acid fraction was reduced in the acne group. As seen in Figure 4 the relative composition of sebum in acne subjects has 34% more squalene, 19% more triglycerides, 16% less wax esters and 53% less free fatty acids compared to the control subjects.
Figure 4.
Qualitative lipid analysis.
Figure 5 shows a comparison of acne patients (15–20 years old) and age matched controls with control subjects of different age, males (25–35 years old) and females (40–50 years old). Although the overall amount of sebaceous lipid decreases with age the amount of squalene does not. The overall amount of squalene does not correlate with the total amount of sebum as the older male group has similar levels of squalene. However the amount of squalene changes significantly once the subjects have acne.
Figure 5.
Comparative analysis of acne affected and control groups of different age.
Discussion
In this study we analyzed the sebum of individuals affected and not affected by acne. We chose a young male population to ensure adequate levels of sebum and to avoid possible effects of the menstrual cycle on sebum output.
As expected, the subjects with acne secreted larger amounts of sebum than the control subjects (59%). The only class of lipids that was reduced in the acne subjects was free fatty acids. In contrast, triglycerides and wax/cholesterol esters were increased in the acne group. The specific lipid that differed the most between the two groups was squalene, 2.2-fold, which also represented a significantly greater proportion of the total sebaceous lipids in acne subjects compared to controls (20% versus 15% respectively).
Squalene is a precursor of cholesterol. Most mammalian cells synthesize cholesterol, which consists of an essential molecule for membrane fluidity and bilayer structure. Squalene as a long unsaturated hydrocarbon does not accumulate in high levels in most of the tissues where it is quickly converted to lanosterol and finally to cholesterol.10,17 The uniqueness in human sebum is that this non-polar hydrocarbon accumulates in unusually high levels (12–15%) compared to the levels of any other tissue or organ.6,7,10 On the other hand cholesterol accounts for less than 2% of the total sebaceous lipids.
Squalene, as a long and highly unsaturated hydrocarbon in nature, is a lubricant and has high penetration efficiency suggesting its potential roles in addition to cholesterol synthesis. Past reports demonstrated possible roles of squalene oxidation products in UV protection.18 However it is not known if squalene’s ability to scavenge free radicals is of a benefit or rather the opposite, since metabolites of this oxidation process can also cause irritation.19 These squalene oxidation products, together with unsaturated free fatty acids, have been reported to be comedogenic in experimental models.20,21 Moreover a recent study reported that peroxidated squalene induces the production of inflammatory mediators in HaCaT keratinocytes, which could support the involvement of lipid peroxides in establishing the inflammatory process in acne.22
Squalene could potentially alter the rheological properties of sebum, as it is the most non-polar molecule in sebum, but the most unsaturated as well. This molecule could serve as a lipid marker for acne prone skin, as it is far more upregulated than the rest of the lipids and the total sebum level. In contrast the sapienic acid, the major sebaceous fatty acid, is indicative of the respective total quantity of sebum. It is noteworthy to mention that unaffected males had similar levels of squalene regardless of age. However subjects with acne had 1.6-fold higher sebum but also 2.2-folds higher amount of squalene.
We performed a comparative analysis of lipid classes in young males with and without acne. We identified squalene to be significantly increased in acne patients, thus representing a lipid marker associated with acne. It would be of interest to perform a blind analysis of this marker in larger groups of subjects. It could retrospectively identify acne prone individuals in correlation with differential squalene secretion. Better analytical techniques would help to increase our understanding of squalene, its metabolites and their role in the induction or the maintenance of the acne lessions. The field of “sebum-omics” is open for many new discoveries and is constantly enhanced by advances in analytical techniques. This will eventually shed light on intriguing dermatological diseases such as acne. In conclusion, we documented both quantitative and qualitative differences in sebum of unaffected and acne affected individuals.
Materials and Methods
Nine males, 15–20-years old, with and without severe acne were recruited and inclusion into the study was based on a questionnaire. Photos with regular, polarized and fluorescent lights were recorded for each subject.
Sebutapes were applied for 45 min (to the forehead area above the eye brows) after degreasing with an isopropanol 70% impregnated swab. The sebutapes were subsequently extracted by using the Folch method.23 The extracted organic layer was dried under a gentle stream of nitrogen at 40°C. The dried samples were stored under nitrogen at −20°C until processed for TLC and GC-FID analysis according to a method established in the Mylnefield Lipid Analysis laboratories, Dundee, Scotland.24
In brief the sample (50 ml) was spotted on a glass TLC plate, which was developed with 80:20:2 iso-hexane/diethyl ether/formic acid until the solvent front is a short distance from the top. The plate was air dried and subsequently sprayed with 0.01% Primulin to visualize the FFA, TAG and CE/WE bands under UV light. Each marked band was removed from the glass TLC plate and was placed into a test tube where 1 ml toluene and 2 ml 1% sulphuric acid in methanol were added. The tubes were capped and were left overnight at 50°C. Afterwards 5 ml of 5% NaCl solution was added and the samples were extracted with 2 × 2 ml iso-hexane. The extracts were transfered to a fresh test tubes that were shaken with 3 ml of 2% potassium hydrogen carbonate and subsequently were pashed through a pre-washed (with 3 ml iso-hexane) sodium sulphate column and were post washed with 2 ml iso-hexane. The solvent was removed by nitrogen and iso-hexane + BHT (70 µl) were added before transferring them to a GC vial and injecting 5 µl from the sample. A C17:0 was used as an internal standard on a Cp-Wax 52CB (0.25 mm × 25 m × 0.2 µm; Chrompack, UK) column, flow rate 1 ml/min. The GC was Agilent 6890 and the GC was performed according to the method published by Christie.24
Statistical evaluation was performed using the student t-test.
Abbreviations
- FFA
free fatty acids
- WE
wax esters
- TG
triglycerides
- ChoE
cholesterol esters
- TLC
thin layer chromatography
- GC-FID
gas chromatography-flame ionization detection
Footnotes
Previously published online as a Dermato-Endocrinology E-publication: http://www.landesbioscience.com/journals/dermatoendocrinology/article/8473
References
- 1.Thiboutot DM. Overview of acne and its treatment. Cutis. 2008;81:3–7. [PubMed] [Google Scholar]
- 2.Zouboulis CC. Acne and sebaceous gland function. Clin Dermatol. 2004;22:360–366. doi: 10.1016/j.clindermatol.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Zouboulis CC, Baron JM, Böhm M, Kippenberger S, Kurzen H, Reichrath J, Thielitz A. Frontiers in sebaceous gland biology and pathology. Exp Dermatol. 2008;17:542–551. doi: 10.1111/j.1600-0625.2008.00725.x. [DOI] [PubMed] [Google Scholar]
- 4.Downing DT, Stewart ME, Strauss JS. Changes in sebum secretion and the sebaceous gland. Clin Geriatr Med. 1989;5:109–114. [PubMed] [Google Scholar]
- 5.Smith KR, Thiboutot DM. Sebaceous Gland Lipids: Friend or Foe? J Lipid Res. 2008;49:271–281. doi: 10.1194/jlr.R700015-JLR200. [DOI] [PubMed] [Google Scholar]
- 6.Stewart ME. Sebaceous glands lipids. Seminars in Dermatology. 1992;11:100–105. [PubMed] [Google Scholar]
- 7.Strauss JS, Downing DT, Ebling JF, Stewart ME. Goldsmith LA, Physiology, Biochemistry and Molecular Biology of the Skin. New York,: Oxford University Press, Inc; 1991. Sebaceous glands; pp. 712–740. [Google Scholar]
- 8.Cunliffe WJ. Acne. London: Martin Dunitz; 1989. [Google Scholar]
- 9.Thiboutot D. Regulation of human sebaceous glands. J Invest Dermatol. 2004;123:1–12. doi: 10.1111/j.1523-1747.2004.t01-2-.x. [DOI] [PubMed] [Google Scholar]
- 10.Nicolaides N. Skin lipids: their biochemical uniqueness. Science. 1974;186:19–26. doi: 10.1126/science.186.4158.19. [DOI] [PubMed] [Google Scholar]
- 11.Nicolaides N, Ansari MN. Fatty acids of unusual double-bond positions and chain lengths found in rat skin surface lipids. Lipids. 1968;3:403–410. doi: 10.1007/BF02531278. [DOI] [PubMed] [Google Scholar]
- 12.Harris HH, Downing DT, Stewart ME, Strauss JS. Sustainable rates of sebum secretion in acne patients and matched normal control subjects. J Am Acad Dermatol. 1983;8:200–203. doi: 10.1016/s0190-9622(83)70023-x. [DOI] [PubMed] [Google Scholar]
- 13.Mourelatos K, Eady EA, Cunliffe WJ, Clark SM, Cove JH. Temporal changes in sebum excretion and propionibacterial colonization in preadolescent children with and without acne. Br J Dermatol. 2007;156:22–31. doi: 10.1111/j.1365-2133.2006.07517.x. [DOI] [PubMed] [Google Scholar]
- 14.Nordstrom KM, Schmus HG, McGinley KJ, Leyden JJ. Measurement of sebum output using a lipid absorbent tape. J Invest Dermatol. 1986;87:260–263. doi: 10.1111/1523-1747.ep12696640. [DOI] [PubMed] [Google Scholar]
- 15.Perisho K, Wertz PW, Madison KC, Stewart ME, Downing DT. Fatty acids of acylceramides from comedones and from the skin surface of acne patients and control subjects. J Invest Dermatol. 1988;90:350–353. doi: 10.1111/1523-1747.ep12456327. [DOI] [PubMed] [Google Scholar]
- 16.Morello AM, Downing DT, Strauss JS. Octadecadienoic acids in the skin surface lipids of acne patients and normal subjects. J Invest Dermatol. 1976;66:319–323. doi: 10.1111/1523-1747.ep12482300. [DOI] [PubMed] [Google Scholar]
- 17.Elias PM, Feingold KR. Lipids and the epidermal water barrier: metabolism, regulation and pathophysiology. Semin Dermatol. 1992;11:176–182. [PubMed] [Google Scholar]
- 18.Ohsawa K, Watanabe T, Matsukawa R, Yoshimura Y, Imaeda K. The possible role of squalene and its peroxide of the sebum in the occurrence of sunburn and protection from the damage caused by U.V. irradiation. J Toxicol Sci. 1984;9:151–159. doi: 10.2131/jts.9.151. [DOI] [PubMed] [Google Scholar]
- 19.Chiba K, Yoshizawa K, Makino I, Kawakami K, Onoue M. Comedogenicity of squalene monohydroperoxide in the skin after topical application. J Toxicol Sci. 2000;25:77–83. doi: 10.2131/jts.25.77. [DOI] [PubMed] [Google Scholar]
- 20.Kligman AM, Wheatley VR, Mills OH. Comedogenicity of human sebum. Arch Dermatol. 1970;102:267–275. [PubMed] [Google Scholar]
- 21.Motoyoshi K. Enhanced comedo formation in rabbit ear skin by squalene and oleic acid peroxides. Br. J Derm. 1983;109:191–198. doi: 10.1111/j.1365-2133.1983.tb07080.x. [DOI] [PubMed] [Google Scholar]
- 22.Ottaviani M, Alestas T, Flori E, Mastrofrancesco A, Zouboulis CC, Picardo M. Peroxidated squalene induces the production of inflammatory mediators in HaCaT keratinocytes: a possible role in Acne vulgaris. J Invest Dermatol. 2006;126:2430–2437. doi: 10.1038/sj.jid.5700434. [DOI] [PubMed] [Google Scholar]
- 23.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 24.Christie WW. Gas Chromatography and Lipids: a practical guide The Oily Press Ltd. 1994:68. [Google Scholar]