Abstract
New antifungal agents are urgently required to combat life-threatening infections caused by opportunistic fungal pathogens like Candida albicans. The manipulation of endogenous fungal programmed cell death responses could provide a basis for future therapies. Here we assess the physiology of death in C. albicans in response to environmental stresses (acetic acid and hydrogen peroxide) and an antifungal agent (amphotericin B). Exposure of C. albicans to 40-60 mM acetic acid, 5-10 mM hydrogen peroxide, or 4-8 μg·ml-1 amphotericin B produced cellular changes reminiscent of mammalian apoptosis. Nonviable cells that excluded propidium iodide displayed the apoptotic marker phosphatidylserine (as shown by annexin-V-FITC labeling), were terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL)-positive (indicating nuclease-mediated double-strand DNA breakage), and produced reactive oxygen species. Ultrastructural changes in apoptotic cells included chromatin condensation and margination, separation of the nuclear envelope, and nuclear fragmentation. C. albicans cells treated at higher doses of these compounds showed cellular changes characteristic of necrosis. Necrotic cells displayed reduced TUNEL staining, a lack of surface phosphatidylserine, limited reactive oxygen species production, and an inability to exclude propidium iodide. Necrotic cells lacked defined nuclei and showed extensive intracellular vacuolization. Apoptosis in C. albicans was associated with an accumulation of cells in the G2/M phase of the cell cycle, and under some apoptosis-inducing conditions, significant proportions of yeast cells switched to hyphal growth before dying. This is a demonstration of apoptosis in a medically important fungal pathogen.
Candida albicans is the most prevalent systemic fungal pathogen of man and is associated with a range of clinical conditions ranging from irritating superficial infections of the oral and vaginal mucosa to life-threatening systemic disease in immunocompromised patients (1). Candidemia has been reported to be the fourth most common bloodstream infection of hospitalized patients (2), and the incidence of clinical infections in hospitals in the United Kingdom rose by >200% in the 10 years up to 2001 (3). Candida infections are often recalcitrant to therapy, and resistance to traditional therapies such as fluconazole and amphotericin is rising (4, 5). The development of more effective antifungal therapies is therefore of paramount importance. Understanding the mechanistic basis of cell death decisions in fungi may well provide new developments in the search for novel antifungal agents.
Many organisms display stereotypical patterns of cell death when responding to environmental insults or endogenous signals. In mammals, apoptosis is a physiological mode of cell death that plays a key role in normal development; the perturbation of apoptosis can lead to disease. Apoptotic cells are characterized by a specific series of morphological and biochemical properties that set apoptosis apart from accidental cell death or necrosis (6, 7). Programmed cell death responses have been seen in many higher eukaryotes and have now also been observed in lower eukaryotes and prokaryotes (8, 9).
Recent studies of the bakers' yeast, Saccharomyces cerevisiae, have revealed the existence of a programmed cell death response (reviewed in ref. 10). Programmed cell death has been observed after weak acid stress (11), oxidative stress (12, 13), salt stress (14), and UV irradiation (15). Physiological cell death has also been observed in aging yeast cells (16), after exposure to mating pheromone (17), and in a number of mutants such as CDC48 (18) and ASF1 (19). Cells dying under these conditions display several markers characteristic of apoptosis. These include the rapid exposure of phosphatidylserine (PS) at the outer cell membrane (revealed by annexin binding), the margination of chromatin in nuclei, nuclear fragmentation, and the degradation of DNA [revealed by terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) assays]. Exposure of cells to the protein translation inhibitor cycloheximide prevents these death-associated changes, indicating that the death response requires active protein synthesis.
The characterization of the molecular switches that regulate active death processes in C. albicans would facilitate the development of novel antifungal agents that switch on endogenous cell suicide mechanisms in pathogenic fungi. Here, we describe cellular changes that accompany death in C. albicans after exposure to a range of stimuli, including treatment with the antifungal agent amphotericin B (AMB). While characterizing death responses in C. albicans we have also found an unexpected connection between the commitment of yeast cells to die and the commitment of cells to undergo morphogenesis.
Materials and Methods
Organisms. The following C. albicans strains were used in this study: CaF-2 (URA3/ura3Δ::λimm434) was constructed by Fonzi and Irwin (20) from the clinical isolate SC5314; ΔCPH1, cph1Δ::hisG/cph1Δ::hisG-URA3-hisG ura3Δ::λimm434/ ura3-Δ::λimm434 (21); ΔEFG1, efg1Δ::hisG/efg1Δ::hisG-URA3hisG ura3Δ::λimm434/ura3Δ::λimm434 (22); ΔRIM101, rim101Δ::hisG /rim101Δ::hisG-URA3-hisG ura3Δ::λimm434/ura3Δ::λimm434 (23); and ΔTEC1, tec1Δ::hisG/tec1Δ::hisG-URA3hisG ura3-Δ::λimm434/ura3Δ::λimm434 (24).
Medium and Growth Conditions. C. albicans cells were grown in yeast extract/peptone/dextrose (YPD) broth (25). Typically, 100 ml of medium (in 500-ml Erlenmeyer flasks) was inoculated with Candida cells from fresh YPD plate cultures and incubated with shaking at 30°C until the cell density reached 5 × 106 cells per ml-1. Cells were then harvested, washed once with fresh media, and resuspended in 10 ml of treatment medium at 1 × 107 cells per ml-1. Acetic acid-treated cells were resuspended in YPD (pH 3.0) containing 0-120 mM acetic acid (BDH Laboratories, Poole, U.K.). Hydrogen peroxide-treated cells were resuspended in YPD containing 0-50 mM H2O2 (Sigma). Cells treated with AMB (Sigma) were resuspended in medium at 0-64 μg·ml-1 (1 mg·ml-1 stock in dimethyl sulfoxide). After a 200-min incubation at 30°C with shaking, cells were harvested and assessed for viability and cell death markers. All assays were carried out in triplicate on at least three different days.
Growth and Viability Assays. Growth assays were performed in 96-well plates at 30°C by using an iEMS (Labsystems, Chicago) plate reader (OD620). Viability was assessed by using clonogenic assays: cells at 1 × 107 cells per ml-1 were diluted in series and plated out in triplicate on YPD and then incubated at 30°C for 48 h.
Analysis of Apoptotic Markers. Protoplasts of C. albicans were stained with propidium iodide (PI) and FITC-labeled annexin-V by using the Annexin-V-FLUOS kit (Roche Applied Science) to assess cellular integrity and the externalization of PS. Cells were protoplasted by washing twice in PBS and incubating at 30°C for 30 min in 0.5 ml of 50 mM K2HPO4/5 mM EDTA/50 mM DTT (pH 7.2), then digested for 45 min at 30°C in 0.5 ml of 50 mM KH2PO4/40 mM 2-mercaptoethanol/3 μg·ml-1 chitinase (Sigma)/1.8 μg·ml-1 lyticase (stock 200-1,000 units·g-1)/12 μl β-glucuronidase/0.15 mg·ml-1 zymolyase/20 μl of glusulase in 2.4 M sorbitol (pH 7.2). SDS sensitivity of protoplasts was >99% in all experiments. Protoplasts were washed in modified annexin binding buffer (10 mM Hepes/NaOH, pH 7.4/40 mM NaCl/50 mM CaCl2/1.2 M sorbitol). Annexin-V binding assays were performed according to the protocol of Madeo et al. (12) in modified annexin binding buffer containing 20 μl·ml-1 annexin reagent and 20 μg·ml-1 PI. The annexin and PI status of a minimum of 100 protoplasts were recorded for each treatment, and each assay was repeated on at least three separate occasions.
For TUNEL assays, cells were washed twice in PBS and then fixed in 3.6% paraformaldehyde. Cells were stored at 4°C until required. Fixed cells were washed twice in PBS and protoplasted as described earlier. Cell permeabilization and the TUNEL reaction were carried out by using the In Situ Death Detection kit (Roche Applied Science) as described by Madeo et al. (12).
Intracellular reactive oxygen species (ROS) were assessed by spiking treated cells with dihydrorhodamine (DHR) 123 (5 μg/ml) 30 min before the end of each experiment. Cells were then harvested and examined by fluorescence microscopy or readings were taken of total fluorescence (FLUOstar OPTIMA microplate reader, BMG Laboratories, Offenburg, Germany; excitation 485 nm, emission 520 nm).
Electron Microscopy. Cells (108) were washed in phosphate/ magnesium buffer (40 mM K2HPO4/KH2PO4, pH 6.5/0.5 mM MgCl2) and fixed overnight in 2.5% glutaraldehyde in phosphate/magnesium buffer. Cells were washed twice for 15 min in 0.1 M sodium phosphate buffer (pH 6.0) and postfixed for 2 h in 2% osmium tetroxide. Cells were washed twice for 15 min in distilled water and then en bloc stained with 1% uranyl acetate (aqueous) for 30 min. After two further washes, cells were dehydrated in 95% and 100% ethanol. Cells were exposed to propylene oxide for 2 × 10 min and infiltrated for 1 h in 1:1 propylene/epoxy embedding material (Epon) mixture and then overnight in fresh Epon. After polymerization for 48 h at 60°C, ultrathin sections were cut and stained with uranyl acetate and lead citrate. Sections were examined with a Philips (Eindhoven, The Netherlands) 301 transmission electron microscope at 80 kV (magnification ×19,000).
Respiration Rates. Polarigraphic measurements of oxygen consumption were made of cells washed and resuspended at 107 cells per ml-1 in phosphate-buffered 50 mM glucose (pH 6.5). Oxygen consumption was assessed for triplicate 1-ml samples at 30°C by using an Oxygraph Clark-type oxygen electrode (Hansatech Instruments, Pentney King's Lynn, U.K.).
Single-Cell Death Assay. After treatment, cells were stained with PI at 20 μg·ml-1, washed once in water, and resuspended at 2.5 × 106 cells per ml-1. Cell suspensions were spread on synthetic complete medium (25) solidified with 2% agarose and 1 μg/ml PI. One hundred single cells from each treatment were micromanipulated onto a prescored grid (MSM, Singer Instruments, Somerset, U.K.). Cells were examined under a Zeiss Axioplan 2 epifluorescence microscope, and the cell cycle stage and PI status of each cell were recorded. Plates were then incubated at 30°C and reexamined by microscopy after 24 and 48 h for the formation of microcolonies, hyphae, and changes in the cell cycle status of nongrowing yeast cells.
Statistical Analysis. All experiments were performed in triplicate, and at least three independent experiments were done on separate occasions. Data comparing cell cycle distributions between treatments were analyzed for homogeneity by using standard contingency tests. The morphology and mortality of mutants were compared with the controls by using a two-tailed t test performed on arcsine-transformed data (26).
Results
Activities of Death-Inducing Agents Against C. albicans. Treatments required to kill C. albicans (CaF-2) were examined by assessing clonogenic survival. Minimum doses found to be fungicidal after 200-min exposure were 40 mM acetic acid (pH 3.0) and 2.5 mM hydrogen peroxide. Treatments at or above the minimum fungicidal dose were used in all subsequent experiments to examine the phenotypic characteristics of cell death.
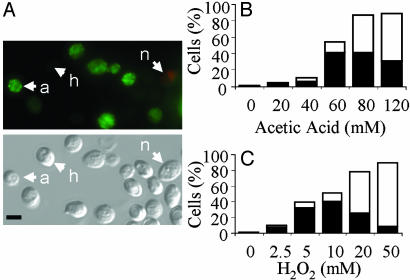
PS Exposure Examined in C. albicans by Using Annexin-V-FITC. PS is distributed asymmetrically in the lipid bilayer of the plasma membrane in yeasts and mammalian cells (27). Exposure of PS on the outer surface of the plasma membrane is an early marker of apoptosis in mammalian cells. Hence, we examined the extent of PS externalization in C. albicans by using the annexin-V-FITC assay. Protoplasts with peripheral, plasma membrane-associated annexin-FITC (green) labeling were detected in cells treated with fungicidal doses of acetic acid and hydrogen peroxide (Fig. 1A). The proportion of annexin(+) PI(-) protoplasts was greatest at the lower fungicidal doses (Fig. 1 B and C). Overall, the number of PI(+) (red) protoplasts increased with drug dose. Annexin(+) and PI(+) cells were only observed rarely in protoplasts obtained from untreated control cells.
Fig. 1.
Exposure of PS in C. albicans.(A) Fluorescence and DIC micrographs showing healthy (h) annexin(-) PI(-) protoplasts, apoptotic (a) annexin(+) PI(-) protoplasts, and necrotic (n) annexin(+) PI(+) protoplasts after treatment with acetic acid. (Scale bar: 5 μm.) (B and C) Percentage of cells that are classified as apoptotic [annexin(+) PI(-); black bars] and necrotic [annexin(+/-) PI(+); white bars] after treatment with acetic acid (B) or hydrogen peroxide (C).
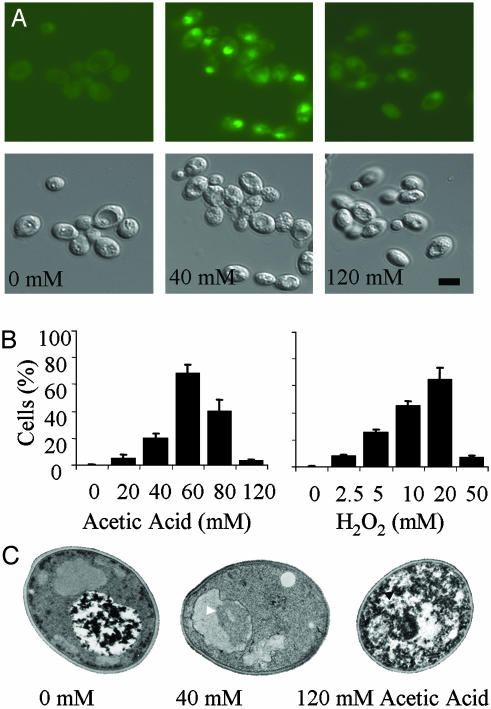
Nuclear Changes Associated with Cell Death in C. albicans. DNA damage was detected in cells exposed to fungicidal doses of acetic acid and hydrogen peroxide. By TUNEL assay (Fig. 2), the percentage of cells displaying double-strand DNA breakage was highest when cells were treated at 40 mM acetic acid and 5-10 mM hydrogen peroxide (Fig. 2B). Although staining was also apparent in some of the cells treated at the very highest doses, the staining was significantly less intense than that seen at the lower fungicidal doses. Staining was only rarely observed in untreated control cells.
Fig. 2.
DNA damage revealed by the TUNEL assay in C. albicans. (A) Fluorescence and DIC micrographs showing TUNEL(+) protoplasts and TUNEL(-) protoplasts after treatment with acetic acid. (Scale bar: 5 μm.) (B) Percentage of cells that contain damaged DNA as revealed by the TUNEL assay after treatment with acetic acid and hydrogen peroxide. (C) Transmission electron micrographs show morphological changes characteristic of apoptosis (chromatin condensation and margination, white arrows) and necrosis (organellar swelling and membrane disintegration, black arrows) in cells treated with different doses of acetic acid. (Scale bar: 5 μm.)
Treated cells were also examined by transmission electron microscopy (Fig. 2C). Untreated cells and cells treated with subinhibitory levels of acetic acid or hydrogen peroxide were indistinguishable. Cells treated at low fungicidal doses of these agents exhibited chromatin condensation and chromatin margination (58-68%), nuclear envelope separation, nuclear fragmentation (38% at 40 mM acetic acid), and limited vacuolization. On rare occasions, membrane blebbing was apparent at the junction of the plasma membrane and cell wall. Cells treated at high fungicidal doses of acetic acid (120 mM) displayed granular cytoplasm with little evidence of an organized internal structure, and nuclear bodies were only rarely observed (4% of cells). Intermediate doses were characterized by increasing levels of vacuolization and the presence of autophagic bodies. High doses of hydrogen peroxide (50 mM) appeared to be less destructive than high doses of acetic acid, causing less dramatic changes in cellular organization. Nevertheless, necrotic cells generated by both treatments were typically characterized by an overall increase in cytoplasmic granularity and a reduction in the number of cells with distinct organellar profiles.
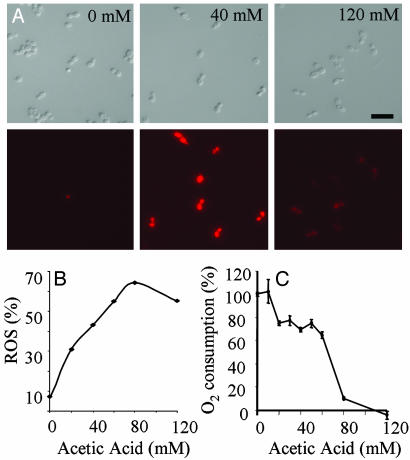
Dying Cells Produce Reactive Oxygen Species. The appearance of apoptotic markers in S. cerevisiae is accompanied by the production of ROS (10). ROS production can be monitored by using DHR123, which is oxidized to a fluorescent derivative by intracellular ROS. Cells treated with 40 mM acetic acid showed elevated DHR staining compared with the controls (Fig. 3 A and B). Oxygen consumption rates in apoptotic cells were 20% lower than the rates seen in the controls (Fig. 3C), whereas oxygen consumption in necrotic cells was negligible. Hydrogen peroxide-treated cells inevitably displayed elevated ROS levels and erratic oxygen consumption readings (not shown).
Fig. 3.
Apoptotic C. albicans cells produce ROS. (A) Fluorescence and DIC micrographs showing DHR123 staining in cells treated with acetic acid. (Scale bar: 5 μm.) (B) Percentage of cells that contain ROS revealed by DHR staining after treatment with acetic acid for 200 min at 30°C. (C) Oxygen consumption rates of cells treated with acetic acid.
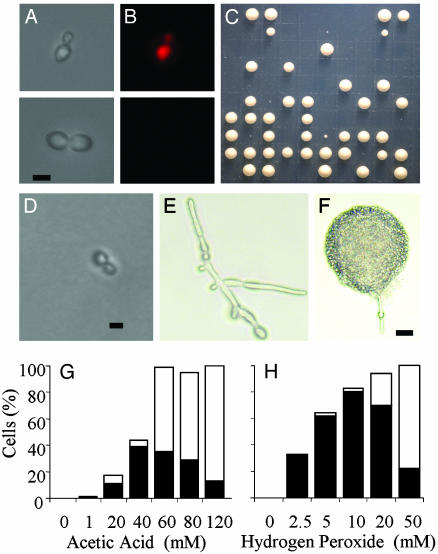
Single-Cell Analysis of Death Responses in C. albicans. Madeo et al. (12) and Ludovico et al. (11) demonstrated that apoptotic death in S. cerevisiae is attenuated by cycloheximide, indicating that apoptosis requires active protein synthesis. Analogous experiments were not possible in C. albicans because it is relatively resistant to cycloheximide (28). As an alternative, we used a single-cell screen that relies on the ability of metabolically active cells to exclude PI (Fig. 4). In mammals, the exclusion of PI due to the sustained activity of a glycine-sensitive anion death channel in the plasma membrane (29) is yet another characteristic of apoptosis. We therefore set out to determine whether individual C. albicans cells could exclude PI even when they were destined to die. After treatment with acetic acid and hydrogen peroxide, individual cells were scored for the ability to exclude PI, their cell cycle status, and the ability to form colonies. After incubation for 24-48 h at 30°C, the cell cycle status and morphology of nongrowing cells was reassessed (Fig. 4 B-F). The highest proportion of PI(-), nonviable cells (36%) occurred after treatment with 40 mM acetic acid (Fig. 4G). Higher doses of acetic acid increased the proportion of cells that were PI-positive. Similar patterns of PI staining and death were recorded for hydrogen peroxide-treated cells (Fig. 4H). The results of the screen correlated well with the findings of the earlier TUNEL and annexin assays (i.e., cell death at 40 mM acetic acid and 5 mM hydrogen peroxide was apoptotic, and necrosis predominated at higher doses).
Fig. 4.
Single-cell manipulations reveal treatments (40 mM acetic acid shown) that maximize the production of PI(-), nonviable cells (nominally apoptotic). (A and B) Representative micrographs showing individual PI(+) and PI(-) cells after micromanipulation onto a grid. (C) Plate showing colonies after single-cell manipulation. (D-F) Cells that failed to form a colony (D), switched to the production of hyphae and then arrested (E), or switched and then formed a colony (F). (Scale bars: D and E, 5 μm; F, 20 μm.) (G and H) Percentage of apoptotic PI(-), nongrowing cells (black bars) and the percentage of necrotic PI(+), nongrowing cells (white bars).
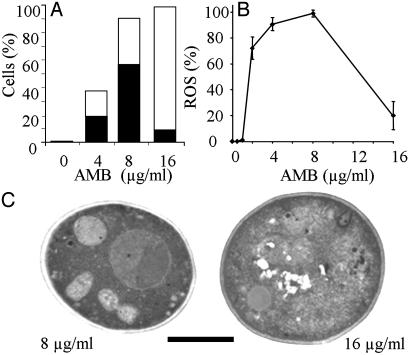
An Antifungal Drug Can Stimulate Apoptosis in C. albicans. AMB is commonly used for the treatment of systemic C. albicans infections (30). Using the single-cell assay, we assessed the death attributes of AMB-treated cells and then compared the results with other assays of cell death. A preliminary clonogenic assay curve revealed that 4 μg·ml-1 AMB or greater was fungicidal. Single-cell assays indicated that 8 μg·ml-1 produced the greatest numbers (57%) of PI(-), nonviable cells (Fig. 5A). Apoptotic changes induced by AMB were confirmed by electron microscopy and by the extensive production of ROS as measured by DHR staining (Fig. 5 B and C). Most cells were PI(+), nonviable at 16 μg·ml·1 AMB. Necrosis was confirmed by the observed ultrastructural changes and the reduced levels of ROS production. Together, these observations imply that low fungicidal doses of AMB are proapoptotic, killing C. albicans cells by inducing apoptosis.
Fig. 5.
C. albicans cells treated with AMB display apoptotic phenotypes. (A) Single-cell manipulation data showing the percentage of apoptotic PI(-), nongrowing cells (black bars) and the percentage of necrotic PI(+), nongrowing cells (white bars) for different doses of AMB. (B) Percentage of cells that contain ROS (revealed by DHR staining) after treatment with AMB. (C) Transmission electron micrographs reveal morphological changes characteristic of apoptosis and necrosis in C. albicans cells treated with AMB. (Scale bar: 5 μm.)
Cell Cycle Changes and Morphogenesis Associated with Dying Cells. All proapoptotic treatments were associated with G2/M phase cell cycle arrest [43% G2/M in the PI(-), nonviable subpopulation after 40 mM acetic acid treatment; t = 2.501, P ≤ 0.0140]. Cells exposed to pronecrotic treatments displayed cell cycle distributions indistinguishable from untreated control cells (28% G2/M at 120 mM acetic acid compared with 26% G2/M in the controls; t = 0.313, P ≤ 0.755). Proapoptotic doses of acetic acid and hydrogen peroxide induced cells to switch from yeast to hyphae in 5-11% and 40-60% of cells, respectively. In the majority of cases, the hyphae arrested and failed to develop into a colony. Hyphae were not seen at lower or higher doses of acetic acid (<20 mM or 120 mM) or hydrogen peroxide (<1 mM or 50 mM), or after treatment with AMB. After treatment with 40 mM acetic acid, mutants with defects in morphogenesis-related signaling (Δegf1, Δcph1, Δtec1, and Δrim101) were tested for their ability to switch in the single-cell assay. No decrease was observed in either the rate of switching or the mortality of the mutants when compared with the wild-type controls (Table 1).
Table 1. Mode of death and production of hyphae in morphological mutants of C. albicans after treatment with 40 mM acetic acid.
| Strain
|
||||||
|---|---|---|---|---|---|---|
| Character | Value | efg1 | cph1 | rim101 | tec1 | CaF-2 |
| Necrotic | Cells, % | 22 | 28 | 10 | 28 | 20 |
| t value† | 0.104 | 0.397 | 0.600 | 0.397 | 0.253 | |
| P ≤ | 0.918 | 0.692 | 0.550 | 0.692 | 0.800 | |
| Significance | NSD | NSD | NSD | NSD | NSD | |
| Apoptotic | Cells, % | 35 | 13 | 32 | 24 | 36 |
| t value | 0.021 | 1.096 | 0.151 | 0.514 | 0.064 | |
| P ≤ | 0.983 | 0.276 | 0.881 | 0.609 | 0.949 | |
| Significance | NSD | NSD | NSD | NSD | NSD | |
| Hyphae | Hyphae, % | 31 | 6 | 16 | 9 | 5 |
| t value | 3.841 | 0.789 | 2.248 | 1.298 | 0.521 | |
| P ≤ | 0.0002 | 0.432 | 0.027 | 0.197 | 0.603 | |
| Significance | *** | NSD | * | NSD | NSD | |
Data were compared against the population mean ± 1 SEM for the pooled control data collected for CaF-2 treated with 40 mM acetic acid (quadruplicate measures, each with n = 100). *, P<0.05; ***, P<0.001; NSD, not significantly different.
Critical tα(2),99 = 1.984 for P < 0.05; n = 100 for each mutant
Discussion
We have found that dying C. albicans cells exhibit many key markers of mammalian programmed cell death (apoptosis). After exposure to low fungicidal doses of acetic acid, hydrogen peroxide, and AMB, C. albicans displayed chromatin margination and condensation, nuclear envelope separation, and nuclear fragmentation. Furthermore, DNA damage exposing free 3′-OH groups was detected by using the TUNEL assay. Intracellular ROS levels were greatest after proapoptotic treatments. Finally, PS was exposed on the outer surface of the plasma membrane when cells were still metabolically active and able to exclude the vital dye PI. Apoptosis in microorganisms has only recently come to light (reviewed in refs. 8-10). The fungal pathogen C. albicans can now be added to the growing list of eukaryotic microbes known to display apoptosis.
The relationship between the regulation of the cell cycle and the induction of apoptosis in higher eukaryotes is complex and poorly understood. We found that all proapoptotic treatments in C. albicans lead to arrest at the G2/M phase of the cell cycle. Proapoptotic HIV-Vpr or adenoviral E4orf4 expression in S. cerevisiae also leads to G2/M phase arrest (31, 32), as does apoptosis induced by mutation of ASF1 (19). The G2/M phase of the cell cycle coincides with the DNA damage repair checkpoint (33). Treatments used to induce apoptosis in yeasts could damage DNA directly through the production of ROS, making G2/M arrest a coincident. Because apoptosis is also associated with the orchestrated degradation of nuclear DNA, accumulation of cells at the DNA-damage repair checkpoint might be essential. In S. cerevisiae, stimulation of protein kinase B (PKB) inhibits ROS-stimulated apoptosis, whereas inhibition of PKB promotes apoptosis (34). Because PKB levels normally increase during the G2/M phase of the cell cycle, it may be that apoptosis is independent of the cell cycle per se. Without further experimental evidence, the precise nature of the link between the cell cycle and the death response in C. albicans will remain unclear.
The observation that C. albicans cells treated with proapoptotic doses of acetic acid and hydrogen peroxide stimulated yeast to hypha switching was unexpected. Regulation of morphogenesis in C. albicans is complex, but to date all signals appear to converge on a small number of regulatory molecules, CPH1, TEC1, RIM101, and EFG1 (35, 36). The morphogenetic switches stimulated by acetic acid and hydrogen peroxide in our experiments were independent of these regulators; in fact, the RIM101 and EFG1 mutants were hyperfilamentous. The signaling pathways responsible for apoptosis and the morphogenetic switch must diverge at some point because switching was not observed in the AMB-treated cells. Despite the morphogenetic switch, most cells were still destined to die, even after transfer to more favorable conditions, but some did survive. It is conceivable that hyphal growth could provide an escape route for cells away from a toxic environment.
Weak acids are widely used as preservatives to prevent the growth of microorganisms on foodstuffs (37) and have also been used in the topical treatment of C. albicans infections (38). Although the sensitivity of C. albicans to acetic acid has been reported (39), this is, to our knowledge, the first report showing that acetic acid promotes apoptosis at low concentrations and necrosis at higher concentrations. Exposure to low doses of acetic acid has previously been shown to induce apoptosis in S. cerevisiae and Zygosaccharomyces bailii (11, 40). The mechanistic basis of weak-acid-induced cell death in C. albicans has not been characterized; but in common with many other yeast species, the intracellular pH remains constant over a wide range of weak-acid doses (41). Bracey et al. (42) found that the maintenance of a constant intracellular pH in the face of an influx of undissociated weak acid in S. cerevisiae is energetically expensive. An increase in the intracellular ADP/ATP ratio under such circumstances might be expected and is typical of mammalian cells undergoing apoptosis (43).
Oxidative stress responses in yeast have been well documented (reviewed in ref. 44), but this is, to our knowledge, the first report of the morphological changes associated with ROS-induced death in C. albicans. A major element in the host response to C. albicans infection is the initiation of a neutrophil attack (45, 46). Killing of the pathogen is mediated by the production of ROS, it is therefore possible that C. albicans cells die by apoptosis in the host. Surprisingly, cell suicide may be advantageous, because C. albicans is an opportunistic pathogen that may be best served in evolutionary terms if the host survives infection. Recently, it has been discovered that the malarial parasite Plasmodium bergei regulates its population size inside its mosquito vector by undergoing apoptosis (47). In the future, it may be pertinent to assess how C. albicans cells die in immunocompetent hosts not showing clinical symptoms of candidiasis.
Oxidative stresses in S. cerevisiae cause protein and lipid peroxidation, reduced mitochondrial enzyme activities, and direct DNA damage (44). Because the effects are pleiotropic, it is difficult to ascertain whether H2O2-induced apoptosis in C. albicans is initiated by a general damage response or by the alteration of a specific cellular component. Madeo et al. (12) and Narasimhan et al. (48) have suggested that ROS are intracellular messengers stimulating some unknown proapoptotic regulatory machinery in S. cerevisiae. ROS accumulation is required for the killing of C. albicans cells by histatin (49), so it would be of interest to determine whether the mechanism of death is apoptotic. Recent work on Aspergillus nidulans (50) indicates that intracellular ROS may be dispensable for fungal apoptosis. Intracellular ROS accumulation was apparent in every proapoptotic treatment we examined. However, we do not know whether it plays a causal role or is a secondary effect of the cellular changes associated with apoptotic death.
The primary mode of action of the antifungal drug AMB is well established. AMB binds to sterols, such as ergosterol, disrupting the osmotic integrity of the fungal membrane and resulting in the leakage of intracellular ions and metabolites. The physiological changes accompanying the death process are not well characterized. S. cerevisiae cells show a reduced intracellular pH and accumulate intracellular ROS (42) after treatment with AMB, providing some parallels with the mode of action of acetic acid and hydrogen peroxide. Liao et al. (51) examined physiological changes in C. albicans cells treated with AMB and described three patterns of death. Death was always accompanied by a drop in ATP level but could be subdivided on the basis of plasma membrane integrity and mitochondrial membrane potential. Nonviable cells that excluded SYBR green probably coincide with the apoptotic PI(-), nonviable cells we have identified. Examination of single cells revealed that apoptotic cells could not be resuscitated even after 48 h incubation on ”favorable” medium. Serum AMB levels used in the treatment of patients with C. albicans infections are typically 0.5-2.0 μg·ml-1 (52), close to the 4-8 μg·ml-1 AMB level that induced apoptosis in C. albicans. Normal treatment of systemic candidiasis may therefore reduce infection loads by initiating apoptosis of the pathogen.
The study of apoptosis in fungi presents new and exciting challenges. It is clear from BLAST searches of fungal genome databases that few regulatory molecules thought to be essential for mammalian apoptosis are present. Functional homologues of mammalian caspases have recently been identified in S. cerevisiae and A. nidulans and are thought to be involved with the apoptotic response (48, 53, 54). Differences in the architecture of the death-regulating machineries of mammals and fungi exist, making the chances of finding novel targets for antifungal therapy all of the more likely.
Acknowledgments
We thank Kevin Mackenzie for technical assistance. A.J.P. was supported by the Medical Research Council, I.S. was supported by a Society for General Microbiology Summer Fellowship, and M.R. was supported by the Lloyd's Tercentenary Foundation.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: AMB, amphotericin B; DHR, dihydrorhodamine; PI, propidium iodide; PS, phosphatidylserine; ROS, reactive oxygen species; TUNEL, terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling.
References
- 1.Odds, F. C. (1988) Candida and Candidosis (Baillière Tindall, London), 2nd Ed.
- 2.Banerjee, S. N., Emori, T. G., Culver, D. H., Gaynes, R. P., Jarvis, W. R., Horan, T., Edwards, J. R., Tolson, J., Henderson, T. & Martone, W. J. (1991) Am. J. Med. 91, 86S-89S. [DOI] [PubMed] [Google Scholar]
- 3.Lamagni, T. L., Evans, B. G., Shigematsu, M. & Johnson, E. M. (2001) Epidemiol. Infect. 126, 397-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pfaller, M. A. (1996) Clin. Infect. Dis. 22, S89-S94. [DOI] [PubMed] [Google Scholar]
- 5.Mah, T. F. & O'Toole, G. A. (2001) Trends Microbiol. 9, 34-39. [DOI] [PubMed] [Google Scholar]
- 6.Kerr, J. F. R., Wyllie, A. H. & Currie, A. R. (1972) Br. J. Cancer 26, 239-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wyllie, A. H., Kerr, J. F. R. & Currie, A. R. (1980) Int. Rev. Cytol. 68, 251-306. [DOI] [PubMed] [Google Scholar]
- 8.Ameisen, J. C. (1996) Science 272, 1278-1279. [DOI] [PubMed] [Google Scholar]
- 9.Cornillon, S., Foa, C., Davoust, J., Buonavista, N., Gross, J. D. & Golstein, P. (1994) J. Cell Sci. 107, 2691-2704. [DOI] [PubMed] [Google Scholar]
- 10.Madeo, F., Engelhardt, S., Herker, E., Lehmann, N., Maldener, C., Proksch, A., Wissing, S. & Fröhlich, K.-U. (2002a) Curr. Genet. 41, 208-216. [DOI] [PubMed] [Google Scholar]
- 11.Ludovico, P., Sousa, M. J., Silva, M. T., Leão, C. & Côrte-Real, M. (2001) Microbiology 147, 2409-2415. [DOI] [PubMed] [Google Scholar]
- 12.Madeo, F., Fröhlich, E., Ligr, M., Grey, M., Sigrist, S. J., Wolf, D. H. & Fröhlich, K.-U. (1999) J. Cell Biol. 145, 757-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fröhlich, K.-U. & Madeo, F. (2000) FEBS Lett. 473, 6-9. [DOI] [PubMed] [Google Scholar]
- 14.Huh, G. H., Damsz, B., Matsumoto, T. K., Reddy, M. P., Rus, A. M., Ibeas, J. I., Narasimhan, M. L., Bressan, R. A. & Hasegawa, P. M. (2002) Plant J. 29, 649-659. [DOI] [PubMed] [Google Scholar]
- 15.Carratore, R. D., Della Croce, C., Simili, M., Taccini, E., Scavuzzo, M. & Sbrana, S. (2002) Mutat. Res. 513, 183-191. [DOI] [PubMed] [Google Scholar]
- 16.Laun, P., Pichova, A., Madeo, F., Fuchs, J., Ellinger, A., Kohlwein, S., Dawes, I., Fröhlich, K.-U. & Breitenbach, M. (2001) Mol. Microbiol. 39, 1166-1173. [PubMed] [Google Scholar]
- 17.Severin, F. F. & Hyman, A. A. (2002) Curr. Biol. 12, R233-R235. [DOI] [PubMed] [Google Scholar]
- 18.Madeo, F., Fröhlich, E. & Fröhlich, K.-U. (1997) J. Cell Biol. 139, 729-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamaki, M., Umehara, T., Chimura, T. & Horikoshi, M. (2001) Genes Cells 6, 1043-1054. [DOI] [PubMed] [Google Scholar]
- 20.Fonzi, W. A. & Irwin, M. Y. (1993) Genetics 134, 717-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu, H., Kohler, J. R. & Fink, G. R. (1994) Science 266, 1723-1726. [DOI] [PubMed] [Google Scholar]
- 22.Lo, H. J., Kohler, J. R., Di Domenico, B., Loebenberg, D., Cacciapuoti, A. & Fink, G. R. (1997) Cell 90, 939-949. [DOI] [PubMed] [Google Scholar]
- 23.Ramon, A. M., Porta, A. & Fonzi, W. A. (1999) J. Bacteriol. 181, 7524-7530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schweizer, A., Rupp, S., Taylor, B. N., Rollinghoff, M. & Schroppel, K. (2000) Mol. Microbiol. 38, 435-445. [DOI] [PubMed] [Google Scholar]
- 25.Guthrie, C. & Fink, G. R. (1991) Guide to Yeast Genetics and Molecular Biology (Academic, San Diego), pp. 3-21.
- 26.Zar, J. H. (1996) Biostatistical Analysis (Prentice-Hall, Upper Saddle River, NJ), 3rd Ed., pp. 1-662.
- 27.Cerbón, J. & Calderón, V. (1991) Biochim. Biophys. Acta 1067, 139-144. [DOI] [PubMed] [Google Scholar]
- 28.Yamada-Okabe, T. & Yamada-Okabe, H. (2002) FEMS Microbiol. Lett. 212, 15-21. [DOI] [PubMed] [Google Scholar]
- 29.Nishimura, Y. & Lemasters, J. J. (2001) Cell Death Differ. 8, 850-858. [DOI] [PubMed] [Google Scholar]
- 30.Filler, S. G. & Kullberg, B. J. (2002) in Candida and Candidiasis, ed. Calderone, R. A. (Am. Soc. Microbiol., Washington, DC), pp. 341-348.
- 31.Kornitzer, D., Sharf, R. & Kleinberger, T. (2001) J. Cell Biol. 154, 331-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elder, R. T., Yu, M., Chen, M., Zhu, X., Yanagida, M. & Zhao, Y. (2001) Virology 287, 359-370. [DOI] [PubMed] [Google Scholar]
- 33.Rhind, P. & Russell, P. (1998) Curr. Opin. Cell Biol. 10, 749-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeon, B. W., Kim, K. T., Chang, S. I. & Kim, H. Y. (2002) J. Biochem. 131, 693-699. [DOI] [PubMed] [Google Scholar]
- 35.Liu, H. (2001) Curr. Opin. Microbiol. 4, 728-735. [DOI] [PubMed] [Google Scholar]
- 36.Sohn, K., Urban, C., Brunner, H. & Rupp, S. (2003) Mol. Microbiol. 47, 89-102. [DOI] [PubMed] [Google Scholar]
- 37.Fleet, G. (1992) Crit. Rev. Biotechnol. 12, 1-44. [DOI] [PubMed] [Google Scholar]
- 38.Jain, S. K. & Agrawal, S. C. (1994) Mycoses 37, 299-301. [DOI] [PubMed] [Google Scholar]
- 39.Shimokawa, O. & Nakayama, H. (1999) Antimicrob. Agents Chemother. 43, 100-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ludovico, P., Sansonetty, F., Silva, M. T. & Côrte-Real, M. (2003) FEMS Yeast Res. 3, 91-96. [DOI] [PubMed] [Google Scholar]
- 41.Stewart, E. (1988) Ph.D. dissertation (Univ. of Aberdeen, Aberdeen, Scotland), pp. 1-137.
- 42.Bracey, D., Holyoak, C. D. & Coote, P. J. (1998) J. Appl. Microbiol. 85, 1056-1066. [DOI] [PubMed] [Google Scholar]
- 43.Bradbury, D. A., Simmons, T. D., Slater, K. J. & Crouch, S. P. M. (2000) J. Immunol. Methods 240, 79-92. [DOI] [PubMed] [Google Scholar]
- 44.Costa, V. & Moradas-Ferreira, P. (2001) Mol. Aspects Med. 22, 217-246. [DOI] [PubMed] [Google Scholar]
- 45.Romani, L., Menacci, A., Cenci, E., Puccetti, P. & Bistoni, F. (1996) Res. Immunol. 147, 512-518. [DOI] [PubMed] [Google Scholar]
- 46.Peltroche-Llacsahuanga, H., Schnitzer, N., Schmidt, S., Tintelnot, K., Lutticken, R. & Haase, G. (2000) FEMS Microbiol. Lett. 191, 151-155. [DOI] [PubMed] [Google Scholar]
- 47.Al-Olayan, E. M., Williams, G. T. & Hurd, H. (2003) Int. J. Parasitol. 32, 1133-1143. [DOI] [PubMed] [Google Scholar]
- 48.Narasimhan, M. L., Damsz, B., Coca, M. A., Ibeas, J. I., Yun, D. J., Pardo, J. M., Hasegawa, P. M. & Bressan, R. A. (2001) Mol. Cell 8, 921-930. [DOI] [PubMed] [Google Scholar]
- 49.Helmerhorst, E. J., Troxler, R. F. & Oppenheim, F. G. (2001) Proc. Natl. Acad. Sci. USA 98, 14637-14642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheng, J., Park, T.-S., Chio, L.-C., Fiscl, A. S. & Yi, X. (2003) Mol. Cell. Biol. 23, 163-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liao, R. S., Rennie, R. P. & Talbot, J. A. (1999) Antimicrob. Agents Chemother. 43, 1034-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bennett, J. (1990) in Principles and Practice of Infectious Diseases, eds. Mandell, G. L., Douglas, R. G. & Bennett, J. E. (Churchill Livingstone, New York), pp. 404-406.
- 53.Madeo, F., Herker, E., Maldener, C., Wissing, S., Lächelt, S., Herlan, M., Fehr, M., Lauber, K., Sigrist, S. J., Wesselborg, S. & Fröhlich, K.-U. (2002) Mol. Cell 9, 911-917. [DOI] [PubMed] [Google Scholar]
- 54.Uren, G. A., O'Rourke, K., Aravind, L., Pisabarro, T. M., Seshagiri, S., Koonin, V. E. & Dixit, M. V. (2000) Mol. Cell 6, 961-967. [DOI] [PubMed] [Google Scholar]