Abstract
Background:
Central or peripheral stress may induce the development of clinical inflammation in the pilosebaceous unit (PSU) leading to the development or to exacerbation of preexisting acne. The presence of a complete corticotropin-releasing hormone (CRH) system has been confirmed in human sebocytes in vitro. CRH is capable to induce lipid synthesis, steroidogenesis and interact with testosterone and growth hormone. α-Melanocyte-stimulating hormone (α-MSH) and its receptors can regulate melanogenesis as well as affect inflammation, apoptosis and sebogenesis.
Objectives:
The purpose of the study was to investigate by immunohistochemistry if changes of CRH/CRH-binding protein (CRHBP)/CRH receptors (CRHR) as well as melanocortin-1 receptor (MC-1R) expression are detectable in acne lesions vs. normal skin, especially in the sebaceous gland (SG).
Results:
Very strong expression of CRH was observed in acne-involved skin in SG cells comparing with weaker expression in non-involved and normal skin SG. The strongest reaction for CRHBP in acne-involved SG was in differentiating sebocytes. CRHR-1 and -2 exhibited the strongest expression in sweat glands and SG, respectively. Sebocytes and cells of the ductus seboglandularis (DSG) of acne-involved and non-involved skin showed very intense MC-1R expression in contrast to less intense scattered immunoreactivity in normal skin samples.
Methods:
33 patients with acne vulgaris and 8 age-matched volunteers without acne participated in the study. Skin biopsies were taken from acne-involved face, the non-involved thigh skin of the same patients and from normal human skin.
Conclusions:
These data suggest that NP, such as the complete CRH system and MC-1R, are involved in the pathogenesis of acne.
Key words: neuropeptides, corticotropin releasing hormone, melanocortin receptor, sebaceous gland, acne
Introduction
Along with eczema and psoriasis, acne vulgaris is one of the most commonly seen chronic inflammatory skin diseases. The classical aspects of multifactorial acne pathogenesis encompasses abnormal follicular differentiation and increased cornification, androgen-mediated enhanced sebaceous gland (SG) activity and seborrhea, bacterial hypercolonization, inflammation and immunological host reaction like being the major contributors.1,2 Acne patients are not endocrine misfits. The SG is the organ conferring on the skin an independent peripheral endocrine function.3 While androgens have a proliferative effect on cultured human sebocytes, they have only a minimal effect on differentiation of sebocytes in culture, and this effect pales beside that of peroxisome proliferator-activated receptors agonists. Insulin growth factor and insulin, glucocorticoids, estrogen and thyroid hormone play roles in pilosebaceous unit (PSU) growth and development, but their exact roles remain to be elucidated.4–7
Increased sebum production may propagate an inflammatory tissue response of the PSU involving the release of proinflammatory cytokines including interleukin (IL)-1α, IL-1β and tumor necrosis factor-α8,9 as well as pro-inflammatory mediators, such as leukotrienes and prostaglandins.10
Psychoemotional stress, which may induce the development of clinical inflammation in the PSU is one of recently confirmed pathogenetic aspect in acne vulgaris.11,12 Activation of the hypothalamic-pituitary-adrenal (HPA) axis with release of stress neuropeptides (NP) is essential for biological homeostasis and responses to external and internal challenges.13,14 It has been confirmed that facial skin from acne patients is characterized by increased numbers of substance P-containing nerves and mast cells, and by strong expression of neutral endopeptidase in SG compared with normal skin.15 SG expresses receptors for β-endorphin, corticotrophin-releasing factor (CRH), urocortin, propiomelanocortin, vasoactive intestinal polypeptide, neuropeptide Y, calcitonin gene-related peptide. After ligand binding, NP receptors modulate the production of inflammatory cytokines, proliferation, differentiation, lipogenesis and androgen metabolism in sebocytes. By means of their autocrine, paracrine and endocrine actions, these neuroendocrine factors appear to mediate centrally and topically induced stress towards the SG, ultimately affecting the clinical course of acne.12–18
CRH, its binding protein (CRHBP) and CRH receptors (CRHR) act as a central regulatory system of the HPA axis.19 CRH is a 41-amino acid polypeptide.20 The biological effect of CRH and related peptides involves interactions with membrane-bound CRH receptors CRHR-1 and CRHR-2 and can be modified by CRHBP on the central, local or systemic levels.21,22 CRH/CRHR system can be involved in the clinical development of acne.23 CRH is an important autocrine hormone in sebocytes with a homeostatic pro-differentiation activity. It directly, independently from the HPA axis, induces lipid synthesis and steroidogenesis.12,23–25 The presence of a complete CRH/CRH-receptor system in human sebocytes has also been confirmed.23
Alpha-melanocyte stimulating hormone (α-MSH), was evaluated not only as a sebotropin and pigmentation hormone but also as a modulator of inflammatory and immune tissue responses within the PSU.26–28 The effects of α-MSH is mediated via binding to MC receptors (MC-R) on the cell surface of target cells. These receptors belong to the superfamily of G-protein coupled receptors.29 Recently, the presence of MC-Rs in human sebocyte cultures established from facial skin as well as in the immortalized human sebocyte cell line SZ95 was reported.26,30 In SZ95 sebocytes, α-MSH partially abrogated the inductive effect of IL-1β on the secretion of IL-8, an important chemokine directing neutrophils to sites of inflammation including the SG.26
In comparison with a variety of immunohistochemical investigations in case of other inflammatory skin diseases, there are only few studies in the field of acne. We have evaluated the expression of CRH system and MC-1R in SG of acne-involved facial skin, in comparison with the non-involved skin of the same patients with acne and with normal human skin suggesting that CRH system and MC-1R are involved in acne pathogenesis.
Results
CRH/CRHR system.
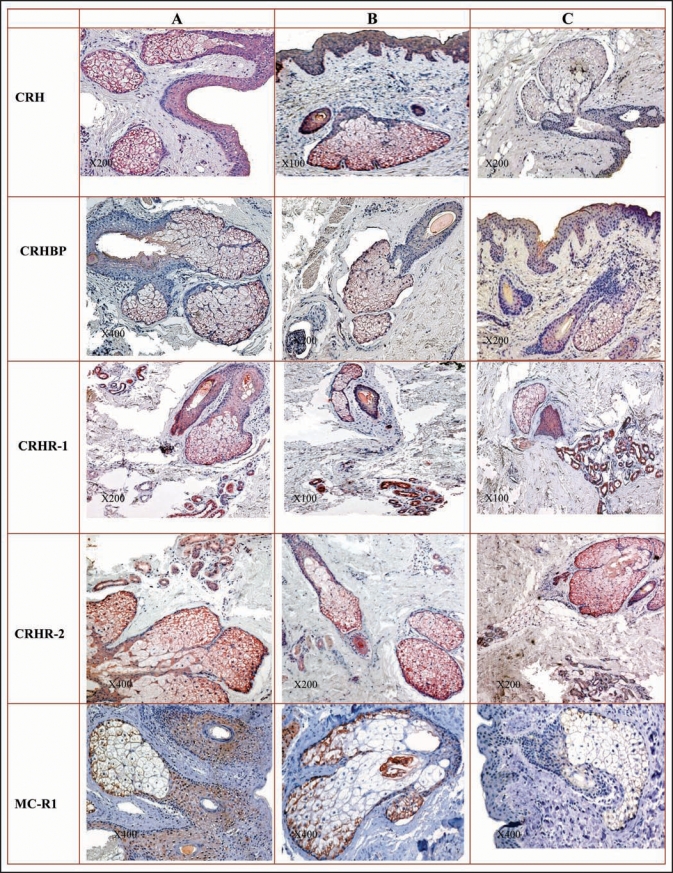
In acne-involved skin, sebocytes in all differentiation stages showed very strong positive reaction for CRH. Keratinocytes of ductus seboglandulares exhibited strong CRH positivity. The staining within SGs in acne non-involved and normal skin was dependent on the sebocyte differentiating stage. While basal and differentiating sebocytes demonstrated a strong immunoreaction to CRH, mature sebocytes and ductal keratinocytes revealed a weak and apoptotic sebocytes a barely discernible staining. Differentiating sebocytes showed the most distinct reaction for CRH binding protein (CRHBP) within SG in acne-involved skin. Keratinocytes of ductus seboglandulares showed no or a very week, homogeneous staining. SGs of acne-uninvolved and normal skin were stained homogeneously for CRHBP. When comparing CRHBP staining in differentiating sebaceous cells, acne-involved skin showed a stronger signal than acneuninvolved and normal skin. Eccrine sweat glands revealed for CRHR-1 a strong homogeneous immunostaining, which represented the most intense labeling in all sample groups. The most intensive very strong CRHR-2 expression was detected within SG in all sample groups (Fig. 1).
Figure 1.
(See previous page). Detection of CRH system (CRH, CRHBP, CRHR-1, CRHR-2) and MC-R1 in the different compartments of the human skin. (A) acne-involved skin; (B) uninvolved skin of acne patients (C) skin of healthy controls. Immunohistochemistry was performed as described in Materials and Methods. Mayer’s hematoxylin. CRH: All types of sebocytes and keratinocytes of ductus seboglandularis in acne skin show very intensive immunoexpression (A); significant weaker and dependent upon sebocytes differentiation stage immunoreaction of sebaceous gland in acne-uninvolved skin of acne patients (B) and normal skin (C). CRHBP: The most distinct and stronger than in acne-uninvolved and normal skin reaction of differentiating sebocytes, moderate to weak staining of basal and mature sebocytes and faint reaction of apoptotic sebocytes within sebaceous gland in acne-involved skin; no or very weak homogeneous staining of keratinocytes of ductus seboglandularis in acne-involved skin (A). Independent from sebocyte differentiation stage, homogeneous staining of sebaceous gland of acne-uninvolved (B) and normal skin (C). CRHR-1: Eccrine sweat glands exhibit the most intense labeling in all sample groups (A–C); the as very strong and strong assessed immunostaining within basal and differentiating sebocytes in acne-involved skin is weaker that the one detected in eccrine glands (A); strong reaction within mature sebocytes in acne-involved skin (A) and a weak reaction in acne non-involved (B) and normal (C) skin; barely discernible or negative staining of apoptotic sebocytes in all groups (A–C). CRHR-2: All sebocytes, except apoptotic ones reveal very strong immunoreaction in all sample groups (A–C); the faintest positive immunoexpression in sweat glands among all skin appendages in all sample groups (A–C). MC-1R: Very strong and strong reaction in almost all of the cells of sebaceous gland in acne-involved (A) and acne-uninvolved (B) skin; reactivity is most prominent in basal and differentiating peripheral sebocytes with less intense staining of mature cells; apoptotic sebocytes are not labeled; less intense immunoreactivity in sebaceous gland of normal skin (C). Very strong immunostaining in keratinocytes of ductus seboglandularis of acne-involved (A) and acne-uninvolved (B) skin is observed in contrast to a scattered reactivity of cells in ductus seboglandularis of normal skin samples (C).
MC-1R.
SGs of acne-involved and uninvolved skin appeared to express very strong and strong staining of MC-1R in almost all, except apoptotic cells. In SGs of normal skin immunoreactivity of MC-1R was less intense than in the biopsies of acne patients. Keratinocytes of the ductus seboglandulares showed very intense MC-1R immunostaining in acne-involved as well in uninvolved skin in contrast to the scattered reactivity in normal skin samples (Fig. 1).
Discussion
In the light of the underlying biological basis of acne we have tried to come more close to characteristics of multifactorial acne pathogenesis which stays questionable and intriguing until now. In the present study we have extended the existing immunohistochemical investigations and have confirmed for the first time the presumptions that NPs of HPA axis like the CRH/CRHR system and α-MSH, can be active in SGs and can be involved directly in the clinical development of acne vulgaris, a disease that obviously exacerbates under the stress.
The immunoexpression for CRH gene in human SG of acneinvolved, non-involved and normal skin is in accordance with the previous detection of CRH and CRHBP genes in situ,31 CRH, CRHBP and CRHR expression in SZ95 sebocytes in vitro.23 It is interesting that a strong CRH immunoreaction in sebocytes was irrespective of their differentiation stage in acne-involved skin, while the weaker CRH expression in acne non-involved and normal skin was dependent upon the sebocyte differentiation stage. CRH directly induces lipid synthesis and enhances mRNA expression of Δ5-3β-hydroxysteroid dehydrogenase, the enzyme that converts dehydroepiandrosterone to testosterone in human sebocytes.23–25 Testosterone and growth hormone, which upregulates sebaceous differentiation,32 were found to antagonize CRH by modifying CRHR mRNA levels.23 It has been also confirmed the property of CRH to modulate inflammation when secreted in inflammatory sites with direct pro-inflammatory paracrine effects,33 exerted via degranulation of the mast cells, a major immune target of CRH.34 In central nervous system, cytokines regulate expression of CRH.35 The stronger immunoexpression of CRH in the SG in acne skin samples compared to acne non-involved and normal skin in our study suggests that CRH may interact with inflammatory mediators and immune factors in acne, especially, taking in to account the PSU as an immunocompetent organ and an organ involved in responses to stress.10
Investigations with CRHBP have revealed a secondary buffering system role for this peptide, which exhibits a negative regulation of the local availability of CRH, prevents it from entering systemic circulation, becoming inactive and unable to bind it receptors.36 The most distinct CRHBP immunoexpression was in acneinvolved skin within differentiating sebocytes, which are the most active sebaceous cells in sebogenesis. If pituitary CRHBP mRNA levels increase in response to acute restraint stress, an intense CRHBP expression in differentiating sebocytes of acne-involved skin may be indicative of a local stress reaction in acne.
CRHR-1 has important role in skin physiology and pathology, most likely through the regulation of local pigmentary, epidermal, adnexal, secretory, vascular and dermal activity.36 Our findings are in agreement with the reported detection of CRHR-1 and expression of functional CRHR-1 in SG and cultured sebocytes.23 Noteworthy we noticed that CRHR-1 exhibits the most intensive expression in the secretory part of eccrine sweat glands in all (acne, non-acne and normal) skin samples studied. Knowing that eccrine glands are under the regulation of stress hormones we can presume that CRHR-1 is an important receptor for the eccrine sweat glands and may have a direct role in the regulation of local dermal secretory activity. The finding of a stronger immunoexhibition of CRHR-1 in keratinocytes of ductus seboglandularis in acne-involved then in non-involved or normal skin can also be attributed to a stress response system in the skin. In further agreement our findings correlate with data suggesting that CRHR-1 is the major coordinator of the stress response at the central and local level, while CRHR-2 only plays a modulatory role. Our study detected the most significant immunoexpression for CRHR-2 within the SG in all sebocytes, except apoptotic cells in all skin samples of acne-involved, non-involved and normal skin. A much more distinct expression of this peptide within keratinocytes of DSG was detected in acne-involved skin compared with non-involved and normal skin. It is possible that, similarly, to the responsibility of CRH-2 receptor in hair growth,36 it can locally regulate SG functions and have a direct influence on sebum production. It seems likely that CRHR-2 can be the most important receptor for SG biological response to CRH. These findings correlate with the investigations of the expression of CRHR in SZ95 sebocytes in vitro, whereas SZ95 sebocytes express mRNA levels for both CRH receptors.23
In accordance with previous data on healthy skin26,37,38 adnexal structures in both involved and uninvolved skin of acne patients expressed prominent immune signals for MC-1R. In the present study, MC-1R immunoreaction was more accentuated in the SG of involved and non-involved skin from acne patients than in SG from healthy individuals. Moreover, the reactivity of MC-1R was most distinct in basal and differentiating peripheral sebocytes, which are the biologically most active cells of the SG. Several explanations may account for the increased in situ expression of MC-1R in SGs of acne patients. Firstly, MC-1R expression has been shown to be upregulated by proinflammatory signals.39 Since proinflammatory cytokines are upregulated in acne lesions,40 sebocytes may respond to these signals with increased MC-1R expression thereby generating a negative feedback mechanism for α-MSH, which exerts direct anti-inflammatory actions.26 Preliminary findings suggest that IL-1 increases MC-1R expression and the number of detectable α-MSH binding sites in SZ95 sebocytes, respectively (Böhm and Schiöth, unpublished). Secondly, α-MSH itself can upregulate its own receptor.41 Therefore, aberrant α-MSH levels may induce increased MC-1R expression in SGs under situations of increased systemic or cutaneous stress of acne patients. Expression of CRH, a key regulator of the pituitary and cutaneous propiomelanocortin system, in acne skin in our study and in SZ95 sebocytes17,18,23 indicates an increased synthesis of α-MSH and upregulation of MC-1R in patients with acne. In addition to mediating anti-inflammatory actions, increased MC-1R expression by sebocytes in acne may confer cytoprotection from harmful cytotoxic stimuli released during inflammation.42,43 The relevance of increased MC-1R expression in sebocytes of acne patients may not only be a negative feedback mechanism. Recent studies confirmed a lipogenic effect of α-MSH and related peptides in primary cultures of human sebocytes derived from facial skin.30 There are very surprising and interesting differences of MC-1R immunoreactivity in the PSU between acne patients and healthy individuals in the ductus seboglandularis, the anatomical structure which play a crucial role in the pathogenesis of acne due to increased cellular turnover or keratinization.44 In contrast to SZ95 sebocytes in which α-MSH does not augment proliferation,45 this NP can increase the metabolic activity in HaCaT keratinocytes.46 Benign and malignant adnexal neoplasms of the skin display prominent MC-1R immunoreactivity independently of their apocrine, eccrine, sebaceous or follicular differentiation as well as a non-neoplastic hyperproliferative skin disease, acanthosis nigricans, characterized by increased keratinocyte turnover and excessive hyperkeratosis, which supports the potential role of MC-1R as a mediator of increased cellular turnover of the cells situated in the ductus seboglandularis of acne patients.43,47 These findings suggest an intimate relation between keratinocyte proliferation, differentiation and the α-MSH/MC-1R system.
In summary, we can presume that stress response system mechanisms are initiated in response to the inflammation and to the altered tissue environment like to the local stress situation in the PSU in acne. The results of this study provide evidence that NP, such as the CRH system and MC-1R, emerge among the key regulators of SG activity and are involved in the pathogenesis of acne. Further studies will have to clarify the precise mechanism of increased CRH and α-MSH/MC-1R systems expression in sebocytes of patients with acne with a hope that these peptidergic systems may represent targets for novel therapeutic strategies in acne vulgaris.
Materials and Methods
Biopsies (3–5 mm) were obtained from acne lesions of the facial (acne-involved skin) and the thigh (acne-uninvolved skin) of 33 patients with active acne (18 male and 15 female, aged 15–22 years) of the Outpatient Clinic “Seskines Poliklinika” and Centre of Dermatovenereology, Vilnius University Hospital, Santariskiu Clinics (Vilnius, Lithuania), as well as from 8 age-matched healthy individuals undergoing routine plastic or post-traumatic surgery. This study was conducted according to the ethical standards of the Lithuanian Bioethical Committee for studies involving human subjects (authorization # 78, protocol # 1, version # 1). Characteristics of severity grade of acne and type characteristics of acne lesions are shown in Tables 1 and 2.
Table 1.
Characteristics of severity grade of acne in the examined patients
| Acne grade | Frequeny | Percent |
| 2.0 | 6 | 18.2 |
| 3.0 | 8 | 24.2 |
| 4.0 | 16 | 48.5 |
| 5.0 | 3 | 9.1 |
| Total | 33 | 100.0 |
Table 2.
Type characteristics of acne lesions in the examined patients
| Acne lesions | Frequency | Percent |
| Opened comedo | 2 | 6.1 |
| Closed comedo | 4 | 12.1 |
| Open inflamed comedo | 12 | 36.4 |
| Closed inflamed comedo | 7 | 21.2 |
| Inflamed papule | 7 | 21.2 |
| Pustule | 1 | 3.0 |
| Total | 33 | 100.0 |
Materials.
IHC was performed with a commercially available antibodies against CRH, CRHBP, CRHR-1, CRHR-2, MC-R1 (Table 3). Secondary anti-mouse, anti-rabbit, rabbit/anti-goat immunoglobulins (depending on the origin of primary antibodies), biotin, antibody diluent, alkaline phosphatase anti-alkaline phosphatase complex, streptavidine enzyme conjugate and diaminobenzidine tetrachloride reagent (En-Vision System Kit) were from Dako (Hamburg, Germany). Other supplements and reagents were from Biochrom (Berlin, Germany) or Sigma (Deisenhofen, Germany).
Table 3.
Antibodies against CRH, CRHBP, CRHR-1, CRHR-2, MC-R1
| Primary antibodies | Dilution | Incubation time and temperature | Prevention of non-specific binding | Retrieval procedure | Manufacturer |
| CRH (C-20)—goat polyclonal IgG (cat# sc-1759, Lot# 1071) | 1:50 | 30 min room | – | – | Santa Cruz Biotechnology Inc., Santa Cruz, CA |
| CRHBP (C-19)—goat polyclonal IgG (cat# sc-1822, Lot# E 211) | 1:50 | 30 min room | – | – | # |
| CRH-R1 (C-20)—goat polyclonal IgG (cat# sc-1757, Lot# B 1903) | 1:50 | 30 min room | – | – | # |
| CRH-R2 (N-20)—goat polyclonal IgG (cat# sc-1826, Lot# C 0303) | 1:50 | 30 min room | – | – | # |
| MC-R1—synthetic anti-hMC-1R polycolnal antibody corresponding to the amino acids 2–18 of the N-terminal, extracellular domain of the human MC-1R | 2 μg/ml | 30 min room | Quenching of endogenous peroxidase activity; blocking with 2% bovine serum albumin | High temperature antigen unmasking pretreatment | Münster, Germany. |
Immunohistochemistry.
After deparaffinization the tissue slices were incubated with primary and secondary antibodies. The optimal concentration of both the primary and secondary antibody was predetermined by titration assay. The antigen retrieval (boiling with the high pressure in unmasking solution), blocking and staining technique, incubation time and temperature was choused considering the strongest specific antigen staining reaction with the lowest non-specific background. The biotin/avidin block in combination with a blocking protein solution (fetal calf serum) was applied to tissue sections for the prevention of non-specific bindings. The negative controls consisted of tissues incubated with antibody diluent instead of the primary antiserum. The specimens were incubated with a streptavidine enzyme conjugate, reacted with a fuchsine substrate-chromogen system and were counterstained with Mayer’s hematoxylin. Immunostaining of SG cells at different stages of differentiation was evaluated semiquantitatively on a scale of 0 to 3: 0, negative; 0.5, barely discernible; 1, moderate intensive; 2, strong staining; 3, very strong uniform immunostaining. For evaluation of specific signals, a microscopic view analyzing system (Olympus BX51, Japan) was used. Tissues showing membrane or cytoplasmic staining of any cells were assessed as positive.
Statistical analysis.
Statistical significance of the results of the immunohistological studies was calculated by the Mann-Whitney U test. Mean differences were considered to be significant when p < 0.05.
Acknowledgements
Ruta Ganceviciene is a scholarship holder of the Berlin Foundation for Dermatology. We are grateful to associates of the Research Group for Biogerontology, Dermato-Pharmacology and Dermato-Endocrinology, Institute of Clinical Pharmacology and Toxicology, Campus Benjamin Franklin, Charité Universitaetsmedizin Berlin and the Institute of Experimental and Clinical Medicine of Vilnius University, Vilnius, Lithuania for friendly advices and for excellent technical assistance.
Footnotes
Previously published online as a Dermato-Endocrinology E-publication: http://www.landesbioscience.com/journals/dermatoendocrinology/article/8496
References
- 1.Brown SK, Shalita AR. Acne vulgaris. Lancet. 1998;351:1871–1876. doi: 10.1016/S0140-6736(98)01046-0. [DOI] [PubMed] [Google Scholar]
- 2.Zouboulis CC, Eady A, Philpott M, Goldsmith LA, Orfanos CE, Cunliffe WC, et al. What is the pathogenesis of acne? Exp Dermatol. 2005;14:143–152. doi: 10.1111/j.0906-6705.2005.0285a.x. [DOI] [PubMed] [Google Scholar]
- 3.Zouboulis CC. Human skin: an independent peripheral endocrine organ. Horm Res. 2000;54:230–242. doi: 10.1159/000053265. [DOI] [PubMed] [Google Scholar]
- 4.Chen W, Yang C-C, Sheu H-M, Seltmann H, Zouboulis CC. Expression of peroxisome proliferator-activated receptor and CCAAT/enhancer binding protein transcription factors in cultured human sebocytes. J Invest Dermatol. 2003;121:441–447. doi: 10.1046/j.1523-1747.2003.12411.x. [DOI] [PubMed] [Google Scholar]
- 5.Zouboulis CC, Akamatsu H, Stephanek K, Orfanos CE. Androgens affect the activity of human sebocytes in culture in a manner dependent on the localization of the sebaceous glands and their effect is antagonized by spironolactone. Skin Pharmacol. 1994;7:33–40. doi: 10.1159/000211271. [DOI] [PubMed] [Google Scholar]
- 6.Rosenfield RL, Deplewski D, Greene ME. Peroxisome proliferator-activated receptors and skin development. Horm Res. 2000;54:269–274. doi: 10.1159/000053270. [DOI] [PubMed] [Google Scholar]
- 7.Zouboulis CC. Acne and sebaceous gland function. Clin Dermatol. 2004;22:360–366. doi: 10.1016/j.clindermatol.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Ingham E, Eady EA, Goodwin CE, Cove JH, Cunliffe WJ. Pro-inflammatory levels of interleukin-1alpha-like bioactivity are present in the majority of open comedones in Acne vulgaris. J Invest Dermatol. 1992;98:895–901. doi: 10.1111/1523-1747.ep12460324. [DOI] [PubMed] [Google Scholar]
- 9.Boehm KD, Yun JK, Strohl KP, Elmets CA. Messenger RNAs for the multifunctional cytokines interleukin-1alpha, interleukin-1beta and tumor necrosis factor-alpha are present in adnexal tissues and in dermis of normal human skin. Exp Dermatol. 1995;4:335–341. doi: 10.1111/j.1600-0625.1995.tb00057.x. [DOI] [PubMed] [Google Scholar]
- 10.Alestas T, Ganceviciene R, Fimmel S, Muller-Decker K, Zouboulis CC. Enzymes involved in the biosynthesis of leukotriene B(4) and prostaglandin E(2) are active in sebaceous glands. J Mol Med. 2006;84:75–87. doi: 10.1007/s00109-005-0715-8. [DOI] [PubMed] [Google Scholar]
- 11.Chiu A, Chon SY, Kimball AB. The response of skin disease to stress: changes in the severity of Acne vulgaris as affected by examination stress. Arch Dermatol. 2003;139:897–900. doi: 10.1001/archderm.139.7.897. [DOI] [PubMed] [Google Scholar]
- 12.Zouboulis CC, Bohm M. Neuroendocrine regulation of sebocytes—a pathogenetic link between stress and acne. Exp Dermatol. 2004;13:31–35. doi: 10.1111/j.1600-0625.2004.00254.x. [DOI] [PubMed] [Google Scholar]
- 13.Lotti T, Bianchi B, Panconesi E. Neuropeptides and skin disorders. The new frontiers of neuro-endocrine-cutaneous immunology. International J Dermatol. 1999;38:673–675. doi: 10.1046/j.1365-4362.1999.00767.x. [DOI] [PubMed] [Google Scholar]
- 14.Slominski A, Mihm M. Potential mechanism of skin response to stress. Int J Dermatol. 1996;35:849–851. doi: 10.1111/j.1365-4362.1996.tb05049.x. [DOI] [PubMed] [Google Scholar]
- 15.Toyoda M, Nakamura M, Makino T, Kagoura M, Morohashi M. Sebaceous glands in acne patients express high levels of neutral endopeptidase. Experimental Dermatol. 2002;1:241–247. doi: 10.1034/j.1600-0625.2002.110307.x. [DOI] [PubMed] [Google Scholar]
- 16.Slominski A, Wortsman J. Neuroendocrinology of the skin. Endocr Rev. 2000;21:457–487. doi: 10.1210/edrv.21.5.0410. [DOI] [PubMed] [Google Scholar]
- 17.Ganceviciene R, Marciukaitiene I, Graziene V, Rimkevicius A, Zouboulis CC. New accents in the pathogenesis of Acne vulgaris. Acta Medica Lituanica. 2006:13. [Google Scholar]
- 18.Wollina U, Abdel-Naser MB, Ganceviciene R, Zouboulis CC. Receptors of eccrine, apocrine and holocrine skin glands. Dermatol Clin. 2007;25:577–588. doi: 10.1016/j.det.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Slominski A, Wortsman J, Paus R, Luger T, Salomon S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev. 2000;80:979–1020. doi: 10.1152/physrev.2000.80.3.979. [DOI] [PubMed] [Google Scholar]
- 20.Slominski A, Ermak G, Mazurkiewicz JE, Baker J, Wortsman J. Characterization of corticotropin releasing hormone (CRH) in human skin. J Clin Endocrinol Metab. 1998;83:1020–1024. doi: 10.1210/jcem.83.3.4650. [DOI] [PubMed] [Google Scholar]
- 21.Grammatopoulos DK, Chrousos GP. Functional characteristics of CRH receptors and potential clinical applications of CRH-receptor antagonists. Trends Endocrinol Metab. 2002;13:436–444. doi: 10.1016/s1043-2760(02)00670-7. [DOI] [PubMed] [Google Scholar]
- 22.Seasholtz AF, Valverde RA, Denver RJ. Corticotropin-releasing hormone-binding protein: biochemistry and function from fishes to mammals. J Endocrinol. 2002;175:89–97. doi: 10.1677/joe.0.1750089. [DOI] [PubMed] [Google Scholar]
- 23.Zouboulis CC, Seltmann H, Hiroi N, et al. Corticotropin-releasing hormone: An autocrine hormone that promotes lipogenesis in human sebocytes. Proc Natl Acad Sci. 2002;99:7148–7153. doi: 10.1073/pnas.102180999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slominski A, Zbytek B, Szczesniewski A, Semak I, Kaminski J, Sweatman T, et al. CRH stimulation of corticosteroids production in melanocytes is mediated by ACTH. Am J Physiol Endocrinol Metab. 2005;288:701–706. doi: 10.1152/ajpendo.00519.2004. [DOI] [PubMed] [Google Scholar]
- 25.Slominski A, Zbytek B, Semak I, Sweatman T, Wortsman J. CRH stimulates POMC activity and corticosterone production in dermal fibroblasts. J Neuroimmunol. 2005a;162:97–102. doi: 10.1016/j.jneuroim.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 26.Böhm M, Schiller M, Ständer S, Seltmann H, Li Z, Brzoska T, et al. Evidence for expression of melanocortin-1 receptor in human sebocytes in vitro and in situ. J Invest Dermatol. 2002;118:533–539. doi: 10.1046/j.0022-202x.2001.01704.x. [DOI] [PubMed] [Google Scholar]
- 27.Lipton JM, Catania A. Antiinflammatory actions of the neuroimmunomodulator α-MSH. Immunol Today. 1997;18:140–145. doi: 10.1016/s0167-5699(97)01009-8. [DOI] [PubMed] [Google Scholar]
- 28.Bhardwaj RS, Schwarz A, Becher E, Mahnke K, Aragane Y, Schwarz T, et al. Proopiomelanocortin-derived peptidesinduce IL-10 production in human monocytes. J Immunol. 1996;156:2517–2521. [PubMed] [Google Scholar]
- 29.Cone RD, Lu D, Koppula S, Vage DI, Klungland H, Boston B, et al. The melanocortin receptors: agonists, antagonists and the hormonal control of pigmentation. Recent Prog Horm Res. 1996;51:287–318. [PubMed] [Google Scholar]
- 30.Zhang L, Anthonavage M, Huang Q, Li W, Eisinger M. Proopiomelanocortin peptides and sebogenesis. Ann NY Acad Sci. 2003;994:154–161. doi: 10.1111/j.1749-6632.2003.tb03175.x. [DOI] [PubMed] [Google Scholar]
- 31.Kono M, Nagata H, Umemura S, Kawana S, Osamura RY. In situ expression of corticotropin-releasing hormone (CRH) and proopiomelanocortin (POMC) genes in human skin. FASEB J. 2001;15:2297–2299. doi: 10.1096/fj.01-0254fje. [DOI] [PubMed] [Google Scholar]
- 32.Deplewski D, Rosenfield RL. Growth hormone and insulin-like growth factors have different effects on sebaceous cell growth and differentiation. Endocrinology. 1999;140:4089–4094. doi: 10.1210/endo.140.9.6957. [DOI] [PubMed] [Google Scholar]
- 33.Webster EL, Torpy DJ, Elenkov IJ, Chrousos GP. Corticotropin-releasing hormone and inflammation. Ann N Y Acad Sci. 1998;840:21–32. doi: 10.1111/j.1749-6632.1998.tb09545.x. [DOI] [PubMed] [Google Scholar]
- 34.Theoharides T, Singh L, Boucher W, Pang X, Letourneau R, Webster E, et al. Corticotropin-releasing hormone induces skin mast cell degeneration and increased vascular permebility, a possible explanation for its proinflammatory effects. Endocrinol. 1998;139:403–413. doi: 10.1210/endo.139.1.5660. [DOI] [PubMed] [Google Scholar]
- 35.Boehm KD, Yun JK, Strohl KP, Elmets CA. Messenger RNAs for the multifunctional cytokines interleukin-1alpha, interleukin-1beta and tumor necrosis factor-alpha are present in adnexal tissues and in dermis of normal human skin. Exp Dermatol. 1995;4:335–341. doi: 10.1111/j.1600-0625.1995.tb00057.x. [DOI] [PubMed] [Google Scholar]
- 36.Slominski A, Pisarchik A, Tobin DJ, Mazurkiewicz JE, Wortsman J. Differential expression of a cutaneous corticotropin-releasing hormone system. Endocrinology. 2004;145:941–950. doi: 10.1210/en.2003-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Böhm M, Metze D, Schulte U, Becher E, Luger TA, Brzoska T. Detection of melanocortin-1 immunoreactivity in human skin cells in culture and in situ. Exp Dermatol. 1999;8:453–461. doi: 10.1111/j.1600-0625.1999.tb00303.x. [DOI] [PubMed] [Google Scholar]
- 38.Böhm M, Schulte U, Schiller M, Brzoska T, Luger TA. Characterization of a polyclonal antibody against the human melanocortin-1 receptor. Ann NY Acad Sci. 1999;885:372–382. doi: 10.1111/j.1749-6632.1999.tb08693.x. [DOI] [PubMed] [Google Scholar]
- 39.Hartmeyer M, Sholzen T, Becher E, Bhardwaj R, Schwarz T, Luger T. Human dermal microvascular endothelial cells express the melanocortin receptor type 1 and produce increased levels of IL-8 upon stimulation with alphamelanocyte-stimulating hormone. J Immunol. 1997;159:1930–1937. [PubMed] [Google Scholar]
- 40.Jeremy A, Holland D, Roberts S, Thomson K, Cunliffe W. Inflammatory events are involved in acne lesion initiation. J Invest Dermatol. 2003;121:20–27. doi: 10.1046/j.1523-1747.2003.12321.x. [DOI] [PubMed] [Google Scholar]
- 41.Rouzaud F, Annereau JP, Valencia JC, Costin GE, Hearing VJ. Regulation of melanocortin 1 receptor expression at the mRNA and protein levels by its natural agonist and antagonist. FASEB J. 2003;17:2154–2156. doi: 10.1096/fj.03-0206fje. [DOI] [PubMed] [Google Scholar]
- 42.Hill RP, Wheeler P, MacNeil S, Haycock JW. Alpha-melanocyte stimulating hormone cytoprotective biology in human dermal fibroblast cells. Peptides. 2005;26:1150–1158. doi: 10.1016/j.peptides.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 43.Ständer S, Böhm M, Brzoska T, Zimmer KP, Luger T, Metze D. Expression of melanocortin-1 receptor (MC-1R) in normal, malformed and neoplastic skin glands and hair follicles. Exp Dermatol. 2002;11:42–51. doi: 10.1034/j.1600-0625.2002.110105.x. [DOI] [PubMed] [Google Scholar]
- 44.Toyoda M, Morohashi M. Patogenesis of acne. Med Electron Microsc. 2001;34:29–40. doi: 10.1007/s007950100002. [DOI] [PubMed] [Google Scholar]
- 45.Zouboulis CC, Xia L, Akamatsu H, Seltmann H, Fritsch M, Hornemann S, et al. The human sebocytes culture model provides new insights into development and management of seborrhoea and acne. Dermatol. 1998;196:21–31. doi: 10.1159/000017861. [DOI] [PubMed] [Google Scholar]
- 46.Orel L, Simon MM, Karlseder J, Bhardwaj R, Trautinger F, Schwarz T, et al. Alphamelanocyte stimulating hormone downregulates differentiation driven heat shock protein 70 expression in keratinocytes. J Invest Dermatol. 1997;108:401–405. doi: 10.1111/1523-1747.ep12289699. [DOI] [PubMed] [Google Scholar]
- 47.Kleikamp S, Böhm M, Frosch P, Brinkmeier T. Acanthosis nigricans, Papillomatosis mucosae und “tripe palms” bei einem Patienten mit metastasiertem Adenokarzinom des Magens. Fallbericht und immunhistochemische Untersuchung zur Pathogenese der kutanen Hyperpigmentierung und Hyperplasie. Dtsch Med Wochschr. 2006. (Ger). [DOI] [PubMed]