Abstract
Approximately five million people suffer with Alzheimer disease (AD) and more than twenty-four million people are diagnosed with AD, pre-senile dementia, and other disorders of cognitive loss worldwide. Furthermore, the annual cost per patient with AD can approach $200,000 with an annual population aggregate cost of $100 billion. Yet, complete therapeutic prevention or reversal of neurovascular injury during AD and cognitive loss is not achievable despite the current understanding of the cellular pathways that modulate nervous system injury during these disorders. As a result, identification of novel therapeutic targets for the treatment of neurovascular injury would be extremely beneficial to reduce or eliminate disability from diseases that lead to cognitive loss or impairment. Here we describe the capacity of intrinsic cellular mechanisms for the novel pathways of erythropoietin and forkhead transcription factors that may offer not only new strategies for disorders such as AD and cognitive loss, but also function as biomarkers for disease onset and progression.
Key words: aging, Alzheimer disease, angiogenesis, apoptosis, cognitive loss, diabetes, erythropoietin, forkhead transcription factors, immune system, ischemia, neurodegeneration, oxidative stress, vascular disease, Wnt, wingless
Introduction
Alzheimer disease, cognitive loss, novel cellular pathways.
For the population in the United States, the National Institute on Aging estimates that almost five million people have Alzheimer’s disease (AD). Furthermore, more than twenty-four million people suffer from AD, pre-senile dementia, and other disorders of cognitive loss worldwide. If one then includes other related degenerative disorders of the central nervous system (CNS), the scope of these illnesses approach 370 million people throughout the globe. With these disorders of cognition, the cost of physician services, hospital and nursing home care, and medications continues to rise dramatically. In addition, the medical costs parallel a progressive loss of economic productivity with rising morbidity and mortality, ultimately resulting in an annual deficit to the economy that is greater than $400 billion. Interestingly, the most significant portion of this economic loss is composed of only a few neurodegenerative disease entities, such as ischemic disease and AD. The annual cost per patient with AD is estimated at greater than $174,000 with an annual population aggregate cost of $100 billion.1,2
Despite the current understanding of the cellular pathways that modulate CNS injury during AD and cognitive disorders, complete therapeutic prevention or reversal of neurovascular injury during AD or dementia is not achievable. As a result, identification of novel therapeutic targets for the treatment of neurovascular injury would be extremely beneficial to reduce or eliminate disability from diseases that lead to cognitive loss or impairment. Current studies have begun to focus on pathways of oxidative stress that involve a variety of cellular pathways in the neurovascular systems. Here we describe the capacity of intrinsic cellular mechanisms that may offer novel therapy for disorders such as AD. Oxidative stress leads to apoptotic injury that involves early loss of cellular membrane asymmetry as well as the eventual destruction of genomic DNA. These dynamic stages of oxidative stress and apoptosis can be governed by cytokines such as erythropoietin (EPO) and transcription factors such as forkhead. Further understanding of these pathways may provide new insight for novel strategies that can treat AD and cognitive disorders as well as the complications associated with these disorders.
Oxidative stress and neurovascular injury.
Release of reactive oxygen species (ROS) that consist of oxygen free radicals and other chemical entities can result in the development of oxidative stress in the body. Oxygen free radicals can be generated in elevated quantities during the reduction of oxygen and lead to cell injury. ROS can involve superoxide free radicals, hydrogen peroxide, singlet oxygen, nitric oxide (NO) and peroxynitrite.3–5 Most species are produced at low levels during normal physiological conditions and are scavenged by endogenous antioxidant systems that include superoxide dismutase (SOD), glutathione peroxidase, catalase and small molecule substances such as vitamins C and E. Other closely linked pathways to oxidative stress may be tempered by different vitamins, such as vitamin D3,6 and the amide form of niacin or vitamin B3, nicotinamide.7–13
Oxidative stress leads to the destruction of multiple cell types through apoptotic pathways.14–16 Apoptotic induced oxidative stress in conjunction with processes of mitochondrial dysfunction17–19 can contribute to a variety of disease states such as diabetes, ischemia, cognitive loss, Alzheimer’s disease and trauma.3,20–23 Oxidative stress can lead to apoptosis in neurons, endothelial cells (ECs), cardiomyocytes and smooth muscle cells that involve separate as well as overlapping pathways.21,24–28
Apoptosis is a dynamic process that consists of both the early exposure of membrane phosphatidylserine (PS) residues and the late destruction of genomic DNA.29,30 Externalization of membrane PS residues is an early event during cell apoptosis31,32 and can become a signal for the phagocytosis of cells.16,33,34 The loss of membrane phospholipid asymmetry leads to the exposure of membrane PS residues on the cell surface and assists microglia to target cells for phagocytosis.13,26,35–37 This process occurs with the expression of the phosphatidylserine receptor (PSR) on microglia during oxidative stress.38,39 It has been shown that blockade of PSR function in microglia prevents the activation of microglia.36,40 Externalization of membrane PS residues occurs in neurons, vascular cells and inflammatory microglia in conjunction with AD and cognitive loss during reduced oxygen exposure,16,41–44 β-amyloid (Aβ) exposure45,46 during AD progression, nitric oxide exposure,47–51 and during the administration of agents that induce the production of ROS, such as 6-hydroxydopamine. 52 Membrane PS externalization on platelets also has been associated with clot formation in the vascular system.53
The cleavage of genomic DNA into fragments43,54,55 usually occurs after membrane PS exposure56 and is considered to be a later event during apoptotic injury.26,55,57,58 Several enzymes responsible for DNA degradation include the acidic, cation independent endonuclease (DNase II), cyclophilins, and the 97 kDa magnesium—dependent endonuclease.3,59 Three separate endonuclease activities also have been found in neurons that include a constitutive acidic cation-independent endonuclease, a constitutive calcium/magnesium-dependent endonuclease, and an inducible magnesium dependent endonuclease.60,61
During oxidative stress, mitochondrial membrane transition pore permeability also is increased,12,26,62,63 a significant loss of mitochondrial NAD+ stores occurs, and further generation of superoxide radicals leads to cell injury.13,64 Mitochondria are a significant source of superoxide radicals that are associated with oxidative stress.3,65 Blockade of the electron transfer chain at the flavin mononucleotide group of complex I or at the ubiquinone site of complex III results in the active generation of free radicals which can impair mitochondrial electron transport and enhance free radical production.38,59 Furthermore, mutations in the mitochondrial genome have been associated with the potential development of a host of disorders, such as hypertension, hypercholesterolemia and hypomagnesemia.66,67 ROS also may lead to cellular acidosis and subsequent mitochondrial failure.20 Disorders, such as hypoxia,68 diabetes69,70 and excessive free radical production61,71,72 can result in the disturbance of intracellular pH.
Erythropoietin (EPO) and its Receptor
EPO and the EPO receptor.
The EPO gene is located on chromosome 7, exists as a single copy in a 5.4 kb region of the genomic DNA, and encodes a polypeptide chain containing 193 amino acids. During the production and secretion of EPO, a circulatory mature protein of 165 amino acids is produced.73,74 The principal organs of EPO production and secretion are the kidney, liver, brain and uterus. EPO production and secretion occurs foremost in the kidney.75
Interestingly, increased levels of EPO in the fetal plasma and amniotic fluid during gestation may function as a biomarker of intrauertine hypoxia.76 For biological systems, a “biomarker” can consist of any entity that occurs in the body and that can be measured to predict the diagnosis, onset or progression of a disease process.77 Novel pathways that involve the cytokine and growth factor EPO may indicate that the increased presence of this agent during periods of oxidative stress may lead to cellular mechanisms to protect against ROS.74,78,79 Recent studies have demonstrated that EPO is not only required for erythropoiesis, but also functions in other organs and tissues, such as the brain, heart and vascular system that can be relevant for the treatment of AD40,80–84 (Fig. 1). EPO production is believed to occur throughout the body5,74,85 and can be detected in the breath of healthy individuals.86 In addition, it has been suggested that EPO may provide developmental cognitive support. In experimental animal models, EPO may reduce apoptotic pathways during periods of hyperoxia in the developing brain.87,88 Furthermore, clinical disorders may have periods of hyperoxia followed by cerebral hypoperfusion and hypoxia that can lead to cerebral injury with associated oxidative stress.89 In these circumstances, EPO also may be protective since it can promote neurite outgrowth90 and also may regulate hemoglobin levels that have recently been associated with cognitive decline.91 In other work, elevated EPO concentrations during infant maturation have been correlated with increased Mental Development Index scores92 and EPO may prevent toxic effects of agents used to control cognitive function such as haloperidol.93
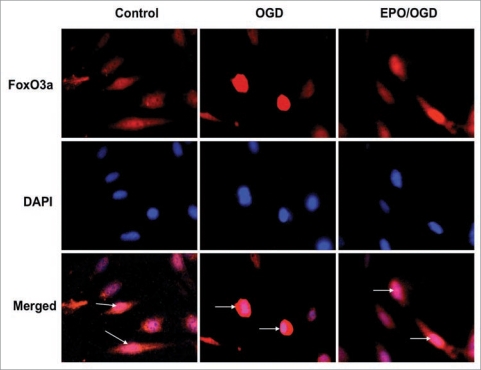
Figure 1.
Erythropoietin (EPO) regulates the intracellular trafficking of the forkhead transcription factor FoxO3a in endothelial cells (ECs) during oxygen glucose deprivation (OGD). EPO (10 ng/ml) was administered to ECs 1 hour prior to exposure of OGD for an 8 hour period. Immunofluorescent staining for FoxO3a at 6 hours following OGD was performed with primary rabbit anti-FoxO3a antibody followed by Texas red conjugated antirabbit secondary antibody. Nuclei of ECs were counterstained with DAPI. Control cells were untreated and not exposed to OGD. In control cells, FoxO3a remains primarily in the cytoplasm of cells with the nuclei visible in merged images and indicated by the white arrows. In contrast, OGD activates FoxO3a to translocate to the nucleus demonstrating FoxO3a in the cytoplasm and nuclei of these cells in merged images. However, EPO prevents nuclear translocation of FoxO3a by retaining FoxO3a in the cytoplasm similar to control cells with nuclei visible in merged images and indicated by the white arrows.
In addition, knowledge that EPO and its receptor are present in the neurovascular systems has generated great enthusiasm for the potential clinical applications of EPO for AD and related cardiac insufficiency94,95 and cardiac transplantation.96,97 In the nervous system, primary sites of EPO production and secretion are in the hippocampus, internal capsule, cortex, midbrain, cerebral endothelial cells (ECs) and astrocytes.73,74,98,99 Further work has revealed several other organs as secretory tissues for EPO that include peripheral ECs,100 myoblasts,101 insulinproducing cells102 and cardiac tissue.74,75 The EPOR also is expressed in primary cerebral ECs63,103 as well as in human umbilical veins, bovine adrenal capillaries and rat brain capillaries.100,104
Despite the fact that EPO is a critical modulator of erythropoiesis, the presence of a diminished oxygen tension is required rather than a low concentration of red blood cells.5,78,79,105 Gene transcription of EPO is mediated by the transcription enhancer located in the 3′-flanking region of the EPO gene that specifically binds to hypoxia-inducible factor 1 (HIF-1).73,74 Yet, hypoxia is not the only condition that can alter the expression of EPO and the EPOR. A variety of cellular disturbances may lead to either increased or decreased EPO expression through the control of HIF, such as hypoglycemia, cadmium exposure, raised intracellular calcium, or intense neuronal depolarizations generated by mitochondrial ROS.99,106,107 Anemic stress, insulin release and several cytokines, including insulin-like growth factor, tumor necrosis factor-α (TNFα),108 interleukin-1β (IL-1β) and interleukin-6 (IL-6)109 also can lead to increased expression of EPO and the EPOR73,74 and may provide a feed-back loop that is regulated by EPO such as TNFα.110
FoxO Transcription Factors
FoxO proteins and their regulation.
Mammalian forkhead transcription factors of the O class (FoxOs) function to either block or activate target gene expression.111 At least 100 forkhead genes and 19 human subgroups that range from FOXA to FOXS are now known to exist since the initial discovery of the fly Drosophila melanogaster gene forkhead.112 The original nomenclature for these proteins, such as forkhead in rhabdomyosarcoma (FKHR), the Drosophila gene fork head (fkh) and Forkhead RElated ACtivator (FREAC)-1 and -2, has been replaced.113 The current nomenclature for human Fox proteins places all letters in uppercase, otherwise only the initial letter is listed as uppercase for the mouse, and for all other chordates the initial and subclass letters are in uppercase.114
FoxO proteins also may function as biomarkers. The activation of FoxO transcription factors during tumor invasion may suggest the initiation of cell pathways that are attempting to restrict neoplastic growth and represent a positive prognostic factor.111,115,116 However, reliance on any single biomarker may be imperfect and lead to initially unpredicted outcomes78,79,117 or the onset of detrimental apoptotic programs with forkhead transcription factors.30 A number of other pathways that occur in combination with a particular biomarker during oxidative stress also may also influence outcome. In the case of breast cancer, studies suggests that the release of androgens, cytokines or even changes in body mass and exercise can influence outcome as well as alter the predictability of a specific biomarker.118,119
FoxO proteins (FoxO1, FoxO3, FoxO4 and FoxO6) are present throughout the body and are expressed in tissues of the reproductive system of males and females, skeletal muscle, the cardiovascular system, lung, liver, pancreas, spleen, thymus and the nervous system.105,111,115,116,120–127 Post-translational control of FoxO proteins employs pathways associated with ubiquitylation and acetylation.128,129 IκB kinase (IKK) can phosphorylate and block the activity of FoxO proteins, such as FoxO3a.113,115 This leads to the proteolysis of FoxO3a via the Ubdependent protea-some pathway.113,115,126,130,131 FoxOs also are acetylated by histone acetyltransferases that include p300, the CREB-binding protein (CBP), and the CBP-associated factor. FoxO proteins are deacetylated by histone deacetylases.115
In addition to acetylation, and ubiquitylation, post-translational modulation of FoxO proteins also involves pathways associated with phosphorylation.113,115,126,130,131 Protein phosphorylation is a critical pathway in the scheme for protein regulation. 132 Akt is a primary mediator of phosphorylation of FoxO1, FoxO3a and FoxO4 that can block activity of these proteins.113,133 Akt phosphorylation of FoxO proteins not only retains these transcription factors in the cytoplasm, but also leads to ubiquitination and degradation through the 26S proteasome.129,130 Interestingly, activation of Akt in pathways that involve EPO or FoxOs is usually cytoprotective, but may mediate other processes. For example, Akt either alone or through EPO can lead to cell proliferation,134 blood-brain barrier permeability,135 or cell protection during inflammation,136,137 neurodegeneration,138 hyperglycemia,139 hypoxia,80 Aβ toxicity,45,140–143 excitotoxicity,144 cardiomyopathy,145 cellular aging146 and oxidative stress.24,26,36 In addition, Akt can prevent cellular apoptosis through the phosphorylation of FoxO proteins.5 Posttranslational phosphorylation of FoxOs, such as during EPO administration, will maintain FoxO transcription factors in the cytoplasm by association with 14-3-3 proteins and prevent the transcription of pro-apoptotic target genes74,81 (Fig. 1).
Modulation of Akt activity also controls apoptotic pathways of caspases that may offer an alternative mechanism to regulate FoxO proteins.116 Caspases are a family of cysteine proteases that are synthesized as inactive zymogens that are proteolytically cleaved into subunits at the onset of apoptosis.38,147,148 The caspases 1 and 3 have been linked to the apoptotic pathways of genomic DNA cleavage, cellular membrane PS exposure and activation of inflammatory cells.40,56,63 Caspase pathways may be tied to the forkhead transcription factor FoxO3a since increased activity of FoxO3a can result in cytochrome c release and caspase-induced apoptotic death.81,149–151 Pathways that can inhibit caspase 3 appear to offer a unique regulatory mechanism. For example, studies suggests that cell death pathways that rely upon FoxO3a also appear to involve caspase 3 activation.46 FoxO3a activity promotes caspase-induced apoptotic death,81,149–151 but inhibition of caspase 3 also can maintain the phosphorylated “inactive” state of FoxO3a to prevent cell injury.81,149,150 Other work has shown that caspase 3 activity and cleavage is promoted during transfection of a triple mutant FoxO3a expression in which three phosphorylation sites have been altered to prevent inactivation of FoxO3a.152 Furthermore, FoxO3a may control early activation and subsequent apoptotic injury in microglia during Aβ exposure through caspase 3.46 Since Aβ exposure can facilitate the cellular trafficking of FoxO3a from the cytoplasm to the cell nucleus to potentially lead to “pro-apoptotic” programs by this transcription factor,46 one program in particular that may be vital for apoptotic injury appears to involve the activation of caspase 3. Aβ exposure leads to a rapid and significant increases in caspase 3 activity with 6 hours following Aβ administration, but that this induction of caspase 3 activity by Aβ requires FoxO3a, since loss of FoxO3a through gene silencing prevents the induction of caspase 3 activity by Aβ.
EPO, FoxOs, Nervous System Metabolism and Cognitive Impairment
Both EPO and FoxOs play a significant role during brain metabolism and metabolic disorders that can alter the progression of AD, such as during diabetes mellitus (DM). DM is a significant health concern for both young and older populations.153,154 Patients with DM can develop immune dysfunction,155 cognitive disorders, 155,156 hepatic dysfunction,157 renal disease,158 hematological disease,159 neurodegenerative disorders4,105,160 and cardiovascular disease.160,161 Interestingly, the development of insulin resistance and the complications of DM can be the result of cellular oxidative stress.153,160 Furthermore, acute glucose swings in addition to chronic hyperglycemia can trigger oxidative stress mechanisms, illustrating the importance for therapeutic interventions during acute and sustained hyperglycemic episodes.153,160
In regards to EPO during metabolic disorders, EPO administration has been shown both in diabetics as well as non-diabetics with severe, resistant congestive heart failure to decrease fatigue, increase left ventricular ejection fraction, and significantly decrease the number of hospitalization days.162 In vitro studies with vascular cells exposed to elevated glucose also have demonstrated that EPO can significantly improve EC survival in a 1.0 ng/ml range.163 EPO administration in patients also can significantly increase plasma levels of EPO well above this range of 1.0 ng/ml that has been associated with potential EPO cellular protection in patients with cardiac or renal disease,164,165 suggesting that the effects of EPO observed during in vitro studies may parallel the cellular processes altered by EPO in patients with metabolic disorders.92 Furthermore, EPO during elevated glucose and similar to other models of oxidative stress can block neuronal degeneration166 and apoptotic DNA degradation in ECs in vascular cell models.63,80,81,83,167 Protection by EPO also is related to the maintenance of mitochondrial membrane potential (ΔΨm). Loss of ΔΨm through the opening of the mitochondrial permeability transition pore represents a significant determinant for cell injury and the subsequent induction of apoptosis.22,65 EPO has the capacity to prevent the depolarization of the mitochondrial membrane that also affects the release of cytochrome c.47,80,168
Additional work suggests that proteins derived from the Drosophila Wingless (Wg) and the mouse Int-1 genes may be associated with cellular metabolic complications.30 The Wnt proteins are secreted cysteine-rich glycosylated proteins that can control cell proliferation,169,170 differentiation, survival and tumorigenesis.39,171 These genes are present in several cellular populations,172 such as neurons, cardiomyocytes, endothelial cells, cancer cells and preadipocytes.4 Abnormalities in the Wnt pathway, such as with transcription factor 7-like 2 gene, may impart increased risk for type 2 diabetes in some populations173–175 as well as have increased association with obesity.176 Yet, intact Wnt family members may offer glucose tolerance and increased insulin sensitivity177 as well as protect glomerular mesangial cells from elevated glucose induced apoptosis.178 These observations suggest a potential protective cellular mechanism for EPO through Wnt signaling. Cell culture studies demonstrate that the Wnt1 protein is necessary and sufficient to impart cellular protection during elevated glucose exposure.163 EPO maintains the expression of Wnt1 during elevated glucose exposure and prevents loss of Wnt1 expression that would occur in the absence of EPO during elevated glucose. In addition, blockade of Wnt1 with a Wnt1 antibody can neutralize the protective capacity of EPO, illustrating that Wnt1 is a critical component in the cytoprotection of EPO during elevated glucose exposure.163
In regards to FoxO proteins, analysis of the genetic variance in FOXO1a and FOXO3a on metabolic profiles, age-related diseases, fertility, fecundity and mortality in patients have observed higher HbA1c levels and increased mortality risk associated with specific haplotypes of FOXO1a.179 These clinical observations may coincide with the demonstration in human endothelial progenitor cells that elevated glucose levels can reduce post-translational phosphorylation of FOXO1, FOXO3a and FOXO4 and allow for the nuclear translocation of these proteins to initiate an apoptotic program in endothelial progenitor cells.180 In experimental models, FoxO proteins may prevent the toxic effects of high serum glucose levels.113,115 Interferon-gamma driven expression of tryptophan catabolism by cytotoxic T lymphocyte antigen 4 may activate Foxo3a to protect dendritic cells from injury in nonobese diabetic mice.181 Additional studies have demonstrated that adipose tissue-specific expression of Foxo1 in mice improved glucose tolerance and sensitivity to insulin during an elevated fat diet.182 FoxO proteins also may protect against diminished mitochondrial energy levels known to occur during insulin resistance such as in the elderly populations.153,154,160 In caloric restricted mice that have decreased energy reserves, Foxo1, Foxo3a and Foxo4 mRNA levels were noted to progressively increase over a two year course.122 These observations complement studies in Drosophila and mammalian cells that demonstrate an increase in insulin signaling to regulate cellular metabolism during the upregulation of FoxO1 expression.183
It should be noted that the ability for FoxO proteins to maintain proper physiologic controls over cellular metabolism might be limited and occur only during specific circumstances. For example, mice with a constitutively active Foxo1 transgene have increased microsomal triglyceride transfer protein and elevated plasma triglyceride levels.184 Studies in cardiomyocytes also suggest detrimental results with enhanced FoxO activity. Increased transcriptional activity of FoxO1, such as by the Sirt1 activator resveratrol, can diminish insulin mediated glucose uptake and result in insulin resistance.185 Overexpression of Foxo1 in skeletal muscles of mice also can lead to reduced skeletal muscle mass and poor glycemic control,186 illustrating that activation of FoxO proteins also may impair cellular energy reserves. Other studies that block the expression of Foxo1 in normal and cachectic mice187 or reduce FoxO3 expression188 show the reverse with an increase in skeletal muscle mass or resistance to muscle atrophy. With this in mind, one potential agent to consider for the maintenance of cellular metabolism in patients is nicotinamide,13,38 an agent that also can inhibit FoxO protein activity.150 In patients with DM, oral nicotinamide protects β-cell function, prevents clinical disease in islet-cell antibody-positive first-degree relatives of type-1 DM, and can reduce HbA1c levels.13,38,153 Nicotinamide, which is closely linked to cell longevity pathways,189,190 may derive its protective capacity through two separate mechanisms of posttranslational modification of FoxO3a. Nicotinamide not only can maintain phosphorylation of FoxO3a and inhibit its activity, but also preserve FoxO3a integrity to block FoxO3a proteolysis that can yield pro-apoptotic amino-terminal fragments.150
EPO, FoxOs and Neurovascular Survival
EPO and FoxO proteins can directly govern cell survival that can affect the progression of AD and cognitive loss. With EPO, it can prevent cell injury during AD and Aβ cell injury,45,143,191,192 hypoxia,40,80,193–196 excitotoxicity,197–199 parasitic disease,200–202 endotoxin shock,203,204 free radical exposure,47,63,198 cardiac disease,205,206 amyloid toxicity143,192 and pulmonary disease.207,208 EPO also represents a potential option for the prevention of retinal degeneration or neovascularization209–212 as well as glaucoma. 213 In the CNS, systemic application of EPO also can improve functional outcome and reduce cell loss during spinal cord injury,214,215 traumatic cerebral edema,216 cortical trauma217 and epileptic activity.82,218,219
EPO also can reduce cytokine gene expression in endothelial cells exposed to tumor necrosis factor,167 prevent ulcer progression in cases of scleroderma,220 reduce inflammation in murine arthritis models,221 and block primary microglial activation and proliferation24,26,33 during oxidative stress40,143 to prevent phagocytosis of injured cells through pathways that involve cellular membrane PS exposure, protein kinase B,24 and the regulation of caspases.40,63,222 EPO can directly inhibit several pro-inflammatory cytokines, such as IL-6, TNFα and monocyte chemoattractant protein 1,74,223 and reduce leukocyte inflammation.224 EPO also may foster the preservation of microglial cells for neuronal and vascular restructuring by preventing apoptotic injury in microglia.34,225
In contrast to EPO cytoprotection, FoxO transcription factors usually lead to apoptosis during oxidative stress.5 For example, forkhead transcription factors such as FoxO1 and FoxO3a must be present for oxidative stress to result in apoptotic cell injury.226 FoxO3a in conjunction with JNK also has been shown to modulate an apoptotic ligand activating a Fasmediated death pathway in cultured motoneurons,227 to lead to apoptosis through tumornecrosis-factor-related apoptosis-inducing ligand (TRAIL) and BH3-only proteins Noxa and Bim in neuroblastoma cells,151 and to promote pro-apoptotic activity of p53.228 In addition, loss of FoxO expression during oxidative stress is protective to cells. Protein inhibition or gene knockdown of FoxO1 or FoxO3a can lead to reduction in ischemic infarct size in the brain,229 mediate protection of metabotropic glutamate receptors during vascular injury,149 enhance pancreatic β-cell or neuronal survival through NAD+ precursors during oxidative stress,150 and provide trophic factor protection with EPO81 and neurotrophins.230
Furthermore, similar to pathways tied to EPO and Wnt, the canonical Wnt pathway231,232 that involves β-catenin39,171 also appears to link FoxO proteins and Wnt signaling together.30 For example, in relation to AD,233 Aβ is toxic to cells45,143,234 and is associated with the phosphorylation of FoxO1 and FoxO3a that can be blocked with ROS scavengers.235 A common denominator in the pathways linked to Aβ toxicity involves Wnt signaling45,236 and β-catenin. β-catenin may increase FoxO transcriptional activity and competitively limit β-catenin interaction with members of the lymphoid enhancer factor/T cell factor family.237 This may lead to cell injury, since β-catenin has been demonstrated to be necessary for protection against Aβ toxicity in neuronal cells.45
However, not all conditions with FoxOs may lead to cell demise. Some studies suggest that the loss of FoxO1, FoxO3a and FoxO4 protein expression may actually lead to an increase in free radical release that can be responsible for oxidative stress.238 In addition, FoxO proteins also may influence early apoptotic membrane PS externalization.25,34 The ability to regulate early apoptotic membrane PS exposure40 and inflammatory cell activity26 can ultimately affect cell survival since activated immune cells can lead to the phagocytic removal of injured cells.33,59 Furthermore, FoxO proteins may be protective during aging and exercise, since FoxO3a activity may enhance vascular smooth muscle antioxidant properties in aged animals and be beneficial to the cardiovascular system during physical exertion.239
Future Perspectives
As biomarkers for disease onset and progression as well as candidates for the treatment of numerous disorders, EPO and FoxO transcription factors generate excitement for the potential to yield new strategies for the treatment of neurovascular injury and cognitive disorders. Yet, some considerations for EPO exist. In addition to the problems associated with EPO abuse and gene doping,240–242 EPO has been correlated with the alteration of red cell membrane properties leading to a cognitive decrement in rodent animal models.73,74,223 Development of potentially detrimental side-effects during EPO therapy, such as for cerebral ischemia with increased metabolic rate and blood viscosity,243 could also severely limit the use of EPO for neurovascular diseases. As a result, alternate strategies have been suggested. New proposals examine the role of targeted bioavailability for EPO such as in bone marrow stromal cells genetically engineered to secrete EPO244 and controlled release of EPO from encapsulated cells.245,246 The passage of EPO entry into the CNS continues to attract significant interest247 as well as does the use of novel intranasal routes for EPO administration.196 The development of derivations of EPO to reduce erythropoietic activity and the potential associated vascular complications197 have also been put forth as new directions for treatment. Yet, these lines of investigation are not without limitations, since chemical derivatives of EPO can become absent of clinical efficacy73,74 as well as possibly loose the ability to promote sustainable cytoprotective effects, such as neurogenesis248 and angiogenesis.249–252
Other work also offers additional support for the use of FoxO proteins as biomarkers of neurovascular injury that can occur during AD and cognitive loss. Down regulation of the phosphatidylinositol 3 kinase and Akt pathways have been associated with increased transcript levels for FOXO1a and FOXO3a in cell loss scenarios.253 The known mutations in FoxO proteins that exist in several disease entities may provide novel insights for the new treatment strategies. Future analysis in larger populations of patients with metabolic disease and cognitive loss could strengthen our understanding of the role of FoxO proteins in these disorders. In addition, targeting the activity of FoxO1, FoxO3a or FoxO4 in vascular cells may prevent the onset of pathological neointimal hyperplasia that may result in atherosclerosis and cognitive loss. Recent studies also suggest that the utilization and combination of multiple biomarkers may improve risk assessment for patients suffering from a number of disorders.254 These studies illustrate that FoxO proteins may serve as biomarkers of disease activity such as in individualswith imminent cardiac failure.255
As combined therapeutic entities and biomarkers, EPO and FoxO proteins share a number of pathways to offer novel therapeutic strategies for a broad range of disorders. Future studies that involve basic research as well as clinical trials are warranted for EPO and FoxO proteins. Yet, critical to this process is the clear focus upon the intricate cellular pathways governed by EPO and FoxOs to uncover the benefits and risks of these agents for development of proper therapies to prevent the onset or progression of AD and cognitive loss.
Acknowledgements
We apologize to our colleagues whose work we were unable to cite as a result of article space limitations. This research was supported by the following grants to K.M.: American Diabetes Association, American Heart Association (National), Bugher Foundation Award, Janssen Neuroscience Award, LEARN Foundation Award, MI Life Sciences Challenge Award, Nelson Foundation Award, NIH NIEHS (P30 ES06639), NIH NIA and NIH NINDS.
Footnotes
Previously published online: www.landesbioscience.com/journals/oximed/article/9990
References
- 1.Mendiondo MS, Kryscio RJ, Schmitt FA. Models of progression in AD: predicting disability and costs. Neurology. 2001;57:943–944. doi: 10.1212/wnl.57.6.943. [DOI] [PubMed] [Google Scholar]
- 2.McCormick WC, Hardy J, Kukull WA, Bowen JD, Teri L, Zitzer S, et al. Healthcare utilization and costs in managed care patients with Alzheimer’s disease during the last few years of life. J Am Geriatr Soc. 2001;49:1156–1160. doi: 10.1046/j.1532-5415.2001.49231.x. [DOI] [PubMed] [Google Scholar]
- 3.Chong ZZ, Li F, Maiese K. Oxidative stress in the brain: Novel cellular targets that govern survival during neurodegenerative disease. Prog Neurobiol. 2005;75:207–246. doi: 10.1016/j.pneurobio.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Maiese K. Triple play: Promoting neurovascular longevity with nicotinamide, WNT and erythropoietin in diabetes mellitus. Biomed Pharmacother. 2008;62:218–232. doi: 10.1016/j.biopha.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maiese K, Chong ZZ, Hou J, Shang YC. Erythropoietin and oxidative stress. Curr Neurovasc Res. 2008;5:125–142. doi: 10.2174/156720208784310231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Regulska M, Leskiewicz M, Budziszewska B, Kutner A, Jantas D, Basta-Kaim A, et al. Inhibitory effects of 1,25-dihydroxyvitamin D(3) and its low-calcemic analogues on staurosporine-induced apoptosis. Pharmacol Rep. 2007;59:393–401. [PubMed] [Google Scholar]
- 7.Chlopicki S, Swies J, Mogielnicki A, Buczko W, Bartus M, Lomnicka M, et al. 1-Methylnicotinamide (MNA), a primary metabolite of nicotinamide, exerts anti-thrombotic activity mediated by a cyclooxygenase-2/prostacyclin pathway. Br J Pharmacol. 2007;152:230–239. doi: 10.1038/sj.bjp.0707383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chong ZZ, Lin SH, Maiese K. Nicotinamide Modulates Mitochondrial Membrane Potential and Cysteine Protease Activity during Cerebral Vascular Endothelial Cell Injury. J Vasc Res. 2002;39:131–147. doi: 10.1159/000057762. [DOI] [PubMed] [Google Scholar]
- 9.Feng Y, Paul IA, LeBlanc MH. Nicotinamide reduces hypoxic ischemic brain injury in the newborn rat. Brain Res Bull. 2006;69:117–122. doi: 10.1016/j.brainresbull.2005.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hara N, Yamada K, Shibata T, Osago H, Hashimoto T, Tsuchiya M. Elevation of cellular NAD levels by nicotinic acid and involvement of nicotinic acid phosphoribosyltransferase in human cells. J Biol Chem. 2007;282:24574–24582. doi: 10.1074/jbc.M610357200. [DOI] [PubMed] [Google Scholar]
- 11.Ieraci A, Herrera DG. Nicotinamide Protects against Ethanol-Induced Apoptotic Neurodegeneration in the Developing Mouse Brain. PLoS Med. 2006;3:101. doi: 10.1371/journal.pmed.0030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin SH, Vincent A, Shaw T, Maynard KI, Maiese K. Prevention of nitric oxide-induced neuronal injury through the modulation of independent pathways of programmed cell death. J Cereb Blood Flow Metab. 2000;20:1380–1391. doi: 10.1097/00004647-200009000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Maiese K, Chong ZZ. Nicotinamide: necessary nutrient emerges as a novel cytoprotectant for the brain. Trends Pharmacol Sci. 2003;24:228–232. doi: 10.1016/S0165-6147(03)00078-6. [DOI] [PubMed] [Google Scholar]
- 14.Chong ZZ, Li F, Maiese K. Attempted Cell Cycle Induction in Post-Mitotic Neurons Occurs in Early and Late Apoptotic Programs Through Rb, E2F1 and Caspase 3. Curr Neurovasc Res. 2006;3:25–39. doi: 10.2174/156720206775541741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Felice FG, Velasco PT, Lambert MP, Viola K, Fernandez SJ, Ferreira ST, et al. Abeta oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J Biol Chem. 2007;282:11590–11601. doi: 10.1074/jbc.M607483200. [DOI] [PubMed] [Google Scholar]
- 16.Lin SH, Maiese K. The metabotropic glutamate receptor system protects against ischemic free radical programmed cell death in rat brain endothelial cells. J Cereb Blood Flow Metab. 2001;21:262–275. doi: 10.1097/00004647-200103000-00010. [DOI] [PubMed] [Google Scholar]
- 17.He XL, Wang YH, Gao M, Li XX, Zhang TT, Du GH. Baicalein protects rat brain mitochondria against chronic cerebral hypoperfusion-induced oxidative damage. Brain Res. 2009;1249:212–221. doi: 10.1016/j.brainres.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Maiese K, Chong ZZ, Shang YC, Hou J. Therapeutic promise and principles: Metabotropic glutamate receptors. Oxid Med Cell Longev. 2008;1:1–14. doi: 10.4161/oxim.1.1.6842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plecita-Hlavata L, Lessard M, Santorova J, Bewersdorf J, Jezek P. Mitochondrial oxidative phosphorylation and energetic status are reflected by morphology of mitochondrial network in INS-1E and HEP-G2 cells viewed by 4Pi microscopy. Biochim Biophys Acta. 2008;1777:834–846. doi: 10.1016/j.bbabio.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Chong ZZ, Li F, Maiese K. Stress in the brain: novel cellular mechanisms of injury linked to Alzheimer’s disease. Brain Res Brain Res Rev. 2005;49:1–21. doi: 10.1016/j.brainresrev.2004.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris SE, Fox H, Wright AF, Hayward C, Starr JM, Whalley LJ, et al. A genetic association analysis of cognitive ability and cognitive ageing using 325 markers for 109 genes associated with oxidative stress or cognition. BMC Genet. 2007;8:43. doi: 10.1186/1471-2156-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leuner K, Hauptmann S, Abdel-Kader R, Scherping I, Keil U, Strosznajder JB, et al. Mitochondrial dysfunction: the first domino in brain aging and Alzheimer’s disease? Antioxid Redox Signal. 2007;9:1659–1675. doi: 10.1089/ars.2007.1763. [DOI] [PubMed] [Google Scholar]
- 23.Okouchi M, Ekshyyan O, Maracine M, Aw TY. Neuronal apoptosis in neurodegeneration. Antioxid Redox Signal. 2007;9:1059–1096. doi: 10.1089/ars.2007.1511. [DOI] [PubMed] [Google Scholar]
- 24.Chong ZZ, Kang JQ, Maiese K. Akt1 drives endothelial cell membrane asymmetry and microglial activation through Bcl-x(L) and caspase 1, 3 and 9. Exp Cell Res. 2004;296:196–207. doi: 10.1016/j.yexcr.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 25.Chong ZZ, Li F, Maiese K. The pro-survival pathways of mTOR and protein kinase B target glycogen synthase kinase-3beta and nuclear factor-kappaB to foster endogenous microglial cell protection. Int J Mol Med. 2007;19:263–272. [PMC free article] [PubMed] [Google Scholar]
- 26.Kang JQ, Chong ZZ, Maiese K. Critical role for Akt1 in the modulation of apoptotic phosphatidylserine exposure and microglial activation. Mol Pharmacol. 2003;64:557–569. doi: 10.1124/mol.64.3.557. [DOI] [PubMed] [Google Scholar]
- 27.Karunakaran S, Diwakar L, Saeed U, Agarwal V, Ramakrishnan S, Iyengar S, et al. Activation of apoptosis signal regulating kinase 1 (ASK1) and translocation of death-associated protein, Daxx, in substantia nigra pars compacta in a mouse model of Parkinson’s disease: protection by alpha-lipoic acid. Faseb J. 2007;21:2226–2236. doi: 10.1096/fj.06-7580com. [DOI] [PubMed] [Google Scholar]
- 28.Verdaguer E, Susana Gde A, Clemens A, Pallas M, Camins A. Implication of the transcription factor E2F-1 in the modulation of neuronal apoptosis. Biomed Pharmacother. 2007;61:390–399. doi: 10.1016/j.biopha.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 29.Chong ZZ, Li F, Maiese K. Employing new cellular therapeutic targets for Alzheimer’s disease: a change for the better? Curr Neurovasc Res. 2005;2:55–72. doi: 10.2174/1567202052773508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maiese K, Chong ZZ, Shang YC, Hou J. Rogue proliferation versus restorative protection: where do we draw the line for Wnt and forkhead signaling? Expert opinion on therapeutic targets. 2008;12:905–916. doi: 10.1517/14728222.12.7.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maiese K, Vincent A, Lin SH, Shaw T. Group I and Group III metabotropic glutamate receptor subtypes provide enhanced neuroprotection. J Neurosci Res. 2000;62:257–272. doi: 10.1002/1097-4547(20001015)62:2<257::AID-JNR10>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 32.Mari C, Karabiyikoglu M, Goris ML, Tait JF, Yenari MA, Blankenberg FG. Detection of focal hypoxicischemic injury and neuronal stress in a rodent model of unilateral MCA occlusion/reperfusion using radiolabeled annexin V. Eur J Nucl Med Mol Imaging. 2004;31:733–739. doi: 10.1007/s00259-004-1473-5. [DOI] [PubMed] [Google Scholar]
- 33.Chong ZZ, Kang J, Li F, Maiese K. mGluRI Targets Microglial Activation and Selectively Prevents Neuronal Cell Engulfment Through Akt and Caspase Dependent Pathways. Curr Neurovasc Res. 2005;2:197–211. doi: 10.2174/1567202054368317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li F, Chong ZZ, Maiese K. Microglial integrity is maintained by erythropoietin through integration of Akt and its substrates of glycogen synthase kinase-3beta, beta-catenin and nuclear factor-kappaB. Curr Neurovasc Res. 2006;3:187–201. doi: 10.2174/156720206778018758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chong ZZ, Kang JQ, Maiese K. Metabotropic glutamate receptors promote neuronal and vascular plasticity through novel intracellular pathways. Histol Histopathol. 2003;18:173–189. doi: 10.14670/HH-18.173. [DOI] [PubMed] [Google Scholar]
- 36.Kang JQ, Chong ZZ, Maiese K. Akt1 protects against inflammatory microglial activation through maintenance of membrane asymmetry and modulation of cysteine protease activity. J Neurosci Res. 2003;74:37–51. doi: 10.1002/jnr.10740. [DOI] [PubMed] [Google Scholar]
- 37.Mallat M, Marin-Teva JL, Cheret C. Phagocytosis in the developing CNS: more than clearing the corpses. Curr Opin Neurobiol. 2005;15:101–107. doi: 10.1016/j.conb.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 38.Li F, Chong ZZ, Maiese K. Cell Life Versus Cell Longevity: The Mysteries Surrounding the NAD(+) Precursor Nicotinamide. Curr Med Chem. 2006;13:883–895. doi: 10.2174/092986706776361058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li F, Chong ZZ, Maiese K. Winding through the WNT pathway during cellular development and demise. Histol Histopathol. 2006;21:103–124. doi: 10.14670/hh-21.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chong ZZ, Kang JQ, Maiese K. Erythropoietin fosters both intrinsic and extrinsic neuronal protection through modulation of microglia, Akt1, Bad and caspase-mediated pathways. Br J Pharmacol. 2003;138:1107–1118. doi: 10.1038/sj.bjp.0705161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin SH, Chong ZZ, Maiese K. Cell cycle induction in post-mitotic neurons proceeds in concert with the initial phase of programmed cell death in rat. Neurosci Lett. 2001;310:173–177. doi: 10.1016/s0304-3940(01)02118-8. [DOI] [PubMed] [Google Scholar]
- 42.Maiese K. The dynamics of cellular injury: transformation into neuronal and vascular protection. Histol Histopathol. 2001;16:633–644. doi: 10.14670/HH-16.633. [DOI] [PubMed] [Google Scholar]
- 43.Maiese K, Ahmad I, TenBroeke M, Gallant J. Metabotropic glutamate receptor subtypes independently modulate neuronal intracellular calcium. J Neurosci Res. 1999;55:472–485. doi: 10.1002/(SICI)1097-4547(19990215)55:4<472::AID-JNR7>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 44.Vincent AM, Maiese K. Direct temporal analysis of apoptosis induction in living adherent neurons. J Histochem Cytochem. 1999;47:661–672. doi: 10.1177/002215549904700508. [DOI] [PubMed] [Google Scholar]
- 45.Chong ZZ, Li F, Maiese K. Cellular demise and inflammatory microglial activation during beta-amyloid toxicity are governed by Wnt1 and canonical signaling pathways. Cell Signal. 2007;19:1150–1162. doi: 10.1016/j.cellsig.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shang YC, Chong ZZ, Hou J, Maiese K. The fork-head transcription factor FoxO3a controls micro-glial inflammatory activation and eventual apoptotic injury through caspase 3. Curr Neurovasc Res. 2009;6:20–31. doi: 10.2174/156720209787466064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chong ZZ, Lin SH, Kang JQ, Maiese K. Erythropoietin prevents early and late neuronal demise through modulation of Akt1 and induction of caspase 1, 3 and 8. J Neurosci Res. 2003;71:659–669. doi: 10.1002/jnr.10528. [DOI] [PubMed] [Google Scholar]
- 48.Chong ZZ, Lin SH, Kang JQ, Maiese K. The tyrosine phosphatase SHP2 modulates MAP kinase p38 and caspase 1 and 3 to foster neuronal survival. Cell Mol Neurobiol. 2003;23:561–578. doi: 10.1023/A:1025158314016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maiese K, Boccone L. Neuroprotection by peptide growth factors against anoxia and nitric oxide toxicity requires modulation of protein kinase C. J Cereb Blood Flow Metab. 1995;15:440–449. doi: 10.1038/jcbfm.1995.55. [DOI] [PubMed] [Google Scholar]
- 50.Maiese K, Boniece IR, Skurat K, Wagner JA. Protein kinases modulate the sensitivity of hippocampal neurons to nitric oxide toxicity and anoxia. J Neurosci Res. 1993;36:77–87. doi: 10.1002/jnr.490360109. [DOI] [PubMed] [Google Scholar]
- 51.Maiese K, TenBroeke M, Kue I. Neuroprotection of lubeluzole is mediated through the signal transduction pathways of nitric oxide. J Neurochem. 1997;68:710–714. doi: 10.1046/j.1471-4159.1997.68020710.x. [DOI] [PubMed] [Google Scholar]
- 52.Salinas M, Diaz R, Abraham NG, Ruiz de Galarreta CM, Cuadrado A. Nerve growth factor protects against 6-hydroxydopamine-induced oxidative stress by increasing expression of heme oxygenase-1 in a phosphatidylinositol 3-kinase-dependent manner. J Biol Chem. 2003;278:13898–13904. doi: 10.1074/jbc.M209164200. [DOI] [PubMed] [Google Scholar]
- 53.Leytin V, Allen DJ, Mykhaylov S, Lyubimov E, Freedman J. Thrombin-triggered platelet apoptosis. J Thromb Haemost. 2006;4:2656–2663. doi: 10.1111/j.1538-7836.2006.02200.x. [DOI] [PubMed] [Google Scholar]
- 54.Maiese K, Vincent AM. Critical temporal modulation of neuronal programmed cell injury. Cell Mol Neurobiol. 2000;20:383–400. doi: 10.1023/A:1007070311203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maiese K, Vincent AM. Membrane asymmetry and DNA degradation: functionally distinct determinants of neuronal programmed cell death. J Neurosci Res. 2000;59:568–580. doi: 10.1002/(SICI)1097-4547(20000215)59:4<568::AID-JNR13>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 56.Chong ZZ, Kang JQ, Maiese K. Essential cellular regulatory elements of oxidative stress in early and late phases of apoptosis in the central nervous system. Antioxid Redox Signal. 2004;6:277–287. doi: 10.1089/152308604322899341. [DOI] [PubMed] [Google Scholar]
- 57.Dombroski D, Balasubramanian K, Schroit AJ. Phosphatidylserine expression on cell surfaces promotes antibody-dependent aggregation and thrombosis in beta2-glycoprotein Iimmune mice. J Autoimmun. 2000;14:221–229. doi: 10.1006/jaut.2000.0365. [DOI] [PubMed] [Google Scholar]
- 58.Jessel R, Haertel S, Socaciu C, Tykhonova S, Diehl HA. Kinetics of apoptotic markers in exogeneously induced apoptosis of EL4 cells. J Cell Mol Med. 2002;6:82–92. doi: 10.1111/j.1582-4934.2002.tb00313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chong ZZ, Maiese K. The Src homology 2 domain tyrosine phosphatases SHP-1 and SHP-2: diversified control of cell growth, inflammation and injury. Histol Histopathol. 2007;22:1251–1267. doi: 10.14670/hh-22.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vincent AM, Maiese K. Nitric oxide induction of neuronal endonuclease activity in programmed cell death. Exp Cell Res. 1999;246:290–300. doi: 10.1006/excr.1998.4282. [DOI] [PubMed] [Google Scholar]
- 61.Vincent AM, TenBroeke M, Maiese K. Metabotropic glutamate receptors prevent programmed cell death through the modulation of neuronal endonuclease activity and intracellular pH. Exp Neurol. 1999;155:79–94. doi: 10.1006/exnr.1998.6966. [DOI] [PubMed] [Google Scholar]
- 62.Di Lisa F, Menabo R, Canton M, Barile M, Bernardi P. Opening of the mitochondrial permeability transition pore causes depletion of mitochondrial and cytosolic NAD+ and is a causative event in the death of myocytes in postischemic reperfusion of the heart. J Biol Chem. 2001;276:2571–2575. doi: 10.1074/jbc.M006825200. [DOI] [PubMed] [Google Scholar]
- 63.Chong ZZ, Kang JQ, Maiese K. Apaf-1, Bcl-xL, Cytochrome c and Caspase-9 Form the Critical Elements for Cerebral Vascular Protection by Erythropoietin. J Cereb Blood Flow Metab. 2003;23:320–330. doi: 10.1097/01.WCB.0000050061.57184.AE. [DOI] [PubMed] [Google Scholar]
- 64.Chong ZZ, Lin SH, Li F, Maiese K. The sirtuin inhibitor nicotinamide enhances neuronal cell survival during acute anoxic injury through Akt, Bad, PARP and mitochondrial associated “anti-apoptotic” pathways. Curr Neurovasc Res. 2005;2:271–285. doi: 10.2174/156720205774322584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maiese K, Chong ZZ. Insights into oxidative stress and potential novel therapeutic targets for Alzheimer disease. Restor Neurol Neurosci. 2004;22:87–104. [PubMed] [Google Scholar]
- 66.Li F, Chong ZZ, Maiese K. Navigating novel mechanisms of cellular plasticity with the NAD+ precursor and nutrient nicotinamide. Front Biosci. 2004;9:2500–2520. doi: 10.2741/1412. [DOI] [PubMed] [Google Scholar]
- 67.Wilson FH, Hariri A, Farhi A, Zhao H, Petersen KF, Toka HR, et al. A cluster of metabolic defects caused by mutation in a mitochondrial tRNA. Science. 2004;306:1190–1194. doi: 10.1126/science.1102521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roberts E, Jr, Chih CP. The influence of age of pH regulation in hippocampal slices before, during and after anoxia. J Cereb Blood Flow Metab. 1997;17:560–566. doi: 10.1097/00004647-199705000-00010. [DOI] [PubMed] [Google Scholar]
- 69.Cardella F. Insulin therapy during diabetic ketoacidosis in children. Acta Biomed. 2005;76:49–54. [PubMed] [Google Scholar]
- 70.Kratzsch J, Knerr I, Galler A, Kapellen T, Raile K, Korner A, et al. Metabolic decompensation in children with type 1 diabetes mellitus associated with increased serum levels of the soluble leptin receptor. Eur J Endocrinol. 2006;155:609–614. doi: 10.1530/eje.1.02261. [DOI] [PubMed] [Google Scholar]
- 71.Ito N, Bartunek J, Spitzer KW, Lorell BH. Effects of the nitric oxide donor sodium nitroprusside on intracellular pH and contraction in hypertrophied myocytes. Circulation. 1997;95:2303–2311. doi: 10.1161/01.cir.95.9.2303. [DOI] [PubMed] [Google Scholar]
- 72.Vincent AM, TenBroeke M, Maiese K. Neuronal intracellular pH directly mediates nitric oxideinduced programmed cell death. J Neurobiol. 1999;40:171–184. doi: 10.1002/(sici)1097-4695(199908)40:2<171::aid-neu4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 73.Maiese K, Li F, Chong ZZ. Erythropoietin in the brain: can the promise to protect be fulfilled? Trends Pharmacol Sci. 2004;25:577–583. doi: 10.1016/j.tips.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 74.Maiese K, Li F, Chong ZZ. New avenues of exploration for erythropoietin. JAMA. 2005;293:90–95. doi: 10.1001/jama.293.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fliser D, Haller H. Erythropoietin and treatment of non-anemic conditions-cardiovascular protection. Semin Hematol. 2007;44:212–217. doi: 10.1053/j.seminhematol.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 76.Teramo KA, Widness JA. Increased fetal plasma and amniotic fluid erythropoietin concentrations: markers of intrauterine hypoxia. Neonatology. 2009;95:105–116. doi: 10.1159/000153094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Maiese K. Marking the onset of oxidative stress: Biomarkers and novel strategies. Oxid Med Cell Longev. 2009;2:1. doi: 10.4161/oxim.2.1.8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maiese K, Chong ZZ, Li F, Shang YC. Erythropoietin: Elucidating new cellular targets that broaden therapeutic strategies. Prog Neurobiol. 2008;85:194–213. doi: 10.1016/j.pneurobio.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maiese K, Chong ZZ, Shang YC. Raves and risks for erythropoietin. Cytokine Growth Factor Rev. 2008;19:145–155. doi: 10.1016/j.cytogfr.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chong ZZ, Kang JQ, Maiese K. Erythropoietin is a novel vascular protectant through activation of Akt1 and mitochondrial modulation of cysteine proteases. Circulation. 2002;106:2973–2979. doi: 10.1161/01.cir.0000039103.58920.1f. [DOI] [PubMed] [Google Scholar]
- 81.Chong ZZ, Maiese K. Erythropoietin involves the phosphatidylinositol 3-kinase pathway, 14-3-3 protein and FOXO3a nuclear trafficking to preserve endothelial cell integrity. Br J Pharmacol. 2007;150:839–850. doi: 10.1038/sj.bjp.0707161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mikati MA, Hokayem JA, Sabban ME. Effects of a single dose of erythropoietin on subsequent seizure susceptibility in rats exposed to acute hypoxia at p10. Epilepsia. 2007;48:175–181. doi: 10.1111/j.1528-1167.2006.00900.x. [DOI] [PubMed] [Google Scholar]
- 83.Moon C, Krawczyk M, Paik D, Coleman T, Brines M, Juhaszova M, et al. Erythropoietin, modified to not stimulate red blood cell production, retains its cardioprotective properties. J Pharmacol Exp Ther. 2006;316:999–1005. doi: 10.1124/jpet.105.094854. [DOI] [PubMed] [Google Scholar]
- 84.Um M, Gross AW, Lodish HF. A “classical” homodimeric erythropoietin receptor is essential for the antiapoptotic effects of erythropoietin on differentiated neuroblastoma SHSY5Y and pheochromocytoma PC-12 cells. Cell Signal. 2007;19:634–645. doi: 10.1016/j.cellsig.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 85.Arcasoy MO. The non-haematopoietic biological effects of erythropoietin. Br J Haematol. 2008;141:14–31. doi: 10.1111/j.1365-2141.2008.07014.x. [DOI] [PubMed] [Google Scholar]
- 86.Schumann C, Triantafilou K, Krueger S, Hombach V, Triantafilou M, Becher G, et al. Detection of erythropoietin in exhaled breath condensate of nonhypoxic subjects using a multiplex bead array. Mediators Inflamm. 2006;2006:18061. doi: 10.1155/MI/2006/18061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kaindl AM, Sifringer M, Koppelstaetter A, Genz K, Loeber R, Boerner C, et al. Erythropoietin protects the developing brain from hyperoxia-induced cell death and proteome changes. Ann Neurol. 2008;64:523–534. doi: 10.1002/ana.21471. [DOI] [PubMed] [Google Scholar]
- 88.Yis U, Kurul SH, Kumral A, Tugyan K, Cilaker S, Yilmaz O, et al. Effect of erythropoietin on oxygen-induced brain injury in the newborn rat. Neurosci Lett. 2008;448:245–249. doi: 10.1016/j.neulet.2008.10.060. [DOI] [PubMed] [Google Scholar]
- 89.He Z, Huang L, Wu Y, Wang J, Wang H, Guo L. DDPH: improving cognitive deficits beyond its alpha1-adrenoceptor antagonism in chronic cerebral hypoperfused rats. Eur J Pharmacol. 2008;588:178–188. doi: 10.1016/j.ejphar.2008.03.060. [DOI] [PubMed] [Google Scholar]
- 90.Berkingali N, Warnecke A, Gomes P, Paasche G, Tack J, Lenarz T, et al. Neurite outgrowth on cultured spiral ganglion neurons induced by erythropoietin. Hear Res. 2008;243:121–126. doi: 10.1016/j.heares.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 91.Shah RC, Wilson RS, Tang Y, Dong X, Murray A, Bennett DA. Relation of hemoglobin to level of cognitive function in older persons. Neuroepidemiology. 2009;32:40–46. doi: 10.1159/000170905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bierer R, Peceny MC, Hartenberger CH, Ohls RK. Erythropoietin concentrations and neurodevelopmental outcome in preterm infants. Pediatrics. 2006;118:635–640. doi: 10.1542/peds.2005-3186. [DOI] [PubMed] [Google Scholar]
- 93.Pillai A, Dhandapani KM, Pillai BA, Terry AV, Jr, Mahadik SP. Erythropoietin prevents haloperidol treatment-induced neuronal apoptosis through regulation of BDNF. Neuropsychopharmacology. 2008;33:1942–1951. doi: 10.1038/sj.npp.1301566. [DOI] [PubMed] [Google Scholar]
- 94.Assaraf MI, Diaz Z, Liberman A, Miller WH, Jr, Arvanitakis Z, Li Y, et al. Brain erythropoietin receptor expression in Alzheimer disease and mild cognitive impairment. J Neuropathol Exp Neurol. 2007;66:389–398. doi: 10.1097/nen.0b013e3180517b28. [DOI] [PubMed] [Google Scholar]
- 95.Palazzuoli A, Silverberg D, Iovine F, Capobianco S, Giannotti G, Calabro A, et al. Erythropoietin improves anemia exercise tolerance and renal function and reduces B-typenatriuretic peptide and hospitalization in patients with heart failure and anemia. Am Heart J. 2006;152:1096–1099. doi: 10.1016/j.ahj.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 96.Gleissner CA, Klingenberg R, Staritz P, Koch A, Ehlermann P, Wiggenhauser A, et al. Role of erythropoietin in anemia after heart transplantation. Int J Cardiol. 2006;112:341–347. doi: 10.1016/j.ijcard.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 97.Mocini D, Leone T, Tubaro M, Santini M, Penco M. Structure, production and function of erythropoietin: implications for therapeutical use in cardiovascular disease. Curr Med Chem. 2007;14:2278–2287. doi: 10.2174/092986707781696627. [DOI] [PubMed] [Google Scholar]
- 98.Digicaylioglu M, Garden G, Timberlake S, Fletcher L, Lipton SA. Acute neuroprotective synergy of erythropoietin and insulin-like growth factor I. Proc Natl Acad Sci USA. 2004;101:9855–9860. doi: 10.1073/pnas.0403172101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Genc S, Koroglu TF, Genc K. Erythropoietin as a novel neuroprotectant. Restor Neurol Neurosci. 2004;22:105–119. [PubMed] [Google Scholar]
- 100.Anagnostou A, Liu Z, Steiner M, Chin K, Lee ES, Kessimian N, et al. Erythropoietin receptor mRNA expression in human endothelial cells. Proc Natl Acad Sci USA. 1994;91:3974–3978. doi: 10.1073/pnas.91.9.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ogilvie M, Yu X, Nicolas-Metral V, Pulido SM, Liu C, Ruegg UT, et al. Erythropoietin stimulates proliferation and interferes with differentiation of myoblasts. J Biol Chem. 2000;275:39754–39761. doi: 10.1074/jbc.M004999200. [DOI] [PubMed] [Google Scholar]
- 102.Fenjves ES, Ochoa MS, Cabrera O, Mendez AJ, Kenyon NS, Inverardi L, et al. Human, nonhuman primate and rat pancreatic islets express erythropoietin receptors. Transplantation. 2003;75:1356–1360. doi: 10.1097/01.TP.0000062862.88375.BD. [DOI] [PubMed] [Google Scholar]
- 103.Chong ZZ, Kang JQ, Maiese K. Erythropoietin: cytoprotection in vascular and neuronal cells. Curr Drug Targets Cardiovasc Haematol Disord. 2003;3:141–154. doi: 10.2174/1568006033481483. [DOI] [PubMed] [Google Scholar]
- 104.Yamaji R, Okada T, Moriya M, Naito M, Tsuruo T, Miyatake K, et al. Brain capillary endothelial cells express two forms of erythropoietin receptor mRNA. Eur J Biochem. 1996;239:494–500. doi: 10.1111/j.1432-1033.1996.0494u.x. [DOI] [PubMed] [Google Scholar]
- 105.Maiese K, Chong Z, Li F. Reducing oxidative stress and enhancing neurovascular longevity during diabetes mellitus. In: Maiese K, editor. Neurovascular Medicine: Pursuing Cellular Longevity for Healthy Aging. New York, NY: Oxford University Press; 2009. pp. 540–564. [Google Scholar]
- 106.Chong ZZ, Kang JQ, Maiese K. Hematopoietic factor erythropoietin fosters neuroprotection through novel signal transduction cascades. J Cereb Blood Flow Metab. 2002;22:503–514. doi: 10.1097/00004647-200205000-00001. [DOI] [PubMed] [Google Scholar]
- 107.Obara N, Imagawa S, Nakano Y, Suzuki N, Yamamoto M, Nagasawa T. Suppression of erythropoietin gene expression by cadmium depends on inhibition of HIF-1, not stimulation of GATA-2. Arch Toxicol. 2003;77:267–273. doi: 10.1007/s00204-003-0444-0. [DOI] [PubMed] [Google Scholar]
- 108.Li CL, Jiang J, Fan YQ, Fu GS, Wang JA, Fan WM. Knockout of the tumor necrosis factor a receptor 1 gene can up-regulate erythropoietin receptor during myocardial ischemiare perfusion injury in mice. Chin Med J (Engl) 2009;122:566–570. [PubMed] [Google Scholar]
- 109.Nagai A, Nakagawa E, Choi HB, Hatori K, Kobayashi S, Kim SU. Erythropoietin and erythropoietin receptors in human CNS neurons, astrocytes, microglia and oligodendrocytes grown in culture. J Neuropathol Exp Neurol. 2001;60:386–392. doi: 10.1093/jnen/60.4.386. [DOI] [PubMed] [Google Scholar]
- 110.Pregi N, Wenker S, Vittori D, Leiros CP, Nesse A. TNFalpha-induced apoptosis is prevented by erythropoietin treatment on SH-SY5Y cells. Exp Cell Res. 2009;315:419–431. doi: 10.1016/j.yexcr.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 111.Maiese K, Chong ZZ, Shang YC, Hou J. A “FOXO” in sight: targeting Foxo proteins from conception to cancer. Med Res Rev. 2009;29:395–418. doi: 10.1002/med.20139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Weigel D, Jurgens G, Kuttner F, Seifert E, Jackle H. The homeotic gene fork head encodes a nuclear protein and is expressed in the terminal regions of the Drosophila embryo. Cell. 1989;57:645–658. doi: 10.1016/0092-8674(89)90133-5. [DOI] [PubMed] [Google Scholar]
- 113.Maiese K, Chong ZZ, Shang YC. “Sly as a FOXO”: New paths with Forkhead signaling in the brain. Curr Neurovasc Res. 2007;4:295–302. doi: 10.2174/156720207782446306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kaestner KH, Knochel W, Martinez DE. Unified nomenclature for the winged helix/forkhead transcription factors. Genes Dev. 2000;14:142–146. [PubMed] [Google Scholar]
- 115.Maiese K, Chong ZZ, Shang YC. OutFOXOing disease and disability: the therapeutic potential of targeting FoxO proteins. Trends Mol Med. 2008;14:219–227. doi: 10.1016/j.molmed.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Maiese K, Chong ZZ, Shang YC, Hou J. FoxO proteins: cunning concepts and considerations for the cardiovascular system. Clin Sci (Lond) 2009;116:191–203. doi: 10.1042/CS20080113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Maiese K, Li F, Chong ZZ. Erythropoietin and cancer. JAMA. 2005;293:1858–1859. doi: 10.1001/jama.293.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bloomer R, Fisher-Wellman K. Systemic oxidative stress is increased to a greater degree in young, obese women following consumption of a high fat meal. Oxid Med Cell Longev. 2009;2:19–25. doi: 10.4161/oxim.2.1.7860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fisher-Wellman K, Bell H, Bloomer R. Oxidative stress and antioxidant defense mechanisms linked to exercise during cardiopulmonary and metabolic disorders. Oxid Med Cell Longev. 2009;2:43–51. doi: 10.4161/oxim.2.1.7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science. 2003;301:215–218. doi: 10.1126/science.1086336. [DOI] [PubMed] [Google Scholar]
- 121.Furuyama T, Nakazawa T, Nakano I, Mori N. Identification of the differential distribution patterns of mRNAs and consensus binding sequences for mouse DAF-16 homologues. Biochem J. 2000;349:629–634. doi: 10.1042/0264-6021:3490629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Furuyama T, Yamashita H, Kitayama K, Higami Y, Shimokawa I, Mori N. Effects of aging and caloric restriction on the gene expression of Foxo 1, 3 and 4 (FKHR, FKHRL1 and AFX) in the rat skeletal muscles. Microsc Res Tech. 2002;59:331–334. doi: 10.1002/jemt.10213. [DOI] [PubMed] [Google Scholar]
- 123.Hoekman MF, Jacobs FM, Smidt MP, Burbach JP. Spatial and temporal expression of FoxO transcription factors in the developing and adult murine brain. Gene Expr Patterns. 2006;6:134–140. doi: 10.1016/j.modgep.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 124.Lappas M, Lim R, Riley C, Rice GE, Permezel M. Localisation and expression of FoxO1 proteins in human gestational tissues. Placenta. 2009;30:256–262. doi: 10.1016/j.placenta.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 125.Maiese K, Chong Z, Hou J, Shang Y. The “O” Class: Crafting clinical care with FoxO transcription factors. In: Maiese K, editor. Forkhead Transcription Factors: Vital Elements in Biology and Medicine. Austin, TX: Landes Bioscience,; 2009. p. 665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Maiese K, Chong ZZ, Shang YC, Hou J. Clever cancer strategies with FoxO transcription factors. Cell Cycle. 2008;7:3829–3839. doi: 10.4161/cc.7.24.7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Modur V, Nagarajan R, Evers BM, Milbrandt J. FOXO proteins regulate tumor necrosis factor-related apoptosis inducing ligand expression. Implications for PTEN mutation in prostate cancer. J Biol Chem. 2002;277:47928–47937. doi: 10.1074/jbc.M207509200. [DOI] [PubMed] [Google Scholar]
- 128.Matsuzaki H, Daitoku H, Hatta M, Tanaka K, Fukamizu A. Insulin-induced phosphorylation of FKHR (Foxo1) targets to proteasomal degradation. Proc Natl Acad Sci USA. 2003;100:11285–11290. doi: 10.1073/pnas.1934283100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Plas DR, Thompson CB. Akt activation promotes degradation of tuberin and FOXO3a via the protea-some. J Biol Chem. 2003;278:12361–12366. doi: 10.1074/jbc.M213069200. [DOI] [PubMed] [Google Scholar]
- 130.Jagani Z, Singh A, Khosravi-Far R. FoxO tumor suppressors and BCR-ABL-induced leukemia: a matter of evasion of apoptosis. Biochim Biophys Acta. 2008;1785:63–84. doi: 10.1016/j.bbcan.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.van der Horst A, Burgering BM. Stressing the role of FoxO proteins in lifespan and disease. Nat Rev Mol Cell Biol. 2007;8:440–450. doi: 10.1038/nrm2190. [DOI] [PubMed] [Google Scholar]
- 132.Song M, Park JE, Park SG, Lee do H, Choi HK, Park BC, et al. NSC-87877, inhibitor of SHP-1/2 PTPs, inhibits dual-specificity phosphatase 26 (DUSP26) Biochem Biophys Res Commun. 2009;381:491–495. doi: 10.1016/j.bbrc.2009.02.069. [DOI] [PubMed] [Google Scholar]
- 133.Chong ZZ, Li F, Maiese K. Activating Akt and the brain’s resources to drive cellular survival and prevent inflammatory injury. Histol Histopathol. 2005;20:299–315. doi: 10.14670/hh-20.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gayer CP, Chaturvedi LS, Wang S, Craig DH, Flanigan T, Basson MD. Strain-induced proliferation requires the phosphatidylinositol 3-kinase/AKT/glycogen synthase kinase pathway. J Biol Chem. 2009;284:2001–2011. doi: 10.1074/jbc.M804576200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.An J, Zhang C, Polavarapu R, Zhang X, Yepes M. Tissue-type plasminogen activator and the low-density lipoprotein receptor-related protein induce Akt phosphorylation in the ischemic brain. Blood. 2008;112:2787–2794. doi: 10.1182/blood-2008-02-141630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Slaets H, Dumont D, Vanderlocht J, Noben JP, Leprince P, Robben J, et al. Leukemia inhibitory factor induces an antiapoptotic response in oligodendrocytes through Aktphosphorylation and upregulation of 14-3-3. Proteomics. 2008;8:1237–1247. doi: 10.1002/pmic.200700641. [DOI] [PubMed] [Google Scholar]
- 137.Williams R, Dhillon NK, Hegde ST, Yao H, Peng F, Callen S, et al. Proinflammatory cytokines and HIV-1 synergistically enhance CXCL10 expression in human astrocytes. Glia. 2009;57:734–743. doi: 10.1002/glia.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rodriguez-Blanco J, Martin V, Herrera F, Garcia-Santos G, Antolin I, Rodriguez C. Intracellular signaling pathways involved in post-mitotic dopaminergic PC12 cell death induced by 6-hydroxydopamine. J Neurochem. 2008;107:127–140. doi: 10.1111/j.1471-4159.2008.05588.x. [DOI] [PubMed] [Google Scholar]
- 139.Anitha M, Gondha C, Sutliff R, Parsadanian A, Mwangi S, Sitaraman SV, et al. GDNF rescues hyperglycemia-induced diabetic enteric neuropathy through activation of the PI3K/Akt pathway. J Clin Invest. 2006;116:344–356. doi: 10.1172/JCI26295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Burgos-Ramos E, Martos-Moreno GA, Lopez MG, Herranz R, Aguado-Llera D, Egea J, et al. The N-terminal tripeptide of insulin-like growth factor-I protects against beta-amyloidinduced somatostatin depletion by calcium and glycogen synthase kinase 3 beta modulation. J Neurochem. 2009;109:360–370. doi: 10.1111/j.1471-4159.2009.05980.x. [DOI] [PubMed] [Google Scholar]
- 141.Burgos-Ramos E, Puebla-Jimenez L, Arilla-Ferreiro E. Minocycline provides protection against beta-amyloid(25–35)-induced alterations of the somatostatin signaling pathway in the rat temporal cortex. Neuroscience. 2008;154:1458–1466. doi: 10.1016/j.neuroscience.2008.04.036. [DOI] [PubMed] [Google Scholar]
- 142.Burgos-Ramos E, Puebla-Jimenez L, Arilla-Ferreiro E. Minocycline prevents Abeta(25-35)-induced reduction of somatostatin and neprilysin content in rat temporal cortex. Life Sci. 2009;84:205–210. doi: 10.1016/j.lfs.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 143.Chong ZZ, Li F, Maiese K. Erythropoietin requires NFkappaB and its nuclear translocation to prevent early and late apoptotic neuronal injury during beta-amyloid toxicity. Curr Neurovasc Res. 2005;2:387–399. doi: 10.2174/156720205774962683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Campos-Esparza MR, Sanchez-Gomez MV, Matute C. Molecular mechanisms of neuroprotection by two natural antioxidant polyphenols. Cell Calcium. 2009;45:358–368. doi: 10.1016/j.ceca.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 145.Kim KH, Oudit GY, Backx PH. Erythropoietin protects against doxorubicin-induced cardiomyopathy via a phosphatidylinositol 3-kinase-dependent pathway. J Pharmacol Exp Ther. 2008;324:160–169. doi: 10.1124/jpet.107.125773. [DOI] [PubMed] [Google Scholar]
- 146.Tajes M, Yeste-Velasco M, Zhu X, Chou SP, Smith MA, Pallas M, et al. Activation of Akt by lithium: Pro-survival pathways in aging. Mech Ageing Dev. 2009. [DOI] [PubMed]
- 147.Maiese K, Chong ZZ, Li F. Driving cellular plasticity and survival through the signal transduction pathways of metabotropic glutamate receptors. Curr Neurovasc Res. 2005;2:425–446. doi: 10.2174/156720205774962692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Salvesen GS, Riedl SJ. Caspase mechanisms. Adv Exp Med Biol. 2008;615:13–23. doi: 10.1007/978-1-4020-6554-5_2. [DOI] [PubMed] [Google Scholar]
- 149.Chong ZZ, Li F, Maiese K. Group I Metabotropic Receptor Neuroprotection Requires Akt and Its Substrates that Govern FOXO3a, Bim and beta-Catenin During Oxidative Stress. Curr Neurovasc Res. 2006;3:107–117. doi: 10.2174/156720206776875830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chong ZZ, Lin SH, Maiese K. The NAD+ precursor nicotinamide governs neuronal survival during oxidative stress through protein kinase B coupled to FOXO3a and mitochondrial membrane potential. J Cereb Blood Flow Metab. 2004;24:728–743. doi: 10.1097/01.WCB.0000122746.72175.0E. [DOI] [PubMed] [Google Scholar]
- 151.Obexer P, Geiger K, Ambros PF, Meister B, Ausserlechner MJ. FKHRL1-mediated expression of Noxa and Bim induces apoptosis via the mitochondria in neuroblastoma cells. Cell Death Differ. 2007;14:534–547. doi: 10.1038/sj.cdd.4402017. [DOI] [PubMed] [Google Scholar]
- 152.Gomez-Gutierrez JG, Souza V, Hao HY, Montes de Oca-Luna R, Dong YB, Zhou HS, et al. Adenovirus-mediated gene transfer of FKHRL1 triple mutant efficiently induces apoptosis in melanoma cells. Cancer biology & therapy. 2006;5:875–883. doi: 10.4161/cbt.5.7.2911. [DOI] [PubMed] [Google Scholar]
- 153.Maiese K, Chong ZZ, Shang YC. Mechanistic insights into diabetes mellitus and oxidative stress. Curr Med Chem. 2007;14:1729–1738. doi: 10.2174/092986707781058968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Maiese K, Morhan SD, Chong ZZ. Oxidative stress biology and cell injury during type 1 and type 2 diabetes mellitus. Curr Neurovasc Res. 2007;4:63–71. doi: 10.2174/156720207779940653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hao J, Shen W, Tian C, Liu Z, Ren J, Luo C, et al. Mitochondrial nutrients improve immune dysfunction in the type 2 diabetic Goto-Kakizaki rats. J Cell Mol Med. 2009;13:701–711. doi: 10.1111/j.1582-4934.2008.00342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kuhad A, Bishnoi M, Tiwari V, Chopra K. Suppression of NFkappabeta signaling pathway by tocotrienol can prevent diabetes associated cognitive deficits. Pharmacol Biochem Behav. 2009;92:251–259. doi: 10.1016/j.pbb.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 157.Wu SY, Wang GF, Liu ZQ, Rao JJ, Lu L, Xu W, et al. Effect of geniposide, a hypoglycemic glucoside, on hepatic regulating enzymes in diabetic mice induced by a high-fat diet and streptozotocin. Acta Pharmacol Sin. 2009;30:202–208. doi: 10.1038/aps.2008.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Guarnieri G, Zanetti M, Vinci P, Cattin MR, Barazzoni R. Insulin resistance in chronic uremia. J Ren Nutr. 2009;19:20–24. doi: 10.1053/j.jrn.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 159.Gossai D, Lau-Cam CA. The effects of taurine, taurine homologs and hypotaurine on cell and membrane antioxidative system alterations caused by type 2 diabetes in rat erythrocytes. Adv Exp Med Biol. 2009;643:359–368. doi: 10.1007/978-0-387-75681-3_37. [DOI] [PubMed] [Google Scholar]
- 160.Maiese K. Diabetic stress: new triumphs and challenges to maintain vascular longevity. Expert Rev Cardiovasc Ther. 2008;6:281–284. doi: 10.1586/14779072.6.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Donahoe SM, Stewart GC, McCabe CH, Mohanavelu S, Murphy SA, Cannon CP, et al. Diabetes and mortality following acute coronary syndromes. JAMA. 2007;298:765–775. doi: 10.1001/jama.298.7.765. [DOI] [PubMed] [Google Scholar]
- 162.Silverberg DS, Wexler D, Iaina A, Schwartz D. The interaction between heart failure and other heart diseases, renal failure and anemia. Semin Nephrol. 2006;26:296–306. doi: 10.1016/j.semnephrol.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 163.Chong ZZ, Shang YC, Maiese K. Vascular injury during elevated glucose can be mitigated by erythropoietin and Wnt signaling. Curr Neurovasc Res. 2007;4:194–204. doi: 10.2174/156720207781387150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mason-Garcia M, Beckman BS, Brookins JW, Powell JS, Lanham W, Blaisdell S, et al. Development of a new radioimmunoassay for erythropoietin using recombinant erythropoietin. Kidney Int. 1990;38:969–975. doi: 10.1038/ki.1990.299. [DOI] [PubMed] [Google Scholar]
- 165.Namiuchi S, Kagaya Y, Ohta J, Shiba N, Sugi M, Oikawa M, et al. High serum erythropoietin level is associated with smaller infarct size in patients with acute myocardial infarction who undergo successful primary percutaneous coronary intervention. J Am Coll Cardiol. 2005;45:1406–1412. doi: 10.1016/j.jacc.2005.01.043. [DOI] [PubMed] [Google Scholar]
- 166.Chattopadhyay M, Walter C, Mata M, Fink DJ. Neuroprotective effect of herpes simplex virus-mediated gene transfer of erythropoietin in hyperglycemic dorsal root ganglion neurons. Brain. 2009;132:879–888. doi: 10.1093/brain/awp014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Avasarala JR, Konduru SS. Recombinant erythropoietin downregulates IL-6 and CXCR4 genes in TNFalpha-treated primary cultures of human micro-vascular endothelial cells: implications for multiple sclerosis. J Mol Neurosci. 2005;25:183–189. doi: 10.1385/JMN:25:2:183. [DOI] [PubMed] [Google Scholar]
- 168.Miki T, Miura T, Yano T, Takahashi A, Sakamoto J, Tanno M, et al. Alteration in erythropoietin-induced cardioprotective signaling by postinfarct ventricular remodeling. J Pharmacol Exp Ther. 2006;317:68–75. doi: 10.1124/jpet.105.095745. [DOI] [PubMed] [Google Scholar]
- 169.Espada J, Calvo MB, Diaz-Prado S, Medina V. Wnt signalling and cancer stem cells. Clin Transl Oncol. 2009;11:411–427. doi: 10.1007/s12094-009-0380-4. [DOI] [PubMed] [Google Scholar]
- 170.Wilusz M, Majka M. Role of the Wnt/beta-catenin network in regulating hematopoiesis. Arch Immunol Ther Exp (Warsz) 2008;56:257–266. doi: 10.1007/s00005-008-0029-y. [DOI] [PubMed] [Google Scholar]
- 171.Maiese K, Li F, Chong ZZ, Shang YC. The Wnt signaling pathway: Aging gracefully as a protectionist? Pharmacol Ther. 2008;118:58–81. doi: 10.1016/j.pharmthera.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kikuchi A, Yamamoto H, Sato A. Selective activation mechanisms of Wnt signaling pathways. Trends Cell Biol. 2009;19:119–129. doi: 10.1016/j.tcb.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 173.Grant SF, Thorleifsson G, Reynisdottir I, Benediktsson R, Manolescu A, Sainz J, et al. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet. 2006;38:320–323. doi: 10.1038/ng1732. [DOI] [PubMed] [Google Scholar]
- 174.Lehman DM, Hunt KJ, Leach RJ, Hamlington J, Arya R, Abboud HE, et al. Haplotypes of Transcription Factor 7-Like 2 (TCF7L2) Gene and Its Upstream Region Are Associated With Type 2 Diabetes and Age of Onset in Mexican Americans. Diabetes. 2007;56:389–393. doi: 10.2337/db06-0860. [DOI] [PubMed] [Google Scholar]
- 175.Scott LJ, Bonnycastle LL, Willer CJ, Sprau AG, Jackson AU, Narisu N, et al. Association of transcription factor 7-like 2 (TCF7L2) variants with type 2 diabetes in a Finnish sample. Diabetes. 2006;55:2649–2653. doi: 10.2337/db06-0341. [DOI] [PubMed] [Google Scholar]
- 176.Guo YF, Xiong DH, Shen H, Zhao LJ, Xiao P, Guo Y, et al. Polymorphisms of the lowdensity lipoprotein receptor-related protein 5 (LRP5) gene are associated with obesity phenotypes in a large family-based association study. J Med Genet. 2006;43:798–803. doi: 10.1136/jmg.2006.041715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wright WS, Longo KA, Dolinsky VW, Gerin I, Kang S, Bennett CN, et al. Wnt10b Inhibits Obesity in ob/ob and Agouti Mice. Diabetes. 2007;56:295–303. doi: 10.2337/db06-1339. [DOI] [PubMed] [Google Scholar]
- 178.Lin CL, Wang JY, Huang YT, Kuo YH, Surendran K, Wang FS. Wnt/beta-catenin signaling modulates survival of high glucose-stressed mesangial cells. J Am Soc Nephrol. 2006;17:2812–2820. doi: 10.1681/ASN.2005121355. [DOI] [PubMed] [Google Scholar]
- 179.Kim JR, Jung HS, Bae SW, Kim JH, Park BL, Choi YH, et al. Polymorphisms in FOXO gene family and association analysis with BMI. Obesity (Silver Spring, Md) 2006;14:188–193. doi: 10.1038/oby.2006.24. [DOI] [PubMed] [Google Scholar]
- 180.Marchetti V, Menghini R, Rizza S, Vivanti A, Feccia T, Lauro D, et al. Benfotiamine counteracts glucose toxicity effects on endothelial progenitor cell differentiation via Akt/FoxO signaling. Diabetes. 2006;55:2231–2237. doi: 10.2337/db06-0369. [DOI] [PubMed] [Google Scholar]
- 181.Fallarino F, Bianchi R, Orabona C, Vacca C, Belladonna ML, Fioretti MC, et al. CTLA-4-Ig activates forkhead transcription factors and protects dendritic cells from oxidative stress in nonobese diabetic mice. J Exp Med. 2004;200:1051–1062. doi: 10.1084/jem.20040942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Nakae J, Cao Y, Oki M, Orba Y, Sawa H, Kiyonari H, et al. Forkhead transcription factor FoxO1 in adipose tissue regulates energy storage and expenditure. Diabetes. 2008;57:563–576. doi: 10.2337/db07-0698. [DOI] [PubMed] [Google Scholar]
- 183.Puig O, Tjian R. Transcriptional feedback control of insulin receptor by dFOXO/FOXO1. Genes Dev. 2005;19:2435–2446. doi: 10.1101/gad.1340505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kamagate A, Dong HH. Foxo1 integrates insulin signaling to VLDL production. Cell Cycle. 2008;7:3162–3170. doi: 10.4161/cc.7.20.6882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ni YG, Wang N, Cao DJ, Sachan N, Morris DJ, Gerard RD, et al. FoxO transcription factors activate Akt and attenuate insulin signaling in heart by inhibiting protein phosphatases. Proc Natl Acad Sci USA. 2007;104:20517–20522. doi: 10.1073/pnas.0610290104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kamei Y, Miura S, Suzuki M, Kai Y, Mizukami J, Taniguchi T, et al. Skeletal muscle FOXO1 (FKHR) transgenic mice have less skeletal muscle mass, down-regulated Type I (slow twitch/red muscle) fiber genes, and impaired glycemic control. J Biol Chem. 2004;279:41114–41123. doi: 10.1074/jbc.M400674200. [DOI] [PubMed] [Google Scholar]
- 187.Liu CM, Yang Z, Liu CW, Wang R, Tien P, Dale R, et al. Effect of RNA oligonucleotide targeting Foxo-1 on muscle growth in normal and cancer cachexia mice. Cancer Gene Ther. 2007;14:945–952. doi: 10.1038/sj.cgt.7701091. [DOI] [PubMed] [Google Scholar]
- 188.Sandri M, Lin J, Handschin C, Yang W, Arany ZP, Lecker SH, et al. PGC-1alpha protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc Natl Acad Sci USA. 2006;103:16260–16265. doi: 10.1073/pnas.0607795103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Balan V, Miller GS, Kaplun L, Balan K, Chong ZZ, Li F, et al. Life span extension and neuronal cell protection by Drosophila nicotinamidase. J Biol Chem. 2008;283:27810–27819. doi: 10.1074/jbc.M804681200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Chong ZZ, Maiese K. Enhanced Tolerance against Early and Late Apoptotic Oxidative Stress in Mammalian Neurons through Nicotinamidase and Sirtuin Mediated Pathways. Curr Neurovasc Res. 2008;5:159–170. doi: 10.2174/156720208785425666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ma R, Xiong N, Huang C, Tang Q, Hu B, Xiang J, et al. Erythropoietin protects PC12 cells from beta-amyloid(25–35)-induced apoptosis via PI3K/Akt signaling pathway. Neuropharmacology. 2009;56:1027–1034. doi: 10.1016/j.neuropharm.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 192.Sun ZK, Yang HQ, Pan J, Zhen H, Wang ZQ, Chen SD, et al. Protective effects of erythropoietin on tau phosphorylation induced by beta-amyloid. J Neurosci Res. 2008;86:3018–3027. doi: 10.1002/jnr.21745. [DOI] [PubMed] [Google Scholar]
- 193.Liu R, Suzuki A, Guo Z, Mizuno Y, Urabe T. Intrinsic and extrinsic erythropoietin enhances neuroprotection against ischemia and reperfusion injury in vitro. J Neurochem. 2006;96:1101–1110. doi: 10.1111/j.1471-4159.2005.03597.x. [DOI] [PubMed] [Google Scholar]
- 194.Meloni BP, Tilbrook PA, Boulos S, Arthur PG, Knuckey NW. Erythropoietin preconditioning in neuronal cultures: signaling, protection from in vitro ischemia, and proteomic analysis. J Neurosci Res. 2006;83:584–693. doi: 10.1002/jnr.20755. [DOI] [PubMed] [Google Scholar]
- 195.Wei L, Han BH, Li Y, Keogh CL, Holtzman DM, Yu SP. Cell death mechanism and protective effect of erythropoietin after focal ischemia in the whisker-barrel cortex of neonatal rats. J Pharmacol Exp Ther. 2006;317:109–116. doi: 10.1124/jpet.105.094391. [DOI] [PubMed] [Google Scholar]
- 196.Yu YP, Xu QQ, Zhang Q, Zhang WP, Zhang LH, Wei EQ. Intranasal recombinant human erythropoietin protects rats against focal cerebral ischemia. Neurosci Lett. 2005;387:5–10. doi: 10.1016/j.neulet.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 197.Montero M, Poulsen FR, Noraberg J, Kirkeby A, van Beek J, Leist M, et al. Comparison of neuroprotective effects of erythropoietin (EPO) and carbamylerythropoietin (CEPO) against ischemia-like oxygen-glucose deprivation (OGD) and NMDA excitotoxicity in mouse hippocampal slice cultures. Exp Neurol. 2007;204:106–117. doi: 10.1016/j.expneurol.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 198.Yamasaki M, Mishima HK, Yamashita H, Kashiwagi K, Murata K, Minamoto A, et al. Neuroprotective effects of erythropoietin on glutamate and nitric oxide toxicity in primary cultured retinal ganglion cells. Brain Res. 2005;1050:15–26. doi: 10.1016/j.brainres.2005.05.037. [DOI] [PubMed] [Google Scholar]
- 199.Yoo JY, Won YJ, Lee JH, Kim JU, Sung IY, Hwang SJ, et al. Neuroprotective effects of erythropoietin posttreatment against kainate-induced excitotoxicity in mixed spinal cultures. J Neurosci Res. 2009;87:150–163. doi: 10.1002/jnr.21832. [DOI] [PubMed] [Google Scholar]
- 200.Bienvenu AL, Ferrandiz J, Kaiser K, Latour C, Picot S. Artesunate-erythropoietin combination for murine cerebral malaria treatment. Acta Trop. 2008;106:104–108. doi: 10.1016/j.actatropica.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 201.Casals-Pascual C, Idro R, Picot S, Roberts DJ, Newton CR. Can erythropoietin be used to prevent brain damage in cerebral malaria? Trends Parasitol. 2009;25:30–36. doi: 10.1016/j.pt.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 202.Kaiser K, Texier A, Ferrandiz J, Buguet A, Meiller A, Latour C, et al. Recombinant human erythropoietin prevents the death of mice during cerebral malaria. J Infect Dis. 2006;193:987–995. doi: 10.1086/500844. [DOI] [PubMed] [Google Scholar]
- 203.Aoshiba K, Onizawa S, Tsuji T, Nagai A. Therapeutic effects of erythropoietin in murine models of endotoxin shock. Crit Care Med. 2009;37:889–898. doi: 10.1097/CCM.0b013e31819b8371. [DOI] [PubMed] [Google Scholar]
- 204.Wagner F, Baumgart K, Simkova V, Georgieff M, Radermacher P, Calzia E. Year in review 2007: Critical Care-shock. Crit Care. 2008;12:227. doi: 10.1186/cc6949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Chen HH, Tarng DC, Lee KF, Wu CY, Chen YC. Epoetin alfa and darbepoetin alfa: effects on ventricular hypertrophy in patients with chronic kidney disease. J Nephrol. 2008;21:543–549. [PubMed] [Google Scholar]
- 206.Mao W, Iwai C, Liu J, Sheu SS, Fu M, Liang CS. Darbepoetin alfa exerts a cardioprotective effect in autoimmune cardiomyopathy via reduction of ER stress and activation of the PI3K/Akt and STAT3 pathways. J Mol Cell Cardiol. 2008;45:250–260. doi: 10.1016/j.yjmcc.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Tascilar O, Cakmak GK, Tekin IO, Emre AU, Ucan BH, Bahadir B, et al. Protective effects of erythropoietin against acute lung injury in a rat model of acute necrotizing pancreatitis. World J Gastroenterol. 2007;13:6172–6182. doi: 10.3748/wjg.v13.i46.6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Wu H, Dong G, Liu H, Xu B, Li D, Jing H. Erythropoietin attenuates ischemiareperfusion induced lung injury by inhibiting tumor necrosis factor-alpha and matrix metalloproteinase-9 expression. Eur J Pharmacol. 2009;602:406–412. doi: 10.1016/j.ejphar.2008.11.037. [DOI] [PubMed] [Google Scholar]
- 209.Chen J, Connor KM, Aderman CM, Willett KL, Aspegren OP, Smith LE. Suppression of retinal neovascularization by erythropoietin siRNA in a mouse model of proliferative retinopathy. Invest Ophthalmol Vis Sci. 2009;50:1329–1335. doi: 10.1167/iovs.08-2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wang ZY, Shen LJ, Tu L, Hu DN, Liu GY, Zhou ZL, et al. Erythropoietin protects retinal pigment epithelial cells from oxidative damage. Free Radic Biol Med. 2009;46:1032–1041. doi: 10.1016/j.freeradbiomed.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 211.Zhong Y, Yao H, Deng L, Cheng Y, Zhou X. Promotion of neurite outgrowth and protective effect of erythropoietin on the retinal neurons of rats. Graefes Arch Clin Exp Ophthalmol. 2007;245:1859–1867. doi: 10.1007/s00417-007-0671-9. [DOI] [PubMed] [Google Scholar]
- 212.Zhong YS, Liu XH, Cheng Y, Min YJ. Erythropoietin with retrobulbar administration protects retinal ganglion cells from acute elevated intraocular pressure in rats. J Ocul Pharmacol Ther. 2008;24:453–459. doi: 10.1089/jop.2008.0021. [DOI] [PubMed] [Google Scholar]
- 213.Tsai JC, Song BJ, Wu L, Forbes M. Erythropoietin: a candidate neuroprotective agent in the treatment of glaucoma. J Glaucoma. 2007;16:567–571. doi: 10.1097/IJG.0b013e318156a556. [DOI] [PubMed] [Google Scholar]
- 214.King VR, Averill SA, Hewazy D, Priestley JV, Torup L, Michael-Titus AT. Erythropoietin and carbamylated erythropoietin are neuroprotective following spinal cord hemisection in the rat. Eur J Neurosci. 2007;26:90–100. doi: 10.1111/j.1460-9568.2007.05635.x. [DOI] [PubMed] [Google Scholar]
- 215.Okutan O, Solaroglu I, Beskonakli E, Taskin Y. Recombinant human erythropoietin decreases myeloperoxidase and caspase-3 activity and improves early functional results after spinal cord injury in rats. J Clin Neurosci. 2007;14:364–368. doi: 10.1016/j.jocn.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 216.Verdonck O, Lahrech H, Francony G, Carle O, Farion R, Van de Looij Y, et al. Erythropoietin protects from post-traumatic edema in the rat brain. J Cereb Blood Flow Metab. 2007;27:1369–1376. doi: 10.1038/sj.jcbfm.9600443. [DOI] [PubMed] [Google Scholar]
- 217.Cherian L, Goodman JC, Robertson C. Neuroprotection with erythropoietin administration following controlled cortical impact injury in rats. J Pharmacol Exp Ther. 2007;322:789–794. doi: 10.1124/jpet.107.119628. [DOI] [PubMed] [Google Scholar]
- 218.Chu K, Jung KH, Lee ST, Kim JH, Kang KM, Kim HK, et al. Erythropoietin reduces epileptogenic processes following status epilepticus. Epilepsia. 2008;49:1723–1732. doi: 10.1111/j.1528-1167.2008.01644.x. [DOI] [PubMed] [Google Scholar]
- 219.Nadam J, Navarro F, Sanchez P, Moulin C, Georges B, Laglaine A, et al. Neuroprotective effects of erythropoietin in the rat hippocampus after pilocarpineinduced status epilepticus. Neurobiol Dis. 2007;25:412–426. doi: 10.1016/j.nbd.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 220.Ferri C, Giuggioli D, Sebastiani M, Colaci M. Treatment of severe scleroderma skin ulcers with recombinant human erythropoietin. Clin Exp Dermatol. 2007;32:287–290. doi: 10.1111/j.1365-2230.2007.02363.x. [DOI] [PubMed] [Google Scholar]
- 221.Cuzzocrea S, Mazzon E, di Paola R, Genovese T, Patel NS, Britti D, et al. Erythropoietin reduces the degree of arthritis caused by type II collagen in the mouse. Arthritis Rheum. 2005;52:940–950. doi: 10.1002/art.20875. [DOI] [PubMed] [Google Scholar]
- 222.Wu Y, Shang Y, Sun S, Liang H, Liu R. Erythropoietin prevents PC12 cells from 1-methyl-4-phenylpyridinium ion-induced apoptosis via the Akt/GSK-3beta/caspase-3 mediated signaling pathway. Apoptosis. 2007;12:1365–1375. doi: 10.1007/s10495-007-0065-9. [DOI] [PubMed] [Google Scholar]
- 223.Li F, Chong ZZ, Maiese K. Erythropoietin on a Tightrope: Balancing Neuronal and Vascular Protection between Intrinsic and Extrinsic Pathways. Neurosignals. 2004;13:265–289. doi: 10.1159/000081963. [DOI] [PubMed] [Google Scholar]
- 224.Contaldo C, Meier C, Elsherbiny A, Harder Y, Trentz O, Menger MD, et al. Human recombinant erythropoietin protects the striated muscle microcirculation of the dorsal skinfold from postischemic injury in mice. Am J Physiol Heart Circ Physiol. 2007;293:274–283. doi: 10.1152/ajpheart.01031.2006. [DOI] [PubMed] [Google Scholar]
- 225.Vairano M, Dello Russo C, Pozzoli G, Battaglia A, Scambia G, Tringali G, et al. Erythropoietin exerts anti-apoptotic effects on rat microglial cells in vitro. Eur J Neurosci. 2002;16:584–592. doi: 10.1046/j.1460-9568.2002.02125.x. [DOI] [PubMed] [Google Scholar]
- 226.Nakamura T, Sakamoto K. Forkhead transcription factor FOXO subfamily is essential for reactive oxygen species-induced apoptosis. Mol Cell Endocrinol. 2007;281:47–55. doi: 10.1016/j.mce.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 227.Barthelemy C, Henderson CE, Pettmann B. Foxo3a induces motoneuron death through the Fas pathway in cooperation with JNK. BMC Neurosci. 2004;5:48. doi: 10.1186/1471-2202-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.You H, Yamamoto K, Mak TW. Regulation of transactivation-independent proapoptotic activity of p53 by FOXO3a. Proc Natl Acad Sci USA. 2006;103:9051–9056. doi: 10.1073/pnas.0600889103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Won CK, Ji HH, Koh PO. Estradiol prevents the focal cerebral ischemic injury-induced decrease of forkhead transcription factors phosphorylation. Neurosci Lett. 2006;398:39–43. doi: 10.1016/j.neulet.2005.12.060. [DOI] [PubMed] [Google Scholar]
- 230.Caporali A, Sala-Newby GB, Meloni M, Graiani G, Pani E, Cristofaro B, et al. Identification of the prosurvival activity of nerve growth factor on cardiac myocytes. Cell Death Differ. 2008;15:299–311. doi: 10.1038/sj.cdd.4402263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Slotkin TA, Seidler FJ. Protein kinase C is a target for diverse developmental neurotoxicants: transcriptional responses to chlorpyrifos, diazinon, dieldrin and divalent nickel in PC12 cells. Brain Res. 2009;1263:23–32. doi: 10.1016/j.brainres.2009.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Slotkin TA, Seidler FJ, Fumagalli F. Targeting of neurotrophic factors, their receptors, and signaling pathways in the developmental neurotoxicity of organophosphates in vivo and in vitro. Brain Res Bull. 2008;76:424–438. doi: 10.1016/j.brainresbull.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Erol A. Unraveling the Molecular Mechanisms Behind the Metabolic Basis of Sporadic Alzheimer’s Disease. J Alzheimers Dis. 2009. [DOI] [PubMed]
- 234.Lu J, Wu DM, Zheng YL, Sun DX, Hu B, Shan Q, et al. Trace amounts of copper exacerbate beta amyloid-induced neurotoxicity in the cholesterol-fed mice through TNF mediated inflammatory pathway. Brain Behav Immun. 2009;23:193–203. doi: 10.1016/j.bbi.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 235.Smith WW, Norton DD, Gorospe M, Jiang H, Nemoto S, Holbrook NJ, et al. Phosphorylation of p66Shc and forkhead proteins mediates Abeta toxicity. J Cell Biol. 2005;169:331–339. doi: 10.1083/jcb.200410041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Mercado-Gomez O, Hernandez-Fonseca K, Villavicencio-Queijeiro A, Massieu L, Chimal-Monroy J, Arias C. Inhibition of Wnt and PI3K signaling modulates GSK-3beta activity and induces morphological changes in cortical neurons: role of tau phosphorylation. Neurochem Res. 2008;33:1599–1609. doi: 10.1007/s11064-008-9714-9. [DOI] [PubMed] [Google Scholar]
- 237.Hoogeboom D, Essers MA, Polderman PE, Voets E, Smits LM, Burgering BM. Interaction of FOXO with {beta}-Catenin Inhibits {beta}-Catenin/T Cell Factor Activity. J Biol Chem. 2008;283:9224–9230. doi: 10.1074/jbc.M706638200. [DOI] [PubMed] [Google Scholar]
- 238.Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 239.Ferrara N, Rinaldi B, Corbi G, Conti V, Stiuso P, Boccuti S, et al. Exercise Training Promotes SIRT1 Activity in Aged Rats. Rejuvenation Res. 2008;11:139–150. doi: 10.1089/rej.2007.0576. [DOI] [PubMed] [Google Scholar]
- 240.Baoutina A, Alexander IE, Rasko JE, Emslie KR. Potential use of gene transfer in athletic performance enhancement. Mol Ther. 2007;15:1751–1766. doi: 10.1038/sj.mt.6300278. [DOI] [PubMed] [Google Scholar]
- 241.Diamanti-Kandarakis E, Konstantinopoulos PA, Papailiou J, Kandarakis SA, Andreopoulos A, Sykiotis GP. Erythropoietin abuse and erythropoietin gene doping: detection strategies in the genomic era. Sports Med. 2005;35:831–840. doi: 10.2165/00007256-200535100-00001. [DOI] [PubMed] [Google Scholar]
- 242.Segura J, Pascual JA, Gutierrez-Gallego R. Procedures for monitoring recombinant erythropoietin and analogues in doping control. Anal Bioanal Chem. 2007;388:1521–1529. doi: 10.1007/s00216-007-1316-x. [DOI] [PubMed] [Google Scholar]
- 243.Frietsch T, Maurer MH, Vogel J, Gassmann M, Kuschinsky W, Waschke KF. Reduced cerebral blood flow but elevated cerebral glucose metabolic rate in erythropoietin over-expressing transgenic mice with excessive erythrocytosis. J Cereb Blood Flow Metab. 2007;27:469–476. doi: 10.1038/sj.jcbfm.9600360. [DOI] [PubMed] [Google Scholar]
- 244.Eliopoulos N, Gagnon RF, Francois M, Galipeau J. Erythropoietin delivery by genetically engineered bone marrow stromal cells for correction of anemia in mice with chronic renal failure. J Am Soc Nephrol. 2006;17:1576–1584. doi: 10.1681/ASN.2005101035. [DOI] [PubMed] [Google Scholar]
- 245.Orive G, De Castro M, Ponce S, Hernandez RM, Gascon AR, Bosch M, et al. Long-term expression of erythropoietin from myoblasts immobilized in biocompatible and neovascularized microcapsules. Mol Ther. 2005;12:283–289. doi: 10.1016/j.ymthe.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 246.Ponce S, Orive G, Hernandez RM, Gascon AR, Canals JM, Munoz MT, et al. In vivo evaluation of EPO-secreting cells immobilized in different alginate-PLL microcapsules. J Control Release. 2006;116:28–34. doi: 10.1016/j.jconrel.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 247.Doolittle ND, Peereboom DM, Christoforidis GA, Hall WA, Palmieri D, Brock PR, et al. Delivery of chemotherapy and antibodies across the blood-brain barrier and the role of chemoprotection, in primary and metastatic brain tumors: report of the Eleventh Annual Blood-Brain Barrier Consortium meeting. J Neurooncol. 2007;81:81–91. doi: 10.1007/s11060-006-9209-y. [DOI] [PubMed] [Google Scholar]
- 248.Gonzalez FF, McQuillen P, Mu D, Chang Y, Wendland M, Vexler Z, et al. Erythropoietin enhances long-term neuroprotection and neurogenesis in neonatal stroke. Dev Neurosci. 2007;29:321–330. doi: 10.1159/000105473. [DOI] [PubMed] [Google Scholar]
- 249.Li Y, Lu Z, Keogh CL, Yu SP, Wei L. Erythropoietin-induced neurovascular protection, angiogenesis and cerebral blood flow restoration after focal ischemia in mice. J Cereb Blood Flow Metab. 2007;27:1043–1054. doi: 10.1038/sj.jcbfm.9600417. [DOI] [PubMed] [Google Scholar]
- 250.Reinders ME, Rabelink TJ, Briscoe DM. Angiogenesis and endothelial cell repair in renal disease and allograft rejection. J Am Soc Nephrol. 2006;17:932–942. doi: 10.1681/ASN.2005121250. [DOI] [PubMed] [Google Scholar]
- 251.Slevin M, Kumar P, Gaffney J, Kumar S, Krupinski J. Can angiogenesis be exploited to improve stroke outcome? Mechanisms and therapeutic potential. Clin Sci. 2006;111:171–183. doi: 10.1042/CS20060049. [DOI] [PubMed] [Google Scholar]
- 252.Zhang SX, Ma JX. Ocular neovascularization: Implication of endogenous angiogenic inhibitors and potential therapy. Prog Retin Eye Res. 2007;26:1–37. doi: 10.1016/j.preteyeres.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 253.Hellwinkel OJ, Rogmann JP, Asong LE, Luebke AM, Eichelberg C, Ahyai S, et al. A comprehensive analysis of transcript signatures of the phosphatidylinositol-3 kinase/protein kinase B signal-transduction pathway in prostate cancer. BJU Int. 2008;101:1454–1460. doi: 10.1111/j.1464-410X.2008.07540.x. [DOI] [PubMed] [Google Scholar]
- 254.Zethelius B, Berglund L, Sundstrom J, Ingelsson E, Basu S, Larsson A, et al. Use of multiple biomarkers to improve the prediction of death from cardiovascular causes. N Engl J Med. 2008;358:2107–2116. doi: 10.1056/NEJMoa0707064. [DOI] [PubMed] [Google Scholar]
- 255.Hannenhalli S, Putt ME, Gilmore JM, Wang J, Parmacek MS, Epstein JA, et al. Transcriptional genomics associates FOX transcription factors with human heart failure. Circulation. 2006;114:1269–1276. doi: 10.1161/CIRCULATIONAHA.106.632430. [DOI] [PubMed] [Google Scholar]