Abstract
Oxidative stress is currently suggested to play a major role in the development of diabetes mellitus. There is an increasing demand of natural anti-diabetic agents, as continuous administration of existing drugs and insulin are associated with many side effects and toxicity. The present study was aimed to investigate the effect of Momordica charantia (MC) and Trigonella foenum graecum (TFG) extracts (aqueous) on antioxidant status and lipid peroxidation in heart tissue of normal and alloxan induced diabetic rats. In a 30 days treatment, rats were divided into six groups (I-VI) of five animals in each, experiments were repeated thrice. Administration of MC (13.33 g pulp/kg body weight/day) and TFG (9 g seeds powder/kg body weight/day) extracts in diabetic rats has remarkably improved the elevated levels of fasting blood glucose. A significant decrease in lipid peroxidation (p<0.001) and significant increase in the activities of key antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), glutathione-s-transferase (GST) and reduced glutathione (GSH ) contents in heart tissue of diabetic rats were observed (group V and VI) upon MC and TFG treatment. Our studies demonstrate the anti-hyperglycemic and anti-oxidative potential of Momordica charantia and Trigonella foenum graecum, which could exert beneficial effects against the diabetes and associated free radicals complications in heart tissue.
Key words: Alloxan, diabetic rat, heart, antioxidant status, lipid peroxidation, Momordica charantia, Trigonella foenum graecum
Introduction
Diabetes mellitus (DM) is characterized by abnormal glucose metabolism which is usually associated with elevated blood glucose levels due to insulin deficiency or resistance, diminished glucose utilization in tissues that require insulin for glucose uptake, tissues in which glucose transport is not regulated by insulin (cardiac tissue, blood vessels, peripheral nerves, renal medulla and ocular lens) face severe and sustained hyperglycemia.1 One of the major complications of diabetes is the progression of cardiovascular disease in both the macro- and micro circulation. Microvascular complications of diabetes include nephropathy and retinopathy, macrovascular complication results in atherosclerotic cardiovascular disease such as coronary artery disease, cerebrovascular disease and peripheral vascular disease which are the leading cause of death in diabetic population.2-4 Several studies had demonstrated that oxidative stress, mediated mainly by hyperglycemia-induced generation of free radicals, contributes to the development and progression of diabetes and related complications. Oxidative stress describes the condition where the amount of reactive oxygen species (ROS) overpowers the amount of neutralizing agents or antioxidants.5–7 Hyperglycemia can induce oxidative stress through advanced glycation end product (AGEs) formation, increased flux through the polyol pathway, increased activation of protein kinase C (PKC) and increased flux through hexosamine pathway.8
Formation of excess superoxide by the mitochondrial transport chain during hyperglycemic has been reported as the initial factor of diabetes induced oxidative stress.9 The formation of superoxide and the subsequent increase in oxidative stress may lead to endothelial dysfunction and ultimately cardiovascular disease (CVD) through several different mechanisms.10 AGEs can produce ROS, bind to receptors that promote oxidative stress and trigger mechanisms that generates intracellular oxidants.11 An increased polyol pathway flux during hyperglycemia is due to increase in aldose reductase (AR) activity in cardiac tissues which reduces glucose to sorbitol by consuming NADPH. AR is also been reported to metabolize GSH-lipid derived aldehyde adducts which results in decrease in GSH and subsequently increases oxidative stress.12,13
Because the production of ROS leads to endothelium dysfunction, a precursor to the development of CVD thus targeting ROS seems to be a logical approach to combat the vascular complications of diabetes. Our body has variety of antioxidants to counteract the damaging effects of ROS. Enzymes such as superoxide dismutase (SOD) converts superoxide to hydrogen peroxide (H2O2) which is then transformed into water by catalase in lysosomes or by glutathione peroxidase (GPx) in mitochondria.14
The diabetes altered activities of these enzymes and reduced level of GSH have been observed which affect the ability to defend against oxidative stress.15 Non enzymatic antioxidant such as vitamin A, C and E are consumed in every day diet. Several animal and human studies were done using these non enzymatic antioxidant to combat endothelial dysfunction resulted from diabetes induced oxidative stress.16,17 Vitamin C and E have been given particular attention because of their known superoxide scavenger property and they have been tested either alone or in combination.2 Not all studies yielded positive result, as some of the studies indicated that antioxidant vitamin supplementation may actually lead to endothelium dysfunction in both diabetic and normal animals.18,19 Long term nonenzymetic antioxidant therapy in humans has not been very successful. A combination of vitamin C and E was given to patients with both type 1 and type 2 diabetes showed that endothelium dysfunction improved only in patient with type 1 diabetes.20
Clinical trials of antioxidant therapy in diabetes especially in the form of supplemental vitamins have not shown decrease risk of cardiovascular outcomes. Therefore, supplementation with vitamin C, E and other antioxidant such as tetrahydrobiopterin (BH4) and benfotiamine should be recommended with caution because there are no adequate data from large clinical trails are available.
Degenerative changes occur in heart, kidney, brain and other neural tissues during diabetes. These include cardiomyopathy, nephropathy and neuropathy and are common complications of diabetes mellitus. This pathogenicity is believed to be due to oxidative damage of the tissues by oxygen free radicals. Several reports are available about the changes in antioxidant enzymes levels in various tissues (liver, kidney and heart) of diabetic rats.21 Several natural products such as Momordica charantia, Azadirachta indica, Gymnema sylvestre, Pterocarpus marsupim, Coccinia indica, Trigonella foenum graecum, Allium sativum and Ocimum sanctum, etc. are being used in India as well as other parts of the world for the management of diabetes and to overcome its complications. These plants are found to be effective and their low cost and minimal side effects have increased the interest of scientists to develop plant based drugs for managing diabetes. Momordica charantia (MC) and Trigonella foenum graecum (TFG) are reported to have beneficial effects in other disease conditions. MC leaf extracts have been reported to possess antibacterial activities against E. coli, Salmonella paratyphi, Shigella dysenterae and Streptomyces griseus. Fruit extracts of MC are reported to have beneficial effects against Helicobacter pylori infection and gastric ulcers.22,23 Inhibitory activity against the larvae of filarial vector, Culex quinquefasciatus has been found in the seed extracts of MC.24 The TFG seed paste (applied externally) is used to treat abscess, boils, ulcers and burns and consumption of TFG seed powder possess beneficial effects against gastritis and gastric ulcers due to bacterial infections.25,26 Several reports are available regarding the anti-hyperglycemic effects of MC and TFG but the data about their antioxidant potential is scantly in heart tissue from diabetes. Therefore, present study has been planed on MC and TFG to evaluate their effects on cardiac antioxidant status in alloxan induced diabetic rats.
Results
Effect on body weight and fasting blood glucose (FBG) levels.
All the rats in all the six groups chosen in the present study were having almost same body weight. Rats in group I-III and V-VI showed almost similar growth during the period (30 days) of study as almost similar gain in weight was observed in these rats whereas rats in group IV (diabetic) showed a significant decrease in body weight, from 184±1.92 to 147±2.98 gram body weight in Table 1.
Table 1.
Change in body weight in control and experimental groups of rats
| Groups | Body weight (grams) | |
| (0 day) | (30th day) | |
| I (Normal control) | 184.3 ± 3.77 | 201.4 ± 2.3 |
| II (Normal + MC) | 183 ± 1.58 | 197.4 ± 3.64 |
| III (Normal + TFG) | 188.6 ± 1.67 | 201.2 ± 2.77 |
| IV (Diabetic control) | 184.2 ± 1.92 | 147 ± 2.91* |
| V (Diabetic + MC) | 184 ± 1.58 | 198.2 ± 1.92* |
| VI (Diabetic + TFG) | 189.6 ± 1.14 | 199.6 ± 2.4* |
Each values is a mean ± SD (n = 5 in each group). *Values are statistically significant at p < 0.001. Normal + MC treated (groups II), normal + TFG treated (group III) and diabetic control (group IV) were compared with normal control (group I). Diabetic + MC treated (group V) and diabetic + TFG treated (groups VI) were compared with diabetic control (group IV). The experiments were repeated thrice. MC, Momordica charantia; TFG, Trigonella foenum graecum.
An intraperitonial dose (150 mg/kg body weight) of alloxan resulted in increase in fasting blood glucose level from 101.4±2.3 mg/dl to 284±3.16 mg/dl after 96 hours of alloxan injection (group IV to VI), this high level of FBG was maintained in group IV (diabetic) throughout the period of study, treatment of diabetic rat with MC extract (group V) and TFG extract (group VI) showed a time dependent decrease in FBG levels. (Table 2)
Table 2.
Fasting blood glucose levels in control and experimental groups of rats
| Groups | Fasting blood glucose levels (mg/dl) | ||||
| 0 day | 7th day | 14th day | 21st day | 30th day | |
| I (Normal control) | 101.4 ± 2.3 | 103 ± 1.87 | 99.8 ± 1.3 | 103.4 ± 1.34 | 101 ± 1.58 |
| II (Normal + MC) | 102.4 ± 1.94 | 99.6 ± 2.07 | 98.6 ± 1.14 | 98 ± 1.58 | 95.2 ± 0.84 |
| III (Normal + TFG) | 103.8 ± 1.92 | 101.4 ± 2.07 | 96.4 ± 0.89 | 94.4 ± 1.51 | 90.8 ± 1.48 |
| IV (Diabetic control) | 284 ± 3.16* | 282 ± 1.58* | 280 ± 1.58* | 276.2 ± 1.48* | 275.8 ± 1.48* |
| V (Diabetic + MC) | 281.4 ± 2.07 | 234.6 ± 1.67* | 183.2 ± 1.64* | 137.4 ± 2* | 116.4 ± 1.81* |
| VI (Diabetic + TFG) | 283.6 ± 2.3 | 229.6 ± 1.14* | 159.8 ± 1.3* | 127 ± 1.58* | 103 ± 1.58* |
Each values is a mean ± SD (n = 5 in each group). *Values are statistically significant at p < 0.001. Normal + MC treated (groups II), normal + TFG treated (group III) and diabetic control (group IV) were compared with normal control (group I). Diabetic + MC treated (group V) and diabetic + TFG treated (groups VI) were compared with diabetic control (group IV). The experiments were repeated thrice. MC, Momordica charantia; TFG, Trigonella foenum graecum.
Levels of cardiac lipid peroxidation.
The TBARS level were significantly increased (p < 0.001) in cardiac tissue of diabetic rats (group IV) by 2.66-fold when compared with normal rats (group I). However there was no significant change was observed in normal rats treated with MC and TFG (group II and III). Treatment of diabetic rats with MC (group V) resulted in significant decrease (p < 0.001) in TBARS level from 105 nM/g tissue (diabetic rats) to 60.00 nM/g tissue. Similarly, TFG treatment to the diabetic rats (group VI) resulted in significant decrease (p<0.001) in TBARS levels from 105.00 nM/g tissue (diabetic rats) to 45.96 nM/g tissue. (Table 3)
Table 3.
Levels of TBARS and GSH in control and experimental groups of rats
| Groups | TBARS | GSH |
| (nmoles/g of tissue) | (μmoles/mg protein) | |
| I (Normal control) | 39.45 ± 1.89 | 0.86 ± 0.25 |
| II (Normal + MC) | 38.16 ± 1.17 | 0.94 ± 0.6 |
| III (Normal + TFG) | 37.9 ± 1.62 | 0.92 ± 0.1 |
| IV (Diabetic control) | 105.3 ± 12.22* | 0.49 ± 0.02* |
| V (Diabetic + MC) | 60.13 ± 2.84* | 1.01 ± 0.09* |
| VI (Diabetic + TFG) | 45.96 ± 4.0* | 1.1 ± 0.12* |
Each values is a mean ± SD (n = 5 in each group). *Values are statistically significant at p < 0.001. Normal + MC treated (groups II), normal + TFG treated (group III) and diabetic control (group IV) were compared with normal control (group I). Diabetic + MC treated (group V) and diabetic + TFG treated (groups VI) were compared with diabetic control (group IV). The experiments were repeated thrice. MC, Momordica charantia; TFG, Trigonella foenum graecum; TBARS, Thiobarbituric acid reactive substances; GSH , Reduced glutathione.
GSH Content.
The GSH content were significantly decreased (p<0.001) in cardiac tissue of diabetic rats (group IV) from 0.86±0.25 to 0.49±0.02 µM/mg protein when compared with normal rats (group I). However there was no significant change was observed in normal rats treated with MC and TFG (group II and III). Treatment of diabetic rats with MC (group V) resulted in significant increase (p<0.001) in GSH content from 0.49±0.02 µM/mg protein (diabetic rats) to 1.01±0.09 µM/mg protein. Similarly, TFG treatment to the diabetic rats (group VI) resulted in significant increase (p<0.001) in GSH contents from 0.49±0.02 µM/mg protein (diabetic rats) to 1.1±0.12 µM/mg protein. (Table 3)
Activities of cardiac antioxidants enzymes.
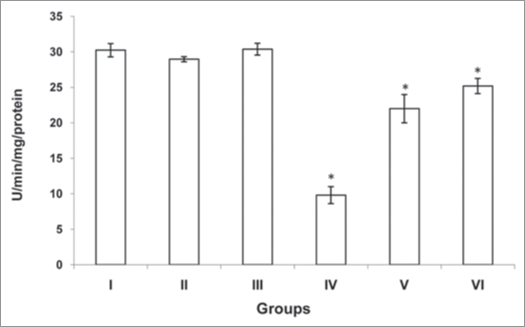
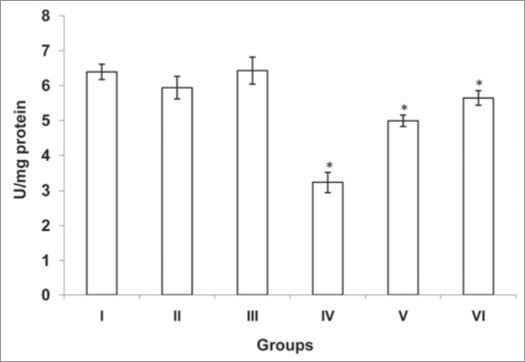
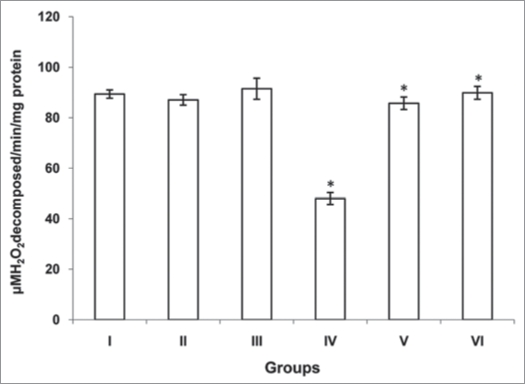
The activities of antioxidants (SOD, CAT and GST) were significantly decreased (p<0.001, p<0.001 and p<0.001) in cardiac tissue of diabetic rats (group IV) when compared with normal rats (group I). However there was no significant change was observed in normal rats treated with MC and TFG (group II and III). Treatment of diabetic rats with MC (group V) resulted in significant increase (p<0.001, p<0.001 and p<0.001) in antioxidants activities. Similarly, TFG treatment to the diabetic rats (group VI) resulted in significant increase (p<0.001, p<0.001 and p<0.001) in antioxidants activities when compared with diabetic rats (group IV) (Figs. 1–3).
Figure 1.
Activity of superoxide dismutase (SOD) in control and experimental groups of rats. SOD activity was measured by the method of Misra and Fridovich.42 The activity was expressed in U/min/mg protein. One unit of enzyme activity has been defined to cause 50% inhibition of auto-oxidation of epinephrine by 1.0 ml of homogenate. Each values is a mean ± SD (n = 5 in each group). *Values are statistically significant at p<0.001. Normal + MC treated (groups II), normal + TFG treated (group III) and diabetic control (group IV) were compared with normal control (group I). Diabetic + MC treated (group V) and diabetic + TFG treated (groups VI) were compared with diabetic control (group IV). The experiments were repeated thrice. MC, Momordica charantia; TFG, Trigonella foenum graecum.
Figure 3.
Activity of glutathione s-tranferase (GST) in control and experimental goups of rats. GST activity was assayed as μmoles of GSH -CDNB conjugate formed /min./mg protein according to the method of Habig et al.43 One unit of GST activity is defined as the amount of enzyme catalyzing the formation of 1.0 μ mole of product per min/mg protein under assay conditions. Each values is a mean ± SD (n = 5 in each group). *Values are statistically significant at p < 0.001. Normal + MC treated (groups II), normal + TFG treated (group III) and diabetic control (group IV) were compared with normal control (group I). Diabetic + MC treated (group V) and diabetic + TFG treated (groups VI) were compared with diabetic control (group IV). The experiments were repeated thrice. MC, Momordica charantia; TFG,Trigonella foenum graecum.
Discussion
Cardiovascular disease is one of the major complications of diabetes. Diabetes induced oxidative stress has been implicated in the progression of pathogenesis of the cardiovascular complications and antioxidants were considered as promising therapeutic strategy.2 In spite of the presence of large number of antidibetic pharmaceuticals agents, there is renewed interest in remedies from plants sources because of their lesser side effects and low cost. In the present study, anti-diabetic and antioxidant potentials of aqueous extracts of Momordica charantia and Trigonella foenum graecum have been evaluated in heart tissue in alloxan induced diabetic rats.
A dose of alloxan 150 mg/kg body weight was chosen in this study as up to 140–200 mg/kg body weight alloxan doses were reported to non lethal. In this range of alloxan dose pancreatic β cells are so damaged that insulin is secreted but not in sufficient amounts to regulate blood glucose levels, resulting in significant increase in fasting blood glucose levels. The suggested mechanism of action of alloxan is generation of oxidative stress in pancreatic tissues.27 Treatment of diabetic rats with aqueous extracts of MC and TFG (13.33 g pulp/kg body weight/day and 9 g paste/kg body weight/day) for 30 days after establishment of hyperglycemia resulted in significant reduction of FBG levels which might be due to enhanced peripheral glucose utilization or these plant extracts potentiate the insulin effect by rejuvenation of damaged pancreatic β cell.
Hypoinsulinemia due to alloxan induced diabetes leads to several biochemical alterations including lipid peroxidation.28 Increased plasma total lipid, triglycerides and total cholesterol are common in diabetics. This may be due to increased activity of lipases (sensitive to insulin) which results in increased lipolysis. The levels of other hormones such as glucagon and catacholamines are also increased in diabetics, compound the effect by stimulating lipolysis. Increased lipid peroxidation impairs membrane functions, its product ate harmful to most of the cells in the body and associated with a variety of diseases.29 Our present study showed a significant (p < 0.001) increase in TBARS content in heart tissue of diabetic rats suggesting that peroxidative injury may be involved in diabetes related cardiac dysfunctions. The aqueous extract of MC and TFG could significantly lower the elevated heart lipid peroxidation products levels (Table 3). This indicates that aqueous extract of MC and TFG may contain potent inhibitory molecule (s) to protect heart tissue from oxidative damage. The significant improvement in serum lipid profile and lipid peroxide formation (in liver and kidney) was observed when diabetic rats were treated with TFG and MC.30,31
Reduced glutathione (GSH) is known to protect the cellular system against the toxic effects of lipid peroxidation.32 GSH function as direct free radical scavenger, as a co-substrate for glutathione peroxidase (GPx) activity and as a cofactor for many enzymes and forms conjugates in endo and xenobiotic reactions.33 Several studies support the hypothesis that prolonged hyperglycemia up regulate the polyol pathways as well as advanced glycation end products formation and free radical generation rates. A relative depletion of NADPH occurs due to AR activation in heart tissue of diabetic rats. Role of AR has also been implicated in detoxifications of lipid peroxidation products such as 4-hydroxynonenal (4-HNE) and malondialdehyde (MDA) in the form of GSH-aldehyde adducts.12 All these may lead to depleted GSH levels in heart tissue. Our results also in accordance to these finding as decreased GSH levels were estimated in heart tissue because of hyperglycemia induced oxidative stress as demonstrated by increased lipid peroxidation and was significantly recovered in diabetic rats who were fed with MC and TFG extracts. These findings are in accordance with previous report where treatment of diabetic rats with MC extract was found to restore the GSH content in RBC.31
Reduced Activities of SOD and CAT in heart tissue of diabetic rats have been observed in our study. The decrease in activities of SOD and CAT in diabetic rats may be due to increased production of reactive oxygen radicals that can themselves reduce the activity of these enzymes.34 SOD is an important defense enzyme which converts superoxide to H2O2.35 CAT is hemeprotein, which decomposes H2O2 and protects the tissues from highly reactive OH·−.36 The reduction of these enzymes in heart tissue may lead to number of deleterious effects. Administration of MC and TFG restores the activities of these enzymes and may help to avoid the deleterious effects of free radicals generated during diabetes.
In conclusion, the present study demonstrates that Momordica charantia and Trigonella foenum graecum exhibit antiperoxidative and antioxidant activities in heart tissue of alloxan induced diabetic rats by decreasing the levels of lipid peroxidation products and increasing the levels/activities of antioxidants. Thus, the role of these plant extracts in management of diabetes of paramount importance as these plant extracts may serve various purposes in diabetics- lower of blood glucose levels, delay complications (atherosclerosis, nephropathy, neuropathy and gastroparesis etc). Their anti-infective properties may be an added benefit as diabetic are known to be more susceptible to infections. Further investigations are necessary to find out the active components (s) present in these plant extracts and their mechanism of action and to establish their therapeutic potential in the treatment of diabetes and related complications.
Methods
Plant material.
MC fruits and TFG seeds were purchased from a local vegetable market in Lucknow. Seeds were clean, dry and finally powdered.
Preparation of extracts.
Aqueous extract of MC. Fresh fruits (250 g) were taken and the seeds were removed. The fleshy parts were cut into small pieces and macerated with 250 ml TDW using electrical blander. This suspension was squeezed through a sterile muslin cloth, and the liquid was centrifuged at 5000 rpm for 30 minutes in the cold. The supernatant was lyophilized at low temperature and reduced pressure by the method of Karunanayaka et al.37 using Christ alpha 1–4, Germany.
Aqueous extract of TFG seed powder.
250g of powdered TFG seeds were boiled in 2500 ml distilled water for 30 mins. Then, the decoction was cooled for 30 mins at room temperature. Next, the cooled decoction was filtered through a coarse sieve twice. Finally, the filtrate was concentrated by rotavapour (Buchi, R-210, Germany) at 50°C to a thick paste.38
Animals and treatments.
Animals. Male albino wistar rats weighing 150 to 200g were purchased from Central Drug Research Institute (CDRI), Lucknow, India, for study and housed at 25±5°C in the animal room in the department. They were provided a standard pellet diet (Hindustan Lever Ltd, Mumbai, India) and had free access to water. Prior permission for animal use and approval of the protocol were obtained from the Institutional Animal Ethical Committee.
Induction of diabetes.
Diabetes in rats was induced with a single injection of alloxan monohydrate (150 mg/kg body weight), dissolved in sterile 0.15 M normal saline, by intraperitoneal route.39 Blood samples to measure FBG were obtained by tail vain puncture of all groups of rats, glucose levels were determined on different days using a glucometer (One touch ultra blood glucose monitoring system from Lifescan, Johonson and Johonson Company). After fifth day the development of diabetes was confirmed, rats with FBG range 250–300 mg/dl were considered as diabetic rats and used for the further experiments.
Treatment.
Rats were divided into six groups.
Group I Normal control
Group II Normal + MC treated (13.33 g pulp/kg body weight/day)
Group III Normal + TFG treated (9 g seed powder/kg body weight/day)
Group IV Diabetic control
Group V Diabetic + MC treated (13.33 g pulp/kg body weight/day)
Group VI Diabetic +TFG treated (9 g seed powder/kg body weight/day)
The body weight was measured on the first (after the diabetes was confirmed) and 30th day of the experiment. The extracts were given orally by gastric intubation to the rats of respective groups (II, III, V and VI) once daily for 30 days. Control animals (groups I and IV) received the same amount of normal saline.
After 30 days of treatment, rats were fasted overnight and sacrificed. Heart tissue was collected separately and stored at −20°C.
Chemicals.
Alloxan monohydrate, 5,5′-Dithio-bis 2-nitrobenzeoic acid (DTNB), Epinephrine, glacial metaphosphoric acid and Glutathione were purchased from Sigma chemical company Inc., St Louis, Mo, USA. All other chemicals used were of analytical grade and obtained from SRL (India), Qualigens fine chemicals (India).
Preparation of homogenate.
Heart tissue was washed thoroughly with ice cold saline. 10% (w/v) homogenate was prepared in a Potter Elvehjem type homogenizer in ice-cold 50mM phosphate buffer pH 7.4 containing mammalian protease inhibitor cocktail. The homogenate was centrifuged at 10,000xg for 30 min at 4oC. The supernatant was used for the assay of antioxidant activities/levels and lipid peroxidation.
Estimation of lipid peroxidation.
Lipid peroxidation was estimated in terms of MDA formed, according to the method of Ohkawa, et al.40 using thiobarbituric acid (TBA) reagent. To 0.2 ml of the homogenate, added 0.2 ml sodium dodecyl sulphate [8.1% (w/v)], 1.5 ml glacial acetic acid and thiobarbituric acid [0.8% (w/v)]. The final volume was made to 3.0 ml. The contents of the tubes were vortexed vigorously, heated in a water bath at 90°C for 1 hr and then immediately cooled under running tap water. To each tube, 1.0 ml of water and 5.0 ml of a mixture of n-butanol and pyridine (15:1, v/v) were added and the tubes were vortexed and centrifuged at 800xg for 20 min. The upper layer was aspirated out to measure the color intensity at 532 nm. The reference used was 1, 1, 3, 3 tetraethoxypropane (TEP).
Catalase assay.
The activity of catalase was determined by the method of Sinha (1971).41 Catalase was assayed colorimetrically at 570 nm. The reaction mixture in a total volume 1.6 ml contains 1.0 ml of 0.01 M, phosphate buffer, pH 7.0, 0.1 ml of tissue homogenate-supernatant and 0.5 ml of 2.0 M H2O2. The reaction was stopped by the addition of 2.0 ml dichromate acetic acid reagent (5% potassium dichromate and glacial acetic acid mixed in a 1:3 ratio). The activity was expressed as μmoles H2O2 decomposed/min/mg protein.
SOD assay.
Superoxide dismutase (SOD) activity was assayed by the method of Misra and Fridovich, 1972.42 3.0 ml reaction mixture contains of 1.5 ml of 0.1 M carbonate-bicarbonate buffer, pH 10.3; 0.1 ml of 30 mM EDTA, suitable aliquot of enzyme preparation and water to make up the volume to 2.94 ml. The reaction was started by addition of 0.06 ml of 15 mM epinephrine. Change in absorbance was recorded at 480 nm for one min at 15 sec interval. Control consisting of all the ingredients, except enzyme preparation, was run simultaneously. One unit of enzyme activity has been defined to cause 50% inhibition of auto-oxidation of epinephrine by 1.0 ml of homogenate.
GST activity.
GST was determined using 1-chloro-2, 4-dinitrobenzene (CDNB) as a substrate. The assay mixture contained 1mM GSH, 1mM CDNB and 100 mM phosphate buffer, pH 6.5. The reaction was started by the addition of enzyme in linearity range, the rate of increase in absorbance due to formation of CDNB conjugate of GSH was monitored at 340 nm for 3 mins. The activity of GST is expressed as μmoles of GSH-CDNB conjugate formed/min/mg protein (Habig et al. 1974).43
GSH contents.
0.2 ml homogenate were mix in 0.3 ml precipitating regent (0.2 M glacial meta-phosphoric acid, 5.1 M NaCl and 5.9 mM EDTA). After centrifugation at 10,000xg for 15 minutes, 0.2 ml supernatant was added to 0.8 ml 0.3 M Na2HPO4, followed by the addition of 0.1 ml 5,5 dithiobis (2- nitrobenzoic acid); DTNB; 0.04% in 1% sodium citrate). The change in optical density at 412 nm was recorded using UV-Vis spectrophotometer. (Chandra et al. 2002).44
Protein estimation.
Protein was estimated by the method of Lowry et al.45 using bovine serum albumin as standard, at 660 nm.
Statistical analysis.
The results are expressed as mean ± SD in each group (n=5). Comparisons were made by means of one way analysis of variance (ANOVA) test. A values of p<0.05 were considered as significant.
Figure 2.
Activity of catalase in control and experimental groups of rats. Catalase activity was estimated by the method of Sinha.41 The activity was expressed as μmoles H2O2 decomposed/min/mg protein. Each values is a mean ± SD (n=5 in each group). *Values are statistically significant at p<0.001. Normal + MC treated (groups II), normal + TFG treated (group III) and diabetic control (group IV) were compared with normal control (group I). Diabetic + MC treated (group V) and diabetic + TFG treated (groups VI) were compared with diabetic control (group IV).The experiments were repeated thrice. MC, Momordica charantia; TFG, Trigonella foenum graecum.
Acknowledgements
UNT is thankful to DST for financial assistance. Grants from UGC and DST under the FIST programme for the development of Department of Biochemistry are gratefully acknowledged.
Abbreviations
- AGEs
advanced glycation end product
- AR
aldose reductase
- CAT
catalase
- CDNB
1-chloro-2, 4-dinitrobenzene
- CVD
cardiovascular disease
- DM
diabetes mellitus
- DTNB
5,5′-Dithio-bis 2-nitrobenzoic acid
- EDTA
ethylene diamine tetra acetic acid
- FBG
fasting blood glucose
- GPx
glutathione peroxidase
- GSH
reduced glutathione
- GST
glutathione-s-transferase
- LPO
lipid peroxidation
- MC
Momordica charantia
- MDA
malondialdehyde
- NADPH
nicotinamide adenine dinucleotide phosphate (reduced)
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- TBARS
thiobarbituric acid reactive substances
- TEP
1, 1, 3, 3 tetraethoxypropane
- TFG
Trigonella foenum graecum
Footnotes
Previously published online: www.landesbioscience.com/journals/oximed/article/9529
References
- 1.Chandra D, Jackson EB, Ramana KV, Kelley R, Srivastava SK, Bhatnagar A. Nitric oxide prevents aldose reductase activation and sorbitol accumulation during diabetes. Diabetes. 2002;51:3095–3101. doi: 10.2337/diabetes.51.10.3095. [DOI] [PubMed] [Google Scholar]
- 2.Yim S, Malhotra A, Veves A. Antioxidants and CVD in Diabetes: Where Do We Stand Now? Curr Diab Rep. 2007;7:8–13. doi: 10.1007/s11892-007-0003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ceriello A. Oxidative stress and glycemic regulation. Metabolism. 2000;49:27–29. doi: 10.1016/s0026-0495(00)80082-7. [DOI] [PubMed] [Google Scholar]
- 4.Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications: A new perspective on an old paradigm. Diabetes. 1999;48:1–9. doi: 10.2337/diabetes.48.1.1. [DOI] [PubMed] [Google Scholar]
- 5.Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40:405–412. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- 6.Chang KC, Chung SY, Chong WS, Suh JS, Kim SH, Noh HK, et al. Possible superoxide radical-induced alteration of vascular reactivity in aortas from streptozotocin-treated rats. J Pharmacol Exp Ther. 1993;266:992–1000. [PubMed] [Google Scholar]
- 7.Young IS, Tate S, Lightbody JH, McMaster D, Trimble ER. The effects of desferrioxamine and ascorbate on oxidative stress in the streptozotocin diabetic rat. Free Radic Biol Med. 1995;18:833–840. doi: 10.1016/0891-5849(94)00202-u. [DOI] [PubMed] [Google Scholar]
- 8.Chakravarthy BR, Wang J, Tremblay R, Atkinson TG, Wang F, Li H, et al. Comparison of the changes in protein kinase C induced by glutamate in primary cortical neurons and by in vivo cerebral ischaemia. Cell Signal. 1998;10:291–295. doi: 10.1016/s0898-6568(97)00131-9. [DOI] [PubMed] [Google Scholar]
- 9.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 10.Creager MA, Luscher TF, Cosentino F, Beckman JA. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: Part I. Circulation. 2003;108:1527–1532. doi: 10.1161/01.CIR.0000091257.27563.32. [DOI] [PubMed] [Google Scholar]
- 11.Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, et al. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- 12.Bhatnagar A, Srivastava SK. Aldose reductase: congenical and injurious profiles of an enigmatic enzyme. Biochem Med Metabol Biol. 1992;48:91–121. doi: 10.1016/0885-4505(92)90055-4. [DOI] [PubMed] [Google Scholar]
- 13.Srivastava S, Chandra A, Ansari NH, Srivastava SK, Bhatnagar A. Identification of cardiac oxidoredutase (s) involved in metabolism of lipid peroxidation derived aldehyde-4-hydroxnonenal. Biochem J. 1998;329:469–475. doi: 10.1042/bj3290469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johansen JS, Harris AK, Rychly DJ, Ergul A. Oxidative stress and the use of antioxidants in diabetes: linking basic science to clinical practice. Cardiovasc Diabetol. 2005;4:5. doi: 10.1186/1475-2840-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maritim AC, Sanders RA, Watkins JB., 3rd Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 16.Stewart-Lee AL, Forster LA, Nourooz-Zadeh J, Ferns GA, Anggard EE. Vitamin E protects against impairment of endothelium mediated relaxations in cholesterol- fed rabbits. Arterioscler Thromb. 1994;14:494–499. doi: 10.1161/01.atv.14.3.494. [DOI] [PubMed] [Google Scholar]
- 17.Cinar MG, Ulker S, Alper G, Evinc A. Effect of dietary vitamin E supplementation on vascular reactivity of thoracic aorta in streptozotocin-diabetic rats. Pharmacology. 2001;62:56–64. doi: 10.1159/000056072. [DOI] [PubMed] [Google Scholar]
- 18.Palmer AM, Thomas CR, Gopaul N, Dhir S, Anggard EE, Poston L, et al. Dietary antioxidant supplementation reduces lipid peroxidation but impairs vascular function in small mesenteric arteries of the streptozotocin- diabetic rat. Diabetologia. 1998;41:148–156. doi: 10.1007/s001250050883. [DOI] [PubMed] [Google Scholar]
- 19.Alper G, Olukman M, Irer S, Caglayan O, Duman E, Yilmaz C, et al. Effect of vitamin E and C supplementation combined with oral antidiabetic therapy on the endothelial dysfunction in the neonatally streptozotocin injected diabetic rat. Diabetes Metab Res Rev. 2006;22:190–197. doi: 10.1002/dmrr.586. [DOI] [PubMed] [Google Scholar]
- 20.Beckman JA, Goldfine AB, Gordon MB, Garrett LA, Keaney JF, Jr, Creager MA. Oral antioxidant therapy improves endothelial function in type 1 but not type 2 diabetes mellitus. Am J Physiol Heart Circ Physiol. 2003;285:H2392–2398. doi: 10.1152/ajpheart.00403.2003. [DOI] [PubMed] [Google Scholar]
- 21.Saxena AK, Srivastava P, Kale RK, Baquer NZ. Impaired antioxidant status in diabeteic rat liver: effect of vanadate. Biochem Pharmacol. 1993;45:539–542. doi: 10.1016/0006-2952(93)90124-f. [DOI] [PubMed] [Google Scholar]
- 22.Grover JK, Yadav SP. Pharmacological actions and potential uses of Momordica charantia: a review. J Ethnopharmacol. 2004;93:123–132. doi: 10.1016/j.jep.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 23.Alam S, Asad M, Asdaq SM, Prasad VS. Antiulcer activity of methanolic extract of Momordica charantia L. in rats. J Ethnopharmacol. 2009;123:464–469. doi: 10.1016/j.jep.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 24.Batabval L, Sharma P, Mohan L, Maurya P, Srivastava CN. Relative toxicity of neem fruit, bitter guard, and castor seed extracts against the larvae of filarial vector, Culex quinquefasciatus. Parasitol Res. 2009;105:1205–1210. doi: 10.1007/s00436-009-1541-7. [DOI] [PubMed] [Google Scholar]
- 25.Abou El-Soud NH, Khalil MY, Hussein JS, Oraby FSH, Farrag ARH. Antidiabetic effects of fenugreek alkaloid extract in streptozotocin induced hyperglycaemic rats. J App Sci Res. 2007;3:1073–1083. [Google Scholar]
- 26.Tripathi UN, Jamal F, Chandra D. Pharmacological potentials of Triogonella foenum-graecum (methi): A review. Nat J Life Sci. 2007;4:205–207. [Google Scholar]
- 27.Lenzen S. The mechanisms of alloxan- and streptozotocin- induced diabetes. Diabetologia. 2008;51:216–226. doi: 10.1007/s00125-007-0886-7. [DOI] [PubMed] [Google Scholar]
- 28.Horie S, Ishii H, Suga T. Changes in peroxisomal fatty acid oxidation in diabetic rat liver. J Biochem. 1981;90:1691–1696. doi: 10.1093/oxfordjournals.jbchem.a133645. [DOI] [PubMed] [Google Scholar]
- 29.Xing FZ, Tan BW. Antihyperglycemic and antioxidant properties of Andrographis paniculatain normal and diabetic rats. Clin Exp Pharmacol Physiol. 2000;27:358–363. doi: 10.1046/j.1440-1681.2000.03253.x. [DOI] [PubMed] [Google Scholar]
- 30.Mahadevi MRV, Raghni M, Baluchnejadmojarad T. Role of Trigonella foenum graecum leaf aqueous extract has been implicated to improve vascular reactivity of the thoracic aorta from STZ diabetic rats. Ind J pharmacol. 2008;40:59–63. doi: 10.4103/0253-7613.41039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chandra A, Mahdi AA, Ahmad S, Singh RK. Indian herbs result in hypoglycemic responses in streptozotocin-induced diabetic rats. Nutr Res. 2007;27:161–168. [Google Scholar]
- 32.Nicotera P, Orrenius S. Role of thiols in protection against biological reactive intermediates. Adv Exp Med Biol. 1986;197:41–49. doi: 10.1007/978-1-4684-5134-4_4. [DOI] [PubMed] [Google Scholar]
- 33.Gregus Z, Fekete T, Halászi E, Klaassen CD. Lipoic acid impairs glycine conjugation of benzoic acid and renal excretion of benzoylglycine. Drug Metab Dispos. 1996;24:682–688. [PubMed] [Google Scholar]
- 34.Wohaieb SA, Godin DV. Alterration of free radical tissue-defense mechanism in streptozotocin-induced diabetic rats. Effect of insulin treatment. Diabetes. 1987;36:1014–1018. doi: 10.2337/diab.36.9.1014. [DOI] [PubMed] [Google Scholar]
- 35.Mc-cord JM, Keele BB, Fridovich I. An enzyme based theory of obligate anaerobiosis; the physiological functions of superoxide dismutase. Proc Natl Acad Sci USA. 1976;68:1024–1027. doi: 10.1073/pnas.68.5.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chance B, Greenstein DS, Roughton RJW. The mechanism of catalase action steady state analysis. Arch Biochem Biophys. 1952;37:301–339. doi: 10.1016/0003-9861(52)90194-x. [DOI] [PubMed] [Google Scholar]
- 37.Karunanayake EH, Jeevathayaparan S, Tennekoon KH. Effect of Momordica charantia fruit juice on streptozotocin-induced diabetes in rats. J Ethnopharmacol. 1990;30:199–204. doi: 10.1016/0378-8741(90)90008-h. [DOI] [PubMed] [Google Scholar]
- 38.Xue WL, Li XS, Zhang J, Liu YH, Wang ZL, Zhang RJ. Effect of Trigonella foenum-graecum (fenugreek) extract on blood glucose, blood lipid and hemorheological properties in streptozotocin-induced diabetic rats. Asia Pac J Clin Nutr. 2007;16:422–426. [PubMed] [Google Scholar]
- 39.Sochor M, Baquer NZ, McLean P. Glucose over and underutilization in diabetes: Comparative studies on the changes in activities of enzymes of glucose metabolism in rat kidney and liver. Mol Physiol. 1985;7:51–68. [Google Scholar]
- 40.Ohkawa H, Oshishi N, Yagi K. Assay of lipid peroxidation in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 41.Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 42.Misra HP, Fridovich I. The role of superoxide anion in the auto oxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- 43.Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 44.Chandra D, Ramana KV, Wang L, Christensen BN, Bhatnagar A, Srivastava SK. Inhibition of fiber cell globulization and hyperglycemia-induced lens opacification by aminopeptidase inhibitor bestatin. Invest Ophthalmol Vis Sci. 2002;43:2285–2292. [PubMed] [Google Scholar]
- 45.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]