Abstract
Background:
Oxidative stress and inflammation may contribute to the disruption of the protective gut barrier through various mechanisms; mitochondrial dysfunction resulting from inflammatory and oxidative injury may potentially be a significant source of apoptosis during necrotizing enterocolitis (NEC). Tumor necrosis factor (TNF)α is thought to generate reactive oxygen species (ROS) and activate the apoptosis signal-regulating kinase 1 (ASK1)-c-Jun N-terminal kinase (JNK)/p38 pathway. Hence, the focus of our study was to examine the effects of TNFα/ROs on mitochondrial function, ASK1-JNK/p38 cascade activation in intestinal epithelial cells during NEC.
Results:
We found (a) abundant tissue TNFα and ASK1 expression throughout all layers of the intestine in neonates with NEC, suggesting that TNFα/ASK1 may be a potential source (indicators) of intestinal injury in neonates with NEC; (b) TNFα-induced rapid and transient activation of JNK/p38 apoptotic signaling in all cell lines suggests that this may be an important molecular characteristic of NEC; (c) TNFα-induced rapid and transient ROs production in RIe-1 cells indicates that mitochondria are the predominant source of ROS, demonstrated by significantly attenuated response in mitochondrial DNA-depleted (RIE-1-ρ°) intestinal epithelial cells; (d) further studies with mitochondria-targeted antioxidant PBN supported our hypothesis that effective mitochondrial ROS trapping is protective against TNFα/ROs-induced intestinal epithelial cell injury; (e) TNFα induces significant mitochondrial dysfunction in intestinal epithelial cells, resulting in increased production of mtROS, drop in mitochondrial membrane potential (MMP) and decreased oxygen consumption; (f) although the significance of mitochondrial autophagy in NEC has not been unequivocally shown, our studies provide a strong preliminary indication that TNFα/ROs-induced mitochondrial autophagy may play a role in NeC, and this process is a late phenomenon.
Methods:
Paraffin-embedded intestinal sections from neonates with NEC and non-inflammatory condition of the gastrointestinal tract undergoing bowel resections were analyzed for TNFα and ASK1 expression. Rat (RIE-1) and mitochondrial DNA-depleted (RIE-1-ρ°) intestinal epithelial cells were used to determine the effects of TNFα on mitochondrial function.
Conclusions:
Our findings suggest that TNFα induces significant mitochondrial dysfunction and activation of mitochondrial apoptotic responses, leading to intestinal epithelial cell apoptosis during NeC. Therapies directed against mitochondria/ROS may provide important therapeutic options, as well as ameliorate intestinal epithelial cell apoptosis during NeC.
Key words: apoptosis, ASK1, intestinal epithelial cells, JNK, mitochondrial membrane potential, mitogen-activated protein kinases, necrotizing enterocolitis, p38, TNFα
Introduction
Necrotizing enterocolitis (NEC) is the most common gastrointestinal surgical emergency in premature low birth-weight neonates, where prematurity is the single most important risk factor. Although several contributing factors for NEC have been identified,- such as ischemia, bacteria, cytokines and enteral feeding, the exact mechanisms for its pathogenesis remain elusive. The clinical presentation of NEC is often nonspecific and unpredictable. NEC frequently involves diffuse areas of bowel necrosis and perforation, necessitating emergency operation. The presence of pneumatosis intestinalis detected on plain abdominal radiographs remains as a pathognomonic clinical feature. Despite extensive research during the past two decades, the exact pathogenesis of NEC for premature neonates remains ill-defined and largely unknown, hence, limiting development of novel preventive strategies.
Reactive oxygen species (ROS), generated as a result of ischemia- reperfusion injury to the gut, have been linked to the development of NEC in premature infants.1,2 However, cytokines are also thought to play a role in ROS generation, contributing to severe gut inflammation and injury during NEC hallmarked by the exaggerated inflammatory responses by the premature immune system.3,4
Tumor necrosis factor (TNF)α, a pro-inflammatory cytokine implicated in various inflammatory diseases of the small intestine,5,6 is thought to contribute to the pathogenesis of NEC. Recent in vivo NEC studies have demonstrated a significant decrease in the severity and incidence of intestinal injury with anti-TNFα therapy.7,8 TNFα-induced oxidative stress via mitochondrial ROS (mtROS) generation has recently emerged as a new mechanism of inducing cellular injury. Several studies have shown that mtROS generated by TNFα can oxidize the reduced thioredoxin-apoptosis signal-regulating kinase 1 (Trx(SH)2-ASK1) complex,9–13 thus initiating its dissociation and inducing activation of ASK1 and its downstream stress signaling targets such as c-Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinase (MAPK) pathways.14–16
ASK1 is a member of the MAPK family, and is an upstream activator of JNK and p38-MAPK signaling cascades.17 ASK1 can be activated in response to TNFα/ROS and triggers various biological responses such as apoptosis, inflammation, differentiation and survival in various cell types.13,18–22 TNFα/mtROS-induced Trx oxidation results in the dissociation of the Trx(SH)2-ASK1 complex, release of ASK1 and activation of p38 MAPK and JNK stress response pathways.16,23–26 Thus, ASK1 is important in the regulation of ROS and inflammation-induced apoptotic signaling in injured cells;16,27,28 however, its role in intestinal epithelial cells during oxidative injury is unknown.
Oxidative stress and inflammation may contribute to the disruption of the protective gut barrier through various mechanisms; however, mitochondrial dysfunction resulting from inflammatory and oxidative injury may potentially be a significant source of apoptosis during NEC. Hence, the focus of our study was to examine the effects of TNFα/ROS on mitochondrial function, and ASK1- JNK/p38 cascade activation in intestinal epithelial cells.
Results
Increased expression of TNFα and ASK1 in intestinal sections from human NEC and ASK1 expression in vitro.
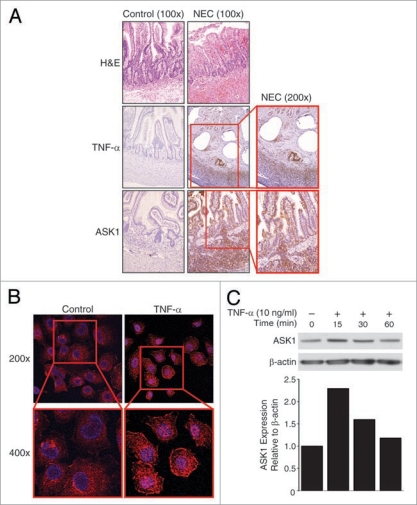
Examination of intestinal sections from NEC showed typical denuded intestinal mucosal structure (Fig. 1A; top row) when compared to controls from a non-inflammatory condition (intestinal atresia) of the gut. In intestinal sections from NEC, we observed abundant tissue TNFα expression throughout all layers of the intestine (Fig. 1A; middle row). Given these findings, we next determined the expression of ASK1 during NEC. Correlative to TNFα, ASK1 levels were also increased throughout all layers of the intestinal sections from NEC (Fig. 1A; bottom row). These increased levels of intestinal TNFα and ASK1 during NEC can be potential markers of intestinal injury in neonates with NEC.
Figure 1.
Increased TNFα and ASK1 expression during NEC. (A) The intestinal sections from neonates with NEC and non-inflammatory condition (intestinal atresia; control) were assessed for TNFα and ASK1 expression. Representative section demonstrates villous tip blunting and inflammatory cell infiltration in NEC sections (top). Increased levels of TNFα (middle) and ASK1 (bottom) were localized to mucosa, submucosa and muscular layers in NEC (brown staining). (B) RIE-1 cells (1 × 104/well) were treated with TNFα (10 ng/mL) for 15 min, incubated with anti-ASK1 antibody, AlexaFluor® 647-labeled goat anti-rabbit IgG (pseudo purple) and Hoechst 33342 nuclear stain (blue), and were visualized using confocal microscopy. TNFα treatment resulted in rapid increase in intracellular ASK1 expression in RIE-1 cells. (C) TNFα treatment also increased ASK1 expression in RIE-1 cells by western blotting. Equal β-actin levels and densitometry indicate even loading.
To examine the effects of TNFα on pool level of ASK1, TNFα-treated RIE-1 cells were analyzed using confocal microscopy and Zeiss LSM 5 image software. We found marked increase in ASK1 expression (pseudo-purple) in both the cytosol and at cell membranes at 15 minutes (min) when compared to control cells (Fig. 1B). These findings suggest an overall pool increase in ASK1 cellular levels, and in particular, increased ASK1 accumulation at cell membranes consistent with ASK1 recruitment to activated TNFα and death receptors. More in-depth studies of TNFα-induced ASK1 accumulation at the cell membrane in RIE-1 cells are warranted. Western blotting also demonstrated early increase in total pool levels of ASK1 after TNFα treatment (Fig. 1C) in intestinal epithelial cells. Future studies targeting TNFα-induced fluctuations in mitochondrial versus cytosolic activated ASK1 protein expression levels in RIE-1 cells, and mitochondrial Trx(SH)2-ASK1 complex dissociation specifically, are necessary.
TNFα-induced mtROS, MMPΔ, autophagy and O2 consumption alterations in vitro and autophagic vacuolization in vivo.
To determine the effects of TNFα/ROS on mitochondrial function in intestinal epithelial cells, we first examined whether mitochondria are the predominant source of intracellular ROS in RIE-1 cells similar to other cell types described by various investigators.13 We also examined the effects of TNFα/ROS on mitochondrial membrane potential (MMP) and oxygen consumption by RIE-1 cells. RIE-1 and mitochondrial DNA (mtDNA)-depleted RIE-1-ρ° cells were used to determine ROS levels.
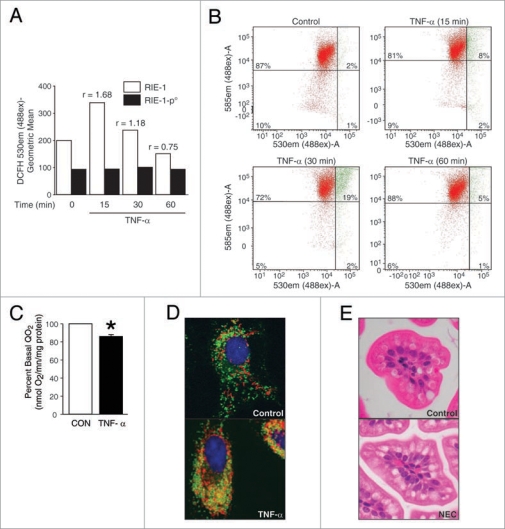
A significant and rapid rise in intracellular ROS levels was noted at 15 and 30 min after TNFα treatment (ratio of stimulated vs. baseline ROS levels, r > 1) in parental RIE-1 cells, whereas RIE-1-ρ° cells did not respond to TNFα stimulation, and the baseline ROS levels remained relatively unchanged (Fig. 2A). These findings demonstrate that mitochondria are the predominant source of TNFα-induced intracellular ROS production in intestinal epithelial cells and concur with other cell type findings, where this effect is observed to be very rapid and transient. Effects of TNFα on MMPΔ in RIE-1 cells were also rapid and transient in nature, demonstrating peak levels of altered MMP within 15 and 30 min after TNFα treatment and recovery of MMP observed at 60 min (Fig. 2B). This finding suggests a significant susceptibility of mitochondria to TNFα injury demonstrated by a rapid and transient drop in MMP, increased permeability and “leakiness” of the mitochondrial membrane which could result in the release of an apoptosis-activating molecule such as cytochrome c into the cytosol. MMP depolarization is an important early indicator of apoptotic signaling activation, and hence, transient and rapid MMPΔ in response to cytokine-induced injury demonstrates mitochondrial susceptibility in RIE-1 cells.
Figure 2.
TNFα induces mitochondrial functional deregulation in intestinal epithelial cells. (A) RIE-1 and RIE-1-ρ° cells (1 × 106) were treated with TNFα, incubated with DCFH-DA for 15 min for ROS level. In RIE-1 cells, TNFα treatment induced rapid, transient rise in intracellular ROS. The ratio of stimulated vs. baseline ROS levels increased (r > 1) in TNFα-treated RIE-1 cells only. (B) RIE-1 cells were treated as before and incubated with JC-1 dye for 15 min in darkness. The collapse of the electrochemical gradient across the mitochondrial membrane (red fluorescence) was analyzed by a FACScan flow cytometer. Mitochondria with intact MMP are represented as green fluorescence. Initiation of MMP decrease is seen at 15 min, and recovery at 60 min. (C) RIE-1 cells (1 × 107) were transferred to QO2 chamber and treated with TNFα (10 ng/mL). O2 consumption was significantly decreased by ∼20% shortly after TNFα treatment (*p < 0.05 vs. control). (D) RIE-1 cells (1 × 104/well) were treated with TNFα and incubated with MitoTracker (red fluorescence, mitochondria) and LysoTracker (green fluorescence, lysosomes) dyes. Merged confocal images demonstrated mitochondrial autophagy (yellow fluorescence) in damaged RIE-1 cells at 24 h. (E) Cross-section of H&E-stained neonatal mouse intestinal villi demonstrate autophagic vacuolization of intestinal epithelial cells in NEC group.
The oxygen consumption level in TNFα-treated RIE-1 cells was measured using a Clark-type electrode. TNFα treatment induced a significant decrease in oxygen consumption level of RIE-1 cells within the first minute of treatment with relatively depressed levels; this effect persisted for 5 min after TNFα treatment (Fig. 2C). This finding demonstrates that mitochondrial functional changes occur rather rapidly in response to TNFα, and that the mitochondrial oxygen consumption is rapidly decreased within the first minute of TNFα exposure. Taken together, these results demonstrate that TNFα induces significant mitochondrial dysfunction in intestinal epithelial cells, resulting in functional derangements such as increased production of mtROS, significantcant alteration in MMP and decreased oxygen consumption.
Organelle autophagy occurs as a result of cellular injury. Hence, we next examined the effects of TNFα treatment on mitochondrial autophagy in RIE-1 cells and sought to evaluate mouse intestinal sections for evidence of autophagy. Initially, we treated RIE-1 cells with TNFα for various time points (15, 30, 60, 90 min and 24 hours), and labeled cells with organelle-specific dyes, MitoTracker (mitochondria, red fluorescence) and LysoTracker (lysosomes, green fluorescence). Laser scanning confocal microscopy did not reveal significant mitochondrial autophagy at early time points (data not shown); however, when cells were treated with TNFα for 24 hours (h), and then labeled, we found significant co-localization of damaged mitochondria with lysosomes (yellow fluorescence) and typical morphologic changes (Fig. 2D) consistent with mitochondrial damage and autophagy of dysfunctional mitochondria. These findings suggest that TNFα-induced mitochondrial autophagy is a late phenomenon in contrast to more rapid and transient mitochondrial ROS production, MMPΔ, reduction in cellular respiration, activation of mitochondrial apoptotic signaling pathways and JNK/p38 stress pathways. Late autophagy may represent cellular inability to cope with overwhelming burden of damaged mitochondria. When taken together with RIE-1 cell death ELISA results (Fig. 3A) after TNFα treatment, these data suggest that mitochondrial autophagy may play a pro-apoptotic role during cytokine-induced injury in intestinal epithelial cells.
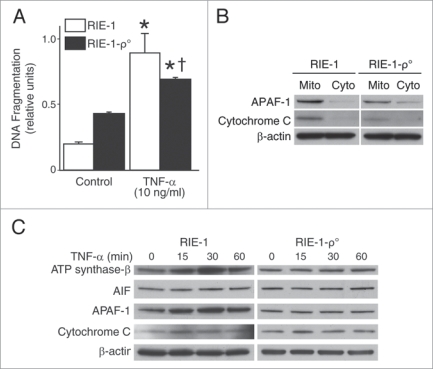
Figure 3.
TNFα activates mitochondrial apoptotic pathways and induces significant intestinal epithelial cell apoptosis. (A) RIE-1 and RIE-1-ρ° cells were treated with TNFα as before. apoptosis was measured by DNA fragmentation ELISA. Data represent triplicate determinations (mean ± SEM; *p < 0.05 vs. control; †p < 0.05 vs. TNFα-treated RIE-1 cells) and experiments were repeated 3 times. TNFα-induced apoptosis was attenuated when compared to RIE-1 cells. (B) RIE-1 and RIE-1-ρ° cells (2 × 107) were plated for 24 h, mitochondria isolated for protein analysis of mitochondrial apoptotic markers, cytochrome c and APAF-1, by western blotting. Mitochondrial DNA-damaged RIe-1-ρ° cells showed attenuated mitochondrial protein levels. (C) TNFα treatment resulted in increased expression of mitochondrial apoptotic markers (AIF, APAF-1, cytochrome c and ATP synthase-β) in RIE-1 cells by western blotting. Mitochondrial DNA-damaged RIe-1-ρ° cells treated with TNFα showed unchanged basal levels of all apoptotic markers except for increased expression of cytochrome c at 15 min.
Cross-sectional views of mouse NEC intestinal villi showed ubiquitous autophagic vacuolization in intestinal epithelial cells in contrast with healthy controls (Fig. 2E). The degree of vacuolization is significant and may represent an adaptive function in stressed intestinal epithelial cells in vivo. Histological evaluation and electron scanning microscopy of human NEC sections would be helpful in examining autophagic vacuolization in intestinal epithelial cells.
TNFα-induced RIE-1 cell apoptosis is mitochondria-dependent.
Next, we sought to examine whether TNFα induces apoptosis in intestinal epithelial cells. We treated RIE-1 and RIE-1-ρ° cells with TNFα and DNA fragmentation was quantitated. TNFα-induced RIE-1 cell death was significantly decreased in TNFα-treated RIE-1-ρ° cells (Fig. 3A). These data imply that TNFα-exerted cellular injury mechanism(s) is predominantly mitochondria-dependent in RIE-1 cells.
When baseline levels of mitochondrial apoptotic markers such as Apoptotic protease activating factor 1 (APAF-1) and cytochrome c in RIE-1 and RIE-1-ρ° cells are compared, the mitochondrial expression of these apoptotic molecules is significantly reduced in mtDNA-silenced RIE-1-ρ° cell line (Fig. 3B). Hence, the effect of cytokine-induced injury may be dependent or independent of mitochondrial apoptotic arsenal. To test this hypothesis, we examined the effects of TNFα on mitochondrial apoptotic pathway activation in intestinal epithelial cells by western blot analysis. The expression of mitochondrial apoptotic markers (apoptosis-inducing factor (AIF), APAF-1, cytochrome c) and ATP synthase-β, a marker for the activity of the electron transport chain, were increased in RIE-1 cells after TNFα treatment (Fig. 3C). This effect peaked at 15 min and returned to basal levels by 60 min. In contrast, the mtDNA-depleted RIE-1-ρ° cells displayed no significant alteration in expression levels of mitochondrial apoptotic markers, with the exception of a minimal increase in cytochrome c release at 15 min. This finding may represent either delayed protein degradation or altered nuclear encoding of mitochondrial proteins and enzymes that is unaffected by mtDNA silencing.
ROS trapping and ASK1 siRNA attenuate TNFα-induced apoptosis and JNK/p38 pathway activation in RIE-1 cells.
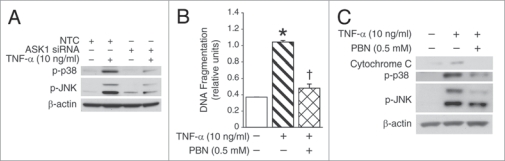
To determine the effectiveness of mitochondria-targeted potential therapies during TNFα/ROS-induced cell injury, we used ASK1 silencing via short interfering RNA (siRNA) treatment and spin-trapping compound, α-phenyl-N-t-butylnitrone (PBN). The dissociation of the Trx(SH)2-ASK1 complex, both cytosolic and mitochondrial, is a crucial step in activating the JNK-mediated apoptotic signaling cascade.22–26 ASK1 inhibition can be protective during TNFα-induced cell injury. Hence, we targeted ASK1 with siRNA silencing method and examined activation of JNK and p38 apoptotic pathways, measured by phosphorylation levels of proteins following TNFα treatment (Fig. 4A). Protein analysis revealed that ASK1 silencing resulted in significant reduction of JNK and p38 phosphorylation levels in TNFα-treated cells. The differential phosphorylation of JNK isoforms was observed in RIE-1 cells, indicating a complex isoformspecific activation process induced by TNFα treatment in intestinal epithelial cells. These findings warrant future studies focusing on mitochondrial ASK1 targeting specifically and examining JNK isoform-specific activation in TNFα-treated intestinal epithelial cells.
Figure 4.
ROS trapping and ASK1 silencing during TNFα-induced activation of apoptotic signaling. (A) ASK1 siRNA- and non-targeted control-transfected RIE-1 cells were treated with TNFα for 15 min and cell lysates were analyzed for phosphorylation of JNK and p38 by western blotting. ASK1 silencing resulted in significant reduction in the levels of JNK and p38 activation during TNFα treatment. Equal β-actin levels indicate even loading. (B) RIE-1 cells (2 × 104/well) were pretreated with PBN for 2 h prior to TNFα treatment. PBN pretreatment dramatically reduced TNFα-induced RIe-1 cell death (*p < 0.05 vs. control; †p < 0.05 vs. TNFα alone). (C) TNFα-induced activation of JNK/p38 pathway and cytochrome c expression was signifi- cantly decreased with PBN pretreatment in RIe-1 cells by western blotting.
Application of spin-trapping of RIE-1 cells showed significant attenuation in TNFα- induced RIE-1 cell death (Fig. 4B), thus demonstrating a protective effect of ROS-trapping by PBN in TNFα/ROS-damaged intestinal epithelial cells. Protein analysis of cell lysates revealed significantly attenuated levels of proapoptotic cytochrome c, and marked reduction in JNK and p38 levels (Fig. 4C). ASK1 inhibition and antioxidant PBN are both effective in protecting RIE-1 cells against TNFα/ROS-induced injury.
Discussion
Cytokines are thought to play a central role in gut inflammation and injury during NEC by inducing exaggerated inflammatory responses, leading to significant intestinal injury in premature neonates.3,4 Previous studies have demonstrated that activation of inflammatory mediators such as TNFα, IL-1, NFκB, toll-like receptors (TLR), IL-8 and inducible NO synthase (iNOS) may play a significant role in the pathogenesis of NEC.7,29,30 Recently, we demonstrated an anti-inflammatory action of peroxisome proliferator-activated receptor (PPAR)γ using in vivo model of NEC, and inhibition of NFκB pathway, a critical transcription factor for the activation of inflammatory mediators and cytokines.31 De Plaen et al. also have demonstrated that inhibition of NFκB pathway during NEC ameliorates bowel injury and improves survival in vivo.29 Recent studies have also focused on molecular signaling mechanism(s) within the TLR signaling pathway32 and cyclooxygenase-2 (COX-2) in intestinal homeostasis and inflammation associated with NEC.33 Modulating early cellular inflammatory pathways during NEC may improve overall survival for neonates.
Previously, we investigated the effects of ROS, generated as a result of ischemia-reperfusion injury to the gut, and activation of apoptotic and survival signaling during NEC. The aim of the present study was to examine the effects of pro-inflammatory TNFα on mitochondrial dysfunction in intestinal epithelial cells during NEC, since premature neonatal gut is thought to be more susceptible to inflammatory cascade activation and oxidative injury.
In this study, we observe significant intestinal expression levels of pro-inflammatory TNFα and apoptotic ASK1 molecules throughout all layers of the intestine. This indicates that intestinal inflammatory process during NEC is in fact transmural. Tissue ASK1 levels also share similar pattern of apoptotic activation and injury in premature neonatal gut when compared with TNFα. One may argue that the human tissue expression levels may not fully reflect the exact extent of apoptotic signaling, and likely represent a late stage of the disease. Early NEC tissue analysis is a limiting factor in understanding early intestinal responses and expression levels of TNFα and ASK1.
Complex relationship between inflammation, oxidative stress and activation of apoptotic signaling in intestinal epithelial cells may largely depend on early mitochondrial responses during cellular injury. We demonstrate significant rise in mtROS production, alteration of mitochondrial function, activation of ASK1-JNK/p38 stress signaling and mitochondrial apoptotic cascade in rat intestinal epithelial cells with TNFα stimulation. Neonatal tissue staining together with our in vitro data demonstrate that inflammation and oxidative stress can induce significant mitochondrial deregulation and activation of mitochondria-selective apoptotic response, and may possibly lead to intestinal epithelial cell death in premature neonatal gut during NEC.
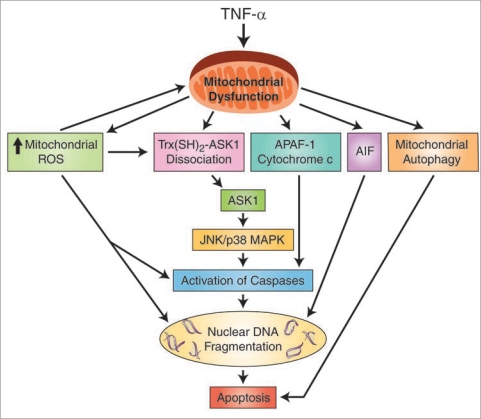
Mitochondrial dysfunction is found in many disease processes, including fulminant hepatic failure in neonates.34,35 Susceptibility of premature neonatal intestine to significant oxidant injury during NEC as a result of mitochondrial dysfunction has not been explored. We have attempted to elucidate some of the early mitochondrial functional derangements in RIE-1 cells with TNFα treatment, and have shown that mitochondrial integrity during inflammation is compromised, leading to early activation of pro-apoptotic and mitochondria-dependent signaling in vitro (Fig. 5).
Figure 5.
A proposed mechanism of TNFα-induced mitochondrial dysfunction and activation of apoptotic signaling pathways in the intestinal epithelial cells during NEC. Apoptosis-inducing factor (AIF); apoptotic protease activating factor 1 (APAF-1); apoptosis signal-regulating kinase 1 (ASK1); c-Jun N-terminal kinase (JNK)/p38 pathway; mitogen-activated protein kinase (MAPK); reactive oxygen species (ROS); thioredoxin-apoptosis signal-regulating kinase 1 (Trx(SH)2-ASK1) complex; tumor necrosis factor-α (TNFα).
It is unclear whether cellular autophagy is a pro-survival or pro-apoptotic cellular mechanism. It serves an important purpose in disease processes requiring the maintenance of healthy population of mitochondria.36,37 Our findings show that mitochondrial damage due to TNFα/ROS elicits late mitochondrial autophagy after TNFα exposure in vitro, suggesting either slow mitochondrial turnover or delayed mitochondrial biogenesis in injured RIE-1 cells. These results suggest that TNFα affects mitochondrial homeostasis and further studies are necessary to gain insight into intestinal mitochondrial dysfunction during NEC. Our previous findings of oxidative stress-induced intestinal cell death and MMP collapse38,39 along with the rapid, transient inflammation-induced mtROS production, early mitochondrial pro-apoptotic response and ASK1-JNK/p38 stress pathway activation in intestinal epithelial cells in the current study, suggest a strong role for ROS-mediated mechanism(s) of cellular damage during NEC.
The selective phosphorylation of JNK isoforms, p56 and p45, in response to TNFα suggests that there may be a differential pathway of activation of downstream signaling proteins in rat intestinal epithelial cells in vitro. For example, although both JNK isoforms are phosphorylated during TNFα stimulation, the predominantly phosphorylated JNK isoform is p45. In contrast, pretreatment with the PBN scavenger specifically decreases p54 phosphorylation and not phosphorylation of p45 JNK isoform. Furthermore, ASK1 silencing with siRNA leads to suppressed phosphorylation levels of both JNK isoforms. These findings suggest a complex and possibly selective (cytosolic or mitochondrial only) responses to the TNFα-mediated activation of ASK1-JNK/p38 signaling pathways in intestinal epithelial cells in vitro.
Regulation of caspase-dependent apoptotic signaling via cytochrome c and APAF-1 release from the mitochondrial matrix appears to be a specific response to TNFα treatment as compared to caspase-independent apoptotic signaling. Though both apoptotic signaling pathways are activated by TNFα, these findings support our hypothesis that injured mitochondria are a significant source of apoptotic signaling in intestinal epithelial cells during TNFα/ROS-induced injury, and can lead to potentially detrimental compromise of the gut mucosal barrier integrity.
Previous in vivo study by Halpern et al. had already demonstrated significant attenuation of NEC in neonatal rat, both in severity and incidence of intestinal injury, with anti-TNFα treatment. In our study, we examined the in vitro effect of TNFα on molecular signaling mechanisms and mitochondrial functional changes in intestinal epithelial cells.
Based on our findings, we propose that developing therapies specifically directed against mitochondria may be beneficial in reducing activation of apoptotic signaling cascades as a result of cytokine-mediated oxidative stress in intestinal epithelial cells during NEC. Increasing ROS production by the electron transport chain of dysfunctional mitochondria can be attenuated by various ROS scavenging compounds. Our successful use of the spin-trapping compound, PBN, which protects RIE-1 cells from TNFα/ROS-mediated death and attenuates activation of ASK1-JNK/p38 apoptotic pathway signaling, suggests that ROS scavengers may be beneficial.
PBN is widely used for ROS scavenging, and most importantly, has been shown to reverse the age-related oxidative changes and to reduce oxidative damage from ischemia/reperfusion injury.40–44 The antioxidant activity of PBN protects biologically important molecules from oxidative damage. Although this effect has not been demonstrated in NEC intestinal tissues, increasing scientific evidence of protective effects of free radical scavengers supports their possible use in clinical application, specifically in conditions requiring the targeting of diseases of mitochondrial dysfunction.
In conclusion, we have demonstrated that: (i) the pro-inflammatory cytokine, TNFα, is abundant in neonatal NEC intestinal sections and can induce significant functional mitochondrial deregulation in intestinal epithelial cells in vitro; (ii) mitochondria are the main source of intracellular ROS during TNFα exposure in intestinal epithelial cells; and (iii) mitochondria are susceptible to TNFα injury; (iv) activation of ASK1-JNK/p38 and mitochondrial apoptotic pathways occurs during inflammation-mediated ROS injury in intestinal epithelial cells, suggesting a central role for mitochondrial dysfunction during TNFα-induced oxidative stress. Therapies directed against mitochondria/ROS may provide important therapeutic options, as well as ameliorate intestinal epithelial cell apoptosis during NEC.
Methods
Human intestinal NEC sections.
Paraffin-embedded intestinal sections from 20 neonates with NEC and 3 neonates with non-inflammatory condition of the gastrointestinal (GI) tract (intestinal atresia; control) undergoing bowel resection were analyzed. Intestinal tissues were fixed and paraffin-embedded for further analysis. Control and NEC sections (5 µm) were prepared for immunohistochemical analysis. Sections were incubated with rabbit anti-TNFα (1:200) and anti-ASK1 (1:100) antibodies overnight at 4°C, then incubated with an anti-rabbit secondary antibody and stained with DAB chromogen (Dako Cytomation EnVision® + System-HRP (DAB) kit, Carpinteria, CA). Slides were washed, counterstained with hematoxylin, dehydrated and cover slipped. For negative control, sections were stained with rabbit IgG (not shown).
In vivo murine NEC model.
Timed pregnant Swiss-Webster mice were purchased (Charles River Labs, Pontage, MI) and pup littermates were randomized to either control or NEC group. All mice in NEC groups were housed in a water bath at 37°C. They were hand-fed KMR liquid milk replacer formula (0.3 cc/g/day; q 3 h) using an animal feeding needle (24 g, ballpoint; Popper & Sons, New Hyde Park, NY). Control mice were maternally reared. To induce NEC, pups were stressed twice daily with hypoxia by placing them in a plexi-glass chamber, breathing 5% oxygen for 10 min. Each mouse was monitored daily for the clinical severity of NEC by assessing the level of activity, oral intake, weight change and abdominal exam findings. Mice that developed abdominal distention, respiratory distress and lethargy during the first 96 h of the experiment were sacrificed. After 96 h, all surviving mice were sacrificed and distal ileum was harvested for analysis. Segments of ileum were fixed in formalin and stored in 70% ethanol for paraffin embedding. Intestinal sections were stained with hematoxylin and eosin for analysis.
Cell lines and culture techniques.
RIE-1 cells (a gift from Dr. Kenneth D. Brown; Cambridge Research Station, Cambridge, UK) were maintained in Dulbecco’s modified Eagle medium (DMEM) supplemented with 5% fetal bovine serum. Cells were maintained at 37°C under an atmosphere containing 5% CO2. Tissue culture media and reagents were obtained from Invitrogen (Carlsbad, CA).
To determine whether mitochondria are the main source of TNFα-induced intracellular ROS production, and that activation of apoptotic pathways is mitochondrial ROS-dependent, we established a RIE-1-ρ° from the parent RIE-1 cell line. Silencing of mitochondrial function was achieved by maintaining RIE-1 cells in isolation medium supplemented with 5% fetal bovine serum, ethidium bromide, EtBr; 0.1 µg/mL, uridine (50 µg/mL), pyruvate (100 µg/mL) and glucose (4.5 g/L). To confirm successful mtDNA depletion in RIE-1-ρ° cell population, cells were evaluated with FACS cell sorting method for EtBr binding after 14–16 passages and cell lysates were analyzed for decreased mitochondrial cytochrome oxidase subunit 1 protein level using western blot analysis (data not shown).
Mitochondrial isolation.
To determine the baseline expression of mitochondrial apoptotic markers in RIE-1 and RIE-1-ρ° (2 × 107) cells, mitochondria were isolated using a mitochondrial isolation kit according to a manufacturer’s protocol (Pierce, Rockford, IL). Mitochondrial lysates were analyzed for mitochondrial apoptotic markers (APAF-1, cytochrome c) by western blot.
Cell death detection ELISA.
RIE-1 and RIE-1-ρ° cells were plated onto 24-well plates for 24 h prior to TNFα treatment. Cells were incubated with TNFα (10 ng/mL). To determine the effects of ROS scavenging on intestinal epithelial cell survival, RIE-1 cells were first pretreated with α-phenyl-N-t-butylnitrone (PBN; 0.5 mM) for 2 h, and then incubated with TNFα (10 ng/mL). DNA fragmentation was evaluated using a Cell Death Detection ELISAPlus kit (Roche Molecular Biochemicals, Indianapolis, IN) according to manufacturer’s instructions.
Western blot analysis.
Cell lysates were clarified with centrifugation (13,200 rpm, 20 min at 4°C) and stored at −80°C. Protein concentrations were determined using the method described by Bradford.45 Equal amounts of total protein (20–30 µg) were loaded onto NUPAGE 4–12% Bis-Tris Gel and transferred to PVDF membranes, incubated in a blocking solution for 1 h (Tris-buffered saline containing 5% nonfat dried milk and 0.1% Tween 20), incubated with primary antibody overnight at 4°C, and then incubated with horseradish peroxidase-conjugated secondary antibody. Anti-β-actin antibody (1:5,000), total JNK (1:1,000) and p38 (1:1,000) were used for protein loading control. All primary antibodies were used in concentration of 1:1,000 to probe membranes. The immune complexes were visualized by ECLPlus (Amersham Biosciences, Piscataway, NJ). Quantitative densitometric analyses of all western blot bands (data not shown) were performed using ImageJ (Image Processing and Analysis in Java software, National Institutes of Health, MD).
ROS trapping with α-phenyl-N-t-butylnitrone (PBN) in RIE-1 cells.
PBN is one of the most widely used spin-trapping compounds that target mitochondrial ROS to reverse age-related oxidative changes and to alleviate oxidative damage from ischemia/ reperfusion injury.40–44 RIE-1 (2 × 104) cells were incubated with 0.5 mM PBN for 2 h at 37°C. To control for the effects of DMSO, a control group of cells were incubated in fresh media with DMSO for 2 h. After incubation, cells were treated with TNFα (10 ng/mL) for 15 min, and protein was harvested for western blot analysis.
ASK1 siRNA transfection of RIE-1 cells.
Rat SMART pool ASK1 and non-targeting control (NTC) siRNA duplexes (Dharmacon, Lafayette, CO) were used for transfection by electroporation (400 V/500 µF) in RIE-1 cells. Cells were maintained in medium for 48–72 h, then treated with TNFα (10 ng/mL) for various time points. Extracted protein was analyzed by western blotting for phospho-JNK and phospho-p38 expression levels.
Dichloro-dihydro-fluorescein (DCFH-DA) flow cytometry for ROS generation analysis.
Dichlorofluorescein (DCF) fluorescence was used to determine intracellular ROS generation based on the oxidation of 2′,7′-DCFH-DA to a fluorescent 2′,7′-DCF. RIE-1 and RIE-1-ρ° (1 × 106) cells were treated with TNFα (10 ng/mL). Cells were trypsinized, washed and incubated with 10 µM DCFH-DA for 15 min at 37°C. To quantify the intensity of DCF fluorescence, the samples were analyzed on a Becton-Dickenson FACScan flow cytometer (488 nm Argon-ion laser, 525 ± 10 nm band pass emission filter).
JC-1 assay for detection of MMP changes.
To determine the effects of TNFα on MMP, we used MitoProbe JC-1 Assay kit (Molecular Probes, Eugene, OR). The collapse in the electrochemical gradient across the mitochondrial membrane was measured using JC-1 fluorescent cationic dye. RIE-1 cells (1 × 106) were treated with TNFα (10 ng/mL), washed with PBS and incubated with 2 µM JC-1 for 15 min at 37°C in darkness. Cells were washed again and analyzed on a FACScan flow cytometer.
Confocal microscopy for ASK1 expression and mitochondrial autophagy.
To examine the effects of TNFα on ASK1 expression and mitochondrial autophagy, RIE-1 cells (2 × 104) were grown in glass chambers overnight, and then treated with TNFα. Cells were first incubated with PBS containing 1% bovine serum albumin, and then incubated with ASK1 antibody and AlexaFluor647-labeled goat anti-rabbit IgG to determine ASK1 expression. For mitochondrial autophagy, treated RIE-1 cells were incubated with organelle-specific dyes from MitoTracker and LysoTracker labeling kits according to the manufacturer’s instructions (Molecular Probes, Eugene, OR). All cells were counterstained with Hoechst 33342 nuclear stain. Zeiss LSM 510 UV Meta laser scanning confocal microscopy was used to visualize mitochondrial and lysosomal co-localization. Data was analyzed using Zeiss LSM 5 Image software.
Mitochondrial oxygen consumption (QO2).
Mitochondrial oxygen consumption was measured using a Strathkelvin Mitocell S200 micro-respirometry system (Strathkelvin; UK), which utilizes a Clark-type oxygen electrode. Respirometry was adapted from Mozo et al.46 Chamber temperature was maintained at 37°C. The respirometer was calibrated prior to experiments using O2-saturated water followed by sodium dithionite to provide 100% O2 and 0% O2 baselines, respectively. RIE-1 cells (1 × 107/ml) were trypsinized, pelleted by centrifugation, and resuspended in culture media lacking fetal bovine serum. Briefly, 1 ml of suspended cells was placed in the respirometry chamber, and oxygen consumption was monitored for 3 min to establish a basal consumption rate. TNFα (10 ng/mL) was added, and O2 consumption was monitored for approximately 5 min. Potassium cyanide (KCN; 2 mM) was added to another aliquot of cells to determine the rate of non-mitochondrial respiration. Rates of O2 consumption were calculated over a linear, one minute interval. All experiments were repeated three times.
Statistical analysis.
Minimum of three sets of experiments were performed to reproduce the results. The data were analyzed separately for each set using Kruskal-Wallis test. For oxygen consumption study, the effect of TNFα was assessed using one-sample t test against 100%. All effects and interactions were assessed at the 0.05 level of significance. Statistical computations were carried out using SAS 9.1®.
Acknowledgements
We would like to thank Karen Martin for assistance with manuscript preparation, Tatsuo Uchida for statistical analysis, Mark Griffin in Flow Cytometry Core Laboratory and Eugene Knutson from Optical Imaging Laboratory for assistance with confocal laser microscopy. This work was supported by grants R01 DK61470, R01 DK48498, P01 DK35608 and T32 DK07639 from the National Institutes of Health and a grant 8580 from Shriners Hospital for Children.
Abbreviations
- TNFα
tumor necrosis factor-α
- ASK1
apoptosis signal-regulating kinase 1
- ROS
reactive oxygen species
- NEC
necrotizing enterocolitis
- JNK
c-Jun N-terminal kinase
- mtROS
mitochondrial ROS
- Trx(SH)2-ASK1
thioredoxin-apoptosis signal-regulating kinase 1
- RIE-1
rat intestinal epithelial cells
- RIE-1-ρ°
mitochondrial DNA-depleted intestinal epithelial cells
- GI
gastrointestinal
- MAPK
mitogen-activated protein kinase
- MMP
mitochondrial membrane potential
- PBN
α-phenyl-N-t-butylnitrone
- NTC
non-targeting control
- DCFH-DA
dichloro-dihydro-fluorescein
- DCF
dichlorofluorescein
Footnotes
Previously published online: www.landesbioscience.com/journals/oximed/article/9541
References
- 1.Clark DA, Fornabaio DM, McNeill H, Mullane KM, Caravella SJ, Miller MJ. Contribution of oxygen-derived free radicals to experimental necrotizing enterocolitis. Am J Pathol. 1988;130:537–542. [PMC free article] [PubMed] [Google Scholar]
- 2.Kelly N, Friend K, Boyle P, Zhang XR, Wong C, Hackam DJ, et al. The role of the glutathione antioxidant system in gut barrier failure in a rodent model of experimental necrotizing enterocolitis. Surgery. 2004;136:557–566. doi: 10.1016/j.surg.2004.05.034. [DOI] [PubMed] [Google Scholar]
- 3.Martin CR, Walker WA. Intestinal immune defences and the inflammatory response in necrotising enterocolitis. Semin Fetal Neonatal Med. 2006;11:369–377. doi: 10.1016/j.siny.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Markel TA, Crisostomo PR, Wairiuko GM, Pitcher J, Tsai BM, Meldrum DR. Cytokines in necrotizing enterocolitis. Shock. 2006;25:329–337. doi: 10.1097/01.shk.0000192126.33823.87. [DOI] [PubMed] [Google Scholar]
- 5.MacDonald TT, Hutchings P, Choy MY, Murch S, Cooke A. Tumour necrosis factor-alpha and interferon-gamma production measured at the single cell level in normal and inflamed human intestine. Clin Exp Immunol. 1990;81:301–305. doi: 10.1111/j.1365-2249.1990.tb03334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reinecker HC, Steffen M, Witthoeft T, Pflueger I, Schreiber S, MacDermott RP, Raedler A. Enhanced secretion of tumour necrosis factor-alpha, IL-6 and IL-1beta by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn’s disease. Clin Exp Immunol. 1993;94:174–181. doi: 10.1111/j.1365-2249.1993.tb05997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halpern MD, Clark JA, Saunders TA, Doelle SM, Hosseini DM, Stagner AM, Dvorak B. Reduction of experimental necrotizing enterocolitis with anti-TNFalpha. Am J Physiol Gastrointest Liver Physiol. 2006;290:757–764. doi: 10.1152/ajpgi.00408.2005. [DOI] [PubMed] [Google Scholar]
- 8.Seitz G, Warmann SW, Guglielmetti A, Heitmann H, Ruck P, Kreis ME, Fuchs J. Protective effect of tumor necrosis factor alpha antibody on experimental necrotizing enterocolitis in the rat. J Pediatr Surg. 2005;40:1440–1445. doi: 10.1016/j.jpedsurg.2005.05.043. [DOI] [PubMed] [Google Scholar]
- 9.Xue X, Piao JH, Nakajima A, Sakon-Komazawa S, Kojima Y, Mori K, et al. Tumor necrosis factor alpha (TNFalpha) induces the unfolded protein response (UPR) in a reactive oxygen species (ROS)-dependent fashion, and the UPR counteracts ROS accumulation by TNFalpha. J Biol Chem. 2005;280:33917–33925. doi: 10.1074/jbc.M505818200. [DOI] [PubMed] [Google Scholar]
- 10.Schulze-Osthoff K, Bakker AC, Vanhaesebroeck B, Beyaert R, Jacob WA, Fiers W. Cytotoxic activity of tumor necrosis factor is mediated by early damage of mitochondrial functions. Evidence for the involvement of mitochondrial radical generation. J Biol Chem. 1992;267:5317–5323. [PubMed] [Google Scholar]
- 11.Goossens V, Grooten J, De Vos K, Fiers W. Direct evidence for tumor necrosis factor-induced mitochondrial reactive oxygen intermediates and their involvement in cytotoxicity. Proc Natl Acad Sci USA. 1995;92:8115–8119. doi: 10.1073/pnas.92.18.8115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandez-Checa JC, Kaplowitz N, Garcia-Ruiz C, Colell A, Miranda M, Mari M, et al. GSH transport in mitochondria: defense against TNF-induced oxidative stress and alcohol-induced defect. Am J Physiol. 1997;273:7–17. doi: 10.1152/ajpgi.1997.273.1.G7. [DOI] [PubMed] [Google Scholar]
- 13.Corda S, Laplace C, Vicaut E, Duranteau J. Rapid reactive oxygen species production by mitochondria in endothelial cells exposed to tumor necrosis factor-alpha is mediated by ceramide. Am J Respir Cell Mol Biol. 2001;24:762–768. doi: 10.1165/ajrcmb.24.6.4228. [DOI] [PubMed] [Google Scholar]
- 14.Tobiume K, Matsuzawa A, Takahashi T, Nishitoh H, Morita K, Takeda K, et al. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2001;2:222–228. doi: 10.1093/embo-reports/kve046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu H, Nishitoh H, Ichijo H, Kyriakis JM. Activation of apoptosis signal-regulating kinase 1 (ASK1) by tumor necrosis factor receptor-associated factor 2 requires prior dissociation of the ASK1 inhibitor thioredoxin. Mol Cell Biol. 2000;20:2198–2208. doi: 10.1128/mcb.20.6.2198-2208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X, Zhang R, Luo D, Park SJ, Wang Q, Kim Y, Min W. Tumor necrosis factor alpha-induced desumoylation and cytoplasmic translocation of homeodomain-interacting protein kinase 1 are critical for apoptosis signal-regulating kinase 1-JNK/p38 activation. J Biol Chem. 2005;280:15061–15070. doi: 10.1074/jbc.M414262200. [DOI] [PubMed] [Google Scholar]
- 17.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 18.Ichijo H, Nishida E, Irie K, ten Dijke P, Saitoh M, Moriguchi T, et al. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- 19.Chang HY, Nishitoh H, Yang X, Ichijo H, Baltimore D. Activation of apoptosis signal-regulating kinase 1 (ASK1) by the adapter protein Daxx. Science. 1998;281:1860–1863. doi: 10.1126/science.281.5384.1860. [DOI] [PubMed] [Google Scholar]
- 20.Takeda K, Hatai T, Hamazaki TS, Nishitoh H, Saitoh M, Ichijo H. Apoptosis signal-regulating kinase 1 (ASK1) induces neuronal differentiation and survival of PC12 cells. J Biol Chem. 2000;275:9805–9813. doi: 10.1074/jbc.275.13.9805. [DOI] [PubMed] [Google Scholar]
- 21.Sayama K, Hanakawa Y, Shirakata Y, Yamasaki K, Sawada Y, Sun L, et al. Apoptosis signal-regulating kinase 1 (ASK1) is an intracellular inducer of keratinocyte differentiation. J Biol Chem. 2001;276:999–1004. doi: 10.1074/jbc.M003425200. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, Min W. Thioredoxin promotes ASK1 ubiquit-ination and degradation to inhibit ASK1-mediated apoptosis in a redox activity-independent manner. Circ Res. 2002;90:1259–1266. doi: 10.1161/01.res.0000022160.64355.62. [DOI] [PubMed] [Google Scholar]
- 23.Machino T, Hashimoto S, Maruoka S, Gon Y, Hayashi S, Mizumura K, et al. Apoptosis signal-regulating kinase 1-mediated signaling pathway regulates hydrogen peroxide-induced apoptosis in human pulmonary vascular endothelial cells. Crit Care Med. 2003;31:2776–2781. doi: 10.1097/01.CCM.0000098027.49562.29. [DOI] [PubMed] [Google Scholar]
- 24.Liu H, Zhang H, Iles KE, Rinna A, Merrill G, Yodoi J, et al. The ADP-stimulated NADPH oxidase activates the ASK-1/MKK4/JNK pathway in alveolar macrophages. Free Radic Res. 2006;40:865–874. doi: 10.1080/10715760600758514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song JJ, Lee YJ. Differential role of glutaredoxin and thioredoxin in metabolic oxidative stress-induced activation of apoptosis signal-regulating kinase 1. Biochem J. 2003;373:845–853. doi: 10.1042/BJ20030275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imoto K, Kukidome D, Nishikawa T, Matsuhisa T, Sonoda K, Fujisawa K, et al. Impact of mitochondrial reactive oxygen species and apoptosis signal-regulating kinase 1 on insulin signaling. Diabetes. 2006;55:1197–1204. doi: 10.2337/db05-1187. [DOI] [PubMed] [Google Scholar]
- 27.Zhang R, He X, Liu W, Lu M, Hsieh JT, Min W. AIP1 mediates TNFalpha-induced ASK1 activation by facilitating dissociation of ASK1 from its inhibitor 14-3-3. J Clin Invest. 2003;111:1933–1943. doi: 10.1172/JCI17790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang W, Zheng S, Storz P, Min W. Protein kinase D specifically mediates apoptosis signal-regulating kinase 1-JNK signaling induced by H2O2 but not tumor necrosis factor. J Biol Chem. 2005;280:19036–19044. doi: 10.1074/jbc.M414674200. [DOI] [PubMed] [Google Scholar]
- 29.De Plaen IG, Liu SX, Tian R, Neequaye I, May MJ, Han XB, et al. Inhibition of nuclear factor-kappaB ameliorates bowel injury and prolongs survival in a neonatal rat model of necrotizing enterocolitis. Pediatr Res. 2007;61:716–721. doi: 10.1203/pdr.0b013e3180534219. [DOI] [PubMed] [Google Scholar]
- 30.Leaphart CL, Cavallo J, Gribar SC, Cetin S, Li J, Branca MF, et al. A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J Immunol. 2007;179:4808–4820. doi: 10.4049/jimmunol.179.7.4808. [DOI] [PubMed] [Google Scholar]
- 31.Baregamian N, Mourot JM, Ballard AR, Evers BM, Chung DH. PPARgamma agonist protects against intestinal injury during necrotizing enterocolitis. Biochem Biophys Res Commun. 2009;379:423–427. doi: 10.1016/j.bbrc.2008.11.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gribar SC, Sodhi CP, Richardson WM, Anand RJ, Gittes GK, Branca MF, et al. Reciprocal expression and signaling of TLR4 and TLR9 in the pathogenesis and treatment of necrotizing enterocolitis. J Immunol. 2009;182:636–646. doi: 10.4049/jimmunol.182.1.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lugo B, Ford HR, Grishin A. Molecular signaling in necrotizing enterocolitis: regulation of intestinal COX-2 expression. J Pediatr Surg. 2007;42:1165–1171. doi: 10.1016/j.jpedsurg.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 34.Lee WS, Sokol RJ. Liver disease in mitochondrial disorders. Semin Liver Dis. 2007;27:259–273. doi: 10.1055/s-2007-985071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sokol RJ, Treem WR. Mitochondria and childhood liver diseases. J Pediatr Gastroenterol Nutr. 1999;28:4–16. doi: 10.1097/00005176-199901000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Lemasters JJ. Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction and aging. Rejuvenation Res. 2005;8:3–5. doi: 10.1089/rej.2005.8.3. [DOI] [PubMed] [Google Scholar]
- 37.Kim I, Rodriguez-Enriquez S, Lemasters JJ. Selective degradation of mitochondria by mitophagy. Arch Biochem Biophys. 2007;462:245–253. doi: 10.1016/j.abb.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baregamian N, Song J, Jeschke MG, Evers BM, Chung DH. IGF-1 protects intestinal epithelial cells from oxidative stress-induced apoptosis. J Surg Res. 2006;136:31–37. doi: 10.1016/j.jss.2006.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou Y, Wang Q, Evers BM, Chung DH. Signal transduction pathways involved in oxidative stress-induced intestinal epithelial cell apoptosis. Pediatr Res. 2005;58:1192–1197. doi: 10.1203/01.pdr.0000185133.65966.4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramadan M, Gamal-Eldeen AM, Abdel-Aziz M, Abuo-Rahma Gel D, Abdel-Nabi H, Nagib AH. C-(2-chloroquinoline-3-yl)-N-phenyl nitrone: new synthetic antioxidant inhibits proliferation and induces apoptosis of breast carcinoma MCF-7 cells. Arch Pharm (Weinheim) 2006;339:242–249. doi: 10.1002/ardp.200500250. [DOI] [PubMed] [Google Scholar]
- 41.Hensley K, Pye QN, Maidt ML, Stewart CA, Robinson KA, Jaffrey F, Floyd RA. Interaction of alpha-phenyl-N-tert-butyl nitrone and alternative electron acceptors with complex I indicates a substrate reduction site upstream from the rotenone binding site. J Neurochem. 1998;71:2549–2557. doi: 10.1046/j.1471-4159.1998.71062549.x. [DOI] [PubMed] [Google Scholar]
- 42.Kim S, de AVGV , Bouajila J, Dias AG, Cyrino FZ, Bouskela E, et al. Alpha-phenyl-N-tert-butyl nitrone (PBN) derivatives: synthesis and protective action against microvascular damages induced by ischemia/reperfusion. Bioorg Med Chem. 2007;15:3572–3578. doi: 10.1016/j.bmc.2007.02.033. [DOI] [PubMed] [Google Scholar]
- 43.Wen JJ, Bhatia V, Popov VL, Garg NJ. Phenyl-alphatert-butyl nitrone reverses mitochondrial decay in acute Chagas’ disease. Am J Pathol. 2006;169:1953–1964. doi: 10.2353/ajpath.2006.060475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee JH, Park JW. The effect of alpha-phenyl-N-tbutylnitrone on ionizing radiation-induced apoptosis in U937 cells. Free Radic Res. 2005;39:1325–1333. doi: 10.1080/10715760500217215. [DOI] [PubMed] [Google Scholar]
- 45.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 46.Mozo J, Ferry G, Studeny A, Pecqueur C, Rodriguez M, Boutin JA, Bouillaud F. Expression of UCP3 in CHO cells does not cause uncoupling, but controls mitochondrial activity in the presence of glucose. Biochem J. 2006;393:431–439. doi: 10.1042/BJ20050494. [DOI] [PMC free article] [PubMed] [Google Scholar]