Abstract
Organic cations (OCs) are substances of endogenous (e.g., dopamine, choline) or exogenous (e.g., drugs like cimetidine) origin that are positively charged at physiological ph. since many of these compounds can not pass the cell membrane freely, their transport in or out of cells must be mediated by specific transport systems. Transport by organic cation transporters (OCTs) can be regulated rapidly by altering their trafficking and/or affinities in response to stimuli. However, for example, a specific disease could lead to modifications in the expression of OCTs. Chronic exposure to oxidative stress has been suggested to alter regulation and functional activity of proteins through several pathways. According to results from a previous work, oxidation-reduction pathways were thought to be involved in intestinal organic cation uptake modulation. The present work was performed in order to evaluate the influence of oxidative stressors, especially glutathione, on the intestinal organic cation absorption. For this purpose, the effect of compounds with different redox potential (glutathione, an endogenous antioxidant, and procyanidins, diet antioxidants) was assessed on MPP+ (1-methyl-4-phenylpyridinium iodide) uptake in an enterocyte cell line (Caco-2). Caco-2 cells were subcultured with two different media conditions (physiological: 5 mM glucose, referred as control cells; and high-glucose: 25 mM glucose, referred as HG cells). In HG cells, the uptake was significantly lower than in control cells. Redox changing interventions affected Mpp+ uptake, both in control and in high-glucose Caco-2 cells. Cellular glutathione levels could have an important impact on membrane transporter activity. The results indicate that modifications in the cellular oxidative state modulate MPP+ uptake by Caco-2 cells. Such modifications may reflect in changes of nutrient and drug bioavailability.
Key words: Caco-2 cells, glucose, MPP+, glutathione, OCTs (organic cation transporters), oxidation-reduction
Introduction
Epithelial cells of the gastrointestinal tract are an important barrier between the “milieu interne” and the luminal content of the gut. They perform the transport of nutrients, salts and water, which is essential for the maintenance of body homeostasis. Additionally, it constitutes the major route for xenobiotic entry and is also an important site of secretion of many compounds. Biological membranes prevent transmembrane diffusion of the majority of organic molecules that bear net charges. Organic cations are polar and positively charged at physiological pH making the involvement of membrane-bound transport systems on its biokinetic, necessary. Thus, intestinal transporters play a crucial role in limiting and/or promoting the bioavailability of organic cations.
It has been demonstrated that the absorption of certain nutrients and drugs and their effects may be influenced by the concomitant ingestion of certain food components. In a previous work,1 the ability of procyanidins, a group of compounds common in diet, a class of polyphenolic polymers composed of flavan-3-ol units (catechin and epicatechin) found specially in red wine, apples, tea and cocoa or chocolate, to modulate (increasing or decreasing) organic cation apical uptake into Caco-2 cells (human colon epithelial cancer cell line used as a model of human intestinal absorption) was observed, and it was suggested that this could be achieved through oxidationreduction pathways.
It has been well established that high glucose environments favour oxidative stress through mechanisms that include nonenzymatic, enzymatic and mitochondrial pathways. As a consequence, cellular components (lipids, proteins and nucleic acids) may undergo significant modifications that can culminate in cell injury. Several reports focusing on aging and diabetes mellitus have implicated high extracellular glucose concentrations in significant alterations in regulatory and functional activity of proteins.2–7 If targets of such alterations are transporters that regulate molecule transfer in exchange surfaces, like the intestinal epithelium, bioavailability of substrates can be compromised. The aim of this study was to assess the effect of compounds with different redox potential (glutathione and procyanidins) on MPP+ (1-methyl-4-phenylpyridinium iodide) uptake in Caco-2 cells. Caco-2 cells were subcultured with two different media conditions: physiological (5.5 mM) or high glucose concentration (25 mM).
Results
In the present work, Caco-2 cells were used. These cells are a human colon epithelial cancer cell line used as a model of human intestinal absorption of drugs and other compounds. When cultured as a monolayer, Caco-2 cells differentiate to form tight junctions between cells to serve as a model of paracellular movement of compounds across the monolayer. In addition, Caco-2 cells express transporter proteins, efflux proteins to model a variety of transcellular pathways as well as metabolic transformation of test substances. In many respects, the Caco-2 cell monolayer mimics the human intestinal epithelium. In vitro permeability assays remain a valuable tool of screening leading scientists for compound optimization. For more than a decade, the Caco-2 screening assay has remained a popular, in vitro system to test compounds for intestinal permeability and efflux liability.
As previously reported,12 uptake of 3H-MPP+ into Caco-2 cells is linear in time for up to 5 min of incubation. Therefore, in order to determine initial rates of uptake, cells were incubated in the presence of 3H-MPP+ (200 nM) for 5 minutes.
Caco-2 cells were preincubated for 60 min with Hanks buffer and incubated with 3H-MPP+ (200 nM) for 5 minutes.
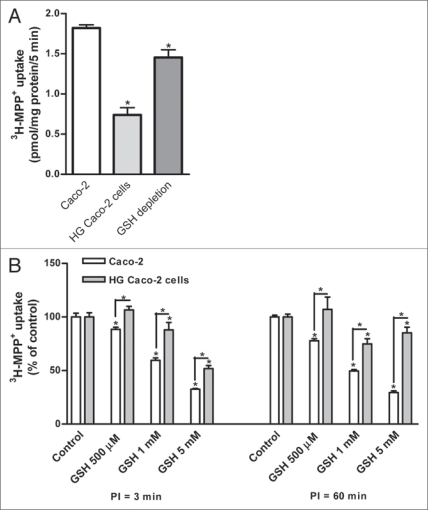
As previously demonstrated,13 3H-MPP+ uptake was significantly reduced in Caco-2 cells treated with high glucose concentration (HG) as compared to control cells (Fig. 1A). Additionally, a reduction of MPP+ uptake in Caco-2 cells by depletion of glutathione was observed (Fig. 1A).
Figure 1.
(A) In the y-axis it is represented 3H-Mpp+ apical uptake (as pmol/mg protein/5 min) of Caco-2, Caco-2 cells maintained with 25 mM glucose (HG Caco-2) and Caco-2 cells depleted of reduced glutathione (GSH depleted Caco-2). Confluent Caco-2 monolayers were preincubated at 37°C for 60 min with Hanks buffer and then incubated with 200 nM 3H-MPP+ for 5 min. (B) Effect of reduced glutathione (GSH) (500 µM, 1 mM and 5 mM) on 3H-MPP+ apical uptake (represented as % of the control treatment) by Caco-2 cells and Caco-2 cells maintained with 25 mM glucose (HG Caco-2). Confluent monolayers were preincubated at 37°C for 3 and 60 min (PI = 3 min and PI = 60 min, respectively) and then incubated with 200 nM 3H-MPP+ for 5 min in the presence of H2O (control) or the tested compounds. each value represents the mean ± SEM (n = 6). *p < 0.05.
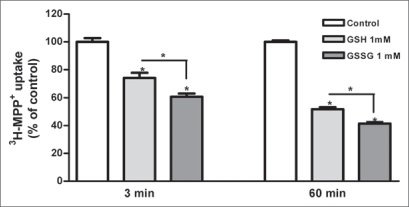
Caco-2 cells, were preincubated with glutathione (500 µM, 1 mM and 5 mM) for 3 and 60 min. Preincubation with reduced glutathione led to a reduction in 3-MPP+ uptake (Fig. 1B). Glutathione inhibited 3-MPP+ uptake in both cells in a concentration- and time dependent manner. When both cell cultures were preincubated (3 and 60 min) with 1 mM oxidized glutathione (GSSG), a significant inhibition of 3-MPP+ uptake was observed. This effect was significantly more pronounced than that of GSH at the same concentration (Fig. 2). Additionally, 60 min of preincubation with GSH and GSSG led to a significant reduction of 3-MPP+ uptake compared with 3 min of preincubation (Fig. 2).
Figure 2.
Effect of reduced (GSH) and oxidized (GSSG) glutathione (1 mM) on 3H-MPP+ apical uptake (represented as % of the control treatment) by Caco-2 cells. Confluent Caco-2 monolayers were preincubated at 37°C for 3 and 60 min and then incubated with 200 nM 3H-MPP+ for 5 min in the presence of H2O (control) or the tested compounds. each value represents the mean ± SEM (n = 6). *p < 0.05.
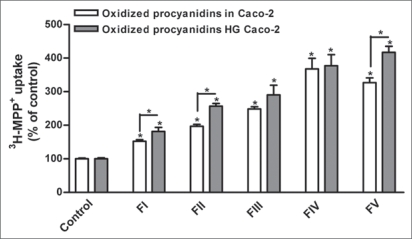
Procyanidins were chromatographically fractionated by molecular weight, according to their structural complexity (Table 1).1 In the present work, all tested procyanidin fractions (FI-FV) increased 3-MPP+ uptake in both control and HG cells. Interestingly, their effect was more marked in HG cells (Fig. 3).
Table 1.
General structure of procyanidins (condensed tannins)
| Recovery | Average Molecular | |
| Time (h) | Weight (Da) | |
| Fraction I | 2:30–4 | 600 |
| Fraction II | 4–4:35 | 800 |
| Fraction III | 4:35–6 | 900 |
| Fraction IV | 6–7 | 1000 |
| Fraction V | 7–8 | 1200 |
Figure 3.
Effect of oxidized procyanidin fractions (FI-FV) on 3H-MPP+ apical uptake (respresented as %, considering control treatment as 100%) by Caco-2 cells and Caco-2 cells maintained with 25 mM glucose (HG Caco-2). Confluent Caco-2 monolayers were preincubated for 60 min with oxidized procyanidins and then incubated with 200 nM 3H-MPP+ for 5 min in the presence of etOh (control) or the tested compounds. each value represents the mean ± SEM (n = 3–27). *p < 0.05.
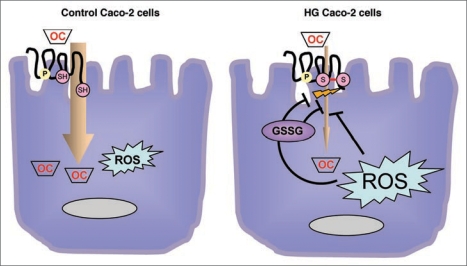
In agreement with our results, our hypothesis is schematically represented in Figure 4. This figure shows the possible pathways involved in OCT modulation according to different oxidative status in two kinds of cultured cells (control cells versus HG, high glucose, cells). It is possible that oxidative stress modifies OCT activity, either directly or through glutathione pathway. Similarly, glutathione, directly, or through indirect pathways, modifies oxidative status of OCT, and consequently its activity.
Figure 4.
Schematic representation of the investigated pathways involved in OCT activity modulation, through changing oxidative cellular status. (A) Control cells. Caco-2 cells were grown in Minimum essential Medium containing 5 mM (physiological) glucose. In these cells the uptake of the organic cations was more pronounced compared to HG cells (B). (B) High Glucose cells (HG). Caco-2 cells were grown in Minimum Essential Medium containing 25 mM (high) glucose. In these cells, uptake was significantly reduced by oxidative stress. It is possible that oxidative stress, increased in these cells, modifies OCTs activity, either directly or indirectly through glutathione pathways. Similarly, glutathione, directly or through indirect pathways, modifies oxidative status of OCTs, and consequently their activity.
Discussion
Organic cations (OCs) are substances of endogenous (e.g., dopamine, choline) or exogenous (e.g., drugs like cimetidine) origin that are positively charged at physiological pH. Since many of these compounds can not pass the cell membrane freely, their transport in or out of cells must be mediated by specific transport systems. Transport by organic cation transporters (OCTs) can be regulated rapidly by altering their trafficking and/or affinities in response to stimuli. However, for example, diabetes is associated with reduced gene and protein expression of renal OCT1, OCT2 and OCT3.14
The purpose of this study was to evaluate the influence of oxidative stress exposure of Caco-2 cells on the functional activity of organic cation absorption, thus, on their bioavailability.
Different mechanisms might have been involved in the observed effect including modification of substrate or transporter protein as a consequence of redox alteration in cellular environment. According to literature,15 organic cation transporter 1 (hOCT1) and organic cation transporter 3 (hOCT3 or hEMT) are the transporters implicated in organic cation uptake at the brush-border membrane of Caco-2 cells. Previous work has shown that hOCT3 transcription was downregulated in HG cells, whereas hOCT1 was not affected by this treatment.13
As to the mechanism of the modulatory effects of high glucose on the transport of MPP+ into Caco-2 cells, the involvement of oxidation-reduction pathways had already been suggested.1 In agreement with this hypothesis, in the present work, a reduction of MPP+ uptake in Caco-2 cells by depletion of glutathione was observed. Glutathione (GSH) is part of endogenous antioxidant defences, and therefore, under these conditions, an alteration in the redox cellular environment, favouring an oxidative surrounding, is expected.16 In the present work, glutathione inhibited 3-MPP+ uptake in both cells in a concentration- and time dependent manner. When both cell cultures were preincubated with 1 mM oxidized glutathione (GSSG), a significant inhibition of 3-MPP+ uptake was observed. This effect was significantly more pronounced than that of GSH at the same concentration. These results are not clear since both reduced and oxidized glutathione were found to inhibit MPP+ uptake in both cells, even though the effect was less pronounced in HG cells. The fact that GSSG exerted a more pronounced inhibition on MPP+ uptake than GSH, and that the inhibitory effects of both GSH and GSSG were more marked after 60 min of preincubation, continues to point to the hypothesis of an involvement of redox changes. As a matter of fact, oxidation of GSH may have occurred during the preincubation. On the other hand, a different effect of glutathione on the transporter, independent of redox changes, can not be excluded.
In a previous work, it has been demonstrated that procyanidins—polyphenolic compounds widespread in nature, commonly present in the human diet, and with well known antioxidant effects, increase 3-MPP+ uptake in Caco-2 cells.1 In the present work, all tested procyanidin fractions (FI-FV) increased 3-MPP+ uptake in both control and HG cells, which was more marked in HG cells. This outcome strengthen the hypothesis of the involvement of redox mechanisms in HG effects on 3-MPP+ uptake.
To our knowledge, there has been no data describing the importance of the redox state in OCT1- or OCT3-mediated transport, although these transporters possess several amino acid residues in intra- and extracellular domains17,18 that are prone to alteration, being readily oxidized upon changes in cellular redox state. Altogether this work’s results are compatible with a modulation of organic cation transport in Caco-2 cells through redox mechanisms.
In conclusion, the data yielded from this study supports the possible influence of redox status on organic cation transport across intestinal epithelial cells. As the maintenance of Caco-2 cells in media with 25 mM glucose is quite common in experimental settings,19–21 results of transmembranar transport modulation must be analysed with great care.
Materials and Methods
Reagents.
3-MPP+ (N-[methyl-3H]-4-phenylpyridinium acetate; specific activity 82 Ci mmol−1) (New England Nuclear Chemicals, Dreieich, Germany); Triton X-100 and D-(+)-glucose (Merck, Darmstadt, Germany); MPP+ (1-methyl-4-phenylpyridinium iodide), DL-buthionine-(S,R)-sulfoximine and N-ethylmaleimide (Sigma, St. Louis, MO, USA).
Cells and culture conditions.
The Caco-2 cell line was obtained from the American Type Culture Collection (ATCC37-HTB, Rockville, MD USA) and was used between passage number 30–41. Caco-2 cells were maintained in a humidified atmosphere of 5% CO2-95% air and were grown in Minimum Essential Medium (Sigma, St. Louis, MO, USA) containing either 25 mM (high) or 5.5 mM (physiological) glucose and supplemented with 15% fetal bovine serum, 25 mmol l−1 HEPES, 100 units ml−1 penicillin, 100 µg ml−1 streptomycin and 0.25 µg ml−1 amphotericin B (all from Sigma). Caco-2 cells were adapted for at least five passages to the high glucose concentration. Osmolarity control was performed with mannitol. Culture medium was changed every 2 to 3 days and the culture was split every 7 days. For subculturing, the cells were removed enzymatically (0.25% trypsin-EDTA), split 1:3, and subcultured in plastic culture dishes (21 cm2; Ø 60 mm; Corning Costar, corning, NY). For the experiments, Caco-2 cells were seeded on 24-well plastic cell culture clusters (2 cm2; Ø 16 mm; corning Costar). For 24 h before the experiment, the cell medium was free of fetal bovine serum. GSH depletion was obtained by pre-treating cells with 1 mM DL-buthionine-(S,R)-sulfoximine (BSO), an inhibitor of GSH synthase, in culture medium free of fetal bovine serum for 22 h and with 30 µM of N-ethylmaleimide (NEM), a cysteine-alkylator for 1 h. After washing the cells with culture medium, they were treated with BSO for an additional hour. Uptake studies were performed 9–11 days after the cells formed a monolayer.
Transport studies.
Transport experiments were performed in Hanks’ medium with the following composition (in mmol l−1): 137 NaCl, 5 KCl, 0.8 MgSO4, 1.0 MgCl2, 0.33 Na2HPO4, 0.44 KH2PO4, 0.25 CaCl2, 0.15 tris.HCl, and 1.0 sodium butyrate, pH 7.4.
Initially, the growth medium was aspirated and the cells were washed with Hanks’ medium at 37°C; then the cell monolayers were preincubated in Hanks’ medium at 37°C. Transport studies were performed in cells cultured on plastic supports, 3-MPP+ being applied to the medium facing the apical cell membrane. Uptake was initiated by the addition of 0.3 ml medium at 37°C containing 200 nM 3-MPP+. Incubation was stopped after 5 min by placing the cells on ice and rinsing them with 0.5 ml icecold Hanks’ medium. The cells were then solubilized with 0.3 ml 0.1% (v/v) triton X-100 (in 5 mmol l−1 tris.HCl, pH 7.4), at room temperature overnight. Radioactivity in the cells was measured by liquid scintillation counting.
Effect of compounds.
Compounds to be tested were present during both the preincubation and incubation periods. Controls for all treatments were run in the presence of the solvent (1%).
Procyanidin fraction.
Condensed tannins were extracted from Vitis vinifera as described elsewhere8 and separated according to the procedure described in the literature.9 The grape seed extract was fractionated through a TSK Toyopearl HW-40(s) gel column (250 mm × 16 mm i.d., with 0.8 ml min−1 methanol as eluent) according to the procedure described in the literature with some modifications.10 Fractions were all obtained after elution with 99.8% (v/v) methanol; the first 120 ml, corresponding to the elution of catechin monomers, were eliminated, and elution was followed over 5 h in order to elute the procyanidin oligomers; all the fractions were mixed with deionised water; the solvent was eliminated using a rotary evaporator under reduced pressure at 30°C and then freeze dried. The resulting solids were analysed by laser secondary ionisation mass spectrometry (LSIMS) (Table 1). Procyanidins were dissolved in ethanol and maintained at −80°C until use. Procyanidin oxidation was achieved by exposure of a small amount of procyanidin solution to air, at room temperature, for 7 days.
Protein determination.
The protein content of cell monolayers was determined using Bradford’s method as described,11 with human serum albumin as standard.
Calculations and statistics.
Values are expressed as the arithmetic mean ± SEM. Statistical significance of the difference between groups was evaluated by one-way analysis of variance (ANOVA) followed by the Bonferroni test. Statistical significance between two groups was evaluated by Student’s t-test. Differences were considered significant when p < 0.05.
Procyanidins were fractionated using low pressure chromatography and recovered during a period of time. Average molecular weights (in Dalton) of procyanidins in grape seeds fractions was determined by Liquid Secondary Ion Mass Spectrometry.
Acknowledgements
This work was supported by Fundação para a Ciência e Tecnologia (POCI, FEDER, Programa Comunitário de Apoio, PTDC/QUI/65501/2006) and SFRH/BD/28160/2006, SFRH/BD/46640/2008 and SFRH/BPD/40110/2007.
Abbreviations
- MPP+
1-methyl-4-phenylpyridinium iodide
- GSH
reduced glutathione
- GSSG
oxidized glutathione
- HG
high glucose
- hOCT1
human organic cation transporter type 1
- hOCT3
human organic cation transporter type 3
- OCs
organic cations
Footnotes
Previously published online: www.landesbioscience.com/journals/oximed/article/9769
References
- 1.Faria A, Mateus N, de Freitas V, Calhau C. Modulation of MPP+ uptake by procyanidins in Caco-2 cells: involvement of oxidation/reduction reactions. FEBS Lett. 2006;580:155–160. doi: 10.1016/j.febslet.2005.11.068. [DOI] [PubMed] [Google Scholar]
- 2.Ha H, Lee HB. Reactive oxygen species as glucose signaling molecules in mesangial cells cultured under high glucose. Kidney Int Suppl. 2000;77:19–25. doi: 10.1046/j.1523-1755.2000.07704.x. [DOI] [PubMed] [Google Scholar]
- 3.Cornford EM, Hyman S, Cornford ME, Clare-Salzler M. Downregulation of blood-brain glucose transport in the hyperglycemic nonobese diabetic mouse. Neurochem Res. 1995;20:869–873. doi: 10.1007/BF00969700. [DOI] [PubMed] [Google Scholar]
- 4.Hahn T, Barth S, Weiss U, Mosgoeller W, Desoye G. Sustained hyperglycemia in vitro downregulates the GLUT 1 glucose transport system of cultured human term placental trophoblast: a mechanism to protect fetal development? Faseb J. 1998;12:1221–1231. doi: 10.1096/fasebj.12.12.1221. [DOI] [PubMed] [Google Scholar]
- 5.Stevens MJ, Hosaka Y, Masterson JA, Jones SM, Thomas TP, Larkin DD. Downregulation of the human taurine transporter by glucose in cultured retinal pigment epithelial cells. Am J Physiol. 1999;277:760–771. doi: 10.1152/ajpendo.1999.277.4.E760. [DOI] [PubMed] [Google Scholar]
- 6.Giannico G, Cortes P, Baccora MH, Hassett C, Taube DW, Yee J. Glibenclamide prevents the increased extracellular matrix formation induced by high glucose concentration in mesangial cells. Am J Physiol Renal Physiol. 2007;292:F57–65. doi: 10.1152/ajprenal.00210.2006. [DOI] [PubMed] [Google Scholar]
- 7.Noyman I, Marikovsky M, Sasson S, Stark AH, Bernath K, Seger R, Madar Z. Hyperglycemia reduces nitric oxide synthase and glycogen synthase activity in endothelial cells. Nitric Oxide. 2002;7:187–193. doi: 10.1016/s1089-8603(02)00106-4. [DOI] [PubMed] [Google Scholar]
- 8.Darné G, Madero-Tamargo J. Mise au point d’une méthode d’extraction des lipides solubles totaux, des glucides solubles totaux et des composés phénoliques solubles totaux des organes de la vigne. Vitis. 1979;18:221–228. (Fre). [Google Scholar]
- 9.Michaud J, Lacaze P, Masquelier J. Fractionnement des oligomères flavanoliques du raisin. Bulletin de laSociété de Pharmacie de Bordeaux. 1971;110:111–116. (Fre). [Google Scholar]
- 10.de Freitas VAP, Glories Y, Bourgeois G, Vitry C. Characterisation of Oligomeric and Polymeric Procyanidins from Grape Seeds by Liquid Secondary Ion Mass Spectrometry. Phytochemistry. 1998;49:1435–1441. [Google Scholar]
- 11.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 12.Martel F, Calhau C, Azevedo I. Characterization of the transport of the organic cation [3H]MPP+ in human intestinal epithelial (Caco-2) cells. Naunyn Schmiedebergs Arch Pharmacol. 2000;361:505–513. doi: 10.1007/s002100000223. [DOI] [PubMed] [Google Scholar]
- 13.Faria AI, Monteiro R, Mateus N, Azevedo I, Calhau C. Influence of extracellular glucose concentration on organic cation transport in Caco-2 cells. FASEB J. 2007;21:701.12. [Google Scholar]
- 14.Nowicki MT, Aleksunes LM, Sawant SP, Dnyanmote AV, Mehendale HM, Manautou JE. Renal and hepatic transporter expression in type 2 diabetic rats. Drug Metab Lett. 2008;2:11–17. doi: 10.2174/187231208783478425. [DOI] [PubMed] [Google Scholar]
- 15.Martel F, Grundemann D, Calhau C, Schomig E. Apical uptake of organic cations by human intestinal Caco-2 cells: putative involvement of ASF transporters. Naunyn Schmiedebergs Arch Pharmacol. 2001;363:40–49. doi: 10.1007/s002100000335. [DOI] [PubMed] [Google Scholar]
- 16.Ferruzza S, Scarino ML, Gambling L, Natella F, Sambuy Y. Biphasic effect of iron on human intestinal Caco-2 cells: early effect on tight junction permeability with delayed onset of oxidative cytotoxic damage. Cell Mol Biol. 2003;49:89–99. [PubMed] [Google Scholar]
- 17.Hayer M, Bonisch H, Bruss M. Molecular cloning, functional characterization and genomic organization of four alternatively spliced isoforms of the human organic cation transporter 1 (hOCT1/SLC22A1) Ann Hum Genet. 1999;63:473–482. doi: 10.1017/S0003480099007770. [DOI] [PubMed] [Google Scholar]
- 18.Grundemann D, Schechinger B, Rappold GA, Schomig E. Molecular identification of the corticosterone-sensitive extraneuronal catecholamine transporter. Nat Neurosci. 1998;1:349–351. doi: 10.1038/1557. [DOI] [PubMed] [Google Scholar]
- 19.Chang TC, Huang SF, Yang TC, Chan FN, Lin HC, Chang WL. Effect of ginsenosides on glucose uptake in human Caco-2 cells is mediated through altered Na+/glucose cotransporter 1 expression. J Agric Food Chem. 2007;55:1993–1998. doi: 10.1021/jf062714k. [DOI] [PubMed] [Google Scholar]
- 20.Bleasby K, Chauhan S, Brown CD. Characterization of MPP+ secretion across human intestinal Caco-2 cell monolayers: role of P-glycoprotein and a novel Na(+)-dependent organic cation transport mechanism. Br J Pharmacol. 2000;129:619–625. doi: 10.1038/sj.bjp.0703078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shirasaka Y, Kawasaki M, Sakane T, Omatsu H, Moriya Y, Nakamura T, et al. Induction of human P-glycoprotein in Caco-2 cells: development of a highly sensitive assay system for P-glycoprotein-mediated drug transport. Drug Metab Pharmacokinet. 2006;21:414–423. doi: 10.2133/dmpk.21.414. [DOI] [PubMed] [Google Scholar]