Abstract
Background
Deep brain stimulation (DBS), an established treatment for some movement disorders, is now being used experimentally to treat psychiatric disorders as well. In a number of recently published case series, DBS yielded an impressive therapeutic benefit in patients with medically intractable psychiatric diseases.
Methods
This review of the use of DBS to treat psychiatric disorders is based on literature retrieved from a selective Pubmed search for relevant keywords, reference works on the topic, and the authors’ own research.
Results
Studies have been performed on the use of DBS to treat medically intractable obsessive-compulsive disorder, depressive disorders, and Tourette syndrome. The case numbers in the cited publications were small, yet at least some of them involved a methodologically sound investigation. Thus, in some studies, the strength of the effect was controlled with a double-blinded interval in which the stimulation was turned off. In general, the primary symptoms were found to improve markedly, by 35% to 70%, although not all patients responded to the treatment. Adverse effects of DBS were very rare in most studies and could usually be reversed by changing the stimulation parameters.
Conclusions
The results of DBS for psychiatric disorders that have been published to date are encouraging. They open up a new perspective in the treatment of otherwise intractable disorders. Nonetheless, the efficacy, mechanism of action, and adverse effects of DBS for this indication still need to be further studied in methodologically adequate trials that meet the highest ethical standard.
In the late 1980s a team of researchers in Grenoble, led by the neurosurgeon A. L. Benabit, introduced the technique of chronic stimulation of subcortical regions of the brain to treat movement disorders (e1, e2). This procedure, known as deep brain stimulation (DBS), involves stereotactic implantation of electrodes that then continuously emit short high-frequency electrical impulses in order to modulate functional neuronal circuits (Figure 1; eSupplement 1). The tip of each electrode contains at least four poles. Postoperatively, this permits a wide range of modes of stimulation from outside the brain. Each electrode is connected via a lead to the impulse generator, which is usually implanted under the collarbone (eSupplement 2).
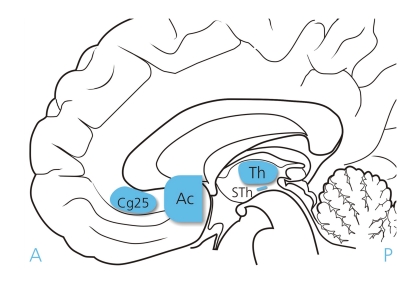
Figure 1.
Target areas for deep brain stimulation in psychiatric diseases: Th, Thalamus; STh, subthalamic nucleus; Ac, nucleus accumbens; Cg25, subgenual area of cingulum, Figure: Jürgen Stoffels, Medizin Foto Köln
Supplement 1: Effect of High-Frequency Stimulation on Brain Structures.
The mechanisms of action of deep brain stimulation (DBS) are not yet fully understood. Reversible functional inhibition of the stimulated target structures by depolarization blockade of the neurons around the electrode, or of the voltage-dependent ion channels on the cell membrane, constitutes one explanation (e50). The inhibition of the physiological activity of stimulated cells, which persists for about 5 minutes after the end of stimulation, correlates with the almost immediate and reversible effect of DBS on Parkinson-plus symptoms and tremor, depending on the functional status of the impulse generator (on/off). Synaptically mediated neuronal inhibition by antidromic excitation of inhibitory GABAergic afferents has also been discussed (e51). A third feasible mechanism is synaptic depression by orthodromic stimulation of efferent axons and consecutive inhibition of transmission by exhaustion of the neurotransmitter pool (e52).
In dystonia and in psychiatric disorders the positive effect of DBS – like that of psychopharmaceuticals when first prescribed – often kicks in only after a number of weeks. This phenomenon cannot be wholly explained by a directly inhibiting mechanism; rather, the system evidently behaves in a plastic manner. Functional adaptation via complex and long-term modulation of neuronal systems seems to enable the effect. Functional magnetic resonance imaging, positron emission tomography (PET), and animal studies show, partially and in certain modalities, an elevation of the hemodynamic response and glucose metabolism of stimulated deep brain structures and their efferent projection areas—a sign of additional excitatory mechanisms of action (e53– e55).
It can be assumed that the action of DBS rests on a large number of effects and interferences, which—depending on stimulation site, stimulation parameters, and the underlying disease—vary in importance and modulate the pathophysiological processes in various ways (e56– e58).
Summarized very simply, DBS influences disordered neuronal networks and circuits by altering the distribution of excitation in the area of the target structure. The aim of DBS is to modulate and ideally eliminate the pathological signal transmission. Reconfiguration of the neuronal activity has a positive impact on the patient’s disease.
Supplement 2: DBS Procedure (Planning and Operation) in the Cologne Study Group.
The procedure for deep brain stimulation (DBS) in patients with psychiatric disorders is analogous to that in movement disorders. Shortly before surgery the patient undergoes cranial magnetic resonance imaging with various weightings and particular modalities—the so-called planning MRI. Furthermore, after application of the stereotactic frame using sedation and local anesthesia, intraoperative computed tomography (CT) is performed. On the basis of the data obtained, and after consulting stereotactic atlases, the target is selected and the electrode pathways visualized in three dimensions.
Following borehole trepanation the electrodes are implanted. Quadripolar (or multipolar) electrodes are used; by selection of the actively stimulating pole, these permit postoperative adjustment and modification of the stimulation site. A previous microdischarge, routine in DBS for movement disorders and serving to check correct positioning of the electrodes, is often performed in psychiatric diseases but is not regularly described. The localization of the electrodes can be checked by postoperative X-ray or cranial CT (and under certain circumstances also MRI). MRI is often not possible after implantation of electrodes because of the potential adverse effects. The initial parameter settings are mostly decided on the basis of empirical experience. The stimulation parameters can vary as follows: amplitude of current from 1 to 6 V, pulse duration from 60 to 200 µs, and stimulation frequency from 120 to 180 Hz.
Surgery is completed in a second session with the infraclavicular implantation of the impulse generator.
In DBS for the treatment of movement disorders the operation is usually performed without symptom-specific medication in order to be able to tell whether the desired effects are achieved with the intraoperative test stimulation. As yet there is no consensus whether medication should be continued or discontinued for DBS of psychiatric diseases, partly because the desired effects of DBS mostly appear only after a considerable interval. If intraoperative exposure and test stimulation are planned, however, it is advisable to reduce the dosage of specific medication as much as possible or interrupt it entirely.
In Parkinson’s disease and essential tremor, DBS has proved so effective that it has been licensed as a treatment option (e3). Furthermore, there have been promising case studies on DBS treatment of certain subtypes of refractory epilepsy (e4, e5), dystonia (e6, e7), and chronic cluster headache (e8, e9). The DBS technique that has been in use for more than 20 years has become well known, and despite its invasive nature is associated with only minor adverse effects.
The idea of extending DBS to the treatment of psychiatric disorders is based on the following considerations:
In various cases, psychiatric adverse effects (induction of depressivity and hypomanic states) were observed in DBS-treated Parkinson’s disease patients. This gave rise to the proposal to employ DBS for primary modulation of psychopathological states (e10, e11).
In recent years knowledge of the mechanisms of origin of psychiatric diseases has grown, largely due to modern imaging procedures. The underlying pathophysiological processes and disordered neuronal networks have to some extent been determined and localized. It is thus now possible to identify potential stimulation sites for DBS.
The lesional procedures employed in the past as a last resort in cases of refractory psychiatric disease—anterior capsulotomy, cingulotomy, limbic leukotomy, etc.—achieved positive results (e12, e13). They do not come into question, however, because of the irreversible brain damage and their severe adverse-effect profile. The DBS technique, which is much less invasive, potentially reversible, and capable of modulation, can be applied to similar anatomic structures and may enable adjustment of the profile of action in the direction of the desired effects.
Methods
The study presented here was based on data retrieved from a search of the Pubmed database for relevant publications in the period from 1980 to January 2009. The search terms were “obsessive compulsive disorder”, “Tourette syndrome”, “depression”, “psychiatric disorder,” “mental disorder,” and “substance abuse” in combination with “deep brain stimulation” (DBS). By using various combinations of these search terms, all Pubmed-listed studies on DBS in psychiatric disease were identified and the reference lists of the relevant publications were screened.
In a further step the search results were limited to studies that explicitly reported the outcome of DBS treatment of primary psychiatric disease in at least three patients. These treatment studies are all discussed below.
Application of DBS in psychiatric disease
Obsessive-compulsive disorders
Obsessive-compulsive disorder is a relatively common psychiatric disease with a lifetime prevalence of around 2% (e14). It manifests clinically in the form of obsessive thoughts and actions with onset between childhood and early adulthood. There is high comorbidity with depression, but also with anxiety and personality disorders (e15). Pathophysiologically, patients with obsessive-compulsive disorder are thought to have a dysequilibrium of the cortico-striato-thalamocortical conduction pathways with a resultant absence of inhibition. With regard to the neurochemistry, the current theory postulates dysregulation of the serotoninergic and dopaminergic systems. These assumptions are based on the known positive effect of the selective serotonin reuptake inhibitors (SSRI), the primarily serotoninergically aligned clomipramine, and some neuroleptics.
Apart from these pharmacological treatment approaches, high rates of success can be achieved with cognitive behavioral therapy, particularly with Exposure and Response Prevention (ERP) (e16).
While 70% to 80% of patients with obsessive-compulsive disorder respond well to cognitive behavioral therapy and pharmacotherapy, most of the remainder display a severe, chronic disease course. These patients were previously candidates for neurosurgical procedures. Among these techniques, all of which involve infliction of irreversible lesions, patients profited most from bilateral anterior capsulotomy, which had the highest success rate at over 60% (e17, e18). These data were obtained in prospective longitudinal studies, some of them over several years, but under non-controlled conditions (e13, e19).
Reports of DBS in the treatment of patients with refractory obsessive-compulsive disorder have been published continuously since 1999. Many of the publications are case reports. We identified five groups of authors who each reported more than three patients whose obsessive-compulsive disorder was treated with DBS (table 1). In the groups led by Nuttin (1, 4–9), Abelson (2), and Mallet (3), as well as our own team in Cologne (11), the study design included a randomized double-blind on–off phase as control mechanism. In other words, in certain segments of the study neither the treating physicians nor the patients knew whether the stimulation was turned on or off (1, 2).
Table 1. Effect of deep brain stimulation in patients with obsessive-compulsive disorders.
| Reference(s) | Patients | Stimulation site | Effect* |
| Nuttin et al., 2003 (1)Gabriels et al., 2003 (4) Nuttin et al., 1999 (5) Nuttin et al., 2003 (6) van Laere et al., 2006 (7) Cosyns et al., 2003 (8) Nuttin et al., 2003 (9) | 6 | Anterior limb of internal capsule, bilateral | Observation for up to 20 months:Reduction in Y-BOCS score: 3 full response 1 partial response 2 no response |
| Abelson et al., 2005 (2) | 4 | Anterior limb of internal capsule, bilateral | Observation 4 to 23 months:Reduction in Y-BOCS score: 2 full response 2 no response |
| Greenberg et al., (2006) (10) | 10 | Anterior limb of internal capsule (7/10), caudal part of nucleus accumbens (8/10), bilateral (8/10) | Observation for 36 months:(data for 8 patients were evaluated): Significant reduktion in Y-BOCS score: 4 full response 2 partial response 2 no response |
| Mallet et al., (2008) (3) | 16 | Subthalamic nucleus, bilateral | Observation for 3 months:Randomized controlled comparison of on-off (off-on) condition: Significant reduction in Y-BOCS score after „on-stimulation“; high rate of adverse effects |
| Huff et al., (in press) (11) | 10 | Nucleus accumbens, right—uniliteral | Observation for 12 months:Significant reduction in Y-BOCS score: 1 full response 4 partial response 5 no response |
*Full response: >35% reduction in Yale-Brown Obsessive Compulsive Scale (Y-BOCS) score; partial response: <35% >25% reduction in Y-BOCS score; no response: <25% reduction in Y-BOCS score (from e13)
For stimulation in the area of the nucleus accumbens/caudate nucleus (11, e20–e22) and the adjacent internal capsule (1, 4– 10) and in the subthalamic nucleus (3), good effects were achieved despite divergent positioning of electrodes.
In all research groups at least 50% of previously refractory patients exhibited improvement within a year in terms of partial response (improvement of =25% on the Yale-Brown Obsessive Compulsive Scale [YBOCS]). Long-term observation showed further improvements in both the extent of symptom reduction and the proportion of patients with obsessive-compulsive disorder who benefit from stimulation. In Cologne we initially restricted ourselves to stimulation of the right nucleus accumbens (figure 2). The decision in favor of unilateral stimulation was based on the findings of preliminary studies, where the best effect was achieved by right-sided stimulation and additional left-sided stimulation produced no essential further improvement (e23). Although we attained significant amelioration of the obsessive-compulsive symptoms, we did not quite match the comparable 1-year results of the other research groups (11).
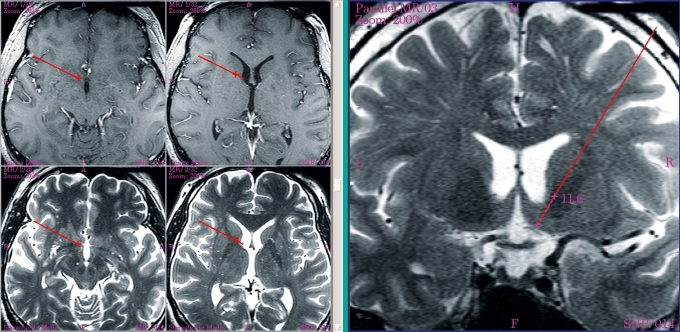
Figure 2.
Magnetic resonance imaging for planning purposes; target: right nucleus accumbens
The preliminary findings of the multicenter study conducted by Mallet’s group (3) are also worthy of note. This team chose to stimulate the subthalamic nucleus, an established target for Parkinson’s disease but a new target for the treatment of obsessive-compulsive disorder. Positive results were achieved in the 3-month on–off phase, but caution is advised in view of the unusually high rate of transient adverse effects (box 1). The intense scientific activity on the subject of DBS is underlined by the recent publication of a further case study with a new target area, the inferior thalamic peduncle. Because this article was published after the end of the chosen search period, it can be mentioned only briefly here. It can be reported, however, that a significant reduction in obsessive-compulsive symptoms within a year was found in patients treated with DBS (e24).
Box 1. Adverse effects.
Although deep brain stimulation (DBS) is an invasive procedure, it causes few adverse effects. The spectrum of unwanted effects can be classified into three types:
Complications of the surgical intervention
Purely technical problems
Adverse effects of the stimulation itself.
Based on the extensive experience of DBS for the treatment of movement disorders (over 55 000 patients worldwide), the risks in the first two categories can be evaluated quite well. The introduction of the electrodes can, in the worst case, result in intracerebral hemorrhage. Depending on surgeon and center, this can be expected in 0.2% to 5% of operations. Intracerebral hemorrhage can lead to focal neurological symptoms such as dysarthria, hemiparesis, or aphasia, or even to death. Postoperative infection via the implanted foreign materials occurs in 2% to 25% of cases, but the risk can be considerably reduced by perioperative administration of systemic antibiotics (e41, e42).
Apparatus-related problems such as lead breakage and failure of the neurostimulator are rapidly decreasing in frequency with technical advances. The rate of 8% per electrode-year found in 2002 is no longer true (e43).
The undesired effects of stimulation vary widely, depending on the anatomical target, but are reversible by cessation of stimulation. Stimulation-related neurological symptoms such as dyskinesia, dysarthria, eyelid apraxia, and, less commonly, unsteady gait often resolve spontaneously, but may particularly regress with modulation of the stimulation.
Close attention is currently being paid to changes in mental state. Alongside descriptions of positive effects on depression and anxiety, the increased use of DBS in Parkinson’s disease in recent years has been accompanied by a growing number of reports of the induction of behavioral abnormalities (e44), depressive states (e45), and manic states (e46). To date, however, these undesired effects have been systematically recorded only for interventions in the subthalamic nucleus. Over a 10-year observation period, a meta-analysis (e47) of DBS in Parkinson’s disease described the following psychiatric adverse effects: depression in 2% to 4% of cases, mania in 0.9% to 1.7%, emotional changes in 0.1% to 0.2%, and suicidality in 0.3% to 0.7%. Subthalamic stimulation may increase the danger of suicide (e48).
Judging from the studies discussed in this article, unwanted effects of DBS as a treatment for psychiatric diseases can be considered unproblematic (with the exception of the approach selected by Mallet et al. [3], who observed an unusually high number of adverse effects with DBS of the subthalamic nucleus). The adverse effects were as a rule only transient, and they mostly resolved with adjustment of the stimulation parameters or the patients tolerated them because of the predominantly positive effects. To date, there are no statistical analyses on this topic. For some of the studies discussed, the eTable lists the transient stimulation-related adverse effects that occurred when DBS was employed for treatment of psychiatric diseases. A number of DBS studies have paid particular attention to the consequences for cognitive performance. To date, no significant cognitive impairments as a result of DBS have been detected (10, 11, 13, 19, 20, 21).
Tourette syndrome
Tourette syndrome is characterized by the chronic but often fluctuating occurrence of vocal (throat clearing, coughing, coprolalia) and motor (blinking, grimacing, jumping) tics. The illness usually manifests at early school age, and around 40% of patients show partial resolution of symptoms or even spontaneous remission with the transition into adulthood. In view of this natural course of the illness, the decision to go ahead with an invasive procedure such as DBS must be weighed up particularly carefully in patients under the age of 21 years.
In almost all cases, Tourette syndrome is comorbid with obsessive-compulsive disorder, attention deficit hyperactivity disorder (ADHD), or depression (e25). The etiology of Tourette syndrome remains to be satisfactorily explained. It was long thought to be a psychogenic disorder, but the prevailing opinion is that Tourette syndrome has been confirmed to have a neurobiological basis. Complex interactions among vulnerability genes and environmental factors are assumed.
The fact that neuroleptics are the class of drugs most effective against tics points to the special role of the dopaminergic system. Imaging procedures have repeatedly shown abnormalities in the ventral striatum (e26). Finally, a dysfunction within specific basal ganglia neuronal circuits in the pathophysiological final pathway is considered probable, involving the thalamus and globus pallidus internus as core structures of motor loops. Based on this knowledge DBS was used in previously treated Tourette patients, with implantation of electrodes in three target areas, all of which have proved effective (table 2):
Table 2. Effect of deep brain stimulation in patients with Tourette syndrome.
| Reference(s) | Patients | Stimulation site | Effect* |
| Visser-Vandewalle et al., 2003 (18) | 3 | Thalamus (ventro-oral nucleus, centromedian nucleus, periventricular substance) | Observation for 8–60 months: between 72% and 90% reduction in tic frequency (video-based measurement) |
| Servello et al., 2007 (12) | 18 | Thalamus (ventro-oral nucleus, centromedian nucleus) | Observation for 3–18 months: 31–95% improvement rates, as measured by YGTSS total tic score |
| Maciunas et al., 2007 (13) | 5 | Thalamus (centromedian nucleus) | Blinded period with on and off phases over 4 weeks: significant (>50%) reduction in tics in 3 of 5 patients in on phase, as measured by a video score (primary target parameter) and the YGTSS (secondary target parameter), compared with off phase; improvement in only 2 of 5 patients after 3 months’ open stimulation |
| Welter et al., 2008 (17) | 3 | Thalamus (centromedian nucleus and parafascicular nucleus) and globus pallidus internus | Observation for 20–60 months (10-month blinded period): 65–96% reduction in symptoms with globus pallidus internus stimulation; 30–64% reduction with thalamic stimulation (as measured by YGTSS) |
| Kuhn et al., 2007 (14)Kuhn et al., 2008 (15) Kuhn et al., 2008 (16) | 8* | Nucleus accumbens (2 patients) Head of caudate nucleus (1 patient) Thalamus (4 patients) Globus pallidus internus (2 patients) | Observation for 3–60 months: improvement in symptoms as measured by YGTSS: Nucleus accumbens: 20–41% Thalamus: 33–80% Globus pallidus internus: 50% (1 patient) No response (1 patient) Caudate nucleus: 33% |
* One patient was initially treated with DBS of the globus pallidus internus, but after failure to respond new electrodes were implanted bilaterally in the thalamus;DBS, deep brain stimulation; YGTSS, Yale Global Tic Severity Scale
The nucleus accumbens as part of the ventral striatum
The globus pallidus internus
The thalamus.
In terms of numbers of patients, the greatest experience in the DBS treatment of Tourette syndrome has been assembled in the thalamus—the internal ventro-oral nucleus, centromedian nucleus, and parafascicular nucleus. In a large open prospective study involving 18 patients, the average improvement rate for tic symptoms was around 70%, as measured using the most frequently employed scale, the Yale Global Tic Severity Scale (YGTSS) (12). It should be pointed out, however, that this study was not carried out under control conditions. Recently—although outside the search period defined above—the same group published the 24-month results of 15 of the 18 patients, documenting continued amelioration of the symptoms (e27).
The first prospective double-blind study to be carried out in patients with Tourette syndrome showed statistically significant improvement in all target parameters in the 4-week blinded phase with stimulation and also after the subsequent 3-month period of open stimulation, although in the long term only two of five patients experienced significant relief (13).
The Cologne group is investigating the problem of response, alongside the absolute degree of efficacy, in various anatomical target structures in the framework of an open prospective pilot study (14–16). In the eight patients enrolled to date, stimulation of the thalamic structures has proved most effective in relieving the Tourette symptoms. In most patients who underwent stimulation, amelioration of the cardinal symptom, the tics, was accompanied by amelioration of the comorbid obsessive-compulsive traits. Nevertheless, the question of the ideal target area for the DBS treatment of Tourette syndrome cannot yet be answered definitively, because the most recent study, carried out in three patients each with four electrodes implanted—bilaterally in the thalamus and in the globus pallidus—has found that pallidal stimulation is more effective than thalamic stimulation (17).
Severe depressive disorders
Severe depressive disorders constitute the most frequent form of psychiatric illness, with a lifetime prevalence of around 15% (e28). In most cases the disease can be effectively treated with a combination of the available drugs such as antidepressants (including augmentation strategies) and psychotherapy. In approximately a tenth of cases, however, the disease becomes chronic and largely refractory (e29). These patients are candidates for non-pharmacological measures, in particular electroconvulsive therapy (ECT) or, in specialized centers, stimulation of the vagal nerve or transcranial magnetic stimulation. ECT is effective but has fundamental disadvantages, e.g., a high recurrence rate and rejection, often vehement, by the patient. DBS could, if proved efficacious, potentially open new therapeutic opportunities as an effective long-term treatment strategy with few adverse effects.
Similar to the illnesses discussed above, the knowledge that severely depressed patients can benefit from lesional neurosurgery motivated the adoption of DBS as a reversible and adaptable form of treatment. Based on the presence of neuronal dysregulation in limbic circuits and positive lesional effects, various target areas for DBS in depressive disorders have been discussed:
Ventral striatum—nucleus accumbens
Subgenual cingulum
Globus pallidus internus
Inferior thalamic peduncle
Rostral cingulate cortex (BA24a)
Lateral habenula.
Research articles have been published on DBS of the ventral striatum—nucleus accumbens and the subgenual cingulum (19– 22) (table 3), but only case reports are available for the inferior thalamic peduncle and the globus pallidus internus (e30– e32).
Table 3. Effect of deep brain stimulation in patients with depressive disorder.
| Reference(s) | Patients | Stimulation site | Effect |
| Schlaepfer et al., 2008 (20) | 3 | Nucleus accumbens | After only 1 week, 42% reduction in symptoms (as measured by HAM-D) |
| Malone et al., 2009 (19) | 15 | Ventral internal capsule and ventral striatum | Observation for 12 months: distinct reduction in symptoms by 57% in this period (as measured by HAM-D) |
| Mayberg et al., 2005 (22) | 6 | Subgenual area of cingulum | Observation for 6 months: 71% reduction in symptoms in 4 of 6 patients (as measured by HAM-D); remission in 2 patients (HAM-D ≤8) |
| Lozano et al., 2008 (21) | 20 | Subgenual area of cingulum | Observation for 12 months: distinct reduction in symptoms by an average 48% in this period, with 2 non-responders; remission in 7 patients (HAM-D ≤7) |
HAM-D, Hamilton Rating Scale for Depression
In depressive disorders the area of the cingulate gyrus below the genu of the corpus callosum displays measurable activity that regresses with antidepressant medication (20). Mayberg’s group therefore chose the subgenual cingulum as target area for DBS. In four of six patients with otherwise refractory depression DBS achieved clear relief of symptoms after 6 months. There was an average 71% reduction in score on the Hamilton Rating Scale for Depression (HAM-D). Furthermore, a drop in the previously elevated cerebral blood flow in the subgenual cingulate gyrus (22) indicated treatment success. No cognitive impairment was detected after 12 months; memory functions, sometimes negatively impacted by ECT, remained unaffected (e33). The recruitment of 14 more refractory patients confirmed the findings of the pilot study: After 6 months there was a reduction of at least 50% in the HAM-D score in 12 of the 20 participants, and seven patients fulfilled the criteria for remission (HAM-D score <7). Analogous to the first investigation, the findings were verified by positron emission tomography (PET). None of the patients exhibited cognitive impairment (21).
The nucleus accumbens constitutes a central interface between emotional, limbic, and motor neuronal circuits and is crucial in the experience of reward and hedonistic stimuli. This motivated the Cologne–Bonn group under the leadership of Sturm and Schläpfer to employ this structure as target area for DBS in depressive disorders (20). After the commencement of stimulation, all three patients spontaneously showed positive effects. Within a week the HAM-D score decreased by an average of 42%. When the stimulation was discontinued under double-blind conditions, two of the three patients deteriorated so much that the study had to be stopped. The correlation between stimulation and depression (HAM-D score) was significant (r = –0.54, p <0.01), demonstrating the efficacy of stimulation in the nucleus accumbens. All three patients responded to the treatment without severe adverse effects. Positron emission tomography (PET) illustrated the modulation of fronto-striatal networks by bilateral stimulation in the nucleus accumbens. This group recently published expanded data on the first ten patients treated in this way. Alongside a 50% reduction in HAM-D score in five patients, there was distinct anxiolysis (measured using the Hamilton Anxiety Scale) in the 1-year observation period. Because the results were published outside the search period defined above, no further detail is given here (e34).
The group led by Malone and Dougherty reported the DBS treatment of 15 depressed patients in a multicenter study. The target area, the ventral internal capsule/ventral striatum, was very similar to that chosen by Schläpfer et al. (e35). In this study, too, the symptoms decreased over the 6-month observation period: the HAM-D score went down by 42%.
Outlook
In addition to the applications discussed above, recent publications have described notable alterations in addictive behavior in patients with substance-related dependence who were subjected to DBS of the nucleus accumbens (23, 24, e36). The treated individuals apparently found it easier to remain abstinent. However, these promising findings, attributed in part to modulation of the addict’s craving, have not yet been supported by studies. Therefore, no substantiated conclusions can yet be drawn.
With regard to the anatomic target structures, ongoing studies are investigating how current and future psychiatric indications can be more clearly defined by means of further image analysis techniques, e.g., diffusion-based tractography to depict cortical and subcortical connectivity (e37, e38).
Conclusions
Although no “global power of effect” can currently be determined, the published results of the treatment of refractory psychiatric diseases with DBS can be considered promising. In the majority of cases there has been a distinct improvement in the psychiatric status of these severely ill and previously untreatable patients. The many case reports have been complemented by an increasing number of pilot studies, some of them with randomized, blinded stimulation phases.
To date, the documented adverse effects of DBS in patients with psychiatric diseases are minor, often reversible by adjustment of the treatment parameters or well tolerated by the patients. Nevertheless, there are no long-term data.
Although no definitive conclusion can be drawn, deep brain stimulation seems to provide new options for the treatment of refractory psychiatric diseases. When deciding whether to implant electrodes for DBS, international recommendations should be taken into consideration (e39) (box 2), and above all the potential benefits of treatment must be weighed against the risks involved in the surgical intervention.
Box 2. Ethical considerations.
Particularly in view of the historical background, above all the notorious era of lesional psychosurgery, it is obligatory to consider critically the ethical issues arising from the increasing application of deep brain stimulation (DBS) for the treatment of psychiatric diseases. Interventions in the brain, which like no other organ represents humanity itself, can lead to changes in personality. The general debate due to fears of this nature in the context of the treatment success achieved by DBS is multilayered and very complex.
The critical-ethical analysis can advantageously be conducted on two levels:
On the one hand, in an application-related, norm-giving sense, criteria for the justifiable application of DBS in research and medical care must be identified and followed. This includes, among other aspects, issues of explanation and consent, study design, and how severe the illness has to be for DBS to be considered.
On the other hand, one has to consider fundamental philosophical-anthropological reflections on the understanding of disease and quality of life and also the human being’s self-concept in his personal identity (e49).
The Cologne center concentrates on both of these levels. Under the coordination of the local Research Center Ethics of the Institute for the History of Medicine and for Medical Ethics in the framework of a binational German–Canadian project funded by the German Federal Ministry of Education and Research, the Cologne group is conducting an empirically based ethical and legal investigation of DBS and its psychosocial impact. An Expert Commission of the European Academy for Research into the Consequences of Scientific and Technical Developments (Europäische Akademie zur Erforschung von Folgen wissenschaftlich-technischer Entwicklungen, Bad Neuenahr-Ahrweiler) is also carrying out an interdisciplinary study of the ethical issues surrounding DBS.
In this context it is important to work towards agreement on guidelines for research and treatment of the kind already drawn up for neurological diseases by the DBS Study Group of the German Neurological Association (Deutsche Gesellschaft für Neurologie). International, interdisciplinary study groups have already taken steps in this direction for individual psychiatric disorders, e.g., obsessive-compulsive disorders and Tourette syndrome (e39, e40).
Key Messages.
The findings on the clinical use of deep brain stimulation (DBS) in psychiatric disorders are encouraging and open up new perspectives for treatment.
For some defined psychiatric diseases, treatment by means of DBS may be an option in cases of chronic and refractory illness.
DBS should always—particularly in psychiatric disease—be accompanied by continuous critical-ethical reflection.
Further research is required into the efficacy, mode of action, and adverse-effect profile of DBS; in particular, there are still no long-term data.
eTable. Selected stimulation-related adverse effects.
| Target | Disease | Transient stimulation-related adverse effects | Reference |
| Nucleus accumbens | Tourette syndrome | Hypomanic episode (1 of 2) | Kuhn et al., 2007 (14) |
| Thalamus | Tourette syndrome | Dizziness (18 of 18 after overstimulation), double vision (4 of 18), abdominal pain (2 of 18), deviation of gaze (1 of 18) | Servello et al., 2008 (12) |
| Thalamus | Tourette syndrome | Psychotic symptoms (1 of 5) | Maciunas et al., 2007 (13) |
| Nucleus accumbens | Obsessive-compulsive disorder | Inner restlessness and anxiety (4 of 12), hypomanic episode (2 of 12), concentration difficulties (1 of 12) | Huff et al., in press (11) |
| Subthalamic nucleus | Obsessive-compulsive disorder | Hypomanic episode (6 of 18), anxiety (2 of 18), dyskinesia and impulsiveness (2 of 18), dysarthria, dysphagia, gait disturbance, and facial asymmetry (1 of 18), depressivity (1 of 18), obsessive-compulsive thoughts (1 of 18), dizziness (1 of 18) | Mallet et al., 2008 (3) |
| Internal capsule/ventral striatum | Obsessive-compulsive disorder | Increased depression with suicidal thoughts (3 of 26), increased intensity of obsessive-compulsive symptoms (3 of 26), hypomanic episode (1 of 26), increased irritability (1 of 26) | Greenberg et al., 2006 (10) |
| Subgenual area of cingulum | Depressive disorder | Increased depression (2 of 20) | Lozano et al., 2008 (21) |
| Internal capsule/ventral striatum | Depressive disorder | Hypomanic episode (1 of 15), increased suicidality (2 of 15), syncope (1 of 15), increased depression (1 of 15) | Malone et al., 2009 (19) |
Acknowledgments
The authors thank Prof. Markus Ullsperger, head of the Max Planck Research Group “Cognitive Neurology” at the Max Planck Institute for Neurological Research in Cologne and Professor of Biological Psychology at Radboud University Nijmegen, for his help in planning and writing this article.
Translated from the original German by David Roseveare.
Footnotes
Conflict of interest statement
Doris Lenartz received financial assistance for travel to congresses from Medtronic AG. Volker Sturm received financial support for studies and travel to congresses and payment for lectures from Medtronic AG and Advanced Neuromodulation Systems, Inc. He is also co-holder of patents on desynchronized brain stimulation and joint founder of ANM-GmbH Jülich, a company that intends to develop new stimulators. Jens Kuhn, Theo Gründler, Joachim Klosterkötter, and Wolfgang Huff declare that no conflict of interest exists according to the guidelines of the International Committee of Medical Journal Editors.
References
- 1.Nuttin BJ, Gabriels LA, Cosyns PR, et al. Long-term electrical capsular stimulation in patients with obsessive-compulsive disorder. Neurosurgery. 2003;52:1263–1272. doi: 10.1227/01.neu.0000064565.49299.9a. discussion 1272-4. [DOI] [PubMed] [Google Scholar]
- 2.Abelson JL., Curtis G, Sagher O, et al. Deep brain stimulation for refractory obsessive-compulsive disorder. Biol Psychiatry. 2005;57:510–516. doi: 10.1016/j.biopsych.2004.11.042. [DOI] [PubMed] [Google Scholar]
- 3.Mallet L, Polosan M, Jaafari N, et al. Subthalamic nucleus stimulation in severe obsessive-compulsive disorder. N Engl J Med. 2008;359:2121–2134. doi: 10.1056/NEJMoa0708514. [DOI] [PubMed] [Google Scholar]
- 4.Gabriels L, Cosyns P, Nuttin B, Demeulemeester H, Gybels J. Deep brain stimulation for treatment-refractory obsessive-compulsive disorder: psychopathological and neuropsychological outcome in three cases. Acta Psychiatrica Scandinavica. 2003;107:275–282. [PubMed] [Google Scholar]
- 5.Nuttin B, Cosyns P, Demeulemeester H, Gybels J, Meyerson B. Electrical stimulation in anterior limbs of internal capsules in patients with obsessive-compulsive disorder. Lancet. 1999;354 doi: 10.1016/S0140-6736(99)02376-4. [DOI] [PubMed] [Google Scholar]
- 6.Nuttin BJ, Gabriels L, van Kuyck K, Cosyns P. Electrical stimulation of the anterior limbs of the internal capsules in patients with severe obsessive-compulsive disorder: anecdotal reports. Neurosurg Clin N Am. 2003;14:267–274. doi: 10.1016/s1042-3680(02)00117-1. [DOI] [PubMed] [Google Scholar]
- 7.van Laere K, Nuttin B, Gabriels L, et al. Metabolic imaging of anterior capsular stimulation in refractory obsessive-compulsive disorder: A key role for the subgenual anterior cingulate and ventral striatum. J Nucl Med. 2006;47:740–747. [PubMed] [Google Scholar]
- 8.Cosyns P, Gabriels L, Nuttin B. Deep brain stimulation in treatment refractory obsessive compulsive disorder. Verh K Acad Geneeskd Belg. 2003;65:385–399. discussion 399-400. [PubMed] [Google Scholar]
- 9.Nuttin B, Gybels J, Cosyns P, Gabriels L, et al. Deep brain stimulation for psychiatric disorders. Neurosurg Clin N Am. 2003;14:xv–xvi. doi: 10.1016/s1042-3680(03)00007-x. [DOI] [PubMed] [Google Scholar]
- 10.Greenberg BD, Malone DA, Friehs GM, et al. Three-year outcomes in deep brain stimulation for highly resistant obsessive-compulsive disorder. Neuropsychopharmacology. 2006;31:2384–2393. doi: 10.1038/sj.npp.1301165. [DOI] [PubMed] [Google Scholar]
- 11.Huff W, Lenartz D, Schormann M, et al. Unilateral deep brain stimulation of the nucleus accumbens in patients with treatment resistant obsessive compulsive disorder—outcomes after one-year stimulation. CNN, in press. doi: 10.1016/j.clineuro.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 12.Servello D, Porta M, Sassi M, Brambilla A, Robertson MM. Deep brain stimulation in 18 patients with severe Gilles de la Tourette syndrome refractory to treatment: the surgery and stimulation. J Neurol Neurosurg Psychiatr. 2008;79:136–142. doi: 10.1136/jnnp.2006.104067. [DOI] [PubMed] [Google Scholar]
- 13.Maciunas RJ, Maddux BN, Riley DE, et al. Prospective randomized double-blind trial of bilateral thalamic deep brain stimulation in adults with Tourette syndrome. J Neurosurg. 2007;107:1004–1014. doi: 10.3171/JNS-07/11/1004. [DOI] [PubMed] [Google Scholar]
- 14.Kuhn J, Lenartz D, Mai JK, et al. Deep brain stimulation of the nucleus accumbens and the internal capsule in therapeutically refractory Tourette-syndrome. J Neurol. 2007;254:963–965. doi: 10.1007/s00415-006-0404-8. [DOI] [PubMed] [Google Scholar]
- 15.Kuhn J, Lenartz D, Heuckmann J, Huff W, Klosterkötter J, Sturm V. Deep brain stimulation of different anatomic structures in therapeutically refractory Tourette-syndrome. Klinische Neurophysiologie. 2008;120:e81–e82. [Google Scholar]
- 16.Kuhn J, Lenartz D, Huff W, et al. Transient manic-like episode follow ing bilateral DBS of the nucleus accumbens and the internal capsule in a patient with Tourette-syndrome. Neuromodulation. 2008;11:128–131. doi: 10.1111/j.1525-1403.2008.00154.x. [DOI] [PubMed] [Google Scholar]
- 17.Welter ML, Mallet L, Houeto JL, et al. Internal pallidal and thalamic stimulation in patients with Tourette syndrome. Arch Neurol. 2008;65:952–957. doi: 10.1001/archneur.65.7.952. [DOI] [PubMed] [Google Scholar]
- 18.Visser-Vandewalle V, Temel Y, Boon P, et al. Chronic bilateral thalamic stimulation: a new therapeutic approach in intractable Tourette syndrome. Report of three cases. J Neurosurg. 2003;99:1094–1100. doi: 10.3171/jns.2003.99.6.1094. [DOI] [PubMed] [Google Scholar]
- 19.Malone DA, Jr, Dougherty DD, Rezai AR, et al. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol Psychiatry. 2009;65:267–275. doi: 10.1016/j.biopsych.2008.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schlaepfer TE, Cohen MX, Frick C, et al. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology. 2008;33:368–377. doi: 10.1038/sj.npp.1301408. [DOI] [PubMed] [Google Scholar]
- 21.Lozano AM, Mayberg HS, Giacobbe P, et al. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2008;64:461–467. doi: 10.1016/j.biopsych.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 22.Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 23.Kuhn J, Bauer R, Pohl S, et al. Deep Brain Stimulation of the nucleus accumbens and its influence on nicotine consumption through cigarette smoking: a retrospective study. Eur Addict Res. 2009;15:196–201. doi: 10.1159/000228930. [DOI] [PubMed] [Google Scholar]
- 24.Kuhn J, Lenartz D, Huff W, et al. Remission of alcohol dependency following deep brain stimulation of the nucleus accumbens: valuable therapeutic implications? J Neurol Neurosurg Psychiatry. 2007;78:1152–1153. doi: 10.1136/jnnp.2006.113092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e1.Benabid AL, Pollak P, Louveau A, Henry S, de Rougemont J. Combined (thalamotomy and stimulation) stereotactic surgery of the VIM thalamic nucleus for bilateral Parkinson disease. Appl Neurophysiol. 1987;50:344–346. doi: 10.1159/000100803. [DOI] [PubMed] [Google Scholar]
- e2.Benabid AL, Pollak P, Gervason C, et al. Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet. 1991;337:403–406. doi: 10.1016/0140-6736(91)91175-t. [DOI] [PubMed] [Google Scholar]
- e3.Benabid AL, Charbardes S, Mitrofanis J, Pollak P. Deep brain stimulation of the subthalamic nucleus for the treatment of Parkinson’s disease. Lancet Neurologie. 2009;8:67–81. doi: 10.1016/S1474-4422(08)70291-6. [DOI] [PubMed] [Google Scholar]
- e4.Theodore W H, Fisher R. Brain stimulation for epilepsy. Acta Neurochir Suppl. 2007;97(Pt 2):261–272. doi: 10.1007/978-3-211-33081-4_29. [DOI] [PubMed] [Google Scholar]
- e5.Schulz-Bonhage A. Deep brain stimulation as a new treatment for epilepsy [Tiefe Hirnstimulation als neuer Therapieansatz bei Epilepsien] Dtsch Arztebl Int. 2009;106(24):407–412. doi: 10.3238/arztebl.2009.0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e6.Kiss ZH, Doig-Beyaert K, Eliasziw M, et al. The Canadian multi-centre study of deep brain stimulation for cervical dystonia. Brain. 2007;130(Pt 11):2879–2886. doi: 10.1093/brain/awm229. [DOI] [PubMed] [Google Scholar]
- e7.Loher TJ, Capelle HH, Kaelin-Lang A, et al. Deep brain stimulation for dystonia: outcome at long-term follow-up. J Neurol. 2008;255:881–884. doi: 10.1007/s00415-008-0798-6. [DOI] [PubMed] [Google Scholar]
- e8.Schoenen J, Di Clemente L, Vandenheede M, et al. Hypothalamic stimulation in chronic cluster headache: a pilot study of efficacy and mode of action. Brain. 2005;128(Pt 4):940–947. doi: 10.1093/brain/awh411. [DOI] [PubMed] [Google Scholar]
- e9.Starr PA, Barbaro NM, Raskin NH, Ostrem JL. Chronic stimulation of the posterior hypothalamic region for cluster headache: technique and 1-year results in four patients. J Neurosurg. 2007;106:999–1005. doi: 10.3171/jns.2007.106.6.999. [DOI] [PubMed] [Google Scholar]
- e10.Bejjani BP, Damier P, Arnulf I, et al. Transient acute depression induced by high-frequency deep-brain stimulation. N Engl J Med. 1999;340:1476–1480. doi: 10.1056/NEJM199905133401905. [DOI] [PubMed] [Google Scholar]
- e11.Mallet L, Mesnage V, Houeto JL, Pelissolo A, et al. Compulsions, Parkinson’s disease, and stimulation. Lancet. 2002;360:1302–1304. doi: 10.1016/S0140-6736(02)11339-0. [DOI] [PubMed] [Google Scholar]
- e12.Temel V, Visser-Vanderwalle V. Surgery in Tourette syndrome. Mov Dis. 2004;19:3–14. doi: 10.1002/mds.10649. [DOI] [PubMed] [Google Scholar]
- e13.Jung HH, Kim CH, Chang JH, Park YG, Chung SS, Chang JW. Bilateral anterior cingulotomy for refractory obsessive-compulsive disorder: Long-term follow-up results. Stereotact Funct Neurosurg. 2006;84:184–189. doi: 10.1159/000095031. [DOI] [PubMed] [Google Scholar]
- e14.Sasson Y, Zohar J, Chopra M, et al. Epidemiology of obsessive-compulsive disorder: a world view. J Clin Psychiatry. 1997 58;(Suppl 12):7–10. [PubMed] [Google Scholar]
- e15.Voderholzer U. Obsessive-compulsive disorders. Fortschr Neurol Psychiatr. 2005;73:526–542. doi: 10.1055/s-2004-830294. [DOI] [PubMed] [Google Scholar]
- e16.Abramowitz JS. Effectiveness of psychological and pharmacological treatments for obsessive-compulsive disorder: a quantitative review. J Consult Clin Psychol. 1997;65:44–52. doi: 10.1037//0022-006x.65.1.44. [DOI] [PubMed] [Google Scholar]
- e17.Jenike MA. Neurosurgical treatment of obsessive-compulsive disorder. Br J Psychiatry Suppl. 1998;35:79–90. [PubMed] [Google Scholar]
- e18.Rauch SL, Dougherty DD, Cosgrove GR, et al. Cerebral metabolic correlates as potential predictors of response to anterior cingulotomy for obsessive compulsive disorder. Biol Psychiatry. 2001;50:659–667. doi: 10.1016/s0006-3223(01)01188-x. [DOI] [PubMed] [Google Scholar]
- e19.Dougherty DD, Baer L, Cosgrove GR, Cassem EH, et al. Prospective long-term follow-up of 44 patients who received cingulotomy for treatment-refractory obsessive-compulsive disorder. Am J Psychiatry. 2002;159:269–275. doi: 10.1176/appi.ajp.159.2.269. [DOI] [PubMed] [Google Scholar]
- e20.Aouizerate B, Cuny E, Martin-Guehl C, et al. Deep brain stimulation of the ventral caudate nucleus in the treatment of obsessive-compulsive disorder and major depression. Case report. J Neurosurg. 2004;101:682–686. doi: 10.3171/jns.2004.101.4.0682. [DOI] [PubMed] [Google Scholar]
- e21.Aouizerate B, Martin-Guehl C, Cuny E, et al. Deep brain stimulation of the ventral striatum in the treatment of obsessive-compulsive disorder and major depression. Med Sci (Paris) 2005;21:811–813. doi: 10.1051/medsci/20052110811. [DOI] [PubMed] [Google Scholar]
- e22.Aouizerate B, Martin-Guehl C, Cuny E, et al. Deep brain stimulation for OCD and major depression. Am J Psychiatry. 2005;162 doi: 10.1176/appi.ajp.162.11.2192. [DOI] [PubMed] [Google Scholar]
- e23.Sturm V, Lenartz D, Koulousakis A, et al. The nucleus accumbens: a target for deep brain stimulation in obsessive-compulsive- and anxiety-disorders. J Chem Neuroanatomy. 2003;26:293–299. doi: 10.1016/j.jchemneu.2003.09.003. [DOI] [PubMed] [Google Scholar]
- e24.Jiménez-Ponce F, Velasco-Campos F, Castro-Farfán G, et al. Preliminary study in patients with obsessive-compulsive disorder treated with electrical stimulation in the inferior thalamic peduncle. Neurosurgery. 2009;65(6 Suppl):203–209. doi: 10.1227/01.NEU.0000345938.39199.90. discussion 209. [DOI] [PubMed] [Google Scholar]
- e25.Jankovic J. Tourette’s syndrome. N Engl J Med. 2001;345:1184–1192. doi: 10.1056/NEJMra010032. [DOI] [PubMed] [Google Scholar]
- e26.Albin RL, Koeppe RA, Bohnen NI, et al. Increased ventral striatal monoaminergic innervation in Tourette syndrome. Neurology. 2003;61:310–315. doi: 10.1212/01.wnl.0000076181.39162.fc. [DOI] [PubMed] [Google Scholar]
- e27.Porta M, Brambilla A, Cavanna AE, Servello D, Sassi M, Rickards H, Robertson MM. Thalamic deep brain stimulation for treatment-refractory Tourette syndrome: two-year outcome. Neurology. 2009;73:1375–1380. doi: 10.1212/WNL.0b013e3181bd809b. [DOI] [PubMed] [Google Scholar]
- e28.Alonso J, Lépine JP E.M.S. Committee . Overview of key data from the European Study of the Epidemiology of Mental Disorders (ESEMeD) The Journal of clinical psychiatry. 2007;68(Suppl 2):3–9. [PubMed] [Google Scholar]
- e29.Keller MB, Lavori PW, Mueller TI, et al. Time to recovery, chronicity, and levels of psychopathology in major depression. A 5-year prospective follow-up of 431 subjects. Arch Gen Psychiatry. 1992;49:809–816. doi: 10.1001/archpsyc.1992.01820100053010. [DOI] [PubMed] [Google Scholar]
- e30.Kosel M, Sturm V, Frick C, et al. Mood improvement after deep brain stimulation of the internal globus pallidus for tardive dyskinesia in a patient suffering from major depression. Journal of psychiatric research. 2007;41:801–803. doi: 10.1016/j.jpsychires.2006.07.010. [DOI] [PubMed] [Google Scholar]
- e31.Jiménez F, Velasco F, Salín-Pascual R, et al. Neuromodulation of the inferior thalamic peduncle for major depression and obsessive compulsive disorder. Acta Neurochir Suppl. 2007;97(Pt 2):393–398. doi: 10.1007/978-3-211-33081-4_44. [DOI] [PubMed] [Google Scholar]
- e32.Jiménez F, Velasco F, Salin-Pascual R, et al. A patient with a resistant major depression disorder treated with deep brain stimulation in the inferior thalamic peduncle. Neurosurgery. 2005;57:585–593. doi: 10.1227/01.neu.0000170434.44335.19. discussion 585-93. [DOI] [PubMed] [Google Scholar]
- e33.McNeely HE, Mayberg HS, Lozano AM, Kennedy SH. Neuropsychological impact of Cg25 deep brain stimulation for treatment-resistant depression: preliminary results over 12 months. J Nerv Ment Dis. 2008;196:405–410. doi: 10.1097/NMD.0b013e3181710927. [DOI] [PubMed] [Google Scholar]
- e34.Bewernick BH, Hurlemann R, Matusch A, et al. Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biol Psychiatry. 2009;67:110–116. doi: 10.1016/j.biopsych.2009.09.013. [DOI] [PubMed] [Google Scholar]
- e35.Schlaepfer TE, Lieb K. Deep brain stimulation for treatment of refractory depression. Lancet. 2005;366:1420–1422. doi: 10.1016/S0140-6736(05)67582-4. [DOI] [PubMed] [Google Scholar]
- e36.Bauer R, Pohl S, Klosterkötter J, Kuhn J. Deep brain stimulation in the context of addiction—a literature-based systematic evaluation. Fortschr Neurol Psychiatr. 2008;76:396–401. doi: 10.1055/s-2008-1038217. [DOI] [PubMed] [Google Scholar]
- e37.Gutman DA, Holtzheimer PE, Behrens TE, Johansen-Berg H, Mayberg HS. A Tractography analysis of two deep brain stimulation white matter targets for depression. Biol Psychiatry. 2009;56:276–282. doi: 10.1016/j.biopsych.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e38.Johansen-Berg H, Gutman DA, Behrens TE, et al. Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cereb Cortex. 2008;18:1374–1383. doi: 10.1093/cercor/bhm167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e39.Kuhn J, Gaebel W, Klosterkoetter J, Woopen C. Deep brain stimulation as a new therapeutic approach in therapy-resistant mental disorders: Ethical aspects of investigational treatment. Eur Arch Psychiatry Clin Neurosci. 2009;259(Suppl. 2):S135–S141. doi: 10.1007/s00406-009-0055-8. [DOI] [PubMed] [Google Scholar]
- e40.Mink JW, Walkup J, Frey KA. Patient selection and assessment recommendations for deep brain stimulation in Tourette syndrome. Mov Disord. 2006;21:1831–1838. doi: 10.1002/mds.21039. [DOI] [PubMed] [Google Scholar]
- e41.The Deep-Brain Stimulation for Parkinson’s Disease Study Group. Deep-brain stimulation of the subthalamic nucleus or the pars interna of the globus pallidus in Parkinson’s disease. N Engl J Med. 2001;345:956–963. doi: 10.1056/NEJMoa000827. [DOI] [PubMed] [Google Scholar]
- e42.Krack P, Batir A, van Blercom N, et al. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced parkinson’s disease. N Engl J Med. 2003;349:1925–1934. doi: 10.1056/NEJMoa035275. [DOI] [PubMed] [Google Scholar]
- e43.Oh MY, Abosch A, Kim SH, Lang AE, Lozano AM. Long-term hardware-related complications of deep brain stimulation. Neurosurgery. 2002;50:1268–1274. doi: 10.1097/00006123-200206000-00017. [DOI] [PubMed] [Google Scholar]
- e44.Schüpbach WM, Maltête D, Houeto JL, et al. Neurosurgery at an earlier stage of Parkinson disease: a randomized, controlled trial. Neurology. 2007;68:267–271. doi: 10.1212/01.wnl.0000250253.03919.fb. [DOI] [PubMed] [Google Scholar]
- e45.Doshi PK, Chhaya N, Bhatt MH. Depression leading to attempted suicide after bilateral subthalamic nucleus stimulation for Parkinson’s disease. Mov Disord. 2002;17:1084–1085. doi: 10.1002/mds.10198. [DOI] [PubMed] [Google Scholar]
- e46.Kulisevsky J, Berthier ML, Gironell A, Pascual-Sedano B, Molet J, Pares P. Mania following deep brain stimulation for Parkinson’s disease. Neurology. 2002;59:1421–1424. doi: 10.1212/wnl.59.9.1421. [DOI] [PubMed] [Google Scholar]
- e47.Appleby BS, Duggan PS, Regenberg A, Rabins PV. Psychiatric and neuropsychiatric adverse events associated with deep brain stimulation: A meta-analysis of ten years’ experience. Mov Disord. 2007;15:1722–1728. doi: 10.1002/mds.21551. [DOI] [PubMed] [Google Scholar]
- e48.Voon V, Krack P, Lang AE, et al. A multicentre study on suicide outcomes following subthalamic stimulation for Parkinson’s disease. Brain. 2008;131(Pt 10):2720–2728. doi: 10.1093/brain/awn214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e49.Woopen C. Ethische Fragen im Zusammenhang mit tiefer Hirnstimulation. Neuro Aktuell. 2009 23;(184):25–28. [Google Scholar]
- e50.Beurrier C, Bioulac B, Audin J, Hammond C. High-frequency stimulation produces a transient blockade of voltage-gated currents in subthalamic neurons. J Neurophysiol. 2001;85:1351–1356. doi: 10.1152/jn.2001.85.4.1351. [DOI] [PubMed] [Google Scholar]
- e51.Dostrovsky JO, Levy R, Wu JP, Hutchison WD, Tasker RR, Lozano AM. Microstimulation-induced inhibition of neuronal firing in human globus pallidus. J Neurophysiol. 2000;84:570–574. doi: 10.1152/jn.2000.84.1.570. [DOI] [PubMed] [Google Scholar]
- e52.Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
- e53.McCracken CB, Grace AA. Nucleus accumbens deep brain stimulation produces region-specific alterations in local field potential oscillations and evoked responses in vivo. J Neurosci. 2009;29:5354–5363. doi: 10.1523/JNEUROSCI.0131-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e54.Jech R, Urgosik D, Tintera J, et al. Functional magnetic resonance imaging during deep brain stimulation: A pilot study in four patients with Parkinson’s disease. Movement Disorders. 2001;16:1126–1132. doi: 10.1002/mds.1217. [DOI] [PubMed] [Google Scholar]
- e55.Haslinger B, Boecker H, Buchel C, et al. Differential modulation of subcortical target and cortex during deep brain stimulation. Neuroscience. 2003;18:517–524. doi: 10.1016/s1053-8119(02)00043-5. [DOI] [PubMed] [Google Scholar]
- e56.Perlmutter JS, Mink JW. Deep brain stimulation. Ann Rev of Neuroscience. 2006;29:229–257. doi: 10.1146/annurev.neuro.29.051605.112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e57.McIntyre CC, Grill WM. Extracellular stimulation of central neurons: influence of stimulus waveform and frequency on neuronal output. J Neurophysiol. 2002;88:1592–1604. doi: 10.1152/jn.2002.88.4.1592. [DOI] [PubMed] [Google Scholar]
- e58.Vitek J. Mechanisms of deep brain stimulation: Excitation or inhibition. Movement Disorders. 2002;17:69–72. doi: 10.1002/mds.10144. [DOI] [PubMed] [Google Scholar]