Abstract
Auxin-binding protein 1 (ABP1) is an auxin receptor for responses not primarily regulated by gene regulation. One fast response is protoplast swelling. By using immunological ABP1 tools we showed that the highly conserved box a is not alone important for auxin binding. Box c is another part of the auxin binding domain.1 Here we present a novel method to analyze auxin-induced, ABP1-mediated effects at the plasma membrane on single cell level in vivo. The fluorescence of FM4-64 in the plasma membrane is reduced by auxin and this response is mediated by ABP1. This method indicates a functional role of ABP1 at the plasma membrane.
Key words: Auxin-binding protein 1, auxin, receptor, protoplast, plasma membrane, FM4-64
Auxins’ Signal Perception-TIR1/AFBs versus ABP1
In spite of the importance of auxin as a plant hormone controlling growth and development, our understanding of auxin signal perception and transduction is still patchy at best. Auxinbinding F-box proteins (AFBs) are a family of well-established intracellular auxin receptors.2–4 The members TIR1 (transport inhibitor response 1), AFB1, AFB2 and AFB3 (auxin signaling F-box) are part of the ubiquitin ligase complex SCFTIR1. Auxin signaling through these intracellular receptors is regulated by degradation of repressors of auxin regulated transcription. Despite the breakthrough discovery of these receptors, there are auxin responses which are not linked to transcriptional regulation through TIR1/AFBs. Some rapid auxin-induced effects like plasma membrane hyperpolarization5 and protoplast swelling6,7 occur within minutes or even seconds and are therefore too fast to be triggered through gene activation. One promising receptor candidate for these fast responses is auxin-binding protein 1 (ABP1) which has a high affinity to auxin and has been discussed for over 36 years as a receptor for auxin.8,9 Protoplast swelling and rapid membrane hyperpolarization are sensitive to immunological tools and synthetic peptides related to ABP1. The biological activity of these probes suggests that ABP1 plays a role in auxin-mediated ion transport and osmoregulation at the plasma membrane.9 Here we report a technique to visualize activation of the ABP1 pathway in a single cell system. Our data shed some light on the rapid membrane effects exerted by auxin via ABP1.
ABP1-A Receptor for Rapid Auxin Effects
The amino acid sequence of ABP1 is highly conserved, particularly the boxes a, b and c and the C-terminus.10–12 It has been suggested that the boxes a and c are important elements of the auxin binding site of the protein.13 Antibodies and antibody fragments raised against domains of ABP1 were shown to induce or inhibit protoplast swelling specifically.1,6 A recent investigation of the biological activity of scFv12, an antibody fragment predominantly directed against box c of ABP1, stressed the role of this particular part of the ABP1 protein in auxin binding and ABP1 activation.1 In most cases investigated so far the activity of the tools in the protoplast swelling test coincide with those in electrophysiological test systems.5,14,15
FM4-64 Fluorescence at the Plasma Membrane is Affected by Auxin and ABP1-Related Antibodies and Peptides
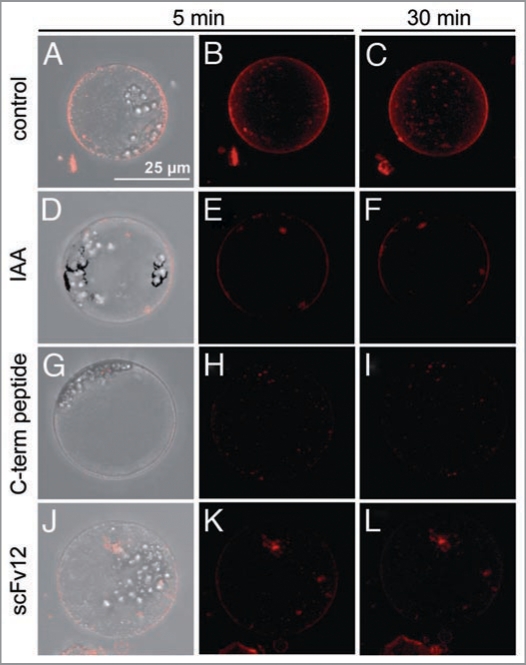
Using the dye FM4-64, we observed another fast ABP1-mediated response at the plasma membrane. FM4-64 is typically used in plants to stain the plasma membrane and to analyze membrane cycling.16,17 In this study, protoplasts of corn coleoptiles were used and stained with FM4-64. The fluorescence of FM4-64 is dependent of its localization and orientation in bilayers like the plasma membrane.18 Protoplasts stained with FM4-64 show a stable fluorescence at the plasma membrane up to one hour (data not shown). Fluorescence also occurred in intracellular compartments (Fig. 1A–C). In the present paper IAA was applied to protoplasts 5 min before FM4-64 treatment. The fluorescence in the plasma membrane of these protoplasts was strongly reduced (Fig. 1D–F). The same effect was detected when the C-terminal peptide of ABP1 consisting of the last 15 amino acids of ZmABP1 (Fig. 1G–I), or scFv12, an antibody fragment predominantly directed against box c of ABP1 (Fig. 1J–L) were applied. It has been previously shown that both C-terminal peptide and the antibody fragment scFv12 induce an IAA-like fast protoplast swelling by triggering the ABP1 pathway.1,6 In this single cell assay IAA strongly reduced FM4-64 fluorescence specifically in the plasma membrane. The same effect was detectable after application of C-terminal peptide and scFv12. In conclusion, IAA-reduced fluorescence in the plasma membrane and IAA-induced protoplast swelling are mediated by ABP1. Using scFv12 in the protoplast system showed that box c is important for auxin binding and signaling. The FM4-64 assay introduced responds to the activation of the ABP1 pathway in a similar way as the electrophysiological systems and the protoplast swelling test.
Figure 1.
IAA and ABP1 tools (C-terminal peptide and scFv12) reduce fluorescence of FM4-64 in the plasma membrane. Protoplasts of corn coleoptiles (isolation method in1) were preincubated for 5 min with 10 µM IAA (D-F), 1 µM C-terminal peptide (G-I) or 0.1 µM scFv12 (J-L), respectively. After preincubation, 8 µM FM4-64 was applied and protoplasts were detected up to 30 min. (A–C) control protoplast were stained with FM4-64. Typical pictures of one protoplast out of four to 14 single protoplasts is shown. The pictures were taken with a CCD camera on a CLSM (TCS SP, Leica, Wetzlar, Germany). The pictures are overlays (z-stacks) of 16 single images for the control and IAA-treated protoplasts and 20 single images for the other treatments. Image number was dependent of the size of the protoplast. Bar is equivalent to 25 µm.
Why is the fluorescence of FM4-64 reduced in the plasma membrane of corn coleoptile protoplasts? In protoplasts auxin induces a rapid activation of the ATP dependent proton current,19 and this results in a rapid hyperpolarization of the plasma membrane.5 Both effects respond to ABP1 related probes. It is possible that changes in membrane potential are responsible for the reduced fluorescence of FM4-64 reported here. The fluorescence of the dye depends on its orientation in the plasma membrane, which depends on charge.18 FM4-64 is therefore sensitive to membrane potential, and auxin-induced membrane hyperpolarization may affect the orientation of the dye in the plasma membrane. In mammals FM4-64 fluorescence is used as a probe for measuring action potentials in neurons, and this method is sensitive enough to resolve potential variation within milliseconds.20,21 While we can not directly compare effects of milliseconds and the fluorescence of the dye in the plasma membrane after several minutes incubation reported here, our results demonstrate that FM4-64 fluorescence is auxin- and ABP1-dependent. And to that end, ABP1 signaling is correlated with a rapid effect localized at the plasma membrane.
Acknowledgements
Support by the Deutsche Forschungsgemeinschaft is gratefully acknowledged. We are very indebted to Catherine Perrot-Rechenmann (CNRS, Gif sur Yvette, France) to allocate ABP1 tools.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/10306
References
- 1.Dahlke RI, Luethen H, Steffens B. The auxin-binding pocket of auxin-binding protein 1 comprises the highly conserved boxes a and c. Planta. 2009;230:917–924. doi: 10.1007/s00425-009-0995-2. [DOI] [PubMed] [Google Scholar]
- 2.Dharmasiri N, Dharmasiri S, Estelle M. The F-box protein TIR1 is an auxin receptor. Nature. 2005;435:441–445. doi: 10.1038/nature03543. [DOI] [PubMed] [Google Scholar]
- 3.Dharmasiri N, Dharmasiri S, Weijers D, Lechner E, Yamada M, Hobbie L, et al. Plant development is regulated by a family of auxin receptor F Box proteins. Dev Cell. 2005;9:109–119. doi: 10.1016/j.devcel.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 4.Kepinski S, Leyser O. The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature. 2005;435:446–451. doi: 10.1038/nature03542. [DOI] [PubMed] [Google Scholar]
- 5.Barbier-Brygoo H, Ephritikhine G, Klaembt D, Ghislain M, Guern J. Functional evidence for an auxin receptor at the plasmalemma of tobacco mesophyll protoplasts. Proc Natl Acad Sci USA. 1989;86:891–895. doi: 10.1073/pnas.86.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steffens B, Feckler C, Palme K, Christian M, Boettger M, Luethen H. The auxin signal for protoplast swelling is perceived by extracellular ABP1. Plant J. 2001;27:591–599. doi: 10.1046/j.1365-313x.2001.01103.x. [DOI] [PubMed] [Google Scholar]
- 7.Yamagami M, Haga K, Napier RM, Iino M. Two distinct signaling pathways participate in auxin-induced swelling of pea epidermal protoplasts. Plant Physiol. 2004;134:735–747. doi: 10.1104/pp.103.031294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hertel R, Thomas K, Russo VEA. In vitro auxin binding to particulate cell fractions from corn coleopitles. Planta. 1972;107:325–340. doi: 10.1007/BF00386394. [DOI] [PubMed] [Google Scholar]
- 9.Napier RM, David KM, Perrot-Rechenmann C. A short history of auxin-binding proteins. Plant Mol Biol. 2002;49:339–348. [PubMed] [Google Scholar]
- 10.Brown JM, Jones AM. Mapping the auxin-binding site of auxin-binding protein 1. J of Biol Chem. 1994;269:21136–21140. [PubMed] [Google Scholar]
- 11.Woo EJ, Bauly J, Chen JG, Marshall J, Macdonald H, Lazarus C, et al. Crystallization and preliminary X-ray analysis of the auxin receptor ABP1. Acta Crystallogr D Biol Crystallogr. 2000;56:1476–1478. doi: 10.1107/s0907444900010714. [DOI] [PubMed] [Google Scholar]
- 12.Woo EJ, Marshall J, Bauly J, Napier RM, Pickersgill RW. Crystal structure of auxin-binding protein 1 in complex with auxin. EMBO J. 2002;21:2877–2885. doi: 10.1093/emboj/cdf291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bertoša B, Kojic-Prodic B, Wade RC, Tomic S. Mechnism of auxin interaction with auxin binding protein (ABP1): a molecular dynamics simulation study. J Biophys. 2007;94:27–37. doi: 10.1529/biophysj.107.109025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Venis MA, Napier RM, Barbier-Brygoo H, Maurel C, Guern J. Antibodies to a peptide from the maize auxin binding protein have auxin agonist activity. Proc Natl Acad Sci USA. 1992;89:7208–7212. doi: 10.1073/pnas.89.15.7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.David KM, Carnero-Diaz E, Leblanc N, Monestiez M, Grosclaude J, Perrot-Rechenmann C. Conformational dynamics underlie the activity of the auxin-binding protein, Nt-abp1. J Biol Chem. 2001;276:34517–34523. doi: 10.1074/jbc.M102783200. [DOI] [PubMed] [Google Scholar]
- 16.Meckel T, Hurst AC, Thiel G, Homann U. Endocytosis against high turgor: intact guard cells of Vicia faba constitutively endocytose fluorescently labelled plasma membrane and GFP-tagged K+-channel KAT1. Plant J. 2004;39:182–193. doi: 10.1111/j.1365-313X.2004.02119.x. [DOI] [PubMed] [Google Scholar]
- 17.Murphy AS, Bandyopadhyay A, Holstein SE, Peer WA. Endocytotic cycling of PM proteins. Annu Rev Plant Biol. 2005;56:221–251. doi: 10.1146/annurev.arplant.56.032604.144150. [DOI] [PubMed] [Google Scholar]
- 18.Wu Y, Yeh FL, Mao F, Chapman ER. Biophysical characterization of styryl dye-membrane interactions. Biophys J. 2009;97:101–109. doi: 10.1016/j.bpj.2009.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rueck A, Palme K, Venis MA, Napier RM, Felle RH. Patch-clamp analysis establishes a role for an auxinbinding protein in the auxin stimulation of plasma membrane current in Zea mays protoplasts. Plant J. 1993;4:41–46. [Google Scholar]
- 20.Bouevitch O, Lewis A, Pinevsky I, Wuskell JP, Loew LM. Probing membrane potential with nonlinear optics. Biophys J. 1993;65:672–679. doi: 10.1016/S0006-3495(93)81126-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dombeck DA, Sacconi L, Blanchard-Desce M, Webb WW. Optical recording of fast neuronal membrane potential transients in acute mammalian brain slices by second-harmonic generation microscopy. J Neurophysiol. 2005;94:3628–3636. doi: 10.1152/jn.00416.2005. [DOI] [PubMed] [Google Scholar]