Abstract
Vibrio cholerae is both an inhabitant of estuarine environments and the etiologic agent of the diarrheal disease cholera. Previous work has demonstrated that V. cholerae forms both an exopolysaccharide-dependent biofilm and a Ca2+-dependent biofilm. In this work, we demonstrate a role for the O-antigen polysaccharide of V. cholerae in Ca2+-dependent biofilm development in model and true sea water. Interestingly, V. cholerae biofilms, as well as the biofilms of several other Vibrio species, disintegrate when Ca2+ is removed from the bathing medium, suggesting that Ca2+ is interacting directly with the O-antigen polysaccharide. In the Bay of Bengal, cholera incidence has been correlated with increased sea surface height. Because of the low altitude of this region, increases in sea surface height are likely to lead to transport of sea water, marine particulates, and marine biofilms into fresh water environments. Because fresh water is Ca2+-poor, our results suggest that one potential outcome of an increase is sea surface height is the dispersal of marine biofilms with an attendant increase in planktonic marine bacteria such as V. cholerae. Such a phenomenon may contribute to the correlation of increased sea surface height with cholera.
Vibrio cholerae, a Gram-negative rod-shaped bacterium, is both a natural inhabitant of estuarine environments and the agent of the severe diarrheal disease cholera. It has acquired great fame and infamy as the perpetrator of seven pandemics. During each pandemic, cholera has struck coastal regions before spreading inland. More recently, a correlation has been reported between sea surface height in the Bay of Bengal and cholera (1). This finding has led researchers to suggest that estuaries are the reservoirs of V. cholerae and that changes in estuarine conditions may precipitate cholera epidemics (2).
In aquatic environments, bacteria are generally found in association with surfaces. In fact, V. cholerae has been detected on the surfaces of aquatic organisms such as zooplankton, phytoplankton, insects, crustaceans, and plants (3-5), and we hypothesize that the ability of V. cholerae to attach to biotic and abiotic surfaces in what is termed a biofilm may be relevant to environmental survival.
The lipopolysaccharide (LPS) of Gram-negative bacteria consists of three domains: (i) lipid A, a hydrophobic domain that associates with the outer membrane, (ii) an oligosaccharide core, and (iii) a terminal, immunogenic polysaccharide domain termed the O antigen. The O-antigen serogroup provides a rational basis for the classification of environmental and clinical strains of V. cholerae. While environmental strains of V. cholerae fall into many different O-antigen serogroups, only the O1 and O139 serogroups have been associated with the disease cholera. The monosaccharide compositions of the V. cholerae O1 and O139 O antigens differ considerably. In particular, the O1 polysaccharide contains perosamine (6, 7), whereas the O139 polysaccharide is distinguished by a d-galactose 4,6-cyclophosphate moiety (8). Furthermore, V. cholerae in the O139 serogroup are encapsulated, whereas pathogenic bacteria in the O1 serogroup are not. Several genes are required for the synthesis of both the V. cholerae O139 O antigen and capsule (9, 10). Furthermore, the V. cholerae O139 O antigen consists of a single oligosaccharide unit that is identical to the repeating unit of the capsule (8). Thus, both the O antigen and capsule are composed of O-antigen polysaccharide.
In LB broth, the V. cholerae O1 El Tor strain N16961 requires the mannose-sensitive hemagglutinin (MSHA), a type IV pilus, and the flagellum to associate with abiotic surfaces (11-13), whereas the V. cholerae O139 strain MO10 depends only on the flagellum for surface association (14). In both strains, subsequent development of a three-dimensional biofilm requires the vps genes, which are responsible for the synthesis of an exopolysaccharide-based adhesive extracellular matrix (11, 14, 15).
Spontaneous rugose variants of V. cholerae have been reported (16, 17, †). Whereas many clinical isolates of V. cholerae form smooth colonies on LB agar plates, these variants form colonies with rough surfaces. The rugose colony morphology is the result of increased synthesis of the VPS exopolysaccharide (15, 19, 20), and transcriptional regulation of the vps genes, which are required for synthesis of the VPS exopolysaccharide, is altered in these strains (21). Thus, these variants rapidly form biofilms in LB broth that are much thicker than those formed by smooth-colony variants of V. cholerae.
Recently, we have reported the formation of a vps-independent V. cholerae biofilm in model sea water (22). Whereas monosaccharides are required for formation of the vps-dependent biofilm, Ca2+ is required for development of the vps-independent biofilm. Ca2+ has been reported to play various roles in biofilm formation by diverse bacterial species. In particular, it has been suggested that Ca2+ directly stabilizes intercellular interactions in Pseudomonas aeruginosa and Streptococcus downei biofilms, presumably by the formation of intercellular salt bridges (23-25). The interactions of surface-associated lectins of oral streptococci with galactose and N-acetylgalactose moieties are enhanced by addition of Ca2+ (23). Finally, it has been suggested that the enhanced intercellular cohesion of the myxobacterium Stigmatella aurantiaca in response to Ca2+ is correlated with the altered expression and cellular localization of a number of proteins (26). The mechanisms underlying the Ca2+ dependence of vps-independent V. cholerae biofilm formation have not yet been established.
In this work, our goal was to delineate the genetic requirements for Ca2+-dependent biofilm development, to test Ca2+-dependent biofilm development as a paradigm for biofilm development by estuarine pathogens in natural environments, and to investigate the implications of Ca2+-dependent biofilm development for surface-attached bacteria in the variable estuarine environment.
Materials and Methods
Bacterial Strains, Plasmids, and Media. MO10, a V. cholerae O139 clinical isolate (27), and mutants derived from this strain were used in all experiments except those examining biofilms formed by a V. cholerae O1 El Tor clinical isolate (N1961), environmental V. cholerae isolates displaying various O antigens (gifts of M. K. Waldor, New England Medical Center), Vibrio parahaemolyticus, Vibrio fluvialis, Vibrio alginolyticus, and Vibrio vulnificus (gifts of S. B. Calderwood, Massachusetts General Hospital, Boston). The V. cholerae ΔflaA and ΔmshA mutants used in these studies have previously been described (11). Biofilms were formed in an inorganic base of either commercial artificial sea water (Instant Ocean; Aquarium Systems, Mentor, OH) or DSW medium, a defined mixture of salts based on the composition of artificial sea water as follows: 468 mM NaCl, 55 mM MgSO4, 3.0 mM NaHCO3, 9.9 mM CaCl2, 10.3 mM KCl, 0.0014 mM Na2B4O7, 0.1 mM SrCl2, 0.03 mM NaBr, 0.002 mM NaI, and 0.026 mM LiCl. Both inorganic mixtures were supplemented with 1% vitamin assay Casamino acids (Difco). True fresh water and sea water were obtained from the Charles River (Newton, MA) and the Massachusetts coast, respectively. The Ca2+ ion concentrations of our true fresh water and sea water samples were 0.47 mM and 9.95 mM, respectively. In biofilm assays, true fresh water and sea water were supplemented with 1% vitamin assay Casamino acids.
Transposon-Insertion Mutagenesis and Screening. A previously generated V. cholerae O139 transposon-insertion mutant library was used as follows (22, 28). Mutant biofilms were formed in commercial artificial sea water medium and evaluated by crystal violet staining as previously described (28). All biofilm-altered mutants were evaluated for hemagglutination and flagellar motility as previously described (14).
Construction of ΔwbfF, ΔwbfR, and ΔwaaL Mutants. ΔwbfF and ΔwbfR in-frame deletion mutants were constructed by double homologous recombination using the suicide plasmids pKKΔwbfF and pKKΔwbfR, which were constructed for this study. Each plasmid harbored a large in-frame deletion of the relevant gene. The ΔwaaL mutant was constructed by using plasmid pKEKΔwaaL generously provided by J. Reidl (29). The presence or absence of the lipopolysaccharide O antigen and capsule in wild-type V. cholerae and mutants was confirmed by immunoblot analysis as previously described by using whole cell antiserum generously provided by M. Waldor (9, 29). As expected, immunoblots of wild-type V. cholerae, the ΔwbfF mutant, the ΔwaaL mutant, and the ΔwbfR mutant demonstrated the presence of both O antigen and capsule, the O antigen alone, the capsule alone, and neither the O antigen nor the capsule, respectively (data not shown). In liquid culture, the growth rates of all mutants were indistinguishable from the growth rate of wild-type V. cholerae.
Biofilm Quantifications. Quantification of surface association was performed as described previously (22). Briefly, borosilicate glass tubes were filled with 300 μl of DSW medium and inoculated with the strain under study. After incubation for 24 h, tubes were rinsed and filled with 300 μl of fresh medium. Biofilm-associated cells were dispersed by mixing in a Vortex apparatus for 10 sec in the presence of borosilicate glass beads (Biospec). Finally, the optical density of the biofilm cell suspension was measured.
Phase-Contrast Microscopy, Fluorescence Microscopy, and Quantitative Analysis of Biofilm Structure. For phase-contrast microscopy, biofilms were formed in sterile 24-well polystyrene microtiter dishes containing 300 μl of DSW medium. After 24 h, biofilms were washed three times with fresh medium and visualized with an Eclipse TE-200 microscope (Nikon) equipped with an Orca digital charge-coupled device camera (Hamamatsu). The bathing medium was then removed and replaced with complete DSW medium, DSW medium lacking Ca2+, DSW medium lacking Mg2+, or DSW medium lacking Ca2+ but containing an equivalent concentration of Mn2+. Biofilms were incubated with the relevant fresh medium for the length of time indicated, rinsed with fresh medium, and then revisualized by microscopy.
Biofilms were prepared and visualized by fluorescence microscopy as previously described (22). Various attributes of the biofilm structure were then quantified by using the COMSTAT software developed by Heydorn and colleagues (30). Reported values represent the average of six image stacks (three image stacks collected in two independent experiments).
Results and Discussion
Structures Identified in the Genetic Screen for Biofilm-Altered Mutants. The vps-independent biofilms of 6,000 transposon-insertion mutants were evaluated, and 86 biofilm-altered mutants were identified. Flagellar motility and the MSHA type IV pilus have previously been implicated in V. cholerae O1 El Tor biofilm development. Thus, all biofilm-defective mutants were tested for hemagglutination and motility. Ten biofilm-defective mutants displayed decreased or absent motility, and seven mutants were unable to agglutinate red blood cells. The transposon-insertion junctions of 8 motile, hemagglutination-competent biofilm-altered mutants were successfully sequenced after amplification by the arbitrary PCR technique (14, 31, 32). These mutants were found to have transposon insertions in genes located in operons responsible for either O-antigen polysaccharide synthesis, such as the putative asparagine synthetase wbfR, or export of capsular precursors, such as wbfF. Crystal violet staining of mutant biofilms suggested that surface adherence of mutants lacking the capsule was increased, whereas surface adherence of mutants lacking both the O antigen and capsule was markedly defective.
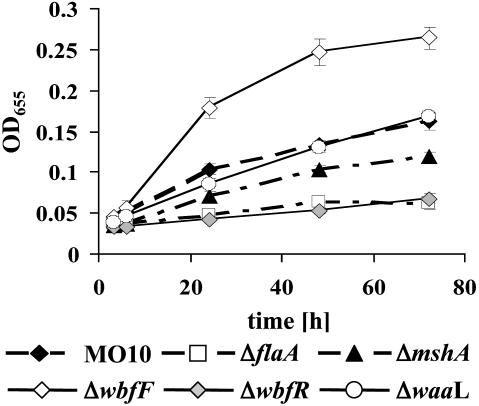
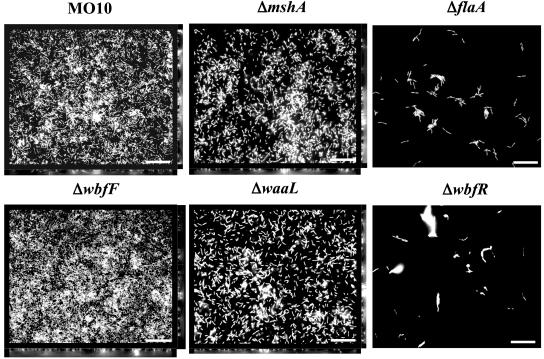
MSHA and the Flagellum Are Required for vps-Independent Biofilm Development. The results of our genetic screen suggested that MSHA and the flagellum are required for vps-independent V. cholerae biofilm development. To confirm these results, previously constructed V. cholerae O139 strains harboring deletions in mshA and flaA were assayed for vps-independent biofilm development over time (11). As shown in Fig. 1, both mutants displayed a defect in biofilm development. However, the ΔmshA mutant was able to accumulate on the surface at a rate just slightly less than that of wild-type V. cholerae, whereas the ΔflaA mutant displayed little surface accumulation. To visualize the three-dimensional structures of the ΔmshA and ΔflaA mutant biofilms, we used fluorescence microscopy. Vertical and horizontal cross sections through wild-type and mutant biofilms formed over 24 h are shown in Fig. 2. Analysis of these images by using the COMSTAT software of Heydorn et al. (30) is shown in Table 1. This analysis demonstrated that the total biomass and substratum coverage of ΔmshA biofilms was not significantly different from that of wild-type V. cholerae. These biofilms differed primarily in average and maximum biofilm thickness, suggesting that intercellular contacts were less efficiently established in the ΔmshA biofilm. In contrast, all parameters of the ΔflaA mutant biofilm were significantly decreased compared with those of the wild-type V. cholerae biofilm.
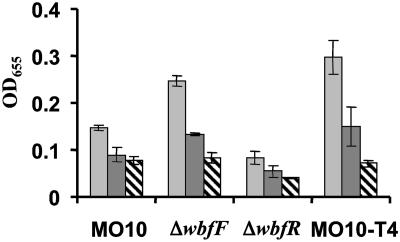
Fig. 1.
Quantification of time-dependent surface association in defined sea water medium (DSW) by wild-type V. cholerae (MO10) and ΔflaA, ΔmshA, ΔwbfF, ΔwbfR, and ΔwaaL mutants.
Fig. 2.
Transverse and vertical cross sections obtained by fluorescence microscopy through DAPI-stained wild-type V. cholerae MO10 and ΔflaA, ΔmshA, ΔwbfF, ΔwbfR, and ΔwaaL mutant biofilms in DSW medium. Transverse sections were taken at the level of the substratum. (Bar = 10 μm.)
Table 1. Quantitative comparison of the architectures of wild-type V. cholerae and ΔmshA, ΔflaA ΔwbfF, ΔwaaL, and ΔwbfR mutant biofilms formed in DSW medium.
| Thickness, μm
|
||||
|---|---|---|---|---|
| V. cholerae | Biomass, μm3/μm2 | Fraction of substratum covered by cells | Mean | Maximum |
| Wild type | 0.18 ± 0.03 | 0.23 ± 0.04 | 0.23 ± 0.03 | 2.45 ± 0.25 |
| ΔmshA | 0.12 ± 0.02 | 0.17 ± 0.01 | 0.14 ± 0.02 | 1.8 ± 0.48 |
| ΔflaA | 0.002 ± 0.001 | 0.019 ± 0.005 | 0 | 1 |
| ΔwbfF | 0.28 ± 0.05 | 0.33 ± 0.05 | 0.32 ± 0.05 | 3.03 ± 0.25 |
| ΔwaaL | 0.13 ± 0.04 | 0.16 ± 0.05 | 0.17 ± 0.04 | 2.03 ± 0.29 |
| ΔwbfR | 0.002 ± 0.001 | 0.02 ± 0.01 | 0 | 1 |
Values were calculated by using the comstat software (30). Results are expressed as the mean ± SD. Each value represents the mean of six image stacks in two independent experiments.
The defects of the V. cholerae O139 ΔmshA and ΔflaA mutants in vps-independent biofilm development differ from those previously reported for vps-dependent biofilm development in LB broth (11). In LB broth, biofilm development by the ΔmshA mutant was indistinguishable from that of wild-type V. cholerae. Furthermore, surface accumulation by the ΔflaA mutant was much less defective in LB broth than in DSW medium. This observation suggests that an additional mediator of surface adhesion, synthesized during growth in LB broth but not DSW medium, masks the attachment defects of the ΔmshA and ΔflaA mutants.
Both the V. cholerae O139 O Antigen and Capsule Promote vps-Independent Biofilm Development. V. cholerae biofilms formed in artificial sea water are independent of the vps genes. Our genetic screen suggested that the capsule and/or O antigen of V. cholerae O139 might provide an alternative adhesive polysaccharide for biofilm development in sea water. In particular, transposon insertion in wbfF, which blocks capsule transport, was found to increase surface association by V. cholerae, whereas transposon insertion in wbfR, which abolishes both O-antigen and capsule synthesis, was found to decrease surface association (33).
To confirm the phenotypes of these transposon-insertion mutants, we engineered mutants carrying in-frame deletions in wbfF or wbfR. Biofilm development by these mutants was compared with that by wild-type V. cholerae in both sea water medium and LB broth. In LB broth, biofilm development by both mutants was quite similar to that developed by wild-type V. cholerae (data not shown). In contrast, in DSW medium, biofilm development by the ΔwbfF mutant was markedly increased, whereas that by the ΔwbfR mutant was markedly defective.
Surface association by wild-type V. cholerae, ΔwbfF, and ΔwbfR mutants was quantified over time. As shown in Fig. 1, the ΔwbfF mutant biofilm accumulated almost 70% more cells than that of wild-type V. cholerae, whereas surface accumulation by the ΔwbfR mutant was negligible. Biofilms formed over 24 h were also visualized by fluorescence microscopy (Fig. 2) and then quantified by using COMSTAT analysis (Table 1). The biomass, substratum coverage, and average thickness of the ΔwbfF mutant biofilm were ≈30% greater than that of wild-type V. cholerae, whereas the maximum thickness was not significantly different. Thus, the ΔwbfF mutant biofilm consisted of more closely packed pillars of cells. In contrast, the ΔwbfR mutant biofilm consisted of scattered attached cells.
Because the ΔwbfF mutant displayed increased surface adhesion, we hypothesized that either the V. cholerae O139 capsule interfered with vps-independent biofilm development by masking the O antigen or that the capsule itself was a mediator, albeit a weaker one, of biofilm development. To distinguish between these two possibilities, we constructed and studied a V. cholerae ΔwaaL mutant. WaaL is a putative O-antigen ligase that attaches the O antigen to the lipid A core. Previous studies, as well as our own immunoblots, demonstrated that the V. cholerae O139 ΔwaaL mutant synthesizes a capsule but no lipopolysaccharide-associated O antigen (29). We predicted that, if the capsule itself had no adhesive properties, this mutant would not form a biofilm. As shown in Fig. 1, this mutant did indeed form a biofilm. Furthermore, quantitation of the ΔwaaL biofilm parameters demonstrated that this biofilm was not significantly different from that of wild-type V. cholerae (Table 1). These results suggest that the V. cholerae O139 capsule itself is able to mediate vps-independent biofilm development.
Because the composition of the O antigen and the repeating unit of the capsule are identical, one might have predicted that biofilm development by a capsule-defective mutant would be indistinguishable from that of the wild-type V. cholerae strain. We postulate that structural constraints imposed by multimerization of the O-antigen polysaccharide destabilizes Ca2+-mediated intercellular contacts. Alternatively, the presence of additional surface-associated structures, exposed only when the capsule is absent, may enhance biofilm development by capsule-deficient mutants.
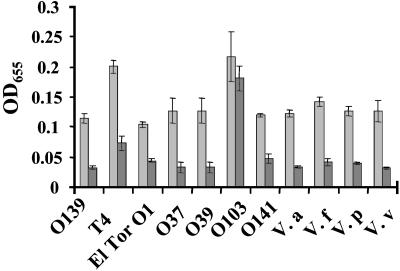
Spontaneous Unencapsulated Variants of V. cholerae O139 also Exhibit Markedly Increased Surface Association. Spontaneous unencapsulated phase variants of V. cholerae O139 have previously been described and isolated (9, 34). Because our studies suggested that unencapsulated mutants display increased vps-independent biofilm development, we chose one such spontaneous mutant, MO10-T4, for further study. In fact, both biofilm quantification and microscopy confirmed that this spontaneous variant displayed increased Ca2+-dependent surface adhesion (see Fig. 5).
Fig. 5.
Biofilm detachment after medium change from DSW medium to DSW medium lacking Ca2+. A quantification of surface association by V. cholerae O139 (MO10), MO10-T4 (T4), V. cholerae O1 El Tor (N16961), V. cholerae O37, V. cholerae O39, V. cholerae O103, V. cholerae O141, V. alginolyticus (V. a), V. fluvialis (V. f), V. parahaemolyticus (V. p), and V. vulnificus (V. v) after incubation for 24 h in DSW medium (gray bars) and 5 h after replacement of the bathing medium with DSW medium lacking Ca2+ (black bars). V. cholerae strain data are labeled with the respective V. cholerae O-antigen designations.
Spontaneous rugose phase variants of V. cholerae have previously been described (16, 17, 19, 20, †). These variants display increased vps-dependent surface adhesion in polysaccharide-rich environments (15). We propose that spontaneous capsule-negative variants of V. cholerae O139 may represent an analogous type of phase variation that results in increased surface association in Ca2+-rich environments such as the sea.
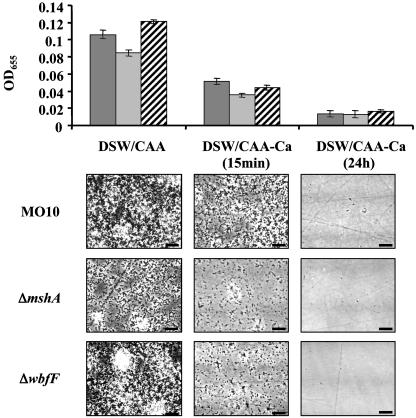
Ca2+ Is an Integral Component of the vps-Independent Extracellular Biofilm Matrix. Previous experiments demonstrated that a vps-independent biofilm would not form in the absence of Ca2+ (22). On the basis of these results, we hypothesized that Ca2+ either was involved in activation of gene transcription or was a direct mediator of cell-cell and/or cell-surface interactions in the biofilm. The V. cholerae O-antigen polysaccharide is present on cells cultured in both DSW medium and LB broth, a Ca2+-poor medium (9, 29). Thus, the discovery that the O-antigen polysaccharide was required for vps-independent biofilm development suggested to us that Ca2+ might serve as an integral component of the vps-independent extracellular matrix. To test this idea, we formed a vps-independent biofilm in DSW medium over 24 h, removed Ca2+ from the bathing medium either by adding the chelator EDTA or by replacing DSW medium with fresh medium lacking Ca2+, and then observed the response of the vps-independent biofilm over time. As shown in Fig. 3, biofilms formed by wild-type V. cholerae as well as the ΔmshA and ΔwbfF mutants disintegrated rapidly after removal of Ca2+ from the medium. In contrast, replacement of the bathing medium with fresh DSW medium containing Ca2+ did not significantly alter the biofilm structure (data not shown).
Fig. 3.
Quantification (Upper) and phase-contrast microscopy (Lower) of wild-type V. cholerae (MO10), ΔmshA mutant, and ΔwbfF mutant biofilms after incubation in DSW medium (including Casamino acids, CAA) for 24 h and then 15 min and 24 h after replacement of bathing medium with DSW medium lacking Ca2+ (-Ca). Black bars, wild-type V. cholerae; gray bars, ΔmshA; striped bars, ΔwbfF. (Scale bars = 4 μm.)
We questioned whether the requirement for Ca2+ in the bathing medium was specific, or whether removal of any divalent cation would have the same effect. Interestingly, replacement of the bathing medium with fresh DSW medium lacking Mg2+ did not result in biofilm dissolution, even though DSW medium contains 5 times more Mg2+ than Ca2+. Furthermore, replacement of DSW medium with DSW containing an equivalent concentration of Mn2+ in place of Ca2+ slowed biofilm dissolution but did not prevent it. These results strongly suggest that Ca2+ is interacting directly and specifically with the O-antigen polysaccharide to maintain biofilm structure. This observation, however, does not preclude a dual role for Ca2+ in activating the synthesis of the O-antigen polysaccharide or other requisite adhesive structures and then forming salt bridges between these structures.
Association of Ca2+ with several bacterial polysaccharides has been demonstrated (35-37). Our results suggest that Ca2+ associates with the O antigen and capsule of V. cholerae O139. These polysaccharides possess a common repeating polysaccharide unit that is negatively charged because of a cyclic phosphate group (8, 38). We hypothesize that Ca2+ interacts with this negatively charged phosphate group to form salt bridges between O-antigen moieties on the same bacterium or on different bacteria.
A V. cholerae Biofilm Formed in True Sea Water Exhibits O-Antigen Polysaccharide Dependence and Disintegrates upon Exposure to True Fresh Water. A major goal of these experiments was to formulate a testable prediction of the fate of a sea water biofilm after transport into a fresh water environment. Because it is difficult to remove components of true sea water, we developed a defined sea water medium for initial experiments. To test the accuracy of our observations in more realistic aquatic environments, we quantitated surface adhesion by wild-type V. cholerae, a ΔwbfF mutant, and a ΔwbfR mutant in true sea water. As shown in Fig. 4, the ΔwbfF mutant demonstrated increased surface association in true sea water, whereas the ΔwbfR mutant demonstrated markedly decreased surface association. Interestingly, the spontaneous unencapsulated mutant, MO10-T4, also demonstrated increased surface association in true sea water. Furthermore, as shown in Fig. 4, we documented dissolution of these true sea water biofilms after replacement of the bathing medium with true fresh water. These data suggest that O-antigen polysaccharide-dependent biofilm development may be operative in true sea water environments. Furthermore, the transport of these sea water biofilms into fresh water environments may result in dispersal of surface-associated bacteria.
Fig. 4.
Quantification of surface adhesion by wild-type V. cholerae (MO10), a ΔwbfF mutant, a ΔwbfR mutant, and a spontaneous unencapsulated mutant (MO10-T4) after incubation for 24 h in true sea water (gray bars) and 1 h (black bars) and 5 h (striped bars) after replacement of the bathing medium with true fresh water.
Many Environmental and Clinical Vibrio Strains Exhibit Ca2+-Dependent Biofilm Dissolution. We were curious to determine whether Ca2+-dependent biofilm dissolution was a feature of other pathogenic and environmental Vibrio strains. To test this possibility, biofilms formed over 24 h by a variety of Vibrio species were quantified before and after replacement of DSW medium with DSW medium lacking Ca2+. As shown in Fig. 5, the vast majority of Vibrio strains exhibited biofilm dissolution upon removal of Ca2+ from the bathing medium. Replacement of the bathing medium with fresh medium containing Ca2+ did not significantly alter the biofilm structure of any of these strains (data not shown). Only one surface adhesion-competent strain, V. cholerae O103, did not follow this pattern. Possible explanations for the resistance of the V. cholerae O103 biofilms to Ca2+ depletion include (i) a requirement for much lower concentrations of Ca2+ in maintenance of the biofilm structure, (ii) dependence of the biofilm structure on an alternative divalent cation, or (iii) dependence of the biofilm structure on a cation-independent adhesive exopolysaccharide matrix.
These results suggested to us that Ca2+-dependent biofilm dissolution is not unique to V. cholerae O139 strain MO10, but is a feature of many Vibrio species. The mechanism of Ca2+-dependent biofilm development and dissolution in these various Vibrio strains has not yet been examined. Cyclic phosphate groups, which we have suggested may play a role in Ca2+-dependent biofilm formation in V. cholerae O139, are a component of the O antigens of a few Vibrio strains (18, 39). However, such a group is not known to be present in the O antigen of V. cholerae O1. We hypothesize that alternative negatively charged groups in the O antigen or capsule of other Vibrio species are able to mediate Ca2+-dependent cell adhesion as well, but this must be investigated further.
Potential Impact of Increases in Sea Surface Height on Ca2+-Dependent Bacterial Biofilms in Estuaries. Our results suggest that Ca2+-dependent biofilm development and dissolution is a common theme among Vibrio species and, potentially, other marine bacteria as well. Furthermore, dissolution of Ca2+-dependent biofilms may occur as the result of transport of biofilm-covered particulates from a marine or estuarine environment into a fresh water environment. We have observed that not all marine bacteria form a Ca2+-dependent biofilm. Thus, we hypothesize that, during times of estuarine instability, a redistribution of the microbial populations associated with surfaces may occur.
Dissolution of Ca2+-dependent biofilms during times of estuarine flux may also have implications for the epidemiology of cholera. Increases in sea surface height in the Bay of Bengal have been positively correlated with cholera (1). One potential outcome of an increase in sea surface height is transport of sea water particulates and attendant adherent V. cholerae into fresh water. The subsequent dissolution of Ca2+-dependent biofilms might result in increased titers of planktonic V. cholerae, which would then be more readily ingested and potentially more virulent. The laboratory-based experiments described here suggest readily testable hypotheses regarding the impact of estuarine ion flux on the microbial ecology of the estuary. Environmental studies to evaluate these hypotheses are necessary.
Acknowledgments
We thank M. K. Waldor, S. B. Calderwood, and J. Reidl for generous sharing of strains and reagents. We also thank Dr. Anne Kane of the Gastrointestinal Research on Absorptive and Secretory Process (GRASP) Center and her staff for their expert advice and preparation of many reagents. This work was supported by an award from the Ellison Medical Foundation, by National Institutes of Health Grant R01 AI50032, and by a pilot project grant from the New England Medical Center GRASP Center, National Institute of Diabetes and Digestive and Kidney Diseases Grant P30 DK34928.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviation: MSHA, mannose-sensitive hemagglutinin.
Footnotes
Crutchley, M. J. (1968) J. Gen. Microbiol. 50, suppl., vii (abstr.)
References
- 1.Lobitz, B., Beck, L., Huq, A., Wood, B., Fuchs, G., Faruque, A. S. G. & Colwell, R. (2000) Proc. Natl. Acad. Sci. USA 97, 1438-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lipp, E. K., Huq, A. & Colwell, R. R. (2002) Clin. Microbiol. Rev. 15, 757-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huq, A., Colwell, R. R., Rahman, R., Ali, A., Chowdhury, M. A., Parveen, S., Sack, D. A. & Russek-Cohen, E. (1990) Appl. Environ. Microbiol. 56, 2370-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huq, A., Small, E. B., West, P. A., Huq, M. I., Rahman, R. & Colwell, R. R. (1983) Appl. Environ. Microbiol. 45, 275-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shukla, B. N., Singh, D. V. & Sanyal, S. C. (1995) FEMS Immunol. Med. Microbiol. 12, 113-120. [DOI] [PubMed] [Google Scholar]
- 6.Redmond, J. W. (1975) FEBS Lett. 50, 147-149. [DOI] [PubMed] [Google Scholar]
- 7.Ito, T., Higuchi, T., Hirobe, M., Hiramatsu, K. & Yokota, T. (1994) Carbohydr. Res. 256, 113-128. [DOI] [PubMed] [Google Scholar]
- 8.Knirel, Y. A., Paredes, L., Jansson, P. E., Weintraub, A., Widmalm, G. & Albert, M. J. (1995) Eur. J. Biochem. 232, 391-396. [DOI] [PubMed] [Google Scholar]
- 9.Waldor, M. K., Colwell, R. & Mekalanos, J. J. (1994) Proc. Natl. Acad. Sci. USA 91, 11388-11392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Comstock, L. E., Maneval, D., Panigrahi, P., Joseph, A., Levine, M. M., Kaper, J. B., Morris, J. G. & Johnson, J. A. (1995) Infect. Immun. 63, 317-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watnick, P. I., Lauriano, C. M., Klose, K. E., Croal, L. & Kolter, R. (2001) Mol. Microbiol. 39, 223-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watnick, P. I., Fullner, K. J. & Kolter, R. (1999) J. Bacteriol. 181, 3606-3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiavelli, D. A., Marsh, J. W. & Taylor, R. K. (2001) Appl. Environ. Microbiol. 67, 3220-3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watnick, P. I. & Kolter, R. (1999) Mol. Microbiol. 34, 586-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yildiz, F. H. & Schoolnik, G. K. (1999) Proc. Natl. Acad. Sci. USA 96, 4028-4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White, P. B. (1938) J. Pathol. Bacteriol. 46, 1-6. [Google Scholar]
- 17.Morris, J. G., Jr., Sztein, M. B., Rice, E. W., Nataro, J. P., Losonsky, G. A., Panigrahi, P. O., Tacket, C. O. & Johnson, J. A. (1996) J. Infect. Dis. 174, 1364-1368. [DOI] [PubMed] [Google Scholar]
- 18.Senchenkova, S. N., Zatonsky, G. V., Shashkov, A. S., Knirel, Y. A., Jansson, P. E., Weintraub, A. & Albert, M. J. (1998) Eur. J. Biochem. 254, 58-62. [DOI] [PubMed] [Google Scholar]
- 19.Mizunoe, Y., Wai, S. N., Takade, A. & Yoshida, S. I. (1999) Infect. Immun. 67, 958-963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wai, S. N., Mizunoe, Y., Takade, A., Kawabata, S. I. & Yoshida, S. I. (1998) Appl. Environ. Microbiol. 64, 3648-3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yildiz, F. H., Dolganov, N. A. & Schoolnik, G. K. (2001) J. Bacteriol. 183, 1716-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kierek, K. & Watnick, P. I. (2003) Appl. Environ. Microbiol. 69, 5097-5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rose, R. K. (2000) Biochim. Biophys. Acta 1475, 76-82. [DOI] [PubMed] [Google Scholar]
- 24.Korstgens, V., Flemming, H. C., Wingender, J. & Borchard, W. (2001) Water Sci. Techol. 43, 49-57. [PubMed] [Google Scholar]
- 25.Turakhia, M. H. & Characklis, W. G. (1989) Biotechnol. Bioeng. 33, 406-419. [DOI] [PubMed] [Google Scholar]
- 26.White, D. & Chang, B. (1992) J. Bacteriol. 174, 5780-5787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waldor, M. K. & Mekalanos, J. J. (1994) J. Infect. Dis. 170, 278-283. [DOI] [PubMed] [Google Scholar]
- 28.Haugo, A. J. & Watnick, P. I. (2002) Mol. Microbiol. 45, 471-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nesper, J., Schild, S., Lauriano, C. M., Kraiss, A., Klose, K. E. & Reidl, J. (2002) Infect. Immun. 70, 5990-5996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heydorn, A., Nielsen, A. T., Hentzer, M., Sternberg, C., Givskov, M., Ersboll, B. K. & Molin, S. (2000) Microbiology 146, 2395-2407. [DOI] [PubMed] [Google Scholar]
- 31.O'Toole, G. A., Pratt, L. A., Watnick, P. I., Newman, D. K., Weaver, V. B. & Kolter, R. (1999) Methods Enzymol. 310, 91-109. [DOI] [PubMed] [Google Scholar]
- 32.Caetano-Annoles, G. (1993) PCR Methods Appl. 3, 85-92. [DOI] [PubMed] [Google Scholar]
- 33.Yamasaki, S., Shimizu, T., Hoshino, K., Ho, S. T., Shimada, T., Nair, G. B. & Takeda, Y. (1999) Gene 237, 321-332. [DOI] [PubMed] [Google Scholar]
- 34.Johnson, J. A., Salles, C. A., Panigrahi, P., Albert, M. J., Wright, A. C., Johnson, R. J. & Morris, J. G., Jr. (1994) Infect. Immun. 62, 2108-2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beveridge, T. J. & Koval, S. F. (1981) Appl. Environ. Microbiol. 42, 325-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Langley, S. & Beveridge, T. J. (1999) Appl. Environ. Microbiol. 65, 489-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cassity, T. R. & Kolodziej, B. J. (1984) Microbios 41, 117-125. [PubMed] [Google Scholar]
- 38.Cox, A. D., Brisson, J. R., Thibault, P. & Perry, M. B. (1997) Carbohydr. Res. 304, 191-208. [DOI] [PubMed] [Google Scholar]
- 39.Landersjo, C., Weintraub, A., Ansaruzzaman, M., Albert, M. J. & Widmalm, G. (1998) Eur. J. Biochem. 251, 986-990. [DOI] [PubMed] [Google Scholar]