Abstract
A diverse, often species-specific, array of herbivore-induced plant volatiles (HIPVs) are commonly emitted from plants after herbivore attack. Although research in the last 3 decades indicates a multi-functional role of these HIPVs, the evolutionary rationale underpinning HIPV emissions remains an open question. Many studies have documented that HIPVs can attract natural enemies, and some studies indicate that neighboring plants may eavesdrop their undamaged neighbors and induce or prime their own defenses prior to herbivore attack. Both of these ecological roles for HIPVs are risky strategies for the emitting plant. In a recent paper, we reported that most branches within a blueberry bush share limited vascular connectivity, which restricts the systemic movement of internal signals. Blueberry branches circumvent this limitation by responding to HIPVs emitted from neighboring branches of the same plant: exposure to HIPVs increases levels of defensive signaling hormones, changes their defensive status, and makes undamaged branches more resistant to herbivores. Similar findings have been reported recently for sagebrush, poplar and lima beans, where intra-plant communication played a role in activating or priming defenses against herbivores. Thus, there is increasing evidence that intra-plant communication occurs in a wide range of taxonomically unrelated plant species. While the degree to which this phenomenon increases a plant’s fitness remains to be determined in most cases, we here argue that withinplant signaling provides more adaptive benefit for HIPV emissions than does between-plant signaling or attraction of predators. That is, the emission of HIPVs might have evolved primarily to protect undamaged parts of the plant against potential enemies, and neighboring plants and predators of herbivores later co-opted such HIPV signals for their own benefit.
Key words: intra-plant signaling, plantplant communication, eavesdropping, systemic wound signals, plant defense, tri-trophic interactions
Plants often emit a unique blend of volatiles in response to herbivore attack. The emission of these herbivore-induced plant volatiles (HIPVs) is an active response to herbivore feeding, producing a blend of volatiles that is distinct from those emitted following mechanical injury alone.1 Their emission can be variable; while some compounds follow a diurnal pattern with increasing amounts during the time of high photosynthesis,2,3 others are emitted primarily at night.4 In some cases, the HIPV blend produced also differs depending on the species of herbivore feeding on the plant.5 This specificity is thought to be due to chemicals in the herbivore’s regurgitant, such as the fatty-acid amino-acid conjugate volicitin, that activate the emission of volatiles in plants.6,7 Furthermore, HIPVs are emitted not only from the site of damage, but also at times from systemically undamaged parts of the plant.8 This and other systemic responses are, however, restricted within a plant such that only parts of the plant that share vascular connections with the damaged tissue receive wound signals and have the potential to respond.9,10
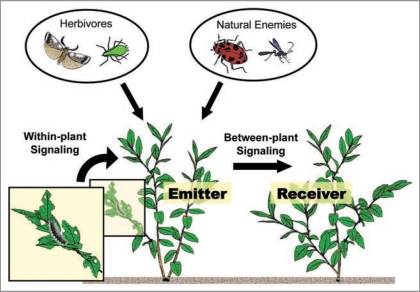
The ecological role of HIPVs has been a subject of fascination and the evolutionary advantage gained for plants by emitting HIPVs remains an unresolved topic of discussion. While some HIPV compounds, and some of their precursors, have sufficient volatility that their release is essentially inevitable after synthesis,11 most tend to be tightly regulated. Assuming that HIPV emissions evolved as a result of trophic interactions among plants, herbivores, and natural enemies, there are four general ecological roles that HIPVs may play: (1) a direct negative effect on the herbivore, (2) a signal to alert natural enemies of the herbivore, (3) a warning signal to nearby undamaged plants, and (4) a systemic warning signal within the damaged plant (Fig. 1). The first two potential roles involve the manipulation of animal behavior, while the last two may alter plant “behavior”.
Figure 1.
Herbivore-induced plant volatiles (HIPVs) play multiple roles in interactions among plants, herbivores, and natural enemies (possible interactions are depicted by arrows). Some of them benefit the HIPV-emitting plant (Emitter); these positive interactions include repellent effects on herbivores, attraction of natural enemies of herbivores, activation or priming of defenses in unwounded parts within the emitting plant (within-plant signaling), and growth inhibitory effects on neighboring plants (Receiver) through allelopathy. On the other hand, HIPVs may negatively affect the emitting plant by attracting herbivores or natural enemies (e.g., certain parasitoids) that result in increased damage. Finally, neighboring plants may “eavesdrop” from the emitting plant by responding to HIPVs (between-plant signaling). This latter interaction may be negative to the emitter if it is outcompeted by neighbors who receive wound signals, but beneficial to the receiving plant. Drawing by Robert Holdcraft.
Scents can have a demonstrable effect on animal behavior. With respect to plant-herbivore interactions, scents can provide information about the status of a plant to herbivores and their natural enemies. For example, HIPVs may repel adults moths searching for oviposition sites,3 which has been interpreted from the perspective of either a plant minimizing damage or, perhaps more realistically, an adult moth searching for an undamaged, high quality resource for her offspring. Conversely, HIPV-emitting plants may increase their chance of being injured if herbivores are attracted to these volatiles.12 The more commonly accepted role of HIPVs in manipulating animal behavior is to attract natural enemies of the herbivores. This tri-trophic “cry for help”13 has a potential evolutionary benefit for both the plant emitting the volatiles and the natural enemies responding to this emission.14–16 Although this idea makes sense in an evolutionary perspective, only a few studies have documented the occurrence of this phenomenon in natural systems.17 Indeed, the effectiveness of a cry for help depends on the presence of a helper and, equally importantly, the ability of the helper to increase plant fitness. In the case of predator attraction, the herbivore may be removed from the plant and consumed, thereby reducing damage for the emitting plant.18 However, insect herbivores infected by parasitoids, which also use HIPV cues to locate hosts,19 may also consume less plant material20 but may also in some cases consume more plant material than unparasitized insect herbivores.21 Since there is currently no evidence that plants can modify HIPV blends to attract selectively predators versus parasitoids, an answered cry for help may not reliably decrease the total amount of damage to an emitting plant. Thus, the fact that natural enemies respond to HIPVs does not imply that these volatiles evolved for this purpose or that there is an adaptive advantage for a plant to use HIPVs to attract natural enemies. Rather, natural enemies of insect herbivores may have learned to co-opt the HIPV signal emitted by plants and, by doing so, increased their fitness irrespective of the ultimate fitness outcome to the plant.
Though more controversial, scents can also have an effect on plant behavior.22 Early work suggested that HIPVs from wounded willows,23 poplars24 and sugar maples24 could trigger defense responses from other neighboring conspecifics. More recent studies have shown that this signaling can occur between different species of plants.25 While these results are intriguing, they appear to have little adaptive function from the perspective of an emitting plant, which could be facilitating the fitness of potential resource competitors. Further, unless the individual within the same plant species shared some degree of kinship,26 an emitting plant would also be at a disadvantage by providing an HIPV wound signal to a conspecific that, in theory, occupies the same competitive niche space. On the other hand, unwounded conspecific should benefit from being able to ‘eavesdrop’ by detecting HIPVs from wounded plants as they share the same herbivore complex and thus are vulnerable to attack. Moreover, from a heterospecific receiver’s perspective, the benefits of eavesdropping can be confounded by the potential of mounting defenses against a signal generated by incompatible herbivores feeding on a different plant species.27 So, eavesdropping may be adaptive for a receiving plant if it realizes increased fitness relative to a conspecific that did not receive the signal. The emitting plant derives no apparent adaptive benefit of using HIPVs to warn neighboring plants. However, the emitting plant may benefit if their HIPVs have inhibitory allelopathic activity on neighboring plants.28
Our recent work1 highlighted another scenario by which an HIPV-emitting plant would derive a direct benefit from the emissions: when HIPVs act as systemic wound signals within damaged plants. We showed that branches of blueberry shrubs lack effective vascular connections and thus cannot transmit wound signals among branches via the vasculature. To compensate, HIPVs can be transmitted among branches and, in so doing, overcome the vascular constraints of the branching life history strategy. Exposure to HIPVs increased levels of defensive signaling hormones in undamaged branches, changed their defensive chemical status, and made them more resistant to herbivores.1 This idea that HIPVs may function in intra-plant communication to activate or prime defenses in other parts of the emitting plant against future attack was first suggested separately by Farmer29 and Orians.9 The hypothesis was first tested with mechanically clipped wild sagebrush,30 and it was further tested with insect herbivores of wild lima bean31 and hybrid poplar.32 Under this scenario, the emitting plant derives a direct benefit from the HIPVs, providing an unambiguous fitness advantage.
So, what is the most beneficial factor to a plant for emitting volatiles in response to herbivore feeding? In terms of maximizing the potential benefit and minimizing the potential risk to the emitting plant, the function of HIPVs in mediating systemic wound signaling clearly provides the greatest potential adaptive advantage. Thus, we propose that the primary adaptive benefit for the evolution of HIPVs is to signal and protect unwounded parts of the attacked plant with high risk of infestation against herbivores. Later, these volatiles provided cues that led to adaptive fitness advantages for neighboring plants and natural enemies of herbivores, which may or may not benefit the HIPV-emitting plant. Indeed, ecologically adaptive advantages have emerged and contribute to a diverse, multi-functional chemical ecology mediated by HIPVs.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/10160
References
- 1.Rodriguez-Saona CR, Rodriguez-Saona LE, Frost CJ. Herbivore-induced volatiles in the perennial shrub, Vaccinium corymbosum, and their role in interbranch signaling. J Chem Ecol. 2009;35:163–175. doi: 10.1007/s10886-008-9579-z. [DOI] [PubMed] [Google Scholar]
- 2.Frost CJ, Mescher MC, Dervinis C, Davis JM, Carlson JE, De Moraes CM. Priming defense genes and metabolites in hybrid poplar by the green leaf volatile cis-3-hexenyl acetate. New Phytol. 2008;180:722–734. doi: 10.1111/j.1469-8137.2008.02599.x. [DOI] [PubMed] [Google Scholar]
- 3.Loughrin JH, Manukian A, Heath RR, Turlings TCJ, Tumlinson JH. Diurnal cycle of emission of induced volatile terpenoids by herbivore-injured cotton plants. Proc Natl Acad Sci USA. 1994;91:11836–1840. doi: 10.1073/pnas.91.25.11836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Moraes CM, Mescher MC, Tumlinson JH. Caterpillar-induced nocturnal plant volatiles repel conspecific females. Nature. 2001;410:577–580. doi: 10.1038/35069058. [DOI] [PubMed] [Google Scholar]
- 5.de Moraes CM, Lewis WJ, Paré PW, Alborn HT, Tumlinson JH. Herbivore-infested plants selectively attract parasitoids. Nature. 1998;393:570–573. [Google Scholar]
- 6.Alborn HT, Jones TH, Stenhagen GS, Tumlinson JH. Identification and synthesis of volicitin and related components from beet armyworm oral secretions. J Chem Ecol. 2000;26:203–220. [Google Scholar]
- 7.Paré PW, Farag MA, Krishnamachari V, Zhang HM, Ryu CM, Kloepper JW. Elicitors and priming agents initiate plant defense responses. Photosynth Res. 2005;85:149–159. doi: 10.1007/s11120-005-1001-x. [DOI] [PubMed] [Google Scholar]
- 8.Paré PW, Tumlinson JH. Cotton volatiles synthesized and released distal to the site of insect damage. Phytochemistry. 1998;47:521–526. [Google Scholar]
- 9.Orians C. Herbivores, vascular pathways and systemic induction: Facts and artifacts. J Chem Ecol. 2005;31:2231–2242. doi: 10.1007/s10886-005-7099-7. [DOI] [PubMed] [Google Scholar]
- 10.Orians CM, Pomerleau J, Ricco R. Vascular architecture generates fine scale variation in systemic induction of proteinase inhibitors in tomato. J Chem Ecol. 2000;26:471–485. [Google Scholar]
- 11.Penuelas J, Llusia J. Plant VOC emissions: making use of the unavoidable. Trends Ecol Evol. 2004;19:402–404. doi: 10.1016/j.tree.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Dicke M, van Loon JJA. Multitrophic effects of herbivore-induced plant volatiles in an evolutionary context. Entomol Exp Appl. 2000;97:237–249. [Google Scholar]
- 13.Dicke M. Behavioural and community ecology of plants that cry for help. Plant Cell Environm. 2009;32:654–665. doi: 10.1111/j.1365-3040.2008.01913.x. [DOI] [PubMed] [Google Scholar]
- 14.Choh Y, Shimoda T, Ozawa R, Dicke M, Takabayashi J. Exposure of lima bean leaves to volatiles from herbivore- induced conspecific plants results in emission of carnivore attractants: Active or passive process? J Chem Ecol. 2004;30:1305–1317. doi: 10.1023/b:joec.0000037741.13402.19. [DOI] [PubMed] [Google Scholar]
- 15.De Boer JG, Posthumus MA, Dicke M. Identification of volatiles that are used in discrimination between plants infested with prey or nonprey herbivores by a predatory mite. J Chem Ecol. 2004;30:2215–2230. doi: 10.1023/b:joec.0000048784.79031.5e. [DOI] [PubMed] [Google Scholar]
- 16.Dicke M. Are herbivore-induced plant volatiles reliable indicators of herbivore identity to foraging carnivorous arthropods? Entomol Exp Appl. 1999;91:131–142. [Google Scholar]
- 17.Kessler A, Baldwin IT. Defensive function of herbivore-induced plant volatile emissions in nature. Science. 2001;291:2141–2144. doi: 10.1126/science.291.5511.2141. [DOI] [PubMed] [Google Scholar]
- 18.Dicke M, De Boer JG, Hofte M, Rocha-Granados MC. Mixed blends of herbivore-induced plant volatiles and foraging success of carnivorous arthropods. Oikos. 2003;101:38–48. [Google Scholar]
- 19.Turlings TCJ, Tumlinson JH, Lewis WJ. Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science. 1990;250:1251–1253. doi: 10.1126/science.250.4985.1251. [DOI] [PubMed] [Google Scholar]
- 20.Hoballah MEF, Turlings TCJ. Experimental evidence that plants under caterpillar attack may benefit from attracting parasitoids. Evol Ecol Res. 2001;3:553–565. [Google Scholar]
- 21.Smallegange RC, van Loon JJA, Blatt SE, Harvey JA, Dicke M. Parasitoid load affects plant fitness in a tritrophic system. Entomol Exp Appl. 2008;128:172–183. [Google Scholar]
- 22.Karban R. Plant behaviour and communication. Ecol Lett. 2008;11:727–739. doi: 10.1111/j.1461-0248.2008.01183.x. [DOI] [PubMed] [Google Scholar]
- 23.Rhoades DF. Responses of alder and willow to attack by tent caterpillars and webworms: evidence for pheromonal sensitivity of willows. In: Hedin PA, editor. Plant Resistance to Insects. Washington DC: American Chemical Society; 1982. [Google Scholar]
- 24.Baldwin IT, Schultz JC. Rapid changes in tree leaf chemistry induced by damage: evidence for communication between plants. Science. 1983;221:277–279. doi: 10.1126/science.221.4607.277. [DOI] [PubMed] [Google Scholar]
- 25.Karban R, Baldwin IT, Baxter KJ, Laue G, Felton GW. Communication between plants: induced resistance in wild tobacco plants following clipping of neighboring sagebrush. Oecologia. 2000;125:66–71. doi: 10.1007/PL00008892. [DOI] [PubMed] [Google Scholar]
- 26.Karban R, Shiojiri K. Self-recognition affects plant communication and defense. Ecol Lett. 2009;12:502–506. doi: 10.1111/j.1461-0248.2009.01313.x. [DOI] [PubMed] [Google Scholar]
- 27.Karban R, Maron J. The fitness consequences of interspecific eavesdropping between plants. Ecology. 2002;83:1209–1913. [Google Scholar]
- 28.Karban R. Experimental clipping of sagebrush inhibits seed germination of neighbours. Ecol Lett. 2007;10:791–797. doi: 10.1111/j.1461-0248.2007.01068.x. [DOI] [PubMed] [Google Scholar]
- 29.Farmer EE. Surface-to-air signals. Nature. 2001;411:854–856. doi: 10.1038/35081189. [DOI] [PubMed] [Google Scholar]
- 30.Karban R, Shiojiri K, Huntzinger M, McCall AC. Damage-induced resistance in sagebrush: Volatiles are key to intra- and interplant communication. Ecology. 2006;87:922–930. doi: 10.1890/0012-9658(2006)87[922:drisva]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 31.Heil M, Silva Bueno JC. Within-plant signaling by volatiles leads to induction and priming of an indirect plant defense in nature. Proc Natl Acad Sci USA. 2007;104:5467–5472. doi: 10.1073/pnas.0610266104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frost CJ, Appel HM, Carlson JE, De Moraes CM, Mescher MC, Schultz JC. Within-plant signalling via volatiles overcomes vascular constraints on systemic signalling and primes responses against herbivores. Ecol Lett. 2007;10:490–498. doi: 10.1111/j.1461-0248.2007.01043.x. [DOI] [PubMed] [Google Scholar]