Abstract
The dramatic movements of some carnivorous plants species are triggered by sensory structures derived from trichomes. While unusual plant species such as the Venus fly trap and sundews may be expected to have elaborate sensors to capture their insect prey, more modest plant species might not be expected to have similar sensory capabilities. Our recent work, however, has revealed that glandular trichomes on tomato (Solanum lycopersicum) appear to have a function similar to trigger hairs of carnivorous species, acting as “early warning” sensors. Using a combination of behavioral, molecular, and biochemical techniques, we determined that caterpillars, moths and mechanical disruption upregulate signaling molecules and defensive genes found in glandular trichomes. Importantly, we discovered that plants whose trichomes have been broken respond more vigorously when their defenses were induced. Taken together, our results suggest that glandular trichomes can act as sensors that detect activity on the leaf surface, and ready plants for herbivore attack.
Key words: glandular trichome, induced responses, jasmonic acid, plant-insect interactions, sensor, Solanum lycopersicum, tomato
Certain plant species are renowned for their ability to respond to contact. The Venus fly trap (Dionaea muscipula) and sundew (Drosera) species come to mind quickly as obviously thigmotropic species. When an insect lands on these carnivorous plant species, dramatic movements ensue once the prey is detected. Some Drosera species respond to contact by bending their “tentacles” toward their trapped prey to further ensnare the victim and begin the process of digestion. These dramatic plant species have captured the attention of many scientists, including Darwin, who remarked on the “extraordinary sensitiveness of [their] glands to slight pressure” and surmised that the tentacles of sundew plants “existed primordially as glandular hairs.”1 As is often the case, Darwin appears to have been quite right. Indeed, morphological and molecular work supports the notion that sundew tentacles and the trigger hairs of the Venus fly trap are homologous sensory structures likely derived from trichomes.2,3
Given Darwin’s appreciation of these trichome-derived sensory organs, he perhaps would not have been surprised by mounting evidence that suggests that trichomes may play even a broader sensory role for plants. We have recently found evidence that glandular trichomes can act as early detection sensors for some plant species.4 These trichomes can be disrupted by the footsteps of walking moths and caterpillars (and other forms of light touching), and this apparently minor plant damage leads to a state of defensive readiness that allows plants to respond to herbivory more quickly than undamaged plants. While this level of trichome-mediated detection does not result in the conspicuous responses of some carnivorous plant species, it still results in significant physiological changes that prepare plants for attack.
In our recent effort, we worked with tomato (Solanum lycopersicum), using a combination of behavioral, molecular, and biochemical techniques to understand the role of trichomes in detecting activity on the leaf surface.4 Defense signaling has been well studied in tomato and there exists a variety of mutants whose defensive responses have been compromised. Moreover, it has been known that tomatoes have a variety of trichome types, including two types of glandular trichomes that burst upon contact with insects, releasing their cellular contents and physically impeding insects (Fig. 1).5,6
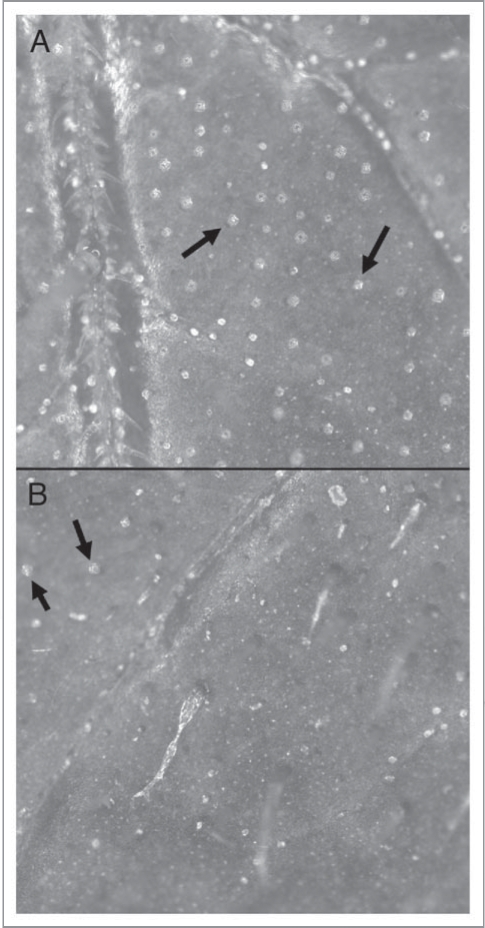
Figure 1.
Surface of a tomato leaf showing (A) intact rounded heads of glandular trichomes (black arrows) and (B) trichomes disrupted with a gloved hand (absence of rounded heads except for a few in the upper left corner [black arrows]). Images were captured at 36x magnification and were taken from different parts of the same leaf.
To determine if plant defense pathways were induced by insect contact, we allowed three species of caterpillar (Manduca sexta, Heliothis virescens and Helicoverpa zea) and one species of moth (H. zea) to crawl over tomato leaves for ten minutes. As a positive control, we also lightly rubbed leaves with a gloved hand or a metal rod. Within time frames ranging from three to twenty-four hours all treatments, insect and otherwise, significantly induced defensive genes as measured by qRT-PCR. Using a combination of RT-PCR and in situ hybridization, we confirmed that JA-signaling and defensive genes are expressed in trichomes. A GC-MS-based technique then confirmed that JA was present in trichomes of undamaged plants and DAB staining, in combination with catalase treatment, provided evidence that hydrogren peroxide and JA are key signals mediating defensegene induction. These conclusions were further reinforced by experiments with def1 mutants, a line of tomato impaired in JA signaling, and accession LA3610, a tomato variety with reduced numbers of trichomes. Lastly, we conducted a factorial experiment both disrupting trichomes and treating tomato plants with methyl jasmonate (MeJA), which induces plant defenses and increases densities of trichomes.7 Results of this final experiment indicated that plants that received both treatments (i.e., MeJA and disruption) had greater defensive gene induction than plants that were only treated with MeJA or plants whose trichomes remained intact, suggesting that increases in trichomes may contribute to greater sensitivity to touch-induced responses.
Taken together, our results are highly suggestive that trichomes can act as “early warning” detectors for plants. Moths seeking to lay eggs on tomato are likely to break trichomes as they explore leaves, upregulating plant defenses in anticipation of egg hatch and feeding by neonate caterpillars. Similarly, herbivores colonizing a new host plant and breaking trichomes on their way across a leaf also appear to “tip the plant off” to impending attack. Considering the drastic response of carnivorous plants to touch, perhaps it should not be surprising that trichomes can function more broadly as sensors. In an evolutionary context, it seems logical that trichomes took on this role. For many plant species, “hairy” varieties receive less herbivory,8 so within a population there could have been a fitness advantage in having more trichomes. Once established, this hairy phenotype could then have been refined via mutation and selection for trichome varieties that had functions adaptive for the plant, perhaps driving the evolution of glandular trichomes and their role as sensors.
Granted, the generalized nature of our results would appear to indicate that plants could be “primed” by nearly any arthropod species that crosses one of their leaves. This would, of course, include natural enemies, which are capable of decreasing herbivore pressure and improving plant fitness.9,10 However, it has been hypothesized that priming evolved due to high fitness costs associated with defensive induction following threats of only minor severity.11 Priming provides an advantage by settling plants into an intermediate “ready” state that allows them to deploy strong defense responses more quickly and the fitness cost associated with being “primed” are lower than full defensive induction.12 Presumably, fitness costs following priming due to natural enemyinduced trichome disruption would also be less than the cost incurred from a bout of unanticipated herbivory and, over the life of the plant, it would be worth the effort to prepare for attack even if the perceived risk is from a natural enemy and not a foe.
Our results build on previously reported priming mechanisms that prepare plants for attack.13,14 And they reveal an additional level of sophistication in the sensory capabilities of plants, which have already been shown to be able to detect nearby threats of herbivory and increase their defenses in response.15,16 It seems that trichomes may have played a much wider role in shaping the nature of plant-animal interaction than previously recognized and we look forward to further work elaborating their function.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/10234
References
- 1.Darwin CR. Insectivorous Plants. London: John Murray; 1875. [Google Scholar]
- 2.Williams SE. Comparative sensory physiology of the Droseraceae-the evolution of a plant sensory system. Proc Am Phil Soc. 1976;120:187–204. [Google Scholar]
- 3.Rivadavia F, Kondo K, Kato M, Hasebe M. Phylogeny of the sundews, Drosera (Droseraceae), based on chloroplast rbcL and nuclear 18S ribosomal DNA sequences. Am J Bot. 2003;90:123–130. doi: 10.3732/ajb.90.1.123. [DOI] [PubMed] [Google Scholar]
- 4.Peiffer M, Tooker JF, Luthe DS, Felton GW. Plants on early alert: glandular trichomes as sensors for insect herbivores. New Phytol. 2009;184:644–656. doi: 10.1111/j.1469-8137.2009.03002.x. [DOI] [PubMed] [Google Scholar]
- 5.Duffey SS. Plant glandular trichomes: their partial role in defence against insects. In: Juniper BE, Southwood TE, editors. Insects and The Plant Surface. London: Arnold; 1986. pp. 151–172. [Google Scholar]
- 6.Steffens JC, Walters DS. Biochemical aspects of glandular trichome-mediated insect resistance in the Solanaceae. ACS Symposium Series. 1991;449:136–149. [Google Scholar]
- 7.Boughton AJ, Hoover K, Felton GW. Methyl jasmonate application induces increased densities of glandular trichomes on tomato, Lycopersicon esculentum. J Chem Ecol. 2005;31:2211–2216. doi: 10.1007/s10886-005-6228-7. [DOI] [PubMed] [Google Scholar]
- 8.Chu CC, Natwick ET, Chen TY, Henneberry TJ. Analyses of cotton leaf characteristics effect on Bemisia tabaci (Homoptera: Aleyrodidae) biotype B colonization on cotton in Arizona and California. Southwest Entomol. 2003;28:235–240. [Google Scholar]
- 9.Gomez JM, Zamora R. Top-down effects in a tritrophic system: parasitoids enhance plant fitness. Ecology. 1994;75:1023–1030. [Google Scholar]
- 10.Tooker JF, Hanks LM. Tritrophic interactions influence reproduction of prairie perennials (Asteraceae: Silphium) Environ Entomol. 2006;35:537–5345. [Google Scholar]
- 11.Conrath U, Beckers GJM, Flors V, Garcia-Agustin P, Jakab G, Mauch F, et al. Priming: Getting ready for battle. Mol Plant Microbe Inter. 2006;19:1062–1071. doi: 10.1094/MPMI-19-1062. [DOI] [PubMed] [Google Scholar]
- 12.van Hulten M, Pelser M, Van Loon LC, Pieterse CMJ, Ton J. Costs and benefits of priming for defense in Arabidopsis. Proc Natl Acad Sci USA. 2006;103:5602–5607. doi: 10.1073/pnas.0510213103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engelberth J, Alborn HT, Schmelz EA, Tumlinson JH. Airborne signals prime plants against insect herbivore attack. Proc Natl Acad Sci USA. 2004;101:1781–1785. doi: 10.1073/pnas.0308037100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kessler A, Halitschke R, Diezel C, Baldwin IT. Priming of plant defense responses in nature by airborne signaling between Artemisia tridentata and Nicotiana attenuata. Oecologia. 2006;148:280–292. doi: 10.1007/s00442-006-0365-8. [DOI] [PubMed] [Google Scholar]
- 15.Arimura G, Ozawa R, Shimoda T, Nishioka T, Boland W, Takabayashi J. Herbivory-induced volatiles elicit defence genes in lima bean leaves. Nature. 2000;406:512–515. doi: 10.1038/35020072. [DOI] [PubMed] [Google Scholar]
- 16.Dolch R, Tscharntke T. Defoliation of alders (Alnus glutinosa) affects herbivory by leaf beetles on undamaged neighbours. Oecologia. 2000;125:504–511. doi: 10.1007/s004420000482. [DOI] [PubMed] [Google Scholar]