Abstract
In nature, plants experience considerable changes in the prevailing illumination, which can drastically reduce photosynthetic efficiency and yield. Such adverse effects are counterbalanced by acclimation responses which ensure high photosynthetic productivity by structural reconfiguration of the photosynthetic apparatus. Those acclimation responses are controlled by reduction-oxidation (redox) signals from two pools of redox compounds, the plastoquinone and the thioredoxin pools. The relative impact of these two redox signaling systems on this process, however, remains controversial. Recently, we showed that photosynthesis controls nuclear gene expression and cellular metabolite states in an integrated manner, thus, stabilizing the varying energetic demands of the plant. Here, we propose a novel model based on a binary redox control mode to explain adaptation of plant primary productivity to the light environment. Plastoquinone and thioredoxin pools are proposed to define specific environmental situations cooperatively and to initiate appropriate acclimation responses controlled by four binary combinations of their redox states. Our model indicates a hierarchical redox regulation network that controls plant primary productivity and supports the notion that photosynthesis is an environmental sensor affecting plant growth and development.
Key words: photosynthetic light acclimation, redox control, sensor function, arabidopsis, plant fitness
Photosynthesis Acts as Environmental Sensor that Integrates Gene Expression and Metabolism
Throughout their life time plants experience highly variable illumination conditions. In growing plants stands, for example, light intensity as well as light quality gradients occur due to light absorption or shading. Alterations in illumination conditions can drastically affect photosynthetic efficiency and accordingly, molecular acclimation mechanisms have evolved to restore photosynthetic homeostasis and primary productivity. These photosynthetic acclimation responses involve adaptation of the photosynthetic apparatus by structural reconfiguration including variations of antenna size via state transitions and readjustment of photosystem stoichiometry.1–3
In the electron transport chain photosystem II (PSII) and photosystem I (PSI), which act electrochemically in series, differ in the absorption maxima of their reaction centers (680 and 700 nm, respectively). Thus, light gradients can induce excitation imbalances between the two photosystems and disturb the redox chemistry of the electron transport chain and, in addition, can affect the Calvin-Benson-Cycle which is central for CO2 fixation and the recovery of the electron end acceptor NADP+. In this way, photosynthesis itself can sense illumination changes and induce appropriate acclimation responses.
In the laboratory, we mimic natural light quality gradients with artificial light sources which preferentially excite either photosystem I or II (PSI- or PSII-light) leading to oxidised or reduced states of the photosynthetic electron transport chain in plants grown under these lights. Recently we showed that long-term photosynthetic acclimation (LTR) involves not only changes in plastid and nuclear gene expression but also distinct adjustment of the metabolism which includes resource allocation between different metabolite pools including starch.4 This adjusts the energetic and metabolic demands of plants to the light-induced changes of the photosynthetic apparatus structure. The distinct metabolic states are referred to as “metabolic state 1” and “metabolic state 2” in accordance with the state of the photosynthetic apparatus induced by the light sources. This metabolic adjustment is controlled by reduction-oxidation (redox) signals from photosynthesis, i.e., from the plastoquinone and thioredoxin pools. The kinetics as well as the relative contribution of the two redox pools, however, are unknown or controversial.
A Universal Model for Regulation of Photosynthetic Acclimation
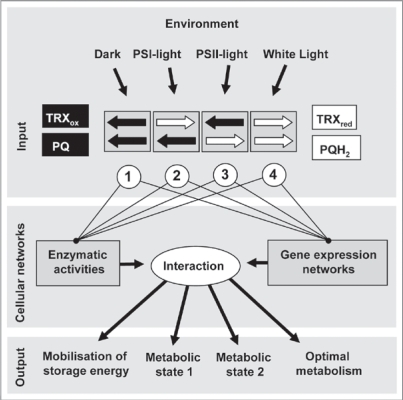
If the “metabolic states 1 and 2” are induced by redox signals from photosynthesis how can this be initiated in a specific and distinct manner and how can photosynthesis distinguish PSI- and PSII-light conditions from dark or white light conditions? Recently determined data on NADP/NADPH and MDH activation state4 allow us to conclude that the thioredoxin redox state becomes more oxidised upon a PSI-II light shift and more reduced upon a PSII-I light shift. Furthermore, recent data on the redox state of PQ demonstrated that shifts between PSI- and PSII-lights induce opposite changes in the redox state of the PQ pool, i.e., plastoquinone being more reduced under PSII-light and more oxidised under PSI-light.5 Combining these observations allows us both to extend existing ideas on photosynthetic redox control and to propose a novel hierarchical regulation mode as working hypothesis for photosynthetic acclimation. So far, PQ and thioredoxin redox states have been regarded as independent regulatory signals, however since they act in parallel it is very likely that they constitute signals which act co-operatively. We thus propose that the light environment induces characteristic relations in the redox balance of plastoquinone and thioredoxin that yield four possible binary conformations. In other words, the light environment of a plant translates into one of four possible binary redox signals/states. These serve as distinct input signals which induce the specific acclimation responses observed hereby affecting enzyme activities and gene expression networks in a co-operative manner (Fig. 1). The induced acclimation processes ultimately result in complex structural and metabolic rearrangements to ensure high photosynthetic efficiency and thus enable plants to cope with variable light environments.
Figure 1.
Working hypothesis of cooperative redox signaling in photosynthetic acclimation. Illumination conditions induce changes in relative redox poises of PQ and Trx pools (black arrows: change towards a more oxidized state, white arrows: change towards a more reduced state). Combinations of them serve as input signals which affect gene expression networks and metabolic enzyme activities in a coordinated and parallel manner. Network interactions then initiate respective output response programmes resulting in defined metabolic states. Indicated redox balances are based on data from this and recent studies.5,23
Our model proposes the following combinations of redox signals and describes their respective implications: (1) Redox balance 1 (oxidized plastoquinone and oxidized thioredoxin (PQ/Trxox)) indicates lack of light energy and activates mobilisation of storage energy (starch). (2) A shift towards redox balance 2 (oxidized plastoquinone and reduced thioredoxin (PQ/Trxred)) signals limitation of PSII and maintains Calvin-Benson-cycle enzymes in an activated state in order to facilitate high CO2 fixation rates and to allow for increased rates of cyclic electron transport for ATP production (metabolic state 1). (3) In redox balance 3 the opposite shift towards PQH2/Trxox signals limitation of PSI preferring linear electron transport to reduce NADP+ (metabolic state 2). (4) Redox balance 4 (PQH2/Trxred) indicates optimal redox balance for efficient photosynthesis, albeit at high reduction pressure there is some need to decrease excitation pressure and to upregulate dissipative pathways as well as the dark reaction. Redox balances (1) to (3) represent regulatory models for light limited conditions. Redox balance (4), however, represents optimal function of photosynthetic light reactions. Further increases in light supply should, therefore, not have an impact on this redox balance and, indeed, experimental evidence in support of this statement has been reported.1 Instead, increasing light intensities will lead to the additional and/or superimposing action of ROS and coupled redox buffer components which would be anticipated to activate stress responses. In addition, action of mechanisms to dissipate excess excitation such as NPQ should be expected. By this means our model provides a universal physiological framework for redox-controlled light responses.
Implications of Cooperative Redox Signaling
As with any model our proposed binary redox control mode is to some extent over-simplified. It must be pointed out that the four basic combinations of redox states from plastoquinone and thioredoxin pools refer to relative changes in the respective redox states since relative changes are important and absolute redox states are difficult to determine. Robust and reliable measures for the redox state of plastoquinone and thioredoxin can be obtained in an indirect manner through 1-qP values and the MDH activation state when observed in the same physiological context. Furthermore, we assume that the redox states of the different thioredoxins in one light condition are the more or less the same which is consistent with current models on thioredoxin.6 Thus, we conclude that a binary mode of redox combinations from two distinct pools of redox compounds; the plastoquinone and thioredoxin pools; is the best way to interpret our data.
The reactions in gene expression and protein/enzyme networks caused by the binary redox signals are most probably acting on many parallel targets rather than initiating a single signaling cascade (such as for example the induction of changes in gene expression followed by changes in enzyme amounts followed by metabolite changes). A number of examples are already known which show that redox control of plastoquinone and thioredoxin affect both gene expression and enzyme activity in a co-operative manner (see also below).7
The observation that the thioredoxin pool(s) appear(s) to be more reduced in PSI-light than in PSII-light was somehow unexpected since it is generally assumed that a more oxidised state of the electron transport chain (as the PQ data indicate) would also result in a more oxidised state of the components at the PSI acceptor side. Thus at first sight our results appear to contradict data from a recent study which reported a lower MDH activity of Arabidopsis in PSI light than in PSII light.8 However, in the respective study plants were shifted from white light into either PSI- or PSII-light which is a physiologically quite distinct situation from the conditions we studied. Furthermore, MDH activity is not the same as the MDH activation state, since the first is essentially a measure of the protein abundance whilst the second provides information concerning its likely in vivo activity, thus the data cannot be directly compared. To date it is unclear how the redox balance we observed is achieved. One possibility would be that the predominantly oxidised PQ pool under PSI light generates a signal which slows down a limiting step in the dark reaction leading to a lower NADPH demand and, in turn, to an increase in the NADPH/NADP ratio.
Cooperative Redox-Signaling Provides a Unifying Framework for Individual Observations and Existing Models
Despite or perhaps even because of the surprising constellation of redox states observed under PSI-light, our novel model of photosynthetic regulation not only explains our data. It could also serve as a universal framework which integrates individual observations and existing models. The model is in good accordance with earlier studies on PQ and thioredoxin regulation of both: light harvesting and gene expression. First, it provides an extended physiological explanation for the combined redox regulation of the LHCII kinase which was observed in vitro. Current models explain this as an activation of the kinase via PQH2 in low light which is overridden in high light leading to inactivation via Trxred.9–12 Our model complements this view by two new situations and explains combined regulation via thioredoxin and plastoquinone also under low-light conditions in which redox balance 3 activates the kinase whilst redox balance 2 inactivates it. Second, the model theoretically integrates two current models on redox regulation of plastid gene expression. In an early study with Chlamydomonas reinhardtii, it was proposed that translation initiation of the psbA gene is activated by light via reduced thioredoxin.13 This redox regulation is mediated by a protein disulfide isomerase.14 Recent data suggest that this mechanism may also be active in higher plants.15 The excitation imbalances caused by the light quality shifts used here are counterbalanced by a long term response (LTR) which involves the increased expression of the respective rate-limiting photosystem depending on the redox state of the PQ pool.16 It is unknown yet whether redox controlled psbA translation initiation contributes to the photosystem stoichiometry adjustment or not. However, according to our model regulation of psbA translation via reduced thioredoxin would work in a direction that supports the LTR, i.e., activation under PSI-light and inactivation under PSII-light.
However, further work on the translation initiation model in Chlamydomonas reinhardtii proposed an additional priming signal originating from the PQ pool as a prerequisite for the action of the thioredoxin mediated activation of psbA translation. This signal is generated by net reduction of the PQ pool allowing gradual translation initiation depending on the redox state of the thioredoxin system.17,18 In these studies 150 µmol photons m−2 s−1 white light have been used which suggests that responses to redox balance 4 were studied. If our model applies to the extremely dynamic photosynthetic apparatus of Chlamydomonas remains to be tested. That said, redox balance 4 would provide optimal translation of psbA under sufficient intensities of white light and, thus, promotes the D1 repair cycle as well as recovery from photoinhibition.
Gene Expression and Metabolite Data Support the Model of Cooperative Redox Signaling
Further support for our model comes from recent investigations with isolated intact chloroplasts from mustard.19 In in organello run on transcription experiments we found that the kinase inhibitor H7 had no effect on plastid transcription while the thiol reagent DTT caused a partial inhibition. Simultaneous application, however, diminished this repression indicating that a phosphorylation-dependent pathway and a thiol-dependent pathway interact at the level of transcription. The PQ redox signal is likely transduced into a phosphorylation signal via the STN7 kinase, thus these data support the proposed action of co-operative redox signaling towards the level of gene expression.
In addition, the model provides an elegant explanation for a still unresolved question in our understanding of the regulation of photosynthetic light reaction. PSI-light is the classical condition under which cyclic electron transport occurs.20,21 However, the additional protons pumped by this mechanism require an active ATPase to produce the extra ATP which, in turn, requires the reduction of regulatory thiol groups in the CF1-γ-subunit.6 An oxidised state of the components at the PSI acceptor side would prevent this. The more reduced Trx pool under PSI-light, as determined here, provides precisely the conditions under which the ATPase is kept in an at least partially activated stage.
Interestingly, our redox regulation model has also implications for metabolite biosynthesis and consequently metabolite pools sizes. After a PSI-II light shift we found slightly increased glutathione amounts as well as decreased amounts of this redox compound after the PSII-I light shift.4 The entry enzyme of glutathione biosynthesis, glutamate-cysteine-ligase (GCL), has been reported to be activated by oxidation and de-activated upon reduction of thiol groups.22 According to our model, therefore, the enzyme should be more active under PSII-light and, indeed, higher amounts of glutathione were found under these conditions. Similarly, our model could provide insight into the regulation of nitrogen compounds. In the metabolite profiles glutamine was found to be increased upon a shift from PSII to PSI light while it is decreased in the opposite shift.4 The ATP-dependent formation of glutamine from glutamate and ammonia is catalysed by the glutamine synthetase, an enzyme which is activated by reduced Trx in plants.6 The observed accumulation of glutamine under PSI-light can only be explained by activation through a more reduced state of Trx pools which is fully consistent with the assumptions in our working hypothesis. Thus, the accumulation patterns of these two metabolites provide further support for our model.
In summary our model explains how a complex photosynthetic control can perform fine-tuning not only of photosynthesis itself but also of metabolic reactions especially under light limiting conditions. This fine-tuning is essential for efficient energy conversion and maximum plant fitness pointing to an important target for plant yield improvements in dense plant populations such as crop fields or forests where strong light gradients persist.
Acknowledgements
This work was supported by grants from the Deutsche Forschungsgemeinschaft to T.P., FOR804 and by the NWP programme of Thuringia.
Abbreviations
- LTR
long-term response to light quality
- MDH
malate dehydrogenase
- PS
photosystem
- PQ
plastoquinone
- TRX
thioredoxin
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/10294
References
- 1.Walters RG. Towards an understanding of photosynthetic acclimation. J Exp Bot. 2005;56:435–447. doi: 10.1093/jxb/eri060. [DOI] [PubMed] [Google Scholar]
- 2.Eberhard S, Finazzi G, Wollman FA. The Dynamics of Photosynthesis. Ann Rev Genet. 2008;42:463–515. doi: 10.1146/annurev.genet.42.110807.091452. [DOI] [PubMed] [Google Scholar]
- 3.Dietzel L, Bräutigam K, Pfannschmidt T. Photosynthetic acclimation: State transitions and adjustment of photosystem stoichiometry—functional relationships between short-term and long-term light quality acclimation in plants. FEBS J. 2008;275:1080–1088. doi: 10.1111/j.1742-4658.2008.06264.x. [DOI] [PubMed] [Google Scholar]
- 4.Bräutigam K, Dietzel L, Kleine T, Ströher E, Wormuth D, Dietz KJ, et al. Dynamic plastid redox signals integrate gene expression and metabolism to induce distinct metabolic states in photosynthetic acclimation in Arabidopsis. Plant Cell. 2009;21:2715–2732. doi: 10.1105/tpc.108.062018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wagner R, Dietzel L, Bräutigam K, Fischer W, Pfannschmidt T. The long-term response to fluctuating light quality is an important and distinct light acclimation mechanism that supports survival of Arabidopsis thaliana under low light conditions. Planta. 2008;228:573–587. doi: 10.1007/s00425-008-0760-y. [DOI] [PubMed] [Google Scholar]
- 6.Schurmann P, Buchanan BB. The ferredoxin/thioredoxin system of oxygenic photosynthesis. Antiox Redox Signal. 2008;10:1235–1273. doi: 10.1089/ars.2007.1931. [DOI] [PubMed] [Google Scholar]
- 7.Pfannschmidt T. Chloroplast redox signals: how photosynthesis controls its own genes. Trends Plant Sci. 2003;8:33–41. doi: 10.1016/s1360-1385(02)00005-5. [DOI] [PubMed] [Google Scholar]
- 8.Piippo M, Allahverdiyeva Y, Paakkarinen V, Suoranta UM, Battchikova N, Aro EM. Chloroplast-mediated regulation of nuclear genes in Arabidopsis thaliana in the absence of light stress. Physiol Genom. 2006;25:142–152. doi: 10.1152/physiolgenomics.00256.2005. [DOI] [PubMed] [Google Scholar]
- 9.Hou CX, Pursiheimo S, Rintamaki E, Aro EM. Environmental and metabolic control of LHCII protein phosphorylation: revealing the mechanisms for dual regulation of the LHCII kinase. Plant Cell Environm. 2002;25:1515–1525. [Google Scholar]
- 10.Rintamaki E, Martinsuo P, Pursiheimo S, Aro EM. Cooperative regulation of light-harvesting complex II phosphorylation via the plastoquinol and ferredoxinthioredoxin system in chloroplasts. Proc Natl Acad Sci USA. 2000;97:11644–11649. doi: 10.1073/pnas.180054297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rochaix JD. Role of thylakoid protein kinases in photosynthetic acclimation. FEBS Letts. 2007;581:2768–2775. doi: 10.1016/j.febslet.2007.04.038. [DOI] [PubMed] [Google Scholar]
- 12.Lemeille S, Willig A, Depège-Fargeix N, Delessert C, Bassi R, Rochaix JD. Analysis of the chloroplast kinase STT7 during state transitions. PLoS Biol. 2009;7:e45. doi: 10.1371/journal.pbio.1000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Danon A, Mayfield SP. Light-regulated translation of chloroplast messenger-RNAs through redox potential. Science. 1994;266:1717–1719. doi: 10.1126/science.7992056. [DOI] [PubMed] [Google Scholar]
- 14.Kim JM, Mayfield SP. Protein disulfide isomerase as a regulator of chloroplast translational activation. Science. 1997;278:1954–1957. doi: 10.1126/science.278.5345.1954. [DOI] [PubMed] [Google Scholar]
- 15.Shen YX, Danon A, Christopher DA. RNA bindingproteins interact specifically with the Arabidopsis chloroplast psbA mRNA 5′ untranslated region in a redox-dependent manner. Plant Cell Physiol. 2001;42:1071–1078. doi: 10.1093/pcp/pce142. [DOI] [PubMed] [Google Scholar]
- 16.Pfannschmidt T, Nilsson A, Allen JF. Photosynthetic control of chloroplast gene expression. Nature. 1999;397:625–628. [Google Scholar]
- 17.Trebitsh T, Levitan A, Sofer A, Danon A. Translation of chloroplast psbA mRNA is modulated in the light by counteracting oxidizing and reducing activities. Molec Cell Biol. 2000;20:1116–1123. doi: 10.1128/mcb.20.4.1116-1123.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trebitsh T, Danon A. Translation of chloroplast psbA mRNA is regulated by signals initiated by both photosystems II and I. Proc Natl Acad Sci USA. 2001;98:12289–12294. doi: 10.1073/pnas.211440698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steiner S, et al. The role of phosphorylation in redox regulation of photosynthesis genes psaA and psbA during photosynthetic acclimation of mustard. Mol Plant. 2009;2:416–429. doi: 10.1093/mp/ssp007. [DOI] [PubMed] [Google Scholar]
- 20.Joliot P, Joliot A. Cyclic electron flow in C3 plants. Biochim Biophys Acta-Bioenergetics. 2006:77. doi: 10.1016/j.bbabio.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 21.Wollman FA. State transitions reveal the dynamics and flexibility of the photosynthetic apparatus. EMBO J. 2001;20:3623–3630. doi: 10.1093/emboj/20.14.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hicks LM, et al. Thiol-based regulation of redoxactive glutamate-cysteine ligase from Arabidopsis thaliana. Plant Cell. 2007;19:2653–2661. doi: 10.1105/tpc.107.052597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kruk J, Karpinski S. An HPLC-based method of estimation of the total redox state of plastoquinone in chloroplasts, the size of the photochemically active plastoquinone-pool and its redox state in thylakoids of Arabidopsis. Biochim Biophys Acta-Bioenergetics. 2006;1757:1669–1675. doi: 10.1016/j.bbabio.2006.08.004. [DOI] [PubMed] [Google Scholar]