Abstract
Streptococcus pneumoniae cause considerable morbidity and mortality, with persistent neurological sequelae, particularly in young children and the elderly. It is widely assumed that carriage occurs through direct mucosal colonization from the environment whereas meningitis results from invasion from the blood. However, the results of published studies can be interpreted that pneumococci may enter the brain directly from the nasal cavity by axonal transport through olfactory nerves. This hypothesis is based on findings that (i) teichoic acid of the pneumococcal cell wall interact with gangliosides (GLS), (ii) the interaction of GLS with cholera toxin leads to axonal transport through the olfactory nerves into the brain, and (iii) viruses enter the brain through axonal transport into olfactory nerves. After nasal inoculation, we observe high numbers of pneumococci in nasal washes and the olfactory nerves and epithelium. Significant numbers of pneumococci also infected the olfactory bulbs, brain, and the trigeminal ganglia. The absence of bacteremia in this model makes it unlikely that the bacteria entered the brain from the blood stream. Recovery of colony-forming units from the brain, lungs, olfactory nerves, and epithelium and nasal washes was inhibited by incubating pneumococci with GLS before nasal inoculation. These findings, confirmed by PCR and immunohistochemistry, support a GLS-mediated process of infection and are consistent with pneumococci reaching the brain through retrograde axonal transport.
Pneumococcal infections are associated with significant mortality and morbidity despite successful treatment with antibiotics (1). The highest incidence of pneumococcal meningitis occurs in young children <2 yr of age (2) and in the elderly (3, 4). The colonization of the nasopharynx is considered a prerequisite to the spread of pneumococci to the lower respiratory tract, sinuses, and the middle ear (5-7). Despite significant progress in recent years, the molecular and cellular mechanisms leading to bacterial meningitis are still poorly understood.
Teichoic and lipoteichoic acids of the cell wall and membrane contain phosphocholine. These molecules are major surface components of all pneumococci (8) but are largely masked by the capsule (9). The teichoic acids are the major component of C-polysaccharides, an autolytic degradation product of the cell wall. Complement-activating Ab specific for phosphocholine offer some protection against i.v. and i.p. challenge with encapsulated pneumococci (10, 11). Teichoic acids in the cell wall of encapsulated pneumococci allow the bacteria to interact with host cells via the cell membrane components asialo-GM1, fucosyl-asialo-GM1, and asialo-GM2 gangliosides (GLS) (12, 13) through a terminal or internal N-acetylgalactosamine-(1-4)-β-galactose (GalNAcβ1-4Gal) sequence in the glycosphingolipids (14). The asialo-GM1 binding to C-polysaccharides depends on phosphocholine (12).
A role for C-polysaccharide-GalNAcβ1-4Gal interactions in attachment is consistent with expression of asialo-GM1 in the lung (15), with in vitro studies inhibiting pneumococcal attachment to tracheal epithelium with asialo-GM1 (16), and with elevated expression of C-polysaccharides (decreased expression of capsule) in transparent variants of Streptococcus pneumoniae (17). Transparent variants dominate in carriage and display increased attachment to epithelial cells when compared with opaque variants (17, 18). Human colostrum and milk inhibit pneumococcal colonization (19), and each contains neolactotetraose and lactotetraose (16, 19). Both sugars contain the GlcNAcβ1-3Galβ disaccharide, which can inhibit attachment of pneumococci to human pharyngeal cells (20) and may contribute to the ability of breast-feeding to reduce pneumococcal colonization and otitis media (21, †).
The B subunits of cholera toxin (CT-B) and Escherichia coli heat-labile toxin I bind to GLS and have been used for tracing of neuronal pathways (22, 23). We have shown that nasal delivery of CT or CT-B targets the olfactory nerves and epithelium (ON/E) and, through retrograde axonal transport, reaches the olfactory bulbs (OB). GM1-dependent, retrograde axonal transport allows CT and CT-B to bypass the blood-brain barrier when given nasally (24).
Because pneumococcal teichoic acids also bind GLS, we hypothesized that GLS not only may be important for carriage but also may permit pneumococci to enter the brain through transport along olfactory neurons, which constitute 47% of the nasal surface of mice, thus bypassing the blood-brain barrier. This study provides direct evidence that nasal colonization by pneumococci results in bacterial entry into the brain in the absence of bacteremia. Pneumococcal colonization of the nasal tract and brain was inhibited by GLS, indicating that nasal challenge with pneumococci results in CNS infection through a GLS-mediated process. The failure of these colonizing bacteria to proceed to cause extensive brain infection makes it clear that the brain has highly effective host immunity against this agent.
Materials and Methods
Pneumococcal Strains. Our studies used two encapsulated strains of S. pneumoniae: EF3030, serotype 19F, and TIGR4 strain, serotype 4, and the avirulent, noncapsular strain R36A, derived from the parent strain D39, serotype 2 (25). The EF3030 strain was chosen because it readily colonizes the respiratory tract in the absence of bacteremia (26) and is incapable of sustained bacteremia after i.v. inoculation. The TIGR4 strain was more virulent but, with a modest nasal inoculum, colonizes without bacteremia.
Mice. The CBA/CAHN/xid (xid) mouse strain was obtained from The Jackson Laboratory. The mutation in the Bruton's tyrosine kinase gene of these mice results in an inability to respond to thymus-independent type II antigens (27, 28) but permits relatively normal T cell-dependent immune responses. These mice fail to respond to capsular polysaccharides and are reproducibly susceptible to pneumococcal infection. The x-linked immunodeficient (xid) mice were maintained under pathogen-free conditions and were used at 7-12 weeks of age. Tissue Collection. The blood was collected into a heparinized capillary tube from the retroorbital plexus. Mice were disinfected with 70% ethanol before collection of nasal wash (NW), kidney, spleen, and lungs. To prevent blood contamination of the NW, an incision was made into the trachea and a 2.0-cm-long Tygon tube with an outer diameter of 0.075 cm (Cole-Parmer) was inserted into the nasopharynx while attached to a syringe filled with Ringer's solution. Fluid from the syringe was expelled through the nose, and three drops were collected.
The nasopharyngeal-associated lymphoreticular tissue (NALT), ON/E, OB, and the remainder of the brain were obtained as described (24, 29). The trigeminal ganglia were carefully excised from the brain with a dissection microscope. The ON/E, OB, trigeminal ganglia, NALT, and cervical lymph nodes (CLNs) were each homogenized in 0.5 ml of Ringer's solution, and the brain and kidney were each homogenized in 1.0 ml of Ringer's solution.
Quantity of Pneumococci in Tissue Minces/Blood/External Excretions. Eight serial, 3-fold dilutions were made of tissues and body fluids in sterile Ringer's solution and plated on blood agar plates containing 4 μg/ml gentamicin sulfate. The cfu were enumerated 24 h after plating and incubation in a candle jar. The results were expressed as cfu per organ, per NW or per ml of blood.
GLS Preincubation of S. pneumoniae Strain EF3030. To block GLS binding sites, 3 × 107 cfu of S. pneumoniae strain EF3030 were incubated for 30 min on ice with either 20 μg of asialo-GM1 from human brain or 125 μg of mixed GLS (18% GM1, 55% GD1a, 15% GD1b, 10% GT1b, and 2% other GLS) from bovine brain (Calbiochem-Novabiochem). GLS were dissolved in PBS and extensively mixed a day before use. The amphiphilic GLS formed micelles in PBS, allowing interaction of pneumococci with the carbohydrate moiety. After incubation, 5 μl per nare was applied nasally to xid mice without further washing. Tissues were analyzed for cfu 4 days later.
Detection of S. pneumoniae Pneumolysin Gene by PCR. To detect S. pneumoniae by PCR, tissues were lysed in 1% SDS with 0.1% deoxycholic acid by freeze-thawing, and incubated at 37°C for 1 h. Proteins were removed by using the cetyltrimethylammoniumbromide/NaCl precipitation method (30). Ten micrograms of DNA was used for PCR amplification. The pneumolysin(ply)-specific primers Ply1, 5′-AT T TCTGTA ACAGCTACCA ACGA-3′, and Ply2, 5′-GA AT TCCCTGTCT T TTCAAAGTC-3′, were added to the PCR mixture to amplify a 400-bp fragment. The PCR involved a 5-min denaturation step at 94°C followed by the amplification cycle: 94°C (1 min), 55°C (1 min), and 72°C (1 min) for 30 cycles. Images of the ethidium bromide-stained PCR fragments were collected on an Alpha-Imager IS-3400 (Alpha Innotech, San Leandro, CA).
Immunofluorescent Staining of OB with PspA-Specific Abs. Mice were nasally challenged with 5 × 105 cfu of the TIGR4 strain. The OB were fixed in 10% buffered formalin. Four-millimeter paraffin sections (24) were stained for PspA family 2 Ab (1:100) by incubating them for 4 h at room temperature in a humidified chamber. Slides were washed in PBS, stained with biotinylated goat F(ab′)2 anti-rabbit IgG (1:200, Southern Biotechnology Associates), washed, and stained with streptavidin-FITC (1:100, BD-Pharmingen). Fluorescent images were collected with a Nikon microscope using a DEI-750 CE digital color video camera (Optronics, Goleta, CA) and processed with SCION IMAGE software (Scion, Frederick, MD).
Statistics. The data are expressed as the mean ± 1 SE, and the results were compared by statistical analysis to determine significant differences in cfu by using the unpaired Mann-Whitney test or the Student t test.
Results
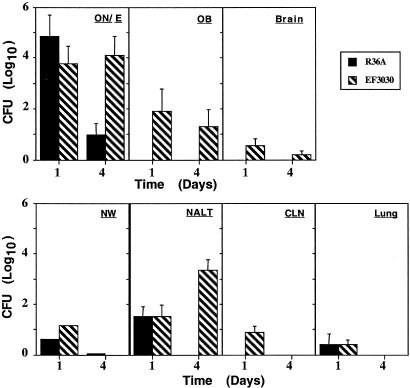
The Role of the Pneumococcal Capsule in Nasal Colonization and CNS Invasion. To examine the uptake of pneumococci through primary sensory olfactory neurons, the ability of EF3030 and a nonencapsulated strain R36A to colonize the nasal tract and enter the CNS were measured at days 1 and 4 (Fig. 1). Although high cfu for both strains were observed in the ON/E on day 1, the R36A were largely absent by day 4 from the ON/E and all other tissues, consistent with earlier results indicating that some capsule is required for prolonged colonization (31). EF3030 showed a clear presence in the OB and brain on both days and were present in high numbers in the NW and NALT on day 4. These findings were consistent with axonal transport of EF3030 pneumococci into the OB and brain after nasal challenge.
Fig. 1.
Nasal delivery of 3 × 106 cfu of either the nonencapsulated R36A strain or the virulent EF3030 strain of S. pneumoniae to xid mice. The neuronal tissues (ON/E, OB, and brain) and the lymphoid tissues (NALT, CLN, and lungs) were collected, minced, and analyzed for the presence of live pneumococci at 1 and 4 days after nasal challenge. Indicated is the mean of log10 cfu ± 1 SE. The 0 value on the y-axis represents the absence of detectable cfu. Indicated are the mean cfu ± SE of five mice per group; data are representative of three different experiments.
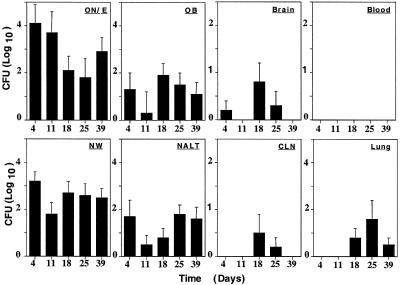
Kinetics of Nasal Colonization and CNS Invasion. EF3030 was maintained in the ON/E, OB, NWs, and NALT at all time points over the 39 days of observation (Fig. 2). Much lower numbers of cfu were seen in the brain and CLN, and those cfu present were generally seen at 18 and 25 days. Interestingly, the lungs did not exhibit pneumococci except at day 1 (Fig. 1) and at days 18, 25, and 39 (Fig. 2). It is highly unlikely that bacteremia contributed to the neuronal tissue distribution because no cfu were detected in the bloodstream of mice during any of the experiments performed with strain EF3030 at the nasal dose used (Fig. 2). Blood was monitored for bacteremia at 1, 3, 6, 12, and 24 h after nasal application and every subsequent day for 1 week. No bacteria were detected in the blood.
Fig. 2.
The kinetics of organ distribution of S. pneumoniae strain EF3030 cfu after nasal challenge. The ON/E, OB, brain, blood, NW, NALT, CLN, and lung tissues were collected on days 4, 11, 18, 25, and 39 and were analyzed for the presence of S. pneumoniae. An aliquot of 3 × 106 cfu of S. pneumoniae resulted in the colonization of the nasal tract and a subsequent infection of the OB. The 0 value on the y-axis represents the absence of detectable cfu. Indicated are the mean cfu ± SE of three separate experiments. Each time point represents 10 mice with the exception of day 39, which represents 5 mice.
S. pneumoniae Infection of Trigeminal Ganglia. The trigeminal neurons innervate the nasopharynx, and thus S. pneumoniae would be expected in the trigeminal ganglia after infection of the nasal mucosa. To test this hypothesis, various tissues and blood were isolated 4 days after inoculation and analyzed for the presence of EF3030 in new experiments. The EF3030 strain was detected in ON/E and OB and in trigeminal ganglia (Table 1). This finding further supported our hypothesis that asialo-GM1 and possibly other GLS function as receptors for neuronal targeting by S. pneumoniae.
Table 1. The distribution of S. pneumoniae strain EF3030 in various tissues after nasal delivery.
| Tissue | Mean cfu (log10) | SE |
|---|---|---|
| Brain | 0 | |
| OB | 1.38 | 0.61 |
| ON/E | 4.93 | 0.42 |
| Blood | 0 | |
| Trigeminal ganglia | 2.08 | (pooled) |
Tissues were isolated on day 4 after nasal application of 1 × 107 cfu of strain EF3030. Blood (50 μl), ON/E, OB, and brain tissue minces were diluted and then plated on blood agar. The trigeminal ganglia were pooled, homogenized, and then plated on this medium. Data are the mean pneumococcal cfu ± SE of five mice and are representative of three separate experiments. In the brain and blood, no pneumococci were detected.
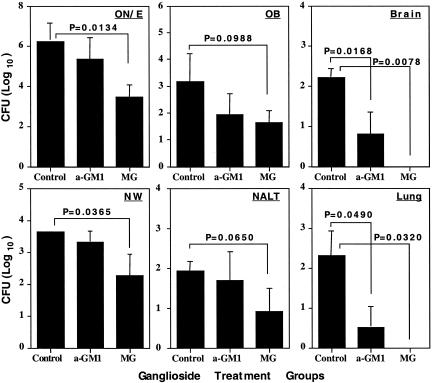
Gangliosides Inhibit Pneumococcal Colonization. To test the hypothesis that binding to GLS by S. pneumoniae may be important for invading the CNS, the EF3030 strain was incubated with asialo-GM1 or mixed GLS micelles in PBS before nasal application. The GLS mixture displayed the strongest inhibitory effect and reduced cfu in NW by 10-fold (P = 0.0365) when assessed 4 days after nasal application. The largest decline in cfu as a result of mixed GLS preincubation was seen in the ON/E (617-fold decline; P = 0.0134). Just as striking were the differences in the lungs (P = 0.0320; Fig. 3B) and CNS tissue (P = 0.0078; Fig. 3A), where an average of 204 and 166 cfu were present in the controls, whereas pneumococci were undetectable (detection limit = 3 cfu) when incubated with GLS. The asialo-GM1 preincubation was less efficient than mixed GLS but still reduced colonization 25- and 63-fold in CNS (Fig. 3A) and lungs (Fig. 3B), respectively. The lungs presumably were infected by inhaled pneumococci, and their attachment to asialo-GM1, relatively abundantly present in lungs, was apparently inhibited by GLS. This result indicates that GLS play a role in the initial attachment to epithelial cells. GLS treatments did not change pneumococcal viability. No pneumococci were detected in the blood during these experiments. Thus, GLS seem to constitute an important target for pneumococcal attachment to neuro-epithelium of the nasal tract and infection of lungs and CNS.
Fig. 3.
The distribution of S. pneumoniae strain EF3030 after preincubation with GLS. Aliquots (3 × 107 cfu) of S. pneumoniae were incubated for 30 min with 20 μg of asialo-GM1 (a-GM1) or 125 μg of mixed GLS (MG) before nasal application. The ON/E, OB, and brain and the NW, NALT, and lungs were collected 4 days later and analyzed for numbers of S. pneumoniae. The 0 value on the y-axis represents the absence of detectable cfu. Indicated are the mean ± 1 SE of five mice, and the P values were obtained after statistical analysis. The data are representative of two separate experiments.
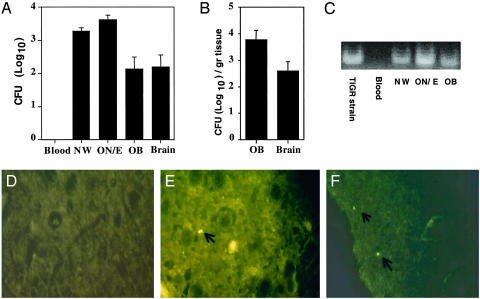
Detection of S. pneumoniae Accumulation in the OB After Nasal Challenge. The numbers of EF3030 in OB were generally too low to make visualization of bacteria by microscopy feasible. To visualize S. pneumoniae in the OB after nasal application, a more virulent strain, TIGR4, was used. Blood samples were tested from representative mice at 1, 3, 6, 12, or 24 h after challenge and on every subsequent day. No bacteremia was observed. The mice were killed 1 week after challenge, and tissues were analyzed for cfu (Fig. 4 A and B). A dose of only 5 × 105 TIGR4 cfu resulted in ≈300 cfu in the OB (Fig. 3). The pneumococci were visualized by staining with PspA-specific Ab in the OB (Fig. 4 D-F). Pneumococci were detected in the OB, i.e., the glomerular layer (Fig. 4F) and the external plexiform layer (Fig. 4E) of challenged mice. Pneumococci were absent in the OB of control mice (Fig. 4D).
Fig. 4.
Detection of the TIGR4 strain of S. pneumoniae in the OB after nasal challenge. An aliquot of 5 × 105 cfu was given nasally, and the blood, NW, ON/E, OB, and brain tissues were analyzed for colonization 1 week after challenge (A and B). These tissues (10 μg of DNA) were also analyzed for the presence of the pneumolysin gene by PCR (C). In addition, the S. pneumoniae were visualized by immunofluorescence with PspA-specific Abs in the OB of control (D) or S. pneumoniae-challenged mice (E and F). Indicated are the mean ± 1 SE. The data are representative of three separate experiments.
The TIGR4 strain was also detected by PCR amplification of the pneumolysin gene from the NWs, ON/E, and OB 6 days after nasal administration (Fig. 4C). No PCR-detectable pneumococci were present in the bloodstream taken at this interval, or in any samples from noninfected mice.
Discussion
We show that nasal colonization with S. pneumoniae results in infection of the OB, the CNS, and trigeminal ganglia in the absence of detectable bacteremia. Infection of CNS in the absence of bacteremia contradicts the assumption that pneumococci induce meningitis only by invasion via the blood. Our results support a direct role of nasal carriage with retrograde axonal transport along olfactory neurons into the CNS. Our results do not suggest that bacteremia is not a major source of meningitis in septic or bacteremic individuals. Nevertheless, our results do show that pneumococci can enter the CNS directly from the nasal cavity and may cause infection by this route in some patients. The use of strain EF3030, which fails to survive in the bloodstream, permitted us to show that pneumococci in the OB and CNS were due to axonal transport rather than infection from the blood. These findings indicate that vaccines that prevent bacteremia, but not carriage, would fail to control any invasion through this route.
A second major observation of this study is that host defense of the brain is active and effective because rapidly progressing brain disease was not observed even after pneumococci entered the OB and brain. Moreover, large numbers of mice have been used in carriage models over the years without the mice commonly giving symptoms of meningitis, paralysis, or death (32, 33).
Infection of the CNS and lungs was largely blocked by preincubation of pneumococci with GLS. We suspect that GLS on human cells are involved in the bacterial attachment at these sites, probably through interactions with the C-polysaccharides of the pneumococcus. Several previous studies (12, 14, 16, 32) have clearly shown that interactions between GLS and C-polysaccharides occur in vitro. Although our studies do not rule out a role for the platelet-activating factor receptor, they suggest that it may not be a primary event in the initial in vivo infections.
Our results with the R36A strain are consistent with the finding that expression of capsular polysaccharide is essential for nasal colonization (31). An 80% reduction of capsule production did not affect nasal colonization (31) and is consistent with the observation that transparent-phase variants, which have thin capsules, are superior in colonizing the nose whereas opaque-phase pneumococci with thick capsules display enhanced virulence in systemic infections (17, 18). C-polysaccharides are expressed at 2-fold higher levels in the transparent-than in the opaque-phase bacteria (17, 18).
GLS are expressed on all neurons, and thus other sensory neurons could also allow pneumococcal infection. Indeed, studies in gerbils showed that CNS infection occurred after middle ear infection with S. pneumoniae (34). This finding is consistent with our data suggesting that GLS-mediated binding of S. pneumoniae causes meningoencephalitis. Preceding influenza infection enhanced subsequent pneumococcal pathogenicity. It was demonstrated that the influenza neuraminidase removed the sialic acids from the epithelium to expose pneumococcal receptors (35) and is consistent with asialo-GM1-mediated pneumococcal attachment (35-37).
In summary, these studies have revealed an in vivo pathway for pneumococci to enter the CNS. Furthermore, this pathway suggests the interaction of pneumococci with neuronal GLS. This interaction allows direct entry of pneumococci into the CNS by passage along olfactory nerves. This pneumococcal-GLS interaction allowed entry into cells of the nasal tract and resulted in a chronic infection of the ON/E and OB, a condition strongly resembling nasal carriage in humans.
Acknowledgments
These studies were supported by National Institutes of Health Grants DC 04976, P30 DK 54781, AI 43197, AI 21548, and AI 18958, the Carsten Cole Buckley Memorial Fund, and National Institute of Allergy and Infectious Diseases Contract NO1 AI 65299.
Abbreviations: cfu, colony forming unit; CLN, cervical lymph node; CT, cholera toxin; GLS, ganglioside; NALT, nasopharyngeal-associated lymphoreticular tissue; NW, nasal wash; OB, olfactory bulb; ON/E, olfactory nerves and epithelium; xid, x-linked immunodeficient.
Footnotes
Deele, D. W., Klein, J. O., Rosner, B. & The Greater Boston Collaborative Otitis Media Program (1980) Pediatr. Res. 14, 494 (abstr.).
References
- 1.Quagliarello, V. & Scheld, W. M. (1992) N. Engl. J. Med. 327, 864-872. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (1997) Morbid. Mortal. Wkly. Rep. 46, RR-8.
- 3.Butler, J. C. & Schuchat, A. (1999) Drugs Aging 15, 11-19. [DOI] [PubMed] [Google Scholar]
- 4.Fedson, D. S., Anthony, J. & Scott, G. (1999) Vaccine 17, Suppl. 1, S11-S18. [DOI] [PubMed] [Google Scholar]
- 5.Musher, D. M. (1992) Clin. Infect. Dis. 14, 801-809. [DOI] [PubMed] [Google Scholar]
- 6.Boulnois, G. J. (1992) J. Gen. Microbiol. 138, 249-259. [DOI] [PubMed] [Google Scholar]
- 7.Stenfors, L.-E. & Raisanen, S. (1992) J. Infect. Dis. 165, 1148-1150. [DOI] [PubMed] [Google Scholar]
- 8.Fischer, W., Behr, T., Hartmann, R., Peter-Katalinic, P. & Egge, H. (1993) Biochemistry 215, 851-857. [DOI] [PubMed] [Google Scholar]
- 9.Yother, J., Forman, C., Gray, B. M. & Briles, D. E. (1982) Infect. Immun. 36, 184-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Briles, D. E., Forman, C., Horowitz, J. C., Volanakis, J. E., Benjamin, W. H., Jr., McDaniel, L. S., Eldridge, J. & Brooks, J. (1989) Infect. Immun. 57, 1457-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Briles, D. E., Forman, C. & Crain, M. (1992) Infect. Immun. 60, 1957-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sundberg-Kovamees, M., Holme, T. & Sjorgen, A. (1996) Microb. Pathog. 21, 223-234. [DOI] [PubMed] [Google Scholar]
- 13.Sundberg-Kovamees, M., Holme, T. & Sjorgen, A. (1994) Microb. Pathog. 17, 63-68. [DOI] [PubMed] [Google Scholar]
- 14.Cundell, D. R., Weiser, J. N., Shen, J., Young, A. & Tuomanen, E. I. (1995) Infect. Immun. 63, 757-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krivan, H. C., Roberts, D. D. & Ginsburg, V. (1988) Proc. Natl. Acad. Sci. USA 85, 6157-6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tong, H. H., McIver, M. A., Fisher, L. M. & DeMaria, T. F. (1999) Microb. Pathog. 26, 111-119. [DOI] [PubMed] [Google Scholar]
- 17.Weiser, J. N., Austrian, R., Sreenivasan, P. K. & Masure, H. R. (1994) Infect. Immun. 62, 2582-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim, J. O. & Weiser, J. N. (1998) J. Infect. Dis. 177, 368-377. [DOI] [PubMed] [Google Scholar]
- 19.Kobata, A. (1977) in The Glycoconjugates, eds. Horowitz, M. I. & Pigman, W. (Academic, London), pp. 423-439.
- 20.Anderson, B., Dahmen, J., Frejd, T., Leffler, H., Magnusson, G., Noori, G. & Svanborg Eden, C. (1983) J. Exp. Med. 158, 559-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cunningham, A. S. (1979) J. Pediatr. 95, 685-689. [DOI] [PubMed] [Google Scholar]
- 22.Trojanowski, J. O. & Schmidt, M. L. (1984) Brain Res. 311, 366-369. [DOI] [PubMed] [Google Scholar]
- 23.Moriizumi, T., Tsukatani, T., Sakashita, H. & Miwa, T. (1994) Neuroscience 61, 733-738. [DOI] [PubMed] [Google Scholar]
- 24.van Ginkel, F. W., Jackson, R. J., Yuki, Y. & McGhee, J. R. (2000) J. Immunol. 165, 4778-4782. [DOI] [PubMed] [Google Scholar]
- 25.Avery, O. T., MacLeod, C. M. & McCarty, M. (1944) J. Exp. Med. 79, 137-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Briles, D. E., Crain, M. J., Gray, B. M., Forman, C. & Yother, J. (1992) Infect. Immun. 60, 111-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amsbaugh, D. F., Hansen, C. T., Prescott, B., Stashak, P. W., Barthold, D. R. & Baker, P. J. (1972) J. Exp. Med. 136, 931-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berning, A. K., Eicher, E. M., Paul, W. E. & Scher, I. (1980) J. Immunol. 124, 1875-1877. [PubMed] [Google Scholar]
- 29.Wu, H.-Y., Nguyen, H. H. & Russell, M. W. (1997) Scand. J. Immunol. 46, 506-513. [DOI] [PubMed] [Google Scholar]
- 30.Ausubel, F. M., Brent, R., Kingston, R. E., Moore, D. D., Seidman, J. G., Smith, J. A. & Struhl, K., eds. (1987) Current Protocols in Molecular Biology (Greene & Wiley, New York), Section 2.4.4.
- 31.Magee, A. & Yother, J. (2001) Infect. Immun. 69, 3755-3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu, H.-Y., Virolainen, A., Mathews, B., King, J., Russell, M. W. & Briles, D. E. (1997) Microb. Pathogen. 27, 127-137. [DOI] [PubMed] [Google Scholar]
- 33.Briles, D. E., Ades, E., Paton, J. C., Sampson, J. S., Carlone, G. M., Huebner, R. C., Virolainen, A., Swiatlo, E. & Hollingshead, S. K. (2000) Infect. Immun. 68, 796-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muffat-Joly, M., Barry, B., Henin, D., Fay, M., Gehanno, P. & Pocidalo, J. J. (1994) Arch. Otolaryngol. Head Neck Surg. 120, 925-930. [DOI] [PubMed] [Google Scholar]
- 35.McCullers, J. A. & Bartmess, K. C. (2003) J. Infect. Dis. 187, 1000-1009. [DOI] [PubMed] [Google Scholar]
- 36.McCullers, J. A. & Rehg, J. E. (2002) J. Infect. Dis. 186, 341-350. [DOI] [PubMed] [Google Scholar]
- 37.Hirano, T., Kurono, Y., Ichimiya, I., Suzuki, M. & Mogi, G. (1999) Otolaryngol. Head Neck Surg. 121, 616-621. [DOI] [PubMed] [Google Scholar]