Abstract
AMP-activated protein kinase (AMPK) protects various tissues and cells from ischemic insults and is activated by many stimuli including mechanical stretch. Therefore, this study investigated if the activation of AMPK is involved in stretch-induced cardioprotection (SIC). Intraventricular balloon and aorto-caval shunt (ACS) were used to stretch rat hearts ex vivo and in vivo, respectively. Stretch preconditioning reduced myocardial infarct induced by ischemia-reperfusion (I/R) and improved post-ischemic functional recovery. Phosphorylation of AMPK and its downstream substrate, acetyl-CoA carboxylase (ACC) were increased by mechanical stretch and ACC phosphorylation was completely blocked by the AMPK inhibitor, Compound C. AMPK activator (AICAR) mimicked SIC. Gadolinium, a blocker of stretch-activated ion channels (SACs), inhibited the stretch-induced phosphorylation of AMPK and ACC, whereas diltiazem, a specific L-type calcium channel blocker, did not affect AMPK activation. Furthermore, SIC was abrogated by Compound C and gadolinium. The in vivo stretch induced by ACS increased AMPK activation and reduced myocardial infarct. These findings indicate that stretch preconditioning can induce the cardioprotection against I/R injury, and activation of AMPK plays an important role in SIC, which might be mediated by SACs.
Keywords: AMP-activated protein kinase, Stretch, Cardioprotection, Ischemia-reperfusion
INTRODUCTION
Ischemic heart disease is characterized by reduced blood supply to the cardiac muscle and is the primary cause of death associated with myocardial infarction usually occurring as a result of damage induced by cardiac ischemia-reperfusion (I/R).
Ischemic preconditioning (IPC) is a phenomenon by which cyclic episodes of brief I/R protect the myocardium against subsequent lethal ischemic injury. It is known to significantly reduce the infarct size, arrhythmia, and post-ischemic contractile dysfunction [1,2].
Numerous studies have shown that IPC can be mimicked by techniques such as pharmacological stimulation [3], heat shock preconditioning [4] and mechanical stretching of the heart [5], etc. Stretch preconditioning (SPC) as shown by many studies, renders the myocardium more resistant to a subsequent sustained ischemic insult. Gysembergh and his coworkers [6] have demonstrated in in vivo studies that myocardial stretch can protect canine and rabbit heart against to I/R injury. In addition, isolated hearts stretched by a transient increase in left ventricular end-diastolic pressure (LVEDP), resulted in a significant enhancement of post-ischemic functional recovery and decrease in infarct size [7,8]. However, the specific cellular signaling pathways of stretch-induced cardioprotection (SIC) remain unclear till date.
It has been reported that stretch has direct effects on the heart, including HR, contractility, gene transcription and protein synthesis. A large number of cellular signal transduction pathways can be activated by stretch, such as the JNK, a group of MAPK, PKC, and JAK/STAT pathway [9-11]. It has also been suggested that stretch can activate the AMP-activated kinase (AMPK) in muscle cells [12,13].
AMPK is known to be the central energy sensor maintaining the energy balance within the cells. It is activated by the phosphorylation at Thr172 in response to different types of energy consuming stress, including hypoxia/ischemia, stretch, glucose deprivation, and exercise [14-16]. In addition, AMPK can be activated in pressure overload-induced hypertrophic rat hearts [17].
AMPK has been reported to exist in many tissues, including liver, heart, brain, and skeletal muscles. In these tissues, AMPK activation has been shown to be related with glycolysis, glucose uptake, fatty acid oxidation, and insulin secretion [18]. AMPK is also reported to preserve myocardial energetics by promoting glucose uptake during I/R and might play a pivotal role in regulating whole-body energy metabolism [19,20]. Activation of AMPK can protect cardiomyocytes against post-ischemic cardiac dysfunction, apoptosis, and ischemic injury, while its deficiency is seen to significantly impair recovery of left ventricular contractile function during I/R [21,22].
Moreover, Gysembergh et al. [6] have indicated in their study that stretch and IPC-induced protection might share a common pathway in the heart. It has also been reported that both IPC and heat shock induced protection occurs via the AMPK activation in liver and heart [16,23,24]. Heart treated with AICAR, an activator of AMPK, affords protection against injury during sustained I/R [25,26]. In summary, these reports suggest that AMPK plays an important role in the process of cardioprotection. It can also be hypothesized that SPC can activate AMPK and protect the heart against I/R damage. To test this hypothesis, isolated rat hearts were subjected to stretch by increasing the left ventricular wall tension using balloon inflation or aorto-caval shunt (ACS) in the in vivo heart following which the mechanism of SIC was studied to understand the role of stretch-activated ion channels (SACs) and AMPK.
METHODS
Ex vivo stretch model
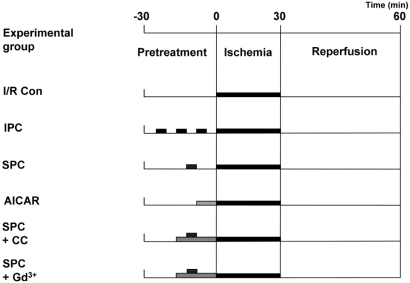
The experimental protocol was approved by Chungbuk National University Medical School Research Institutional Animal Care and Use Committee. Male Sprague-Dawley (SD) rats (7 weeks old, 200~220 g) were anesthetized by the intravenous administration of 30 mg/kg of pentobarbital sodium. Hearts were excised, immediately connected to an aortic cannule, and then perfused at a constant pressure in the nonrecirculating Langendorff apparatus with Krebs-Henseleit buffer (NaCl 118 mM, KCl 4.7 mM, CaCl2 1.25 mM, MgSO2 1.2 mM, glucose 10 mM, NaHCO3 25 mM, KH2PO4 1.2 mM). The buffer solution was saturated with mixture of 95% O2-/ 5% CO2 at 37℃, and the perfusion pressure was maintained at 80 cmH2O. To stretch the left ventricle of the ex vivo rat heart, a plastic catheter with a small balloon tip was inserted into the left ventricle through the mitral valve. The balloon was swollen until the end diastolic pressure reached 3 mmHg. After the cardiac function was stabilized, the left ventricle was subjected to stretch for 5 minutes by expanding the inserted balloon to raise the LVEDP to 40 mmHg. Global ischemia was applied by stopping the perfusion for 30 minutes and then the heart was reperfused for 60 minutes (Fig. 1). Before the ischemia was sustained, the hearts were assigned to a 30 minutes "treatment" period consisting of 1) no pretreatment (I/R control group), 2) three cycles of a 5 minutes period of ischemia (IPC group), 3) 5 minutes of stretch (SPC group), 4) 10 minutes of 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR, 0.5 mM) treatment (AMPK activator group), 5) Compound C (10 µM)+5 minutes of stretch (AMPK inhibitor group), and 6) Gadolinium (Gd3+, 10 µM)+5 minutes of stretch (stretch-activated ion channels inhibitor group).
Fig. 1.
Protocols for each experimental group, showing the components and the time course of various treatments. All the hearts underwent 30 minutes sustained ischemia followed by 1 hour reperfusion. Prior to the sustained ischemia, the hearts were assigned to each experimental group with specific treatment; no treatment, three cycles of a 5 minutes ischemia, 5 minutes stretch, 10 minutes AICAR treatment, CC with 5 minutes stretch, and Gd3+ with 5 minutes stretch. I/R Con, ischemia-reperfusion control; SPC, stretch preconditioning; IPC, ischemia preconditioning; AICAR, 5-Aminoimidazole-4-carboxamide-1-β-D-ribofuranoside; CC, compound C; Gd3+, gadolinium.
In vivo stretch model
To induce mechanical stress in the rat myocardium in vivo, aorto-caval shunt (ACS) surgery was performed as described previously [27]. Rats were anesthetized with ketamine (80 mg/kg, i.p.) and xylazine (5 mg/kg, i.p.), and the inferior vena cava and the abdominal aorta were exposed through a midline abdominal incision under sterile conditions. The ACS was produced using an 18-gauge needle at a point 5 mm caudal to the left renal vein. Creation of a successful shunt was visualized by the pulsatile flow of oxygenated blood into the vena cava from the abdominal aorta. Sham operations were performed in control rats in an identical manner, except for needle insertion. After the abdominal incision was closed, the animals were allowed to recover. The central venous pressure (CVP) and the LVEDP were monitored using catheters inserted into the superior vena cava and the left ventricular lumen, as has previously been reported [28]. They were recorded using a computer data acquisition system (model ML870 PowerLab 8/30, AD Instruments) after blood pressure was stabilized for 10 minutes.
Evaluation of infarct size
Infarct size was measured as described previously [29]. The hearts were sliced in 2 mm thickness perpendicularly to the long axis, and incubated in a 1% solution of 2,3,5-triphenyltetrazolium chloride in phosphate buffer for 10 minutes at 37℃. Each slice area was integrated to calculate total heart volume and each infarct area was integrated to derive total infarct size. Finally, normalized percent infarct size was derived by dividing the calculated total infarct size with the total heart volume.
Measurement of functional recovery
The left ventricular pressure was monitored as described previously [29]. The functional recovery of the heart was evaluated from the functional index which was calculated as the product of heart rate (HR) by beats per minute and left ventricular developed pressure (systolic minus end-diastolic pressure, in mmHg). Coronary blood flow (CBF) was measured by collection of the coronary effluent dripping from the heart.
Immunoblot analyses for AMPK and ACC
Myocardial tissue was homogenized in ice-cold buffer (Tris 50 mM pH 7.4, EDTA 1 mM, DTT 0.5 mM, PMSF 0.4 mM, sodium vanadate 1 mM, sodium orthovanadate 1 mM, sodium fluoride 5 mM) containing a protease inhibitor cocktails (4-(2-Aminoethyl)benzenesulfonyl fluoride hydrochloride, Bestatin hydrochloride, N-(trans-Epoxysuccinyl)-L-leucine 4-guanidinobutylamide, Leupeptin hemisulfate salt, Pepstatin A, 1,10-Phenanthroline). Homogenates were centrifuged at 10,000 g for 15 minutes at 4℃, and the supernatants were used for immunoblot analysis. Twenty micrograms of protein sample was electrophoresed on 12% SDS-polyacrylamide gels under reducing conditions. Proteins on the gels were then transferred to Immobilon-PVDF membranes (Millipore) and incubated with 5% skim milk in Tris-buffered saline for 1 hour, to block nonspecific binding. The membranes were then incubated overnight at 4℃ with a mouse Monoclonal anti-phospho-AMPK and anti-phospho-ACC (Cell Signaling Technology, 1:1,000), or anti-AMPK and anti-ACC (Cell Signaling Technology, 1:1,000) and then further incubated with horseradish peroxidase-conjugated secondary antibodies (1:1,000) for 2 hours. Immunoreactive protein bands were visualized using the enhanced chemiluminescence plus detection system as described previously [27].
Statistical analysis
The data were analyzed with SPSS 12.0 statistical package (SPSS Inc., Chicago, IL, USA) and presented as mean±SEM. The significance of the differences among the means of the experimental groups were determined with one-way analysis of variance (ANOVA) followed by Tukey's HSD test. The level of significance was set at a value of p<0.05.
RESULTS
Protective effects of stretch in the ex vivo heart
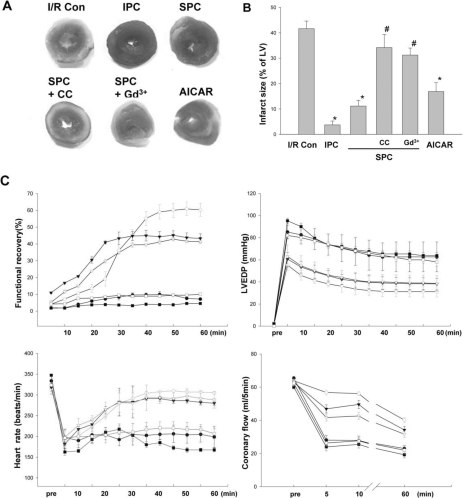
Protective effects of stretch against I/R in the ex vivo hearts were examined by the changes of normalized infarct size and the functional recovery of hearts after I/R (Fig. 2). I/R yielded infarct size to 41.6±3.2% in control hearts. Infarct size was significantly reduced by IPC and SPC to 4.4±0.6% and 8.6±3.5% respectively. When control hearts were treated with Compound C or Gd3+, infarct size did not show any significant change, when compared to the control. However, treatment with Compound C or Gd3+ significantly abolished the infarct-limiting effect of stretch preconditioning (37.2±1.3% and 36.9±1.4% respectively). AICAR alone was effective in reduce infarct size to 15.1±2.6% (Fig. 2A, B). To assess the recovery of heart after ischemia, 4 parameters of cardiac function were evaluated; functional recovery, LVEDP, HR and CBF. Functional recovery induced by SPC was comparable to that by IPC. The heart treated with IPC and SPC showed significantly better recovery of post-ischemic cardiac function (60.38±8.16% and 43.27±4.14%, respectively) than the control group (7.13±2.03%, Fig. 2C). The magnitude and kinetics of functional recovery were not significantly different between IPC and SPC group. AICAR alone also produced similar functional recovery to that produced by SPC. In spite of SPC, Compound C and Gd3+ were able to reduced the functional recovery to the comparable to I/R. A similar pattern of changes were also observed in LVEDP, HR and CBF during reperfusion period. These results indicate that SPC is as effective as IPC in protecting the hearts from the cardiac damage induced by I/R.
Fig. 2.
Effects of stretch preconditioning in ex vivo hearts. (A) After each experimental protocol, slices of heart were stained with TTC and (B) normalized infarct size was calculated and compared. There was no significant difference among IPC, SPC and AICAR group. All data are means±SEM of N≥6 in each experimental group. *p<0.05 compared with I/R Con group, #p<0.05 compared with SPC group. I/R Con, ischemia-reperfusion control; SPC, stretch preconditioning; IPC, ischemia preconditioning; AICAR, 5-Aminoimidazole-4-carboxamide-1-β-D-ribofuranoside; CC, compound C; Gd3+, gadolinium. (C) Functional recovery of ex vivo stretched hearts. During post-ischemic period, cardiac function was evaluated in terms of 4 parameters; cardiac functional recovery, Left ventricular end-diastolic pressure (LVEDP), heart rate, and coronary blood flow. The changes of 4 parameters were traced during 60 minutes post-ischemia-reperfusion period. There was no significant difference among IPC, SPC and AICAR group. All data are means±SEM of N≥6 in each experimental group. ●, ischemia-reperfusion control; ○, ischemic preconditioning; ▾, stretch preconditioning; ▿, AICAR; ■, stretch+Compound C; □, stretch+gadolinium.
Role of AMPK activation in stretch-induced cardioprotection
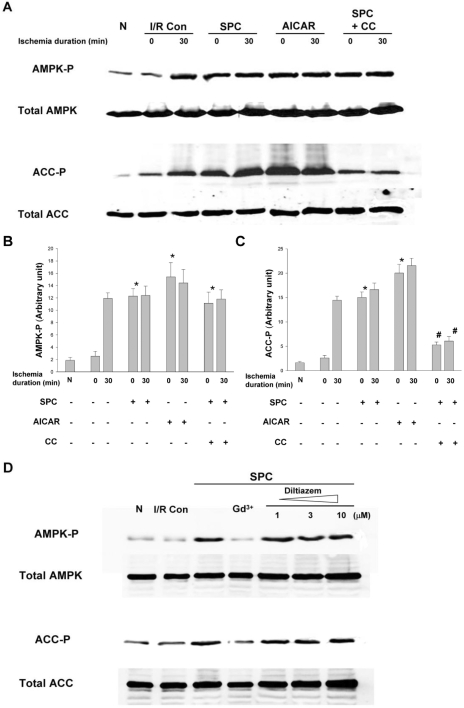
As AMPK is known to be one of the signaling molecules activated by stretch in the skeletal muscle cells, it is implicated that AMPK may also be activated in the heart by stretch. Therefore, phosphorylation of AMPK and its downstream target ACC was examined by Western blot analysis (Fig. 3). Baseline level of the phosphorylation of AMPK and ACC showed no difference between normal control (no ischemia, no stretch) and ischemia control groups. Thirty minutes of sustained ischemia elevated the levels of AMPK phosphorylation by 6-folds and resulted in a corresponding increase of phosphorylated ACC. SPC also produced a similar fold-increase of the phosphorylation of AMPK and ACC, while 30 minutes ischemia after SPC did not further increase the phosphorylation of either of the molecules. AICAR alone induced the phosphorylation of AMPK to the same level by SPC and this phosphoryaltion was not increased further despite 30 minutes of ischemia. The phosphorylation of ACC in AICAR group was also increased to the same level by SPC. Compound C could not abolish the increase in AMPK phosphorylation by stretch and 30 minute ischemia. However, Compound C treatment showed extended inhibitory effect on ACC to block the phosphorylation of ACC to one-third level of phosphorylation by SPC. These results suggested that AMPK activation and/or ACC phosphorylation might play a critical role in the cardioprotection induced by SPC.
Fig. 3.
The phosphorylation of AMPK and ACC in the ex vivo hearts All the hearts underwent 30 minutes sustained ischemia. (A) The phosphorylation of AMPK and ACC was analyzed with immunoblot and (B, C) semi-quantitative densitometry. The phosphorylation level before and after the ischemia with various pretreatment was compared. (D) Effects of gadolinium and diltiazem on AMPK and ACC phosphorylation induced by SPC. Hearts were treated with gadolinium (10 µM) or diltiazem (1, 3, and 10 µM) during SPC. Results are representative from 6 independent experiments and means±SEM. *p<0.05 compared with I/R Con group. #p<0.05 compared with SPC group. N, normal control; I/R Con, ischemia-reperfusion control; SPC, stretch preconditioning; CC, compound C; Gd3+, gadolinium.
Role of SACs in SIC by AMPK activation
In terms of myocardial stretch, stretch-activated ion channels (SACs) are thought to be related to the upstream pathway of stretch-mediated cellular responses and suggested as one of these upstream activators of AMPK [30]. Gadolinium (Gd3+, a blocker of SACs) completely inhibited the increase of AMPK phosphorylation after SPC (Fig. 3D). Consequently, the phosphorylation of ACC was also decreased upto the control baseline level. Although Gd3+ is widely used to inhibit SACs, it is also known to block L-type calcium channels [31]. To exclude the influence of L-type calcium channel in the stretch-induced AMPK activation, stretched heart was treated with a specific L-type calcium channel blocker-diltiazem. Diltiazem did not show any inhibitory effect on the stretch-induced phosphorylation of AMPK and ACC over any concentration used in this experiment (1, 3, and 10 µM). These results suggested that SACs might be required to activate AMPK in the hearts during SPC independent of L-type calcium channels.
Lactate concentration in perfusate of ex vivo stretched heart
In order to rule out the possibility of hypoxic stress on stretched myocardium caused by disturbed microcirculation induced by increased intramural pressure, we measured the lactate concentration by enzymatic assay (Lactate Reagent, Sigma Chemical). Time-matched lactate concentration did not show any difference between normal control and stretched hearts. However, 10 minutes ischemia resulted in more than 6-fold increase of lactate concentration compared to the 0 minute baseline level. These results suggest that mechanical stretch per se was not responsible for ischemic insult to the isolated hearts (Table 1).
Table 1.
Lactate concentration in the langendorff perfusate of ex vivo hearts and in the tissue of in vivo hearts
Each value represents the means±SEM for six to eight hearts.
Effect of SPC on AMPK activation in the in vivo hearts
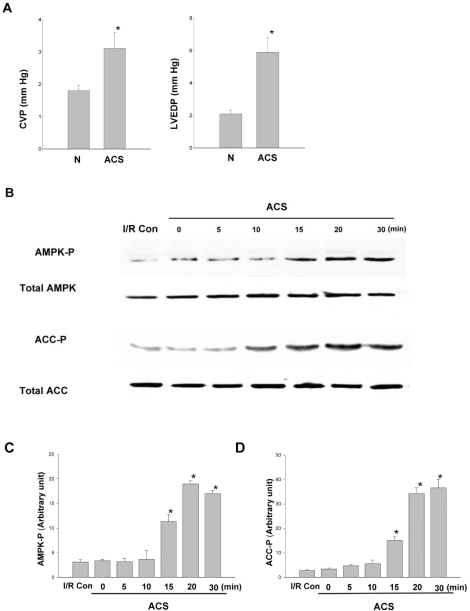
To develop in vivo model for cardiac stretch, aorto-caval shunt (ACS) was used in rats and its validity was examined in two aspects; hemodynamic changes and ischemic insult to the hearts. After ACS was fitted, LVEDP was measured to assess the magnitude of ACS to stretch the heart, while central venous pressure (CVP) was measured to confirm that the shunt was properly created and functioning. After ACS, CVP was increased by 2-folds and LVEDP was elevated by 3-folds (Fig. 4A). Measurements at different time points after surgery (n=6, 5 minutes to 1 hour) revealed no significantly change in the elevated CVP and LVEDP. These results confirmed that ACS was effective in adequately stretching the heart. To evaluate the tissue damage by ACS, tissue lactate concentrations were measured. For the baseline lactate (0 minute in control and stretch group), there was no significant difference between lactate control and stretch group. Lactate concentration did not change significantly during ACS. When the heart was subjected to 10 minutes ischemia, 7-folds increase in lactate concentration was observed (Table 1). Suggesting that ACS was an adequate in vivo model for cardiac stretch and the risk of cardiac tissue damage by ACS was minimal in this experiment. Till date, there are no published data on experimental models for AMPK activation during ACS in rats. Thus, it was necessary to optimize the time course of AMPK activation in this model. AMPK activation by ACS, as evidenced by phosphorylation is detected as early as by 15 minutes and reached a plateau by 20 minutes and maintained in plateau phase for 30 minutes of ACS. The phosphorylation of ACC showed similar time course (Fig. 4B). These results have shown that cardiac stretch by ACS increases the phosphorylation of AMPK and ACC. However, the time course of AMPK phosphorylation after ACS was different to that of isolated hearts. In ACS group, at least 15 minutes of cardiac stretch is required to achieve the phosphorylation of AMPK, while 5 minutes of stretch is sufficient to activate AMPK in isolated hearts.
Fig. 4.
Phosphorylation of AMPK and ACC after aorto-caval shunt. (A) Left ventricular wall stress caused by aorto-caval shunt (ACS). Central venous pressure (CVP) and left ventricular end-diastolic pressure (LVEDP) were monitored after ACS. *p<0.05, N=6. (B) After ACS was fitted to the rats, phosphorylation of AMPK and ACC was analyzed at various time points with immunoblot and (C, D) semi-quantitative densitometry. Results are representative from 6 independent experiments. *p<0.05 compared with I/R Con group. I/R C, ischemia-reperfusion control; ACS, aorto-caval shunt.
Protective effects of stretch in the in vivo hearts
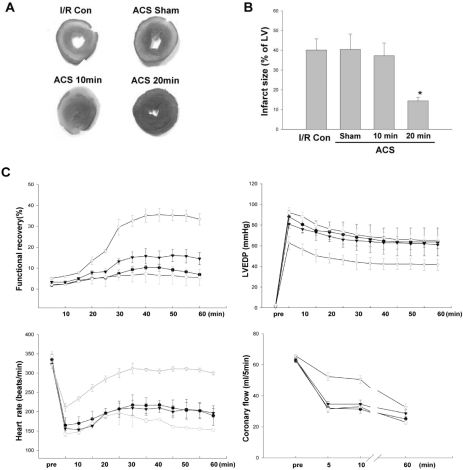
The hearts from control and sham-operated rats underwent I/R and infarct was developed upto 40.2±2.5% and 40.5±3.6% respectively. The hearts from ACS model with different duration of hemodynamic overload (10 minutes and 20 minutes) were subject to I/R in order to ascertain the role of AMPK activation in SIC. After applying ACS on the hearts for 10 minutes ACS, I/R yielded an infarct size of 40.6±1.6%, which is not significantly different from those achieved by the hearts from control and sham-operated rats. On the contrary, 20 minutes ACS exposed hearts were quite resistant to I/R injury and displayed an infarct size that was significantly reduced to 14.4±1.7% (Fig. 5A, B). AMPK phosphorylation level was the main difference between the group treated for 20 minutes ACS and other experimental groups, AMPK activation is likely to be a crucial step to guarantee the proper protection of hearts from I/R.
Fig. 5.
Effects of stretch preconditioning on the infarct size and the functional recovery of in vivo rat hearts. (A) Infarct size was measured using TTC staining after 30 minutes ischemia and 1 hour reperfusion and (B) normalized infarct size was calculated and compared. All data are means±SEM of N≥6 in each experimental group. *p<0.05 compared with I/R Con group. I/R Con, ischemia-reperfusion control; ACS Sham, sham-operated hearts; ACS, aorto-caval shunt. (C) Functional recovery of in vivo stretched rat hearts. Cardiac function was evaluated in terms of 4 parameters; cardiac functional recovery, Left ventricular end-diastolic pressure (LVEDP), heart rate, and coronary blood flow. The changes of 4 parameters were traced during 60 minutes post-ischemic reperfusion period. All data are means±SEM of N≥6 in each experimental group. *p<0.05 compared with control group. ●, ischemia-reperfusion control; ○, Sham; ▾, 10 minutes ACS; ▿, 20 minutes ACS; ACS, aorto-caval shunt.
The recovery of heart after ischemia was also evaluated as in ex vivo hearts experiment (Fig. 5C). The changes in four important parameters, such as cardiac functional recovery, LVEDP, HR and CBF, were evaluated. When cardiac functional recovery of each experimental group was compared on each time point, only the group from 20 minutes ACS showed a significant recovery during the 60 minute post-ischemic period. The cardiac functional recovery was seen during the first 30 minutes and was maintained at the maximal level during the next 30 minutes. The changes of LVEDP, HR and CBF showed similar time trend. The control group, sham-operated group and 10 minutes ACS group did not show any significant differences in the magnitude and time course of cardiac functional recovery, LVEDP, HR and CBF. These results also confirmed that the activation of AMPK is important for protecting hearts from the damages induced I/R and AMPK activation could be achieved in vivo by hemodynamic overload for an optimized duration.
DISCUSSION
In this study, a transient mechanical stretch of the left ventricle significantly reduced infarct size in rat heart. AMPK phosphorylation was enhanced by mechanical stretch in both ex vivo and in vivo (ACS) heart model and was suppressed by Gd3+. Mechanical stretch-induced cardioprotection was blocked by Gd3+ and Compound C. AICAR, an AMPK activator, instead of mechanical stretch protected heart from I/R injury. These results suggest that AMPK plays an important role in the SIC, and this role might be regulated by SACs.
In this study, there were no significant differences in baseline functional parameters, including HR, LVEDP, SP, and CBF, among all groups prior to imparting sustained ischemia (Fig. 2C and Fig. 5C). Therefore, SIC could not be ascribed to stretch induced-hemodynamic changes.
Mechanical stretch in the heart can be produced by increasing the left ventricular wall tension expressed as an increase in LVEDP. In this study, the left ventricular wall tension was increased by in vivo volume overload using an ACS rat heart model. Huang and his coworkers [32] demonstrated two episodes of brief pressure overload of the left ventricle by transverse aortic constriction significantly reduced myocardial infarct size. Although the left ventricular systolic pressure was raised 50% above the baseline and the protection of pressure overload was abolished by blocking stretch-activated ion channels, the study did not provide any evidence of increase in the length of left ventricular segment. In the present study, increase in LVEDP seen in the ACS rats (Fig. 4A) indicates that volume overload adequately increased diastolic wall stress and stretched left ventricular end diastolic length.
On the other hand, there is a possibility that brief volume overload might cause changes in myocardial blood flow and induce transient ischemic stress in the heart. In the present study, myocardial lactate content at baseline and after 30 minutes of mechanical stretch was negligible as compared to the contents after 10 minutes ischemia (Table 1). Overall, there was no evidence of myocardial ischemia during stretch pretreatment and these results suggested that the effect of endocardial ischemia did not seem to play an important role in cardioprotection of mechanical stretch.
Several non-ischemic stimuli can also precondition the myocardium, including pharmacological administration and myocardial stretching. In fact, it was shown that isolated hearts stretched by a transient increase in LVEDP, offered similar protection as classical ischemic preconditioning [7-10]. Clearly, acute volume overload significantly dilated the myocardium, and those hearts that underwent this stretching during the treatment period developed significantly smaller infarcts after a subsequent coronary artery occlusion for 30 minutes than the control hearts. In this study, the protective effect of stretch was confirmed in isolated heart and in vivo volume overload heart. In accordance with previous reports, SPC renders cardioprotection against I/R injury in rats as evidenced by improving cardiac functional recovery during the reperfusion period and resulting reduction in infarct size. Although the exact mechanism is still not known, the protection was abolished by administration of Gd3+, an inhibitor of SACs. Therefore, the protective effect of SPC might be related to activation of SACs.
Mechanical stretch can activate cellular signal transduction pathways, such as the JNK, a group of MAPK, PKC, and JAK/STAT pathway [9-11]. In addition, stretch is also known to activate the AMP-activated protein kinase (AMPK) in muscle cells [12,13]. AMPK is a serine-threonine kinase, which is emerging as an important regulator of diverse cellular pathways in regulating whole-body energy metabolism. AMPK is responsible for the switching on of the catabolic pathways, stimulating the oxidation of FFAs and enhancing glucose uptake and glycolysis that produce ATP while alleviating the ATP-consuming processes [18-20]. Recently, it has been reported that AMPK prevents ischemic injury in the hearts [21,22], and that IPC and heat shock induced protection occurs via AMPK activation in liver and heart [16,23,24]. Furthermore, AICAR affords the protection against I/R injury [25,26]. Therefore, AMPK has emerged as a new pathway that plays an important protective role in the process of cardioprotection. In the present study, SPC-activated AMPK precede the sustained ischemia relative to control (Fig. 3A). Activity of AMPK as measured by the phosphorylation of ACC was inhibited by Compound C, which was accompanied by abrogation of SIC (Fig. 2, 3). Moreover, myocardial stretch time-dependently increased AMPK phosphorylation in vivo. In accordance with the results of AMPK activity, 20 minutes of ACS showed a significant cardioprotection, while 10 minutes of ACS did not (Fig. 5). These results suggested that myocardial stretch can activate AMPK and that AMPK plays an important role in SIC.
AMPK activation could increase energy production during ischemia. However, it augments fatty acid oxidation during ischemia/reperfusion when fatty acid level is high. Studies using isolated rat hearts perfused with glucose and fatty acids suggested that AMPK-dependent acceleration of fatty acid oxidation occurs at the expense of glucose oxidation, and has the potential to be detrimental to the ischemic heart [33]. Since fatty acids were not included in buffer as an energy source in this study, it is possible that untoward effects of AMPK activation that would occur in hearts in situ may have been masked. The question of whether AMPK activation is an ally or enemy to the ischaemic heart remains to be completely elucidated. Long-term experiments in intact animals and in vivo ischaemia-reperfusion models are necessary to clearly define whether AMPK activation is an ally or enemy to the ischaemic heart.
SACs and their relationship to AMPK activation were also studied; Gysembergh et al. [6] indicated that stretch can provide cardioprotection by PKC which is regulated via SACs. Nishino et al. [24] have shown that PKC inhibitors abolished AMPK activation by ischemic episodes, suggesting that AMPK might be activated by IPC in a PKC-dependent manner. Another candidate is LKB1 which has been shown recently to be the upstream kinase phosphorylating AMPK [34]. LKB1 has a fundamental role in cell cycle regulation protein synthesis, suggesting involvement in a number of disorders including cardiac hypertrophy, cancer, and atherosclerosis. AMPK is activated by many stress conditions that deplete cellular ATP and the increased ratio of AMP to ATP seems to render AMPK a better substrate for LKB1. It has been reported that the ratio of AMP/ATP increased 5 times above control which was associated with increased AMPK activity in overload-induced hypertrophied hearts [17]. Together, it is plausible to assume that mechanical stress might cause a fast, transient depletion of intracellular ATP storage, which changes the AMP-to-ATP ratio so that AMPK serves as a better substrate for an activated AMPK kinase, namely LKB1. Further studies are necessary to examine whether SACs can mediate AMPK by PKC or LKB1 in process of SIC.
Despite the fact that SPC is as potent as IPC, we are unable to apply SPC for patient treatments per se. However, SPC may be an important mechanism protecting cardiomyocytes from necrosis and apoptosis at the time of sudden pressure overload, for example, during malignant hypertension, and at the times of acute volume overload as in acute aortic regurgitation. SPC is an obvious tool for analysis of myocyte protection mechanism and future research promises to elucidate the molecular mechanisms responsible for AMPK activation, novel downstream AMPK targets, and the therapeutic potential of targeting AMPK for the development of new therapeutic strategies for treatment of ischemic heart disease.
In conclusion, this study suggests that SPC can induce the cardioprotection against I/R injury, and SIC mediates this action by activation of AMPK with possible association with SACs.
ACKNOWLEDGEMENTS
This work was supported by the research grant of the Chungbuk National University in 2006 and Korea Research Foundation Grant (KRF-2003-002-E00031).
ABBREVIATIONS
- AMPK
AMP-activated protein kinase
- SIC
stretch-induced cardioprotection
- ACS
aorto-caval shunt
- I/R
ischemia-reperfusion
- SACs
stretch-activated ion channels
- IPC
ischemic preconditioning
- SPC
stretch preconditioning
References
- 1.Das DK, Maulik N. Cardiac genomic response following preconditioning stimulus. Cardiovasc Res. 2006;70:254–263. doi: 10.1016/j.cardiores.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 2.Shintani-Ishida K, Nakajima M, Uemura K, Yoshida K. Ischemic preconditioning protects cardiomyocytes against ischemic injury by inducing GRP78. Biochem Biophys Res Commun. 2006;345:1600–1605. doi: 10.1016/j.bbrc.2006.05.077. [DOI] [PubMed] [Google Scholar]
- 3.Kim YH, Kim CH, Kim GT, Kim IK, Park JW, Kim MS. Involvement of adenosine in cardioprotective effect of catecholamine preconditioning in ischemia-reperfused heart of rat. Korean J Physiol Pharmacol. 1998;2:753–761. [Google Scholar]
- 4.Jiao JD, Garg V, Yang B, Hu K. Novel functional role of heat shock protein 90 in ATP-sensitive K+ channel-mediated hypoxic preconditioning. Cardiovasc Res. 2008;77:126–133. doi: 10.1093/cvr/cvm028. [DOI] [PubMed] [Google Scholar]
- 5.Ovize M, Kloner RA, Przyklenk K. Stretch preconditions canine myocardium. Am J Physiol. 1994;266:H137–H146. doi: 10.1152/ajpheart.1994.266.1.H137. [DOI] [PubMed] [Google Scholar]
- 6.Gysembergh A, Margonari H, Loufoua J, Ovize A, André-Fouët X, Minaire Y, Ovize M. Stretch-induced protection shares a common mechanism with ischemic preconditioning in rabbit heart. Am J Physiol. 1998;274:H955–H964. doi: 10.1152/ajpheart.1998.274.3.H955. [DOI] [PubMed] [Google Scholar]
- 7.Mosca SM. Cardioprotective effects of stretch are mediated by activation of sarcolemmal, not mitochondrial, ATP-sensitive potassium channels. Am J Physiol Heart Circ Physiol. 2007;293:H1007–H1012. doi: 10.1152/ajpheart.00051.2007. [DOI] [PubMed] [Google Scholar]
- 8.Nakagawa C, Asayama J, Katamura M, Matoba S, Keira N, Kawahara A, Tsuruyama K, Tanaka T, Kobara M, Akashi K, Ohta B, Tatsumi T, Nakagawa M. Myocardial stretch induced by increased left ventricular diastolic pressure preconditions isolated perfused hearts of normotensive and spontaneously hypertensive rats. Basic Res Cardiol. 1997;92:410–416. doi: 10.1007/BF00796215. [DOI] [PubMed] [Google Scholar]
- 9.Force T, Michael A, Kilter H, Haq S. Stretch-activated pathways and left ventricular remodeling. J Card Fail. 2002;8:S351–S358. doi: 10.1054/jcaf.2002.129272. [DOI] [PubMed] [Google Scholar]
- 10.Pan J, Fukuda K, Saito M, Matsuzaki J, Kodama H, Sano M, Takahashi T, Kato T, Ogawa S. Mechanical stretch activates the JAK/STAT pathway in rat cardiomyocytes. Circ Res. 1999;84:1127–1236. doi: 10.1161/01.res.84.10.1127. [DOI] [PubMed] [Google Scholar]
- 11.Wamel AJ, Ruwhof C, Valk-Kokshoom LE, Schrier PI, Laarse A. The role of angiotensin II, endothelin-1, and transforming growth factor as autocrine/paracrine mediators of stretch-induced cardiomyocyte hypertrophy. Mol Cell Biochem. 2001;218:113–124. doi: 10.1023/a:1007279700705. [DOI] [PubMed] [Google Scholar]
- 12.Smith MA, Moylan JS, Reid MB. Mechanical signal transduction in glucose transport of murine extensor digitorum longus (EDL) muscle. FASEB J. 2007;21:94–98. [Google Scholar]
- 13.Rubin LJ, Magliola L, Feng X, Jones AW, Hale CC. Metabolic activation of AMP-kinase in vascular smooth muscle. J Appl Physiol. 2005;98:296–306. doi: 10.1152/japplphysiol.00075.2004. [DOI] [PubMed] [Google Scholar]
- 14.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: Ancient energy gauge provides clues to modern understanding of metabolism. Cell Metabolism. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Hardie DG, Salt IP, Hawley SA, Davies SP. AMP-activated protein kinase : an ultrasensitive system for monitoring cellular energy charge. Biochem J. 1999;338:717–722. [PMC free article] [PubMed] [Google Scholar]
- 16.Peralta C, Bartrons R, Serafin A, Blazquez C, Guzman M, Prats N, Xaus C, Cutillas B, Gelpí E, Roselló-Catafau J. Adenosine monophosphate activated protein kinase mediates the protective effects of ischemic preconditioning on hepatic ischemia-reperfusion injury in the rat. Hepatology. 2001;34:1164–1173. doi: 10.1053/jhep.2001.29197. [DOI] [PubMed] [Google Scholar]
- 17.Tian R, Musi N, D'Agostino J, Hirshman MF, Goodyear LJ. Increased adenosine monophosphate-activated protein kinase activity in rat hearts with pressure-overload hypertrophy. Circulation. 2001;104:1664–1669. doi: 10.1161/hc4001.097183. [DOI] [PubMed] [Google Scholar]
- 18.Carling D. The AMP-activated protein kinase cascade - a unifying system for energy control. Trends Biochem Sci. 2004;29:18–24. doi: 10.1016/j.tibs.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Bergeron R, Ren JM, Cadman KS, Moore IK, Perret P, Pypaert M, Young LH, Semenkovich CF, Shulman GI. Chronic activation of AMP kinase results in NRF-1 activation and mitochondrial biogenesis. Am J Physiol Endocrinol Metab. 2001;281:E1340–E1346. doi: 10.1152/ajpendo.2001.281.6.E1340. [DOI] [PubMed] [Google Scholar]
- 20.Kukidome D, Nishikawa T, Sonoda K, Imoto K, Fujisawa K, Yano M, Motoshima H, Taguchi T, Matsumura T, Araki E. Activation of AMP-activated protein kinase reduces hyperglycemia-induced mitochondrial reactive oxygen species production and promotes mitochondrial biogenesis in human umbilical vein endothelial cells. Diabetes. 2006;55:120–127. [PubMed] [Google Scholar]
- 21.Terai K, Hiramoto Y, Masaki M, Sugiyama S, Kuroda T, Hori M, Kawase I, Hirota H. AMP-activated protein kinase protects cardiomyocytes against hypoxic injury through attenuation of endoplasmic reticulum stress. Mol Cell Biol. 2005;25:9554–9575. doi: 10.1128/MCB.25.21.9554-9575.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russell I, Li J, Coven DL, Pypaert M, Zechner C, Palmeri M, Giordano FJ, Mu J, Birnbaum MJ, Young LH. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J Clin Invest. 2004;114:495–503. doi: 10.1172/JCI19297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sukhodub A, Jovanovi S, Qingyou DU, Budasl G, Clelland AK, Shen M, Sakamoto K, Tian R, Jovanović A. AMP-activated protein kinase mediates preconditioning in cardiomyocytes by regulating activity and trafficking of sarcolemmal ATP-sensitive K+ channels. J Cell Physiol. 2007;210:224–236. doi: 10.1002/jcp.20862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishino Y, Miura T, Mikia T, Sakamoto J, Nakamura Y, Ikeda Y, Kobayashi H, Shimamoto K. Ischemic preconditioning activates AMPK in a PKC-dependent manner and induces GLUT4 up-regulation in the late phase of cardioprotection. Cardiovasc Res. 2004;61:610–619. doi: 10.1016/j.cardiores.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 25.Walker J, Jijon HB, Diaz H, Salehi P, Churchill T, Madsen KL. 5-Aminoimidazole-4-carboxamide riboside (AICAR) enhances GLUT2-dependent jejunal glucose transport: a possible role for AMPK. Biochem J. 2005;385:485–491. doi: 10.1042/BJ20040694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gaskin FS, Kamada K, Yusof M, Korthuis RJ. 5-AMP-activated protein kinase activation prevents postischemic leukocyteendothelial cell adhesive interactions. Am J Physiol Heart Circ Physiol. 2007;292:H326–H332. doi: 10.1152/ajpheart.00744.2006. [DOI] [PubMed] [Google Scholar]
- 27.Kim CH, Cho YS, Chun YS, Park JW, Kim MS. Early expression of myocardial HIF-1alpha in response to mechanical stresses: regulation by stretch-activated channels and the phosphatidylinositol 3-kinase signaling pathway. Circ Res. 2002;90:E25–E33. doi: 10.1161/hh0202.104923. [DOI] [PubMed] [Google Scholar]
- 28.Nagaya N, Nishikimi T, Yoshihara F, Horio T, Morimoto A, Kangawa K. Cardiac adrenomedullin gene expression and peptide accumulation after acute myocardial infarction in rats. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1019–R1026. doi: 10.1152/ajpregu.2000.278.4.R1019. [DOI] [PubMed] [Google Scholar]
- 29.Kim CH, Choi H, Chun YS, Kim GT, Park JW, Kim MS. Hyperbaric oxygenation pretreatment induces catalase and reduces infarct size in ischemic rat myocardium. Pflugers Arch. 2001;442:519–525. doi: 10.1007/s004240100571. [DOI] [PubMed] [Google Scholar]
- 30.Sadoshima J, Takahashi T, Jahn L, Izumo S. Roles of mechano-sensitive ion channels, cytoskeleton, and contractile activity in stretch-induced immediate-early gene expression and hypertrophy of cardiac myocytes. Proc Natl Acad Sci USA. 1992;89:9905–9909. doi: 10.1073/pnas.89.20.9905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Biagi BA, Enyeart JJ. Gadolinium blocks low and high threshold calcium currents in pituitary cells. Am J Physiol. 1990;259:C515–C520. doi: 10.1152/ajpcell.1990.259.3.C515. [DOI] [PubMed] [Google Scholar]
- 32.Huang CH, Wang JS, Chiang SC, Wang YY, Lai ST, Weng ZC. Brief pressure overload of the left ventricle preconditions rabbit myocardium against infarction. Ann Thorac Surg. 2004;78:628–633. doi: 10.1016/j.athoracsur.2004.01.046. [DOI] [PubMed] [Google Scholar]
- 33.Kudo N, Gillespie JG, Kung L, Witters LA, Schulz R, Clanachan AS, Lopaschuk GD. Characterization of 5-AMP activated protein kinase activity in the heart and its role in inhibiting acetyl-CoA carboxylase during reperfusion following ischemia. Biochim Biophys Acta. 1996;1301:67–75. doi: 10.1016/0005-2760(96)00013-6. [DOI] [PubMed] [Google Scholar]
- 34.Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Makela TP, Alessi DR, Hardie DG. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2:28–32. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]