Abstract
Human tumors, including those of the hepatobiliary system, express a number of specific antigens that can be recognized by T cells, and may provide potential targets for cancer immunotherapy. Dendritic cells (DCs) are rare leucocytes that are uniquely potent in their ability to capture, process and present antigens to T cells. The ability to culture sufficient numbers of DCs from human bone marrow or blood progenitors has attracted a great deal of interest in their potential utilization in human tumor vaccination. CD34+ peripheral blood stem cells (PBSCs) were obtained from a patient with a hepatocellular carcinoma. The PBSCs were cultured in the X-VIVO 20 medium supplemented with the Flt-3 Ligand (FL), GM-CSF, IL-4 and TNF-α for 12 days. The morphology and functions of the cells were examined. The generated cells had the typical morphology of DCs. When the DCs were reinjected into the same patient, an augmentation of the cytotoxic T lymphocyte (CTL) activity was observed. Concomitantly, an increase in the natural killer (NK) cell activity was also detected in the patient. These results suggest that DCs-based cancer immunotherapy may become an important treatment option for cancer patients in the future.
Keywords: Cancer immunotherapy, Dendritic cells (DCs), Peripheral blood stem cells (PBSCs), Natural killer (NK) cells, Hepatocellular carcinoma
INTRODUCTION
Dendritic cells (DCs) are the most potent antigen presenting cells and are unique in their ability to stimulate T cells and initiate adaptive immunity. Recently, there has been relatively slow progress in understanding the biology of DCs because of the paucity of these cells in the blood and other tissues [1]. A major advance has been made by the establishment of ex vivo culture systems, which allows the induction of DCs from precursors [2]. Despite this progress, the total number of DCs available for immunotherapy is still limited [3-6].
It has long been the aim of cancer researchers to increase the patient's own immune response to their cancer, but to date the means to do this has remained elusive. The immunological elimination of tumors occurs primarily from the recognition of tumor-associated antigens, such as carcinoembryonic antigen (CEA), by cytotoxic T lymphocytes (CTLs) capable of lysing cells that express that antigen. Cytokines from CD4+ helper T cells are needed to activate CTLs, natural killer (NK) cells, antigen-presenting cells and other inflammatory cells at the tumor site [7-10].
The current promising strategies seek to produce active immunity through the use of DCs [7-9,11-14]. DCs are rare leucocytes named for their distinctive stellate morphology and are regarded as the most potent antigen-presenting cells in vivo [15]. They present an array of antigenic peptides needed to activate the appropriate antigen-specific T cells, and also produce potent costimulatory signals that drive quiescent T cells into the cell cycle and along the differentiation pathway, producing an expansion of effector cells [16]. In addition, the recent finding that DCs can trigger the function of NK cells directly [17] suggests that DCs are involved in the interaction between the innate and adaptive immune responses. Given these properties, DCs have attracted considerable attention as potential cellular adjuvants for the production of specific tumor vaccines.
This paper describes a simplified and efficient method for preparing DCs from PBSCs for patients with a hepatocellular carcinoma.
METHODS
Materials
Histopaque-1077 and trypsin-EDTA were purchased from Sigma Chemical Co. (St. Louis, MO, USA). The X-VIVO 20 medium was obtained from BioWhittaker (Walkersville, MD, USA). Mouse human-anti CD1a, CD3, CD4, CD8, CD56, CD83 and CD86 antibodies were supplied by Serotec (Raleigh, NC, USA). The Flt3-ligand (FL), GM-CSF (CSF2), IL-4 and TNF-α were acquired from R&D systems (Minneapolis, MN, USA). The plastic tissue culture flasks were purchased from Corning (Corning, NY, USA). All other chemicals used in this study were the highest grade available.
PBSCs collection and processing
The PBSCs were obtained from a patient with a hepatocellular carcinoma. For PBSC mobilization, the patient received granulocyte colony stimulating factor (G-CSF, Leucostim, Dong-A Pharmaceutical Co., Korea) subcutaneously for three consecutive days at a dose of 3 µg/kg/day. Leukapheresis for PBSCs collection began on the 4th day of G-CSF administration. Collection was performed using a CS-3000 Plus (Baxter Inc., Deerfield, IL, USA) cell separator with anticoagulant citrate dextrose formula A (ACD-A).
Generation of DCs
The PBSCs were centrifuged by a density gradient method using Ficoll-Hypaque (Histopaque). The leukocytes were washed twice with phosphate buffered saline (PBS), and resuspended in medium X-VIVO 20 for liquid culture at 37℃ in plastic tissue culture flasks. The purified leukocytes were adjusted to 1.5×106/ml in X-VIVO 20 medium, And supplemented with 50 ng/ml of Flt-3 Ligand (FL), 50 ng/ml of GM-CSF, 25 ng/ml of IL-4 and 10 ng/ml of TNF-α. The cells were incubated in 75 cm2 flasks in a humidified atmosphere containing 5% CO2 at 37℃.
On days 3, 6 and 12 of culture, the total cell counts and viability were assessed by the hemocytometer counts of the dilutions prepared in a trypan blue solution. The DCs were scored as the cells with veils and long projection cell bodies.
Immunofluorescence microscopy
The generated cells were stained with Fluorescein isothiocyanate (FITC)-conjugated murine monoclonal antibodies for 15 minutes at 25℃. After washing with PBS, the cells were resuspended in PBS and 1% paraformaldehyde, and examined using a Olympus Binocular Microscope (BX50F-3). The monoclonal antibodies used were CD1a-FITC, CD83-FITC and CD86-FITC.
DCs vaccination and clinical evaluation
The patient was treated intravenously with the DCs vaccine by mixing the components in 80 ml of normal saline. Constant monitoring was performed while administering the vaccine. No immediate or delayed adverse effects were observed. Four weeks after vaccination, the population of CD3, CD4, CD8 and CD56, the CD8:CD4 ratio and the activity of NK cells in the patient were evaluated at the department of clinical pathology.
RESULTS
Generation of DCs
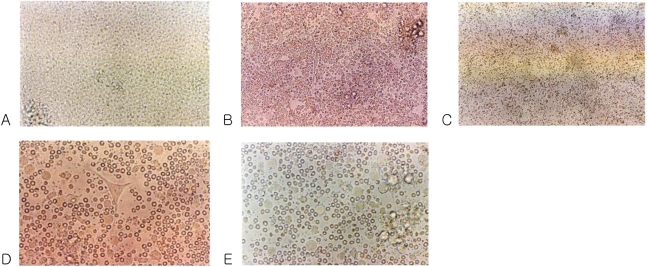
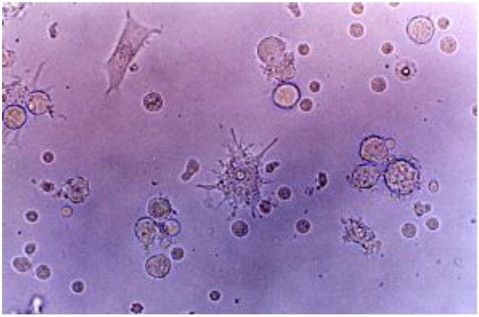
The mobilized PBSCs were cultured in 75 cm2 flasks at a humidified atmosphere containing 5% CO2 and 37℃. Under these conditions, the phase contrast micrograph of the freshly isolated PBSCs showed a homogenous population of equally sized and round cells (Fig. 1A). After 3 days of culture, spindle-shape cells appeared in the flasks (Fig. 1B). Some adherent clustered cells with a speculated and elongated cytoplasmic projection were observed after 6 days of culture (Fig. 1D). These cells were separated, and became motile, veiled and non-adherent cells at 12 days of culture (Fig. 2). The veiled cells were counted at 14 days under an optical microscope. The number of DCs increased up to 8×107 cells.
Fig. 1.
Phase contrast micrograph of freshly isolated peripheral blood stem cells showing a homogenous population of equally sized and round cells. Original magnification ×200 (A). After 3 days of culture with the medium containing GM-CSF, IL-4, TNF-α and FL. Spindle-shape cells are shown in the center. Original magnification ×200 (B). After 3 days of culture with the medium. Original magnification ×100 (C). After 6 days of culture with medium containing GM-CSF, IL-4, TNF-α and FL, cells with large-cell bodies and long dendritic projections were visible. Original magnification ×400 (D). After 6 days of culture with medium. Original magnification ×400 (E).
Fig. 2.
Cells with long cytoplasmic projections were visible at 12 days of culture in a medium containing GM-CSF, IL-4, TNF-α and FL. Original magnification ×400.
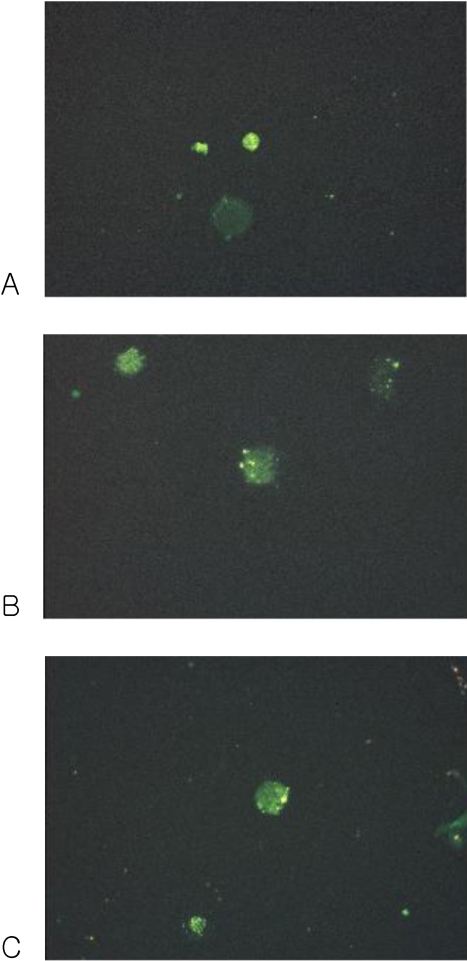
Immunofluorescence microscopy
The cultured DCs expressed CD1a, CD83 and CD86 (Fig. 3).
Fig. 3.
Fluorescence micrograph of cultured DCs showing intense staining with the monoclonal antibodies for the specific cell surface immunophenotype. DCs showed positive FITC staining. (A) CD1a, (B) CD83 and (C) CD86.
DCs vaccination and clinical evaluation
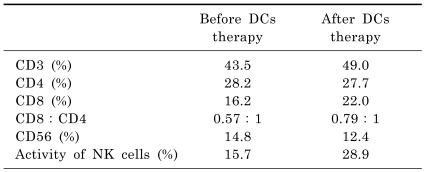
The immunostimulatory activities of the cultured cells were tested using a MLR assay. As shown in Table 1, augmentation of the CTLs activity was observed with a concomitant increase in the NK cells activity in the patient.
Table 1.
Relative fractions of T and NK cells, and NK cells activity in the peripheral blood of a patient with a hepatocellular carcinoma before and after DCs therapy
The DCs vaccine was reinjected into the same patient. Augmentation of CTLs activity was observed. Concomitantly, an increase in NK cells activity was also detected in the patient.
DISCUSSION
This paper describes the induction of DCs from PBSCs purified from a patient with a hepatocellular carcinoma using a one-step process. This one-step process consisted of the extensive amplification of hematopoietic progenitors with the Flt-3 Ligand (FL), and the differentiation of the amplified cells with GM-CSF, IL-4 and TNF-α into functional DCs. The DCs were reinjected into the same patient, and examined to determine if there was some augmentation of the cytotoxic T lymphocytes (CTLs) activity. The patient showed an increase in NK cells activity.
Ideally, the DCs used for vaccines should be CD83+ with high levels of CD86 and HLA-DR expression, as well as strong mixed-lymphocyte response stimulatory activity [12].
More study will be needed to determine if PBMCs or CD34+ cells are the preferred source for DCs generation.
The advantage of this study was that a patient-specific vaccine can be generated by a patient-specific preparation of the mutated antigen because individual cancer patients express unique antigens.
DCs therapy after treatment with conventional anticancer therapies, such as surgery and chemotherapy, will help eliminate the residual cancer cells completely. Therefore DCs-based anticancer therapy is most suitable in the setting of minimal residual disease.
ACKNOWLEDGEMENTS
This work was supported by Grant of Kosin University College of Medicine (2007).
ABBREVIATIONS
- GM-CSF
granulocyte-macrophage-colony stimulating factor
- IL-4
interleukin-4
- TNF-α
tumor necrosis factor-α
- G-CSF
granulocyte-colony stimulating factor
References
- 1.Steinman RM. The dendritic cell system and its role in immunogenicity. Ann Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 2.Lee DH, Park JS, Eo WK, Kim WM, Kang K. Differentiation induction of dendritic cell phenotypes from human leukemic cell lines. Korean J Physiol Pharmacol. 2001;5:79–86. [Google Scholar]
- 3.McKenna HJ, de Vries P, Brasel K, Lyman SD, Williams DE. Effect of flt3 ligand on the ex vivo expansion of human CD34+ hematopoietic progenitor cells. Blood. 1995;86:3413–3420. [PubMed] [Google Scholar]
- 4.Young CJ, Varma A, DiGiusto D, Backer MP. Retention of quiescent hematopoietic cells with high proliferative potential during ex vivo stem cell culture. Blood. 1996;87:545–556. [PubMed] [Google Scholar]
- 5.Piacibello W, Sanavio F, Garetto L, Severino A, Bergandi D, Ferrario J, Fagioli F, Berger M, Aglietta M. Extensive amplification and self-renewal of human primitive hematopoietic stem cells from cord blood. Blood. 1997;89:2644–2653. [PubMed] [Google Scholar]
- 6.Lyman SD, James L, Johnson L, Brasel K, de Vries P, Escobar SS, Downey H, Splett RR, Beckman MP, McKenna HJ. Cloning of the human homologue of the murine flt3 ligand: a growth factor for early hematopoietic progenitor cells. Blood. 1994;83:2795–2801. [PubMed] [Google Scholar]
- 7.Pardoll DM. Cancer vaccines. Nat Med. 1998;4:525–531. doi: 10.1038/nm0598supp-525. [DOI] [PubMed] [Google Scholar]
- 8.Ockert D, Schmitz M, Hampl M, Rieber EP. Advances in cancer immunotherapy. Immunol Today. 1999;20:63–65. doi: 10.1016/s0167-5699(98)01388-7. [DOI] [PubMed] [Google Scholar]
- 9.Nestle FO, Burg G, Dummer R. New perspectives on immunobiology and immunotherapy of melanoma. Immunol Today. 1999;20:5–7. doi: 10.1016/s0167-5699(98)01373-5. [DOI] [PubMed] [Google Scholar]
- 10.Boon T, Coulie PG, Van den Eynde B. Tumor antigens recognized by T cells. Immunol Today. 1997;18:267–268. doi: 10.1016/s0167-5699(97)80020-5. [DOI] [PubMed] [Google Scholar]
- 11.Steinman RM. Dendritic cells and immune-based therapies. Exp Hematol. 1996;24:859–862. [PubMed] [Google Scholar]
- 12.Schuler G, Steinman RM. Dendritic cells as adjuvants for immune-mediated resistance to tumors. J Exp Med. 1997;186:1183–1187. doi: 10.1084/jem.186.8.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lotze MT. Getting to the source: dendritic cells as therapeutic reagents for the treatment of patients with cancer. Ann Surg. 1997;226:1–5. doi: 10.1097/00000658-199707000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colaco C. DC-based cancer immunotherapy: the sequel. Immunol Today. 1999;20:197–198. doi: 10.1016/s0167-5699(98)01407-8. [DOI] [PubMed] [Google Scholar]
- 15.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 16.Bottomly K. Immunology-T cells and dendritic cells get intimate. Science. 1999;283:1124–1125. doi: 10.1126/science.283.5405.1124. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez NC, Lozier A, Flament C. Dendritic cells directly trigger NK cell functions: Cross-talk relevant in innate antitumor immune responses in vivo. Nat Med. 1999;5:405–411. doi: 10.1038/7403. [DOI] [PubMed] [Google Scholar]