Abstract
The serine/threonine kinase Akt has been shown to play a role of multiple cellular signaling pathways and act as a transducer of many functions initiated by growth factor receptors that activate phosphatidylinositol 3-kinase (PI3K). It has been reported that phosphorylated Akt activates eNOS resulting in the production of NO and that NO stimulates soluble guanylate cyclase (sGC), which results in accumulation of cGMP and subsequent activation of the protein kinase G (PKG). It has been also reported that PKG activates PI3K/Akt signaling. Therefore, it is possible that PI3K, Akt, eNOS, sGC, and PKG form a loop to exert enhanced and sustained activation of Akt. However, the existence of this loop in eNOS-expressing cells, such as endothelial cells or astrocytes, has not been reported. Thus, we examined a possibility that Akt phosphorylation might be enhanced via eNOS/sGC/PKG/PI3K pathway in astrocytes in vivo and in vitro. Phosphorylation of Akt was detected in astrocytes after KA treatment and was maintained up to 72 h in mouse hippocampus. 2 weeks after KA treatment, astrocytic Akt phosphorylation was normalized to control. The inhibition of eNOS, sGC, and PKG significantly decreased Akt and eNOS phosphorylation induced by KA in astrocytes. In contrast, the decreased phosphorylation of Akt and eNOS by eNOS inhibition was significantly reversed with PKG activation. The above findings in mouse hippocampus were also observed in primary astrocytes. These data suggest that Akt/eNOS/sGC/PKG/PI3K pathway may constitute a loop, resulting in enhanced and sustained Akt activation in astrocytes.
Keywords: Akt phosphorylation, Astrocyte, Kainic acid, Nitric oxide
INTRODUCTION
Previous studies indicate that phosphorylated Akt activates endothelial nitric oxide synthase (eNOS), leading to the production of nitric oxide (NO) [1,2]. NO stimulates soluble guanylate cyclase (sGC), which results in accumulation of cGMP and subsequent activation of the protein kinase G (PKG) [3,4]. Elevated eNOS activity is sufficient to activate phosphatidylinositol 3-kinase (PI3K)/Akt signaling via PKG [5], and activation of the PI3K/Akt pathway resulting from PKG activation by NO/cGMP can prevent neuronal cell death [6]. In addition, it has been reported that activation of sGC resulted in increased cyclooxygenase 2 (COX-2) expression via the PKG/Akt/NF-kB pathway [7]. Taken these reports together, there might be a loop that consisted of PI3K, Akt, eNOS, sGC, and PKG, which makes Akt activation sustained. However, the existence of this loop in eNOS-expressing cells, such as endothelial cell or astrocytes, has not been reported. Thus, we examined the possibilities that the Akt phosphorylation might be enhanced via eNOS/sGC/PKG/PI3K pathway in astrocytes in vivo and in vitro. Because Akt phosphorylation can be induced in astrocytes of mouse hippocampus by kainic acid injection, and in primary astrocytes by thrombin treatment (unpublished data), we used a model of KA-induced excitotoxicity and primary astrocytes as in vivo and in vitro model, respectively.
METHODS
Animals and reagents
Male ICR mice weighing 23~25 g were obtained from Folas-International, Ltd. (Seoul, Korea). All of the animal experiments were conducted in accordance with the animal care guidelines of the National Institutes of Health (NIH) and Korean Academy of Medical Sciences (KAMS). Mice were housed five per cage in a room maintained at 22+2℃ with an alternating 12/12 h light/dark cycle. Food and water were available ad libitum. KA and L-NAME, aminoguanidine (AG) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). KT-5823, wortmannin, and Guanosine 3',5'-cyclic monophosphate, N2, 2'-O-dibutyryl-sodium salt (db-cGMP) were purchased from Calbiochem (San Diego, CA, USA). 1H-(1,2,4)-oxadiazole[4,3-a]quinoxalin-1-one (ODQ) was purchased from Tocris (Ellisville, MO, USA).
Drug treatments
More than three mice were used for each group: KA (0.1µg/5µl), db-cGMP (2.5µg/5µl) were intracerebroventricularly (i.c.v.) injected according to the procedure established by Laursen and Belknap [8]. Wortmannin (1 mg/kg), L-NAME (50 mg/kg), ODQ (20 mg/kg), and KT-5823 (1 mg/kg) stock solutions were diluted in saline just before intraperitoneal (i.p.) injection, and were administered 1 h prior to KA injection (W+KA, L+KA, O+KA, KT+KA). db-cGMP together with L-NAME was administered 1 h prior to the KA injection (db+L+KA). Vehicle-treated control group (cont) was also prepared. Primary astrocyte, at 70~90% confluence, were treated with thrombin (5 U/ml, Sigma) or SNP (1 mM, Sigma) for 24 h and the following agents were also treated 1 h prior to thrombin or SNP; wortmannin (10µM), ODQ (100µM), KT-5823 (2µM), L-NAME (1 mM), and db-cGMP (500µM).
Immunohistochemistry and double immunofluorescence labeling
All mice were transcardially perfused and post-fixed for 4 h in 4% paraformaldehyde. Brains were cryoprotected in 30% sucrose, sectioned coronally (40µm) on a freezing microtome (MICROM HM 400R, Walldorf, Germany) and collected in cryoprotectant for storage at -20℃. Free floating immunohistochemical staining was performed with anti-rabbit polyclonal Phospho-Akt-Ser473 antibody (1:1,000, Cell Signaling Technology, Beverley, MA, USA) as described previously [9]. For double immunofluorescence labeling, sections were co-incubated with anti-mouse monoclonal GFAP (1:2,500, Sigma) and one of the following antibodies: anti-rabbit polyclonal p-Akt-Ser473, p-eNOS (1:1,000, BD Transduction, Franklin Lakes, NJ, USA). Alexa-488 conjugated goat anti-mouse IgG (green) and Alexa-568 conjugated goat anti-rabbit IgG (red) were used as fluorescent labeled secondary antibodies (Molecular Probes, Eugene, OR, USA).
Analysis of cells exhibiting DNA fragmentation was performed according to the manufacturer's instructions using terminal deoxynucleotidyl transferase using peroxide-12-UTP nick end labeling (TUNEL) (Roche Molecular Biochemicals, Indianapolis, IN, USA) to label double stranded DNA breaks suggestive of aopoptosis as described previously [10].
Immunoblotting
For western blot analysis, the hippocampi were dissected out from KA injected mice, homogenized in 0.25 M Tris-HCl buffer (pH, 6.8) containing 5 mM EGTA, 5 mM EDTA, 1% SDS, and 10% glycerol, with protease inhibitors (2µg/µl aprotinin, 1µg/µl pepstatin, 2µg/µl leupeptin, and 0.1 mM phenylmethylsulfonyl fluoride), and sonicated on ice. The proteins were resolved on SDS-polyacrylamide gels and transferred to nitrocellulose. The blots were incubated with anti-p-Akt, anti-Akt, anti-p-eNOS, respectively. Detection was performed with HRP-conjugated goat anti-rabbit IgG (1:2,000, Jackson Immuno Research, West Grove, PA, USA) or HRP-conjugated goat anti-mouse IgG (1:2,000, Jackson Immuno Research). The blots were visualized with enhanced chemiluminescence (ECL; Amersham, Piscataway, NJ, USA) and exposed on ECL film. Gel images in ECL films were scanned and the digital images were quantified using Image Quant software (Molecular Dynamics, Sunnyvale, CA, USA). Cells, primary astrocyte, were collected in lysis buffer (150 mM NaCl, 10 mM Tris-HCl, 1 mM EGTA, 1 mM EDTA, 0.5% NP-40, with protease inhibitors) and then proceeded as described in western blotting above.
Primary astrocyte culture
Primary astrocyte cultures were derived from 1 to 3 day postnatal SD rat (Daehan Biolink, Seoul, Korea). Briefly, cerebral cortices were dissected out. After removal of the meninges and blood vessels, the cerebral cortices were collected and minced with scalpel. A single-cell suspension was obtained by mechanical dissociation. The dissociated cells were seeded into poly-D-lysine-coated 170 cm2 T-flask at a density of 1×106 cm2 and maintained at 37℃, 5% CO2 and 95% air in Dulbecco's modified Eagle's medium/nutrient F12 (DMEM/F12) (Invitrogen, Carlsbad, CA, USA) containing 10% fetal bovine serum (Invitrogen). Medium was changed after 24 h and then every third day thereafter. When cells reached confluence, flasks were gently shaken for 6 h at 180 rpm to remove microglia and oligodendrocytes. After shaking, astrocytes were detached with 0.125% trypsin-EDTA (Invitrogen), and subcultured at 5×105 cells/60 mm flask. Using this method, we confirmed that these cultures contain over 95% astrocytes, as determined by immunostaining for glial fibrillary acidic protein.
Statistical analysis
Statistical analysis was performed by Kruskal-Wallis one-way ANOVA. Values were considered significantly different when p value was <0.05. The results are expressed as means±SD from at least three independent experiments.
RESULTS
Akt and eNOS activation by KA treatment in mouse hippocampus
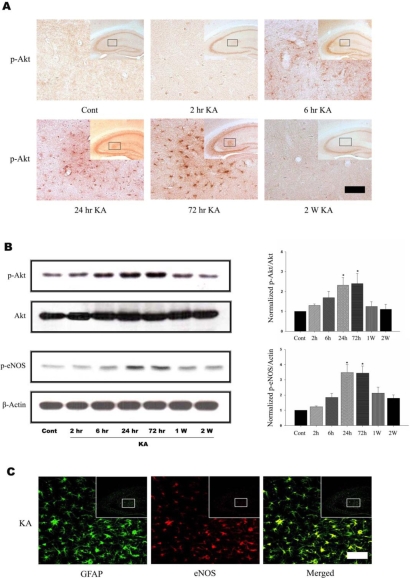
To determine whether Akt and eNOS phosphorylation occurs after KA treatment, we performed immunohistochemistry and immunoblotting after KA treatment. Phosphorylation of Akt was detected in astrocytes 6 h after KA treatment and was maintained up to 72 h (Fig. 1A, 1B). Two weeks after KA treatment, astrocytic Akt phosphorylation was normalized to control. Immunoblottings also showed the phosphorylation of Akt and eNOS, a downstream target, were increased in a time-dependent manner up to 72 h after KA treatment (Fig. 1B) and then decreased to control level. As eNOS immunoreactivity appeared to co-localize with that of GFAP in the hippocampus (Fig. 1C), and the pattern of astrocytic eNOS phosphorylation was in accordance with that of astrocytic Akt phosphorylation (Fig. 1B), it is believed that the observed change of Akt phosphorylation by KA occurs in astrocytes.
Fig. 1.
KA induces Akt and eNOS phosphorylation in mouse hippocampus. (A) Time course of KA-induced Akt phosphorylation in astrocytes. Increased p-Akt immunoreactivity was observed predominantly in astrocytes in the hippocampus 6 h after KA treatment and maintained up to 72 h. Inset at right upper corner shows the position of the enlarged image of CA3 region. (B) Representative immunoblots (left) and quantitative analysis (right) of Akt and eNOS phosphorylation. The levels of phosphorylation of Akt at Ser473 and eNOS at Ser1177 were measured after KA injection. Phosphorylation of Akt and eNOS increased up to 3 days, and then normalized 2 weeks after KA injection. (C) Confocal image of astrocytic eNOS in the hippocampus. Double immunofluorescence staining was carried out with antibodies to eNOS and GFAP, an astrocytic marker. eNOS immunoreactivity appeared to co-localize with that of GFAP. Data represent three independent experiments and were expressed mean±SD. *p<0.05 indicate statistically significant difference from control group. Scale bar: 50µm.
Effects of L-NAME on KA-induced Akt and eNOS phosphorylation
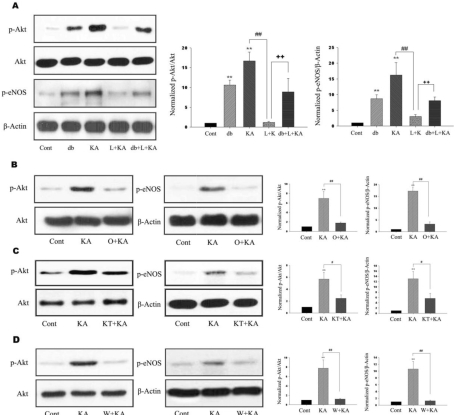
For the elucidation of the relationship between astrocytic Akt phosphorylation and eNOS inhibition by L-NAME, we examined whether Akt and eNOS phosphorylation in astrocytes could be regulated by L-NAME, non-selective NOS inhibitor. Phosphorylation levels of Akt and eNOS were increased at 24 h in KA-treated groups. However, L-NAME administration prior to KA treatment significantly attenuated the KA-induced phosphorylation of Akt and eNOS (Fig. 2A, L+KA).
Fig. 2.
Effects of L-NAME, ODQ, KT-5823, and wortmannin on phosphorylation of Akt and eNOS. (A) Representative immunoblots (left) and quantitative analyses (right) of effects of db-cGMP and L-NAME on phosphorylation of Akt and eNOS. db-cGMP and KA exhibited significantly increased Akt and eNOS phosphorylation (db, KA). However, L-NAME significantly decreased KA-induced phosphorylation of Akt and eNOS (L+KA). Further, co-treatment of db-cGMP attenuated L-NAME-mediated decrease of Akt and eNOS phosphorylation (db+L+KA). (B~D) Effects of ODQ, KT-5823, and wortmannin on phosphorylation of Akt and eNOS. Representative immunoblots (left) and quantitative analyses (right) showed that KA-induced phosphorylation of Akt and eNOS was significantly decreased with ODQ, KT-5823, and wortmannin (O+KA, KT+KA, W+KA). Data represent three independent experiments and were expressed mean±SD. **p<0.01 indicates statistically significant difference from the control group. # indicates p<0.05, ## and ++ indicate p<0.01 between indicated groups.
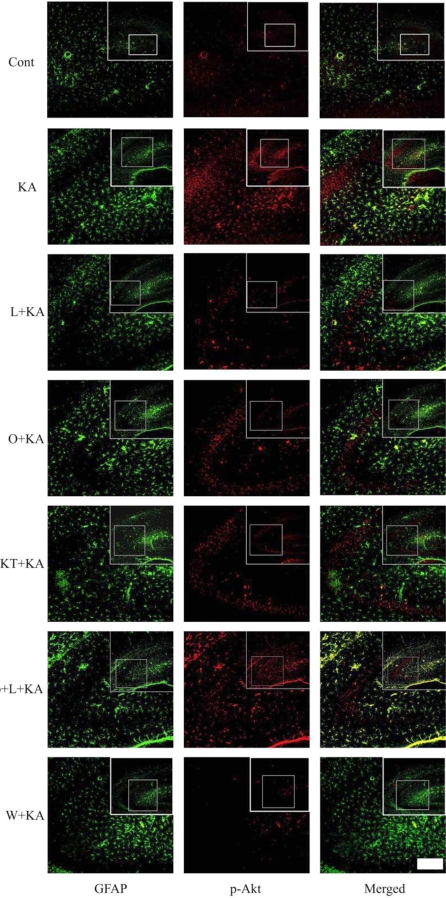
To confirm the astrocytic Akt phosphorylation after L-NAME treatment, double immunofluorescence staining was performed on free-floating brain sections using antibodies to p-Akt and GFAP. When L-NAME and KA were cotreated, p-Akt immunoreactivity was found to be co-localized mainly with the GFAP in the hippocampus (Fig. 3, L+KA).
Fig. 3.
Confocal images of astrocytic p-Akt immunoreactivity indicate in hippocampus upon the absence or presence of chemicals prior to KA treatment. To determine whether Akt phosphorylation occurs in astrocytes, double immunofluorescence staining was carried out with antibodies to phosphor-Akt and GFAP. KA per se exhibited increased Akt phosphorylation (KA), which was found to co-localize mainly with GFAP in astrocytes in the hippocampus. L-NAME, ODQ, and KT-5823 noticeably attenuated KA-induced Akt phosphorylation (L+KA, O+KA, & KT+KA). However, db-cGMP, a PKG activator, exhibited restoration of decreased Akt phosphorylation, which was caused by L-NAME, indicating that PKG is involved in Akt activation (db+L+KA). Wortmannin, a PI3K inhibitor, also decreased Akt phosphorylation in astrocytes (W+KA). Inset at right upper corner shows the position of the enlarged image of CA3 region. Scale bar: 50µm.
Role of sGC on KA-induced neuronal cell death and Akt and eNOS phosphorylation
Given the fact that NO stimulates the formation of cGMP via sGC, it is possible that NO produced from eNOS can regulate the level of Akt phosphorylation and neuronal cell death via sGC. When a specific sGC inhibitor, ODQ, was administered prior to KA treatment, the levels of KA-induced phosphorylation of Akt and eNOS were significantly attenuated (Fig. 2B). When ODQ and KA were cotreated, p-Akt immunoreactivity was decreased and founded to be co-localized mainly with the GFAP in the hippocampus (Fig. 3, O+KA).
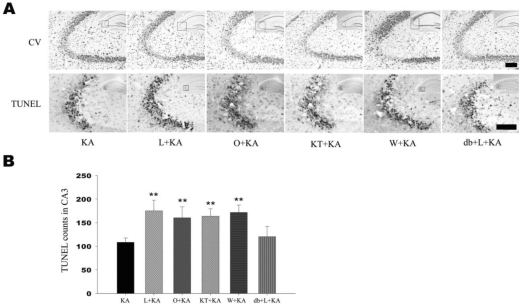
In order to elucidate the effects of the administration of ODQ prior to the KA injection on the KA-induced neuronal cell death, the representative and quantitative analysis of neuronal cell death was determined with cresyl violet and TUNEL staining (Fig. 4, O+KA). TUNEL-positive neurons in the CA3 region were observed more abundantly in mice treated with ODQ prior to KA injection than in KA injection only (Fig. 4B). These results suggest that NO from eNOS might regulate astrocytic Akt phosphorylation via sGC and subsequent inhibition of Akt phosphorylation can aggravate CA3 neuronal death in the hippocampus by KA injection as in L-NAME treatment.
Fig. 4.
Effect of L-NAME, ODQ, KT-5823, and wortmannin on neuronal cell death. Representative (A) and quantitative analysis (B) of neuronal cell death using cresyl violet and TUNEL stainings. In CA3 subfield, KA-induced neuronal loss was aggravated with pretreatment of L-NAME, ODQ, KT-5823, and wortmannin in cresyl violet staining. Considerable number of TUNEL-positive neurons appeared with KA treatment within CA3 region (KA) and the number of TUNEL-positive neurons were significantly increased with pre-treatment of L-NAME, ODQ, KT-5823, and wortmannin (L+KA, O+KA, KT+KA, W+KA). Co-treatment of db-cGMP with L-NAME showed attenuation on L-NAME-induced aggravation of cell death, although not significant (db+L+KA). Inset at right upper corner shows the position of the enlarged image of CA3 region. Data represent three independent experiments and were expressed mean±SD. **p<0.01 indicates statistically significant difference from the KA-only treated group. Scale bar: 100 mm.
Regulation of Akt phosphorylation via NO/cGMP-dependent pathway
sGC, activated by NO, catalyzes the conversion of GTP to the second messenger molecule, cGMP, which activates PKG [5]. We determined whether cGMP-dependent pathway is involved in NO-induced Akt phosphorylation. For the regulation of PKG activity, KT-5823 or db-cGMP were used as a PKG inhibitor or activator, respectively. KT-5823 administration prior to KA treatment significantly attenuated the levels of KA-induced phosphorylation of Akt and eNOS (Fig. 2C). In contrast, db-cGMP treatment per se significantly increased Akt phosphorylation levels. Furthermore, db-cGMP reversed the decreased Akt and eNOS phosphorylation by L-NAME administration (Fig. 2A, db+L+KA). The tendency to p-Akt immunoreactivity in the astrocytes was similar to those of blotting results of the KT-5823 and db-cGMP treatment (Fig. 3). These results suggest that NO from eNOS might regulate astrocytic Akt phosphorylation through NO/cGMP/PKG pathway.
In order to elucidate the effect of PKG inhibition on KA-induced neuronal cell death, the representative and quantitative analysis of neuronal cell death was determined with cresyl violet and TUNEL staining (Fig. 4). TUNEL-positive neurons in the CA3 region were observed more abundantly in mice treated with KT-5823 prior to KA injection than KA injection only (Fig. 4B). However pretreatment with db-cGMP significantly attenuated L-NAME aggravated neuronal cell damage (Fig. 4B). These results suggest that astrocytic PKG activation by sGC may be related to the neuronal cell death.
Role of wortmannin on KA-induced neuronal cell death and Akt and eNOS phosphorylation
In addition, the administration of wortmannin, specific PI3K inhibitor, significantly attenuated the levels of KA-induced phosphorylation of Akt and eNOS (Fig. 2D), which suggests that neuronal and astrocytic Akt phosphorylation is dependent on PI3K activity.
To test the effect of the administration of wortmannin prior to the KA injection on neuronal cell death, the representative and quantitative analysis of neuronal cell death were determined with cresyl violet and TUNEL staining (Fig. 4). TUNEL-positive neurons in the CA3 region were observed more abundantly in the wortmannin pretreated mice than in the KA-only-treated mice (Fig. 4B). These above data suggest that neuronal and astrocytic Akt phosphorylation in i.c.v. KA injection is dependent on PI3K activity. Attenuated neuronal Akt phosphorylation by wortmannin could be a factor that aggravates neuronal death, and also attenuated astrocytic Akt phosphorylation could have influence on neuronal death because of decreased astrocytic support for neuron.
KA-induced astrocytic Akt phosphorylation
Double immunofluorescence labeling experiments with anti-phospho-Akt/anti-GFAP revealed that KA induced astrocytic Akt phosphorylation through PI3K, NO, cGMP, PKG signaling pathways. Several inhibitors, L-NAME, a non-selective NOS inhibitor, ODQ, a specific sGC inhibitor, KT-5823, a PKG inhibitor, db-cGMP, a PKG activator, wortmannin, a PI3K inhibitor, can regulate the level of astrocytic Akt phosphorylation (Fig. 3). Therefore, it is suggested that astrocytic Akt phosphorylation is dependent on NO/cGMP/PKG/PI3K signaling pathways.
In situ verification of a loop for Akt phosphorylation in primary astrocytes
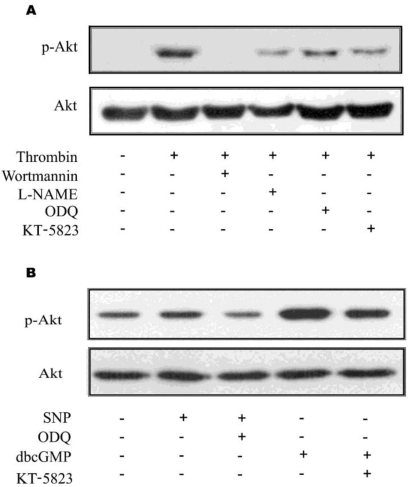
The in vivo data from the present study indicate that NO, produced from astrocytic eNOS, constitutes a loop to achieve enhanced Akt activation in astrocytes after KA-induced neuronal insult in mouse hippocampus. To confirm the proposed pathway exclusively in astrocytes, we blocked pharmacologically each step of Akt/eNOS/sGC/PKG/PI3K pathway in primary astrocytes (Fig. 5). Thrombin was used to induce astrocyte activation leading to Akt phosphorylation in primary astrocytes, because it was the most potent Akt activator in primary astrocytes among tested stimuli in our study (data not shown). Thrombin resulted in increased Akt phosphorylation in primary astrocytes and thrombin-mediated Akt phosphorylation was attenuated with inhibitors, which suppress each step in the proposed loop (Fig. 4A). Furthermore, treatment of SNP resulted in increased phosphorylation of astrocytic Akt and db-cGMP, a PKG activator, also resulted in enhanced Akt activation (Fig. 4B). These results strongly suggest that the loop including Akt/eNOS/sGC/PKG/PI3K is present in astrocytes.
Fig. 5.
In situ verification of Akt activation in primary astrocytes. Akt phosphorylation was evaluated 16 h after thrombin or other treatments. (A) Phosphorylation of Akt and its inhibition were increase in primary culture astrocytes. Thrombin exhibited increased Akt phosphorylation in primary astrocytes and thrombin-mediated increased Akt phosphorylation was attenuated with inhibitors, which suppress each step of Akt/eNOS/sGC/PKG/PI3K pathway. (B) Akt phosphorylation was increased by exogenous NO by SNP. Treatment of SNP resulted in increased phosphorylation of astrocytic Akt and db-cGMP, a PKG activator, resulted in increased Akt activation, suggesting that the pathway of Akt/eNOS/sGC/PKG/PI3K be present in astrocytes.
DISCUSSION
Previously, we showed that KA-induced neuronal death was attenuated by aminoguanidine, selective iNOS inhibitor, but aggravated by L-NAME in ICR mouse hippocampus [10]. There was a limitation that administered L-NAME is not selective eNOS inhibitor. But, given the fact that L-NAME is 20 times more selective to eNOS than iNOS [11] and that iNOS expression was not detected in astrocytes in KA-induced excitotoxicity model (unpublished data), it is reasonable to think that the decrease of Akt phosphorylation in astrocyte by L-NAME administration is due to inhibition of eNOS. eNOS can be activated by Akt-dependent phosphorylation and elevated eNOS activity is sufficient to activate PI3K/Akt signaling via PKG and induce cell migration and angiogenesis through NO/cGMP/PKG in endothelial cells [5]. In PC12 cells, NO exerts its anti-apoptotic effect through a cGMP-dependent activation of the PI3K/Akt signaling pathway [6]. However, the effects and mechanism of astrocytic Akt phosphorylation is largely unknown. Therefore, the hypothesis is intriguing that in a sequential mode, NO activates sGC, sGC increases production of cGMP, cGMP activates PKG, PKG activates PI3K, PI3K phosphorylates Akt, and p-Akt activates eNOS, which can potentially form a loop for enhanced Akt activation.
In the present study, we observed that KA significantly increased astrocytic Akt, eNOS phosphorylation and that NO from eNOS regulates astrocytic Akt phosphorylation through NO/cGMP/PKG/PI3K pathway, suggesting that a loop in the astrocytes is activated to obtain enhanced Akt phosphorylation. Phosphorylated Akt has been shown to play a role in multiple cellular responses, such as cell growth, cell survival, and protein synthesis. Although PI3K is a crucial activator for Akt, increasing evidence has indicated that Akt can be regulated in PI3K independent manners in neurons, such as extracellular signal-regulated kinase 1/2 (ERK1/2) and calmodulin kinase (CaMK) cascades [12,13]. However, our results showed that the administration of PI3K specific inhibitor, wortmannin, significantly attenuated astrocytic Akt phosphorylation, indicating that astrocytic Akt phosphorylation might be regulated mainly via PI3K (Fig. 3).
Several transcription factors are known to play a role in the anti-apoptotic effects of the PI3K/Akt pathway. These include phosphorylated CREB [14,15], and activated NF-kB [16]. Previous studies showed that CREB activation is associated with BDNF and GDNF expression [17,18]. Previously, we showed that melatonin induces Akt phosphorylation and the expression of GDNF in astroglial cells [19]. Enhanced expression of GDNF by melatonin was thought to contribute to the protection of neuronal cell death. Thus, these results indicate that NO/cGMP/PKG pathway-dependent astrocytic Akt phosphorylation can be a critical step in CREB-mediated GDNF expression and neuronal survival. Akt also promotes survival by activating anti-apoptotic NF-kB signaling through IkB kinase (IKK) activation [16]. NF-kB functions as an anti-apoptotic factor through its induction of genes that inhibit apoptosis. NF-kB has been shown to induce the expression of the inhibitor of apoptosis (IAP) protein family (c-IAP1, c-IAP2 and x-chromosome-linked IAP). Recent experimental results of evidence suggest that the transcription of transforming growth factor β (TGF-β) is activated by NF-kB [20]. TGF-β was shown to induce neuroprotection in vitro and in vivo [21,22]. Viven et al. [23] reported that neuroprotective activity of TGF-β1 against NMDA neurotoxicity is associated with the modulation of tissue plasminogen activator (tPA)/plasminogen activator inhibitor-1 (PAI-1). PAI-1, produced by astrocytes, is up-regulated by TGF-β1 treatment [24,25]. This events leads to modulation of the NMDA-evoked calcium influx in neurons and is responsible for the neuroprotective effects of TGF-β1 against NMDA injury. Also, it was shown that bacterial lipopolysaccharide (LPS)-induced ischemic tolerance in the brain is mediated via TGF-β [21,22]. TGF-β can destabilize iNOS mRNA, retard the synthesis of iNOS protein, and accelerate its degradation [26,27]. Also, Pyo et al. [28] reported that wortmannin enhances LPS-induced iNOS expression in microglia in the presence of astrocyte.
In the present study, we have demonstrated that astrocytic Akt phosphorylation might be enhanced through eNOS/sGC/PKG/PI3K pathway, which increased activation of astrocytic Akt may result in the upregulation of pro-survival transcription factor and neuroprotective factor expression. Therefore, astrocytic Akt phosphorylation may play a significant role in pro-survival neuron-glia cross-talks.
ACKNOWLEDGEMENTS
This work was supported by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD, Basic Research Promotion Fund) (KRF-2006-H00010).
ABBREVIATIONS
- eNOS
endothelial nitric oxide synthase
- KA
Kainic acid
- L-NAME
N (G)-nitro-L-arginine methyl ester
- NO
Nitric oxide
- PI3K
Phosphatidylinositol 3-kinase
- PKG
protein kinase G
- sGC
Soluble guanylate cyclase
References
- 1.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 2.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Denninger JW, Marletta MA. Guanylate cyclase and the NO/cGMP signaling pathway. Biochim Biophys Acta. 1999;1411:334–350. doi: 10.1016/s0005-2728(99)00024-9. [DOI] [PubMed] [Google Scholar]
- 4.Schlossmann J, Feil R, Hofmann F. Signaling through NO and cGMP-dependent protein kinases. Ann Med. 2003;35:21–27. doi: 10.1080/07853890310004093. [DOI] [PubMed] [Google Scholar]
- 5.Kawasaki K, Smith RS, Jr, Hsieh CM, Sun J, Chao J, Liao JK. Activation of the phosphatidylinositol 3-kinase/protein kinase Akt pathway mediates nitric oxide-induced endothelial cell migration and angiogenesis. Mol Cell Biol. 2003;23:5726–5737. doi: 10.1128/MCB.23.16.5726-5737.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ha KS, Kim KM, Kwon YG, Bai SK, Nam WD, Yoo YM, Kim PK, Chung HT, Billiar TR, Kim YM. Nitric oxide prevents 6-hydroxydopamine-induced apoptosis in PC12 cells through cGMP-dependent PI3 kinase/Akt activation. Faseb J. 2003;17:1036–1047. doi: 10.1096/fj.02-0738com. [DOI] [PubMed] [Google Scholar]
- 7.Chang MS, Lee WS, Chen BC, Sheu JR, Lin CH. YC-1-induced cyclooxygenase-2 expression is mediated by cGMP-dependent activations of Ras, phosphoinositide-3-OH-kinase, Akt, and nuclear factor-kappaB in human pulmonary epithelial cells. Mol Pharmacol. 2004;66:561–571. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- 8.Laursen SE, Belknap JK. Intracerebroventricular injections in mice. Some methodological refinements. J Pharmacol Methods. 1986;16:355–357. doi: 10.1016/0160-5402(86)90038-0. [DOI] [PubMed] [Google Scholar]
- 9.Baker H, Farbman AI. Olfactory afferent regulation of the dopamine phenotype in the fetal rat olfactory system. Neuroscience. 1993;52:115–134. doi: 10.1016/0306-4522(93)90187-k. [DOI] [PubMed] [Google Scholar]
- 10.Byun J, Lee S, Jeon S, Kwon Y, Lee HJ, Kim S, Kim Y, Kim M, Chun W. Kainic acid-induced neuronal death is attenuated by aminoguanidine but aggravated by L-NAME in mouse hippocampus. Korean J Physiol Pharmacol. 2009;13:265–271. doi: 10.4196/kjpp.2009.13.4.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boer R, Ulrich WR, Klein T, Mirau B, Haas S, Baur I. The inhibitory potency and selectivity of arginine substrate site nitric-oxide synthase inhibitors is solely determined by their affinity toward the different isoenzymes. Mol Pharmacol. 2000;58:1026–1034. [PubMed] [Google Scholar]
- 12.Brami-Cherrier K, Valjent E, Garcia M, Pages C, Hipskind RA, Caboche J. Dopamine induces a PI3-kinase-independent activation of Akt in striatal neurons: a new route to cAMP response element-binding protein phosphorylation. J Neurosci. 2002;22:8911–8921. doi: 10.1523/JNEUROSCI.22-20-08911.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yano S, Tokumitsu H, Soderling TR. Calcium promotes cell survival through CaM-K kinase activation of the protein-kinase-B pathway. Nature. 1998;396:584–587. doi: 10.1038/25147. [DOI] [PubMed] [Google Scholar]
- 14.Du K, Montminy M. CREB is a regulatory target for the protein kinase Akt/PKB. J Biol Chem. 1998;273:32377–32379. doi: 10.1074/jbc.273.49.32377. [DOI] [PubMed] [Google Scholar]
- 15.Pugazhenthi S, Nesterova A, Sable C, Heidenreich KA, Boxer LM, Heasley LE, Reusch JE. Akt/protein kinase B up-regulates Bcl-2 expression through cAMP-response element-binding protein. J Biol Chem. 2000;275:10761–10766. doi: 10.1074/jbc.275.15.10761. [DOI] [PubMed] [Google Scholar]
- 16.Romashkova JA, Makarov SS. NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling. Nature. 1999;401:86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- 17.Cen X, Nitta A, Ohya S, Zhao Y, Ozawa N, Mouri A, Ibi D, Wang L, Suzuki M, Saito K, Ito Y, Kawagoe T, Noda Y, Ito Y, Furukawa S, Nabeshima T. An analog of a dipeptide-like structure of FK506 increases glial cell line-derived neurotrophic factor expression through cAMP response element-binding protein activated by heat shock protein 90/Akt signaling pathway. J Neurosci. 2006;26:3335–3344. doi: 10.1523/JNEUROSCI.5010-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tabuchi A, Sakaya H, Kisukeda T, Fushiki H, Tsuda M. Involvement of an upstream stimulatory factor as well as cAMP-responsive element-binding protein in the activation of brain-derived neurotrophic factor gene promoter I. J Biol Chem. 2002;277:35920–35931. doi: 10.1074/jbc.M204784200. [DOI] [PubMed] [Google Scholar]
- 19.Lee SH, Chun W, Kong PJ, Han JA, Cho BP, Kwon OY. Sustained activation of Akt by melatonin contributes to the protection against kainic acid-induced neuronal death in hippocampus. J Pineal Res. 2006;40:79–85. doi: 10.1111/j.1600-079X.2005.00283.x. [DOI] [PubMed] [Google Scholar]
- 20.Lee KY, Ito K, Hayashi R, Jazrawi EP, Barnes PJ, Adcock IM. NF-kappaB and activator protein 1 response elements and the role of histone modifications in IL-1beta-induced TGF-beta1 gene transcription. J Immunol. 2006;176:603–615. doi: 10.4049/jimmunol.176.1.603. [DOI] [PubMed] [Google Scholar]
- 21.Boche D, Cunningham C, Gauldie J, Perry VH. Transforming growth factor-beta 1-mediated neuroprotection against excitotoxic injury in vivo. J Cereb Blood Flow Metab. 2003;23:1174–1182. doi: 10.1097/01.WCB.0000090080.64176.44. [DOI] [PubMed] [Google Scholar]
- 22.Dhandapani KM, Brann DW. Transforming growth factor-beta: a neuroprotective factor in cerebral ischemia. Cell Biochem Biophys. 2003;39:13–22. doi: 10.1385/CBB:39:1:13. [DOI] [PubMed] [Google Scholar]
- 23.Vivien D, Bernaudin M, Buisson A, Divoux D, MacKenzie ET, Nouvelot A. Evidence of type I and type II transforming growth factor-beta receptors in central nervous tissues: changes induced by focal cerebral ischemia. J Neurochem. 1998;70:2296–2304. doi: 10.1046/j.1471-4159.1998.70062296.x. [DOI] [PubMed] [Google Scholar]
- 24.Buisson A, Nicole O, Docagne F, Sartelet H, Mackenzie ET, Vivien D. Up-regulation of a serine protease inhibitor in astrocytes mediates the neuroprotective activity of transforming growth factor beta1. Faseb J. 1998;12:1683–1691. [PubMed] [Google Scholar]
- 25.Docagne F, Nicole O, Gabriel C, Fernandez-Monreal M, Lesne S, Ali C. Smad3-dependent induction of plasminogen activator inhibitor-1 in astrocytes mediates neuroprotective activity of transforming growth factor-beta 1 against NMDA-induced necrosis. Mol Cell Neurosci. 2002;21:634–644. doi: 10.1006/mcne.2002.1206. [DOI] [PubMed] [Google Scholar]
- 26.Kim WK, Hwang SY, Oh ES, Piao HZ, Kim KW, Han IO. TGF-beta1 represses activation and resultant death of microglia via inhibition of phosphatidylinositol 3-kinase activity. J Immunol. 2004;172:7015–7023. doi: 10.4049/jimmunol.172.11.7015. [DOI] [PubMed] [Google Scholar]
- 27.Vodovotz Y, Geiser AG, Chesler L, Letterio JJ, Campbell A, Lucia MS. Spontaneously increased production of nitric oxide and aberrant expression of the inducible nitric oxide synthase in vivo in the transforming growth factor beta 1 null mouse. J Exp Med. 1996;183:2337–2342. doi: 10.1084/jem.183.5.2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pyo H, Yang MS, Jou I, Joe EH. Wortmannin enhances lipopolysaccharide-induced inducible nitric oxide synthase expression in microglia in the presence of astrocytes in rats. Neurosci Lett. 2003;346:141–144. doi: 10.1016/s0304-3940(03)00505-6. [DOI] [PubMed] [Google Scholar]