Abstract
R-type Cav2.3 high voltage-activated Ca2+ channels in peripheral sensory neurons contribute to pain transmission. Recently we have demonstrated that, among the six Cav2.3 isoforms (Cav2.3a~Cav2.3e), the Cav2.3e isoform is primarily expressed in trigeminal ganglion (TG) nociceptive neurons. In the present study, we further investigated expression patterns of Cav2.3 isoforms in the dorsal root ganglion (DRG) neurons. As in TG neurons, whole tissue RT-PCR analyses revealed the presence of two isoforms, Cav2.3a and Cav2.3e, in DRG neurons. Single-cell RT-PCR detected the expression of Cav2.3e mRNA in 20% (n=14/70) of DRG neurons, relative to Cav2.3a expression in 2.8% (n=2/70) of DRG neurons. Cav2.3e mRNA was mainly detected in small-sized neurons (n=12/14), but in only a few medium-sized neurons (n=2/14) and not in large-sized neurons, indicating the prominence of Cav2.3e in nociceptive DRG neurons. Moreover, Cav2.3e was preferentially expressed in tyrosine-kinase A (trkA)-positive, isolectin B4 (IB4)-negative and transient receptor potential vanilloid 1 (TRPV1)-positive neurons. These results suggest that Cav2.3e may be the main R-type Ca2+ channel isoform in nociceptive DRG neurons and thereby a potential target for pain treatment, not only in the trigeminal system but also in the spinal system.
Keywords: R-type calcium channels, Cav2.3, Voltage-activated calcium channels, DRG neurons
INTRODUCTION
The high voltage-activated Ca2+ channels (HVACCs) play a key role in cellular process under physical, chemical and inflammatory damage in peripheral tissues [1]; they are classified into L-, N-, P/Q- and R-type Ca2+ channels on the basis of pharmacological and electrophysiological properties in sensory neurons such as dorsal root ganglion (DRG) and trigeminal ganglion (TG) neurons [2,3]. HVACCs are formed by one of a number of pore-forming α1 subunits, α1A~F and α1S, in addition to auxiliary subunits. Molecular characterizations have determined that α1C, α1D, α1F, and α1S subunits encode L-type (Cav1.1, 1.2, 1.3 and 1.4) channels [4]; α1A encodes P/Q-type (Cav2.1) channels [5]; α1B encodes N-type (Cav2.2) channels [6]; α1E encodes R-type (Cav2.3) channels [7].
Six isoforms of the R-type Cav2.3 Ca2+ channel (Cav2.3a~Cav2.3f) have been reported in various mammalian species [8-12]. R-type Ca2+ channels are also present in DRG neurons [2], and have been suggested to play a critical role in the pain transmission and neuropathic pain [13,14]. Immunohistochemical and in situ hybridization analysis also showed differential expression of R-type Ca2+ channels in DRG neurons [15,16]. In the previous study, we demonstrated that Cav2.3e is the major Cav2.3 isoform in the nociceptive TG neurons, the counterpart of DRG neurons in the orofacial area [17]. However, to date, expression patterns of R-type Cav2.3 Ca2+ channel in DRG neurons have not been characterized.
In the present study, we determined the Cav2.3 isoforms uniquely expressed in rat nociceptive DRG neurons by using whole-tissue RT-PCR and single-cell RT-PCR. Our results show that Cav2.3 isoform in nociceptive DRG neurons is comparable to that of TG neurons; Cav2.3e is the major R-type Cav2.3 isoform in nociceptive DRG neurons and is preferentially expressed in IB4-negative, trkA-positive and TRPV1-positive neurons.
METHODS
Preparation of DRG neurons
All surgical and experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) in School of Dentistry, Seoul National University. DRG neurons from 2- to 5-day old neonatal rats were prepared as previously described [18]. Briefly, DRGs were rapidly removed from spinal segments and digested sequentially in collagenase (Sigma, St. Louis, MO), dispase (Boehringer Mannheim, Indianapolis, IN), and trypsin (Life Technologies) in HBSS for 10 min at 37℃. The cells were washed in DMEM, triturated with a flame-polished Pasteur pipette to separate cells and remove processes. Subsequently, cells were centrifuged and resuspended, and then placed on a polyornithine-coated glass coverslips (25 mm in diameter). Cells were maintained in an incubator at 37℃ equilibrated with 5% CO2. All experiments were performed with cells cultured for 4~6 h.
Whole tissue Reverse Transcription-Polymerase Chain Reaction (RT-PCR) Analysis
Total RNA was isolated from 2- to 5-day-old rats DRG by using the Trizol reagent (Life Technologies). Following digestion with DNase I, 3µg of total RNA was used for cDNA synthesis using the Superscript First-Strand Synthesis System (Invitrogen, Carlsbad, California). After reverse transcription of total RNA, 1 ng of cDNA was then used as a template for amplification in a reaction mixture. As shown in our previous study [17], primers for PCR were specifically designed to differentiate the presence or the absence of three inserts (inserts I, II and III) on the Cav2.3 transcripts. After a denaturation step of 5 min at 94℃, the amplification was carried out at 94℃ for 30 s, 55℃ for 30 s, and 72℃ for 40 s for 35 cycles. The PCR reaction was completed by maintaining temperatures at 72℃ for 10 min. As a positive control, cDNA from the same preparations was subjected to 35 cycles of PCR with primers for β-actin. All PCR products were resolved on 2% agarose gel.
Single-cell RT-PCR
The entire single cell was aspirated into a pipette under visual control via negative pressure. Pipettes used for entire neuron harvest had a tip diameter range of 12~30µm, and was filled with RNase-free water. The tip of the pipette and its contents were broken into a reaction tube containing reverse transcription (RT) reagents. To avoid genomic DNA contamination, digestion with DNase was performed before RT. RT was carried out for 1 h at 50℃ (Invitrogen) and the cDNA product was used in separate PCR. The forward and reverse primers were chosen from parts specific to the gene to be detected in order to avoid amplification of closely homologous genes. The first round of PCR was preformed in 50µl of PCR buffer containing 0.2 mM dNTPs, 0.2µM "outer" primers, 5µl of RT product, and 0.2µl of platinum Taq DNA polymerase (Invitrogen). The protocol included 5 min of initial denaturation step at 95℃, followed by 40 cycles of 40 s of denaturation at 95℃, 40 s of annealing at 55℃, 40 s of elongation at 72℃, and was completed with 7 min of final elongation. For the second round of amplification, the reaction buffer (20µl) contained 0.2 mM dNTPs, 0.2µM "inner" primers, 5µl of the products from the first round PCR, and 0.1µl of platinum Taq DNA polymerase. The reaction procedure was the same as the first round. "Insert" primers, designed to detect the presence of insert fragments, were also used in second round amplifications. For the positive controls, β-actin primers were used in parallel in each PCR reaction. Negative control was obtained from pipettes that did not harvest any cell contents but were submerged in bath solution. The PCR products were displayed on ethidium bromide-stained 2% agarose gel. Gels were photographed using a digital camera (Bio-print 2000 x-press zoom, Vilber Lourmat, France).
Classification of sensory neurons
DRG neurons are classified into three groups, small-sized (<16µm), medium-sized (16~20µm) and large-sized (20~30µm) neurons [19]. Griffonia simplicifolia isolectin B4 (IB4) was also utilized to classify DRG neurons into either IB4-positive or IB4-negative neurons [20]. Before single cell collection, DRG cells were incubated with 10µg/ml IB4-FITC (Sigma) in a balanced salt solution [(in mM): 145 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES, and 10 glucose] for 10 min and then rinsed.
RESULTS
Presence of Cav2.3 isoforms Cav2.3a and Cav2.3e in DRG neurons
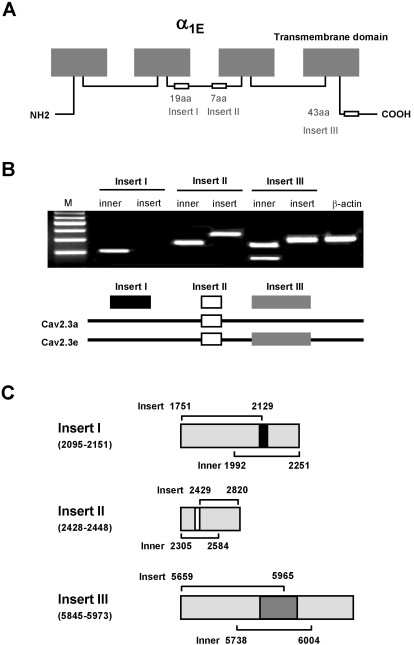
In order to determine Cav2.3 isoform in DRG neurons, we utilized the same strategy, as previously done in TG neurons [17], which examined the presence or the absence of three major inserts: insert I, insert II in the II-III loop, and insert III in the carboxy terminus (Fig. 1A). Using both "inner" primers and "insert" primers, the PCR products were differentiated by size and additionally by the presence of the insert region (Fig. 1C). For the II-III loop, the PCR amplification only yielded Cav2.3 isoforms containing insert II but lacking insert I. However, PCR amplification of the carboxy terminus we could detect both shorter cDNA fragments that lack insert III and longer cDNA fragments that contain insert III. These two isoforms were Cav2.3a and Cav2.3e splice variants, respectively (Fig. 1B).
Fig. 1.
(A) Schematic diagram of α1E (Cav2.3) subunit. The structural variations cover two segments of 19 (insert I) and 7 amino acids (insert II) in the loop between domain II and III, and a third segment of 43 amino acids (insert III) in the proximal carboxy terminus. (B) Two Cav2.3 isoforms were found in DRG. Insert I, II and III was analyzed by whole tissue RT-PCR. Cav2.3 isoforms amplified from DRG neurons have insert II, but not insert I, and either lack or contain insert III depending on the isoform. Cav2.3a contains insert II, but not insert I and insert III, while Cav2.3e has insert II and insert III, but not insert I. (C) Illustrated are the locations of the primers designed to detect insert I, insert II and insert III for RT-PCR analysis in relation to the Cav2.3 subunit.
The prominance of Cav2.3e in DRG neurons
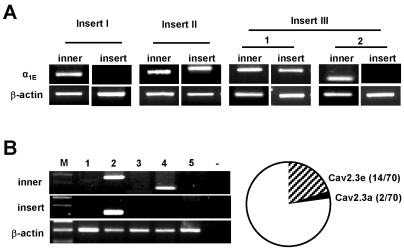
Given that only two isoforms of Cav2.3-Cav2.3a and Cav2.3e splice variants were present in DRG, we then determined the expression patterns of these two isoforms at the single cell level. By using single-cell RT-PCR, we detected Cav2.3a and Cav2.3e mRNA in subpopulations of DRG neurons, which was in good agreement with whole-tissue RT-PCR (Fig. 2A). Since Cav2.3a and Cav2.3e differ only in the presence or absence of insert III, we analyzed insert III to discriminate their expression in individual cells. Cav2.3e and Cav2.3a mRNAs were detected in 20.0% (n=14) and 2.8% (n=2) of neurons, respectively (n=70; Fig. 2B). Likewise in TG neurons, we found that Cav2.3e was the prominant isoform of Cav2.3 in DRG neurons but did not observe any DRG neurons that co-expressed both Cav2.3e and Cav2.3a in the same cells.
Fig. 2.
Expression of Cav2.3a and Cav2.3e isoforms from individual DRG neurons. (A) Single-cell RT-PCR products amplified with nested primer from DRG neurons. PCR products, with or without insert III (larger, 1 and smaller, 2), were produced in each DRG neuron. (B) Representative gels showing single-cell RT-PCR products amplified using insert III-specific primers. The numbers (1-5) indicate five different neurons examined from single-cell RT-PCR reaction. β-actin was used in each reaction as a positive control. Circle diagram shows the distribution of Cav2.3a and Cav2.3e in DRG neurons (total 70 neurons).
Preferential Expression of Cav2.3e isoform in trkA-positive, IB4-negative and TRPV1-positive small-sized DRG neurons
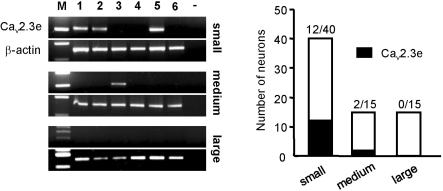
Neonatal DRG neurons can be classified into small-, medium- and large-sized neurons, with the small-sized neurons functioning as nociceptors [21]. Cav2.3e mRNA was detected in 30.0% (n=12/40) of small-sized neurons, 13.3% (n=2/15) of medium-sized neurons, and 0% (n=0/15) of large-sized neurons (Fig. 3). These results indicate that Cav2.3e is prominently expressed in nociceptive DRG neurons as Cav2.3e mRNA was mainly detected in small-sized neurons (n=12/14), but in only a few medium-sized neurons (n=2/14) and not in large-sized neurons.
Fig. 3.
Expression pattern of Cav2.3e was analyzed in three groups: small-sized (<16µm), medium-sized (16~20µm) and large-sized (20~30µm) DRG neurons. Representative gels showing single-cell RT-PCR products obtained from six different neurons. White bars indicate the number of neurons in each group; black bars indicate the number of neurons with expression of Cav2.3e.
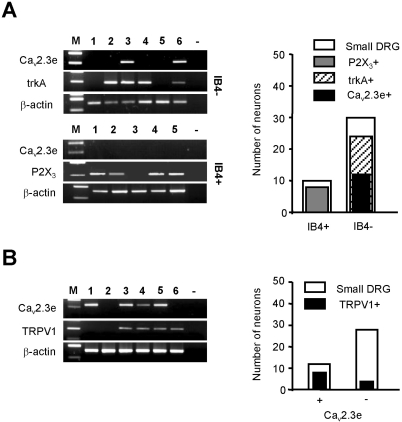
Small-diameter sensory neurons can be divided into two types based on their neurochemical properties [22]. One group contains neuropeptides such as calcitonin-gene related neuropeptide (CGRP) and substance P, and express the high-affinity nerve growth factor (NGF) receptor trkA; the other group lacking neuropeptides instead possess fluoride-resistant acid phosphatase (FRAP) activity, bind the plant lectin isolectin B4 (IB4) and are positive for the ATP-gated ionotropic receptor P2X3 [23-25]. We used an IB4-FITC conjugate to distinguish IB4-positive small-sized DRG neurons (n=40). IB4-positive neurons (80% of which expressed P2X3 mRNA) did not contain Cav2.3e mRNA (n=10). Of the small diameter IB4-negative neurons, 80% expressed trkA mRNA; Cav2.3e (n=12/24) was only detected in trkA-positive neurons (Fig. 4A).
Fig. 4.
(A) Small-sized DRG neurons were divided into IB4-negative and IB4-positive neurons; expression of Cav2.3e was determined in each group with or without trkA and P2X3 expression. The black bar in the graph shows that Cav2.3e is prominent in trkA+/IB4-/TRPV1+ neurons. (B) Expression of Cav2.3e in TRPV1-expressing nociceptive neurons. Representative gels showing RT-PCR products amplified with Cav2.3e, TRPV1, and β-actin-specific primers from six different neurons. The majority of small-sized Cav2.3e-expressing DRG neurons are also TRPV1-positive, though TRPV1 expression is not exclusive to DRG expressing the Cav2.3e subunit (graph; right).
We also examined whether Cav2.3e mRNAs are expressed together with TRPV1 in small neurons. 66.7% (n=8/12) of neurons expressing Cav2.3e mRNA were TRPV1-positive, while 14.3% of neurons lacking Cav2.3e mRNA were TRPV1-negative (Fig. 4B).
DISCUSSION
Our recent study revealed that Cav2.3e expression is highly restricted to small (<16µm) IB4-negative, trkA-positive, and TRPV1 TG neurons [17]. In the present study, we determined expression patterns of Cav2.3 isoform in DRG neurons. We found that Cav2.3a and Cav2.3e isoforms were present in DRG neurons. Among these two isoforms, Cav2.3e was the major isoform preferentially expressed in small-sized TRPV1-positive nociceptive neurons and was restricted to IB4-negative and trkA-positive neurons. These results suggest that the expression patterns of Cav2.3 isoforms in nociceptive DRG neurons are similar to those of TG neurons; the strong presence of Cav2.3e mRNA may correlate with the expression of the R-type Ca2+ channel, which might play a critical role in pain transmission in DRG neurons [26].
Among six isoforms (Cav2.3a, Cav2.3b, Cav2.3c, Cav2.3d, Cav2.3e, Cav2.3f), Cav2.3e is the major endocrine Cav2.3 isoform that was identified in rat and human kidney, insulinoma cell line INS-1 cells and islets of Langerhans [10]. We have shown that Cav2.3e is uniquely expressed in trkA-positive, IB4-negative DRG neurons that contain neuropeptide transmitters such as substance P and CGRP. Based on these results, Cav2.3e might be an important molecular mediator in neuropeptide release from nociceptive nerve terminals, in which case Cav2.3e may be the major Cav2.3 isoform responsible for the nociception mediated by Cav2.3 in the spinal system.
In accordance with our previous work in TG neurons [17], Cav2.3e-expressing DRG neurons also exhibited several properties of nociceptors, such as being of small-diameter, IB4-negative and positive for TRPV1 and trkA. It has been reported that α1E subunits were heterogeneously localized in the cell bodies of the DRG neurons in immunohistochemistry [15] and in situ hybridization [16]. Cav2.3e expression at a higher level in the small-sized DRG neurons suggests that Cav2.3e might have distinctive role in transduction of pain in nociceptive neurons. Also, restricted distribution of Cav2.3e to trkA-positive/IB4-negative nociceptors, but not to P2X3-positive/IB4-positive neuron, suggests that these two populations may transmit pain information to the spinal cord in different manners that are possibly mediated by the participation of different Ca2+ channel isoforms. However, it remains to be determined the exact functional role of preferential Cav2.3e expression in trkA-positive/IB4-negative and TRPV1-positive neurons.
R-type Ca2+ channels are associated with nociceptive processing in various pain conditions. Mathews et al reported that spinal SNX-482, a Cav2.3 Ca2+ channel blocker, inhibited noxious C-fiber and Aδ-fiber-mediated dorsal horn neuronal responses in conditions of neuropathy but not in sham operated rats and that non-noxious Aδ-mediated responses were not affected by SNX-482 [13]. In another study, mice lacking the α1E Ca2+ channel subunit (required for functional expression of Cav2.3 channels) exhibited normal pain behavior against acute mechanical, thermal, and chemical stimuli; however, they showed reduced responses to somatic inflammatory pain [15]. Also, R-type Ca2+ channels are located at primary synapses [27] and contributes to neurotransmitter release [28]. These results suggest that R-type Ca2+ channels may play a dominant role in neuropathic pain rather than in normal physiological pain. Further work is required to elucidate role of Cav2.3e in the pathological pain conditions.
In summary, our study provides the first evidence that two Cav2.3 isoforms are expressed in rat DRG neurons, a major isoform (Cav2.3e) and a minor isoform (Cav2.3a). The expression pattern of Cav2.3 isoforms suggests that Cav2.3e isoform of R-type Ca2+ channels may be important in pain transmission and neuropathic pain both in DRG neurons and TG neurons. Therefore, Cav2.3e isoform in nociceptive neurons would be potential target for the pain treatment not only in the trigeminal system but also in the spinal system.
ACKNOWLEDGEMENTS
This work was supported by a grant (20090086663) from the Basic Research Program funded by the Ministry of Education, Science and Technology, Republic of Korea, Republic of Korea. We thank Alexander J. Davies for the correction of English in this manuscript.
ABBREVIATIONS
- DRG
dorsal root ganglion
- IB4
isolectin B4
- HVACCs
high voltage-activated calcium channels
- RT-PCR
reverse transcription-polymerase chain reaction
- TG
trigeminal ganglion
- trkA
tyrosine kinase A
- TRPV1
transient receptor potential vanilloid 1
References
- 1.Vanegas H, Schaible H. Effects of antagonists to high-threshold calcium channels upon spinal mechanisms of pain, hyperalgesia and allodynia. Pain. 2000;85:9–18. doi: 10.1016/s0304-3959(99)00241-9. [DOI] [PubMed] [Google Scholar]
- 2.Wilson SM, Toth PT, Oh SB, Gillard SE, Volsen S, Ren D, Philipson LH, Lee EC, Fletcher CF, Tessarollo L, Copeland NG, Jenkins NA, Miller RJ. The status of voltage-dependent calcium channels in alpha 1E knock-out mice. J Neurosci. 2000;20:8566–8571. doi: 10.1523/JNEUROSCI.20-23-08566.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ikeda M, Matsumoto S. Classification of voltage-dependent Ca2+ channels in trigeminal ganglion neurons from neonatal rats. Life Sci. 2003;73:1175–1187. doi: 10.1016/s0024-3205(03)00414-4. [DOI] [PubMed] [Google Scholar]
- 4.Williams ME, Feldman DH, McCue AF, Brenner R, Velicelebi G, Ellis SB, Harpold MM. Structure and functional expression of alpha 1, alpha 2, and beta subunits of a novel human neuronal calcium channel subtype. Neuron. 1992;8:71–84. doi: 10.1016/0896-6273(92)90109-q. [DOI] [PubMed] [Google Scholar]
- 5.Bourinet E, Soong TW, Sutton K, Slaymaker S, Mathews E, Monteil A, Zamponi GW, Nargeot J, Snutch TP. Splicing of alpha 1A subunit gene generates phenotypic variants of P- and Q-type calcium channels. Nat Neurosci. 1999;2:407–415. doi: 10.1038/8070. [DOI] [PubMed] [Google Scholar]
- 6.Williams ME, Brust PF, Feldman DH, Patthi S, Simerson S, Maroufi A, McCue AF, Velicelebi G, Ellis SB, Harpold MM. Structure and functional expression of an omega-conotoxin-sensitive human N-type calcium channel. Science. 1992;257:389–395. doi: 10.1126/science.1321501. [DOI] [PubMed] [Google Scholar]
- 7.Zhang JF, Randall AD, Ellinor PT, Horne WA, Sather WA, Tanabe T, Schwarz TL, Tsien RW. Distinctive pharmacology and kinetics of cloned neuronal Ca2+ channels and their possible counterparts in mammalian CNS neurons. Neuropharmacology. 1993;32:1075–1088. doi: 10.1016/0028-3908(93)90003-l. [DOI] [PubMed] [Google Scholar]
- 8.Schneider T, Wei X, Olcese R, Costantin JL, Neely A, Palade P, Perez-Reyes E, Qin N, Zhou J, Crawford GD. Molecular analysis and functional expression of the human type E neuronal Ca2+ channel alpha 1 subunit. Receptors Channels. 1994;2:255–270. [PubMed] [Google Scholar]
- 9.Williams ME, Marubio LM, Deal CR, Hans M, Brust PF, Philipson LH, Miller RJ, Johnson EC, Harpold MM, Ellis SB. Structure and functional characterization of neuronal alpha 1E calcium channel subtypes. J Biol Chem. 1994;269:22347–22357. [PubMed] [Google Scholar]
- 10.Vajna R, Schramm M, Pereverzev A, Arnhold S, Grabsch H, Klockner U, Perez-Reyes E, Hescheler J, Schneider T. New isoform of the neuronal Ca2+ channel alpha1E subunit in islets of Langerhans and kidney--distribution of voltage-gated Ca2+ channel alpha1 subunits in cell lines and tissues. Eur J Biochem. 1998;257:274–285. doi: 10.1046/j.1432-1327.1998.2570274.x. [DOI] [PubMed] [Google Scholar]
- 11.Schramm M, Vajna R, Pereverzev A, Tottene A, Klockner U, Pietrobon D, Hescheler J, Schneider T. Isoforms of alpha1E voltage-gated calcium channels in rat cerebellar granule cells--detection of major calcium channel alpha1-transcripts by reverse transcription-polymerase chain reaction. Neuroscience. 1999;92:565–575. doi: 10.1016/s0306-4522(99)00013-5. [DOI] [PubMed] [Google Scholar]
- 12.Pereverzev A, Leroy J, Krieger A, Malecot CO, Hescheler J, Pfitzer G, Klockner U, Schneider T. Alternate splicing in the cytosolic II-III loop and the carboxy terminus of human E-type voltage-gated Ca2+ channels: electrophysiological characterization of isoforms. Mol Cell Neurosci. 2002;21:352–365. doi: 10.1006/mcne.2002.1179. [DOI] [PubMed] [Google Scholar]
- 13.Matthews EA, Bee LA, Stephens GJ, Dickenson AH. The Cav2.3 calcium channel antagonist SNX-482 reduces dorsal horn neuronal responses in a rat model of chronic neuropathic pain. Eur J Neurosci. 2007;25:3561–3569. doi: 10.1111/j.1460-9568.2007.05605.x. [DOI] [PubMed] [Google Scholar]
- 14.Saegusa H, Kurihara T, Zong S, Minowa O, Kazuno A, Han W, Matsuda Y, Yamanaka H, Osanai M, Noda T, Tanabe T. Altered pain responses in mice lacking alpha 1E subunit of the voltage-dependent Ca2+ channel. Proc Natl Acad Sci USA. 2000;97:6132–6137. doi: 10.1073/pnas.100124197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murakami M, Suzuki T, Nakagawasai O, Murakami H, Murakami S, Esashi A, Taniguchi R, Yanagisawa T, Tan-No K, Miyoshi I, Sasano H, Tadano T. Distribution of various calcium channel alpha(1) subunits in murine DRG neurons and antinociceptive effect of omega-conotoxin SVIB in mice. Brain Res. 2001;903:231–236. doi: 10.1016/s0006-8993(01)02427-1. [DOI] [PubMed] [Google Scholar]
- 16.Yusaf SP, Goodman J, Pinnock RD, Dixon AK, Lee K. Expression of voltage-gated calcium channel subunits in rat dorsal root ganglion neurons. Neurosci Lett. 2001;311:137–141. doi: 10.1016/s0304-3940(01)02038-9. [DOI] [PubMed] [Google Scholar]
- 17.Fang Z, Park CK, Li HY, Kim HY, Park SH, Jung SJ, Kim JS, Monteil A, Oh SB, Miller RJ. Molecular basis of Cav2.3 calcium channels in rat nociceptive neurons. J Biol Chem. 2007;282:4757–4764. doi: 10.1074/jbc.M605248200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim YH, Park CK, Back SK, Lee CJ, Hwang SJ, Bae YC, Na HS, Kim JS, Jung SJ, Oh SB. Membrane-delimited coupling of TRPV1 and mGluR5 on presynaptic terminals of nociceptive neurons. J Neurosci. 2009;29:10000–10009. doi: 10.1523/JNEUROSCI.5030-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murray SS, Cheema SS. Constitutive expression of the low-affinity neurotrophin receptor and changes during axotomy-induced death of sensory neurones in the neonatal rat dorsal root ganglion. J Anat. 2003;202:227–238. doi: 10.1046/j.1469-7580.2003.00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stucky CL, Lewin GR. Isolectin B(4)-positive and -negative nociceptors are functionally distinct. J Neurosci. 1999;19:6497–6505. doi: 10.1523/JNEUROSCI.19-15-06497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- 22.Snider WD, McMahon SB. Tackling pain at the source: new ideas about nociceptors. Neuron. 1998;20:629–632. doi: 10.1016/s0896-6273(00)81003-x. [DOI] [PubMed] [Google Scholar]
- 23.Nagy JI, Hunt SP. Fluoride-resistant acid phosphatase-containing neurones in dorsal root ganglia are separate from those containing substance P or somatostatin. Neuroscience. 1982;7:89–97. doi: 10.1016/0306-4522(82)90155-5. [DOI] [PubMed] [Google Scholar]
- 24.Michael GJ, Averill S, Nitkunan A, Rattray M, Bennett DL, Yan Q, Priestley JV. Nerve growth factor treatment increases brain-derived neurotrophic factor selectively in TrkA-expressing dorsal root ganglion cells and in their central terminations within the spinal cord. J Neurosci. 1997;17:8476–8490. doi: 10.1523/JNEUROSCI.17-21-08476.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bradbury EJ, Burnstock G, McMahon SB. The expression of P2X3 purinoreceptors in sensory neurons: effects of axotomy and glial-derived neurotrophic factor. Mol Cell Neurosci. 1998;12:256–268. doi: 10.1006/mcne.1998.0719. [DOI] [PubMed] [Google Scholar]
- 26.Murakami M, Nakagawasai O, Suzuki T, Mobarakeh II, Sakurada Y, Murata A, Yamadera F, Miyoshi I, Yanai K, Tan-No K, Sasano H, Tadano T, Iijima T. Antinociceptive effect of different types of calcium channel inhibitors and the distribution of various calcium channel alpha 1 subunits in the dorsal horn of spinal cord in mice. Brain Res. 2004;1024:122–129. doi: 10.1016/j.brainres.2004.07.066. [DOI] [PubMed] [Google Scholar]
- 27.Brown SP, Safo PK, Regehr WG. Endocannabinoids inhibit transmission at granule cell to Purkinje cell synapses by modulating three types of presynaptic calcium channels. J Neurosci. 2004;24:5623–5631. doi: 10.1523/JNEUROSCI.0918-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dietrich D, Kirschstein T, Kukley M, Pereverzev A, von der Brelie C, Schneider T, Beck H. Functional specialization of presynaptic Cav2.3 Ca2+ channels. Neuron. 2003;39:483–496. doi: 10.1016/s0896-6273(03)00430-6. [DOI] [PubMed] [Google Scholar]