Congenital diseases of dysmotility are a serious problem in children and, potentially, also in adults. Advances have recently been made in understanding one cause of pediatric dysmotility, Hirschsprung’s disease, which is the most visible congenital defect of the enteric nervous system (ENS).1–5 Although the diagnosis of Hirschsprung’s disease is sometimes missed, and the length of involved gut may vary,6,7 a segment of bowel is always totally aganglionic in patients with Hirschsprung’s disease, which is about an unsubtle as a defect can be. The ENS, however, can be abnormal without being aganglionic. This is true because the function of the ENS is much more sophisticated than that of other regions of the autonomic nervous system.8 The output of the ENS is not a simple binary choice to drive motility or not, or to stimulate secretion or not. The ENS is responsible for the integrative control of behavior, a function that is sufficiently complex as to require all of the classes of neurotransmitter found in the CNS and at least as many neurons as are found in the spinal cord (~108 in humans).4,9–12 The ENS does not simply accept commands from the brain and spinal cord, it also talks back to the CNS. The transfer of information between the bowel and the brain in the vagus nerves is two-way process and in fact, many more vagal fibers are afferent than efferent.13 Vagus nerve stimulation can be employed to affect epilepsy,14–16 treat depression16–18 and even to improve learning and memory.19 To function normally, therefore, the ENS requires a full panoply of neurons of correct phenotypes, proper synaptic connections, and an appropriate interaction with the CNS. More is needed of development, therefore, than just to produce relay ganglia to enable the brain to drive the gut.
Despite the progress made in comprehending the aganglionosis of Hirschsprung’s disease, little is known about subtle abnormalities in subsets of enteric neurons, ENS synapses, or in the establishment of CNS-ENS interactions. Aganglionosis of the type seen in Hirschsprung’s disease cannot be the cause of functional bowel diseases, which are not lethal and are not associated with pseudoobstruction. If there is a developmental defect that causes functional bowel diseases, that defect has to be compatible with propulsive motility because propulsive motility occurs in patients with functional bowel diseases, even if the propulsive motility is so abnormal that the patient is driven to distraction. Abdominal distress from a bowel that propels may be particularly troublesome in children who may not be able to find a constructive means of conveying their difficulty. A congenital defect in the ENS that causes motility to be abnormal but present is likely to be one that affects committed sublineages of neural or glial precursors rather than pluripotent stem cells and thus to occur relatively late in ENS ontogeny.20 Such a late-arising, seemingly limited defect could also stop propulsive motility if it were to disrupt the function of a critical cell, such as the intrinsic primary afferent neurons (IPANs) that are required for the gut to be able to respond to luminal stimuli.21–25 Motility may then be compromised severely even when the pathology of the ENS, examined with conventional stains, is described as normal. This type of defect may be responsible for the still-to-be explained pediatric dysmotilities and chronic intestinal pseudoobstruction (CIP).
This review is designed to examine the development of the ENS, not as a detailed catalogue of all known relevant phenomena, but to provide a conceptual basis for understanding, not only the pathogenesis of Hirschsprung’s disease, but also other disorders of dysmotility that are not associated with aganglionosis. Emphasis will be placed on genes that, when mutated, give rise to defects in the ENS that are not obvious but which nevertheless cause intestinal motility to become subtly or devastatingly abnormal.
The ENS is a unique, large and vital nervous system
Biomedical understanding of the significance of the ENS has changed in recent years. Until the middle of the 20th century, the ENS was considered to be no more than an aggregation of relay ganglia interspersed between the CNS and effectors in the bowel.26 Control and regulation were believed to be entirely the province of “autonomic” centers of the brain, for which the dorsal motor nucleus of the vagus and the sacral parasympathetic preganglionic neurons of the spinal cord served as common conduits for the outflow of information. Change occurred gradually as the independence of the ENS as a center of integrative neuronal activity was re-discovered and the intrinsic complexity of the ENS became apparent.9,21,27,28 The hypothesis that the intrinsic innervation of the bowel can, on its own, determine the behavior of the gut has old roots. This idea was advanced first by Bayliss and Starling29–31 on the basis of experiments performed on the intestines of dogs. Essentially, Bayliss and Starling demonstrated that raising the pressure inside the lumen of the bowel gives rise to propulsive activity, which they called “the law of the intestine,” that consisted of oral contraction and anal relaxation. Because this activity persisted when they cut all of the extrinsic nerves to the gut, Bayliss and Starling concluded that “the law of the intestine” was mediated by the “intrinsic nervous mechanism” of the bowel. They were able to reach this conclusion because Bayliss and Starling were aware of the fact that the gut contains extremely large numbers of interconnected neurons in two major plexuses, the myenteric32,33 and the submucosal,34 the discovery of which dated back to the time of the American Civil War. The hypothesis that the bowel can regulate its own behavior was dramatically verified by Trendelenburg,35 who elicited the same activity seen by Bayliss and Starling in vitro, an experimental design in which the CNS and extrinsic nerves clearly are nonparticipants, and codified by Langley, who in his classical book on the autonomic nervous system, coined the term “enteric nervous system” and emphasized its independence of CNS innervation.36
The hypothesis that the ENS can function without CNS direction had erroneously been abandoned after Langley’s book was published, probably because the sympathetic and parasympathetic nervous systems function entirely under CNS control. The idea was revived when multiple neurotransmitters were found to be present in the ENS37,38 and research was again begun to find the cellular basis of the independence of the ENS. The ultrastructure of the ENS is now known to be different from those of sympathetic or parasympathetic ganglia, enteric neurons are supported by glia rather than by Schwann cells, enteric ganglia lack internal collagen, and in fact, the ENS resembles the CNS more than it resembles other regions of the PNS.9,39–41 The complexity of the ENS transcends that of banal peripheral ganglia. The ENS has many interneurons and intrinsic microcircuits.9,11 Because of the similarities of the CNS and the ENS, moreover, advantage can be taken of principles gleaned from studies of the brain to help guide investigations of ENS ontogeny. It is important to obtain a better understanding of ENS development to learn which gastrointestinal disorders have developmental roots, and ultimately how these conditions can be treated, or better, prevented.
Origins of the ENS
The ENS is formed as the gut becomes colonized by émigrés from the neural crest.42–45 These incoming cells arrive from three defined regions of the crest. The vagal crest (somite levels 3–7) provides the bulk of the precursors of enteric neurons and glia and colonizes the whole gut.46 The postumbilical bowel also receives a late-arriving contribution from the sacral crest,44,45,47–52 and the rostral foregut (primordial esophagus and adjacent stomach) receives cells from the nearby truncal crest.53 Crest-derived cells probably do not migrate as a uniform array of committed or uncommitted precursors, but appear to constitute a heterogeneous population that changes progressively as a function of developmental stage, both as the cells migrate and after they arrive in the target bowel.1,2,20,54,55 The crest-derived precursors have ample opportunity along their route of travel to interact with microenvironmental signaling factors, which include growth factors and elements of extracellular matrix that irreversibly change the precursors and contribute to the determination of their fates.1–4
Hirschsprung’s disease is the best-characterized birth deffect of the ENS
Little is currently known about the development of particular ENS neurons or the roles defects in such neurons play in abnormal ENS function. Progress, however, albeit still not adequate, has been made in understanding the most visible developmental defect of the ENS, Hirschsprung’s disease, in which ganglia are totally absent from variable lengths of the terminal bowel.6,7,56–58 Mutations in multiple genes have been associated with Hirschsprung’s disease, including RET, GDNF, NRTN, EDNRB, EDN3, ECEI, PHOX2b, SOXI0, PAX3, and SMADIPI (SIP1, ZBFX1B),2,3,5,59–61 but even so, these genes account only for ~50% of cases. Penetration, moreover, is incomplete, there is a marked sex difference in clinical expression, and mutant genotypes are associated with pleiotropic effects. It has been suggested that the majority of Hirschsprung’s cases arise from the interactions of multiple susceptibility genes.59 Identified interactions include those of the endothelin B receptor (Ednrb) with the Ret receptor tyrosine kinase59,62 and with Sox10.63 Sox10 is important in maintaining the multipotent nature of precursors that express Phox2b and/or Mash-1.64 Sox10 also interacts with Pax3 to regulate expression of Ret.60
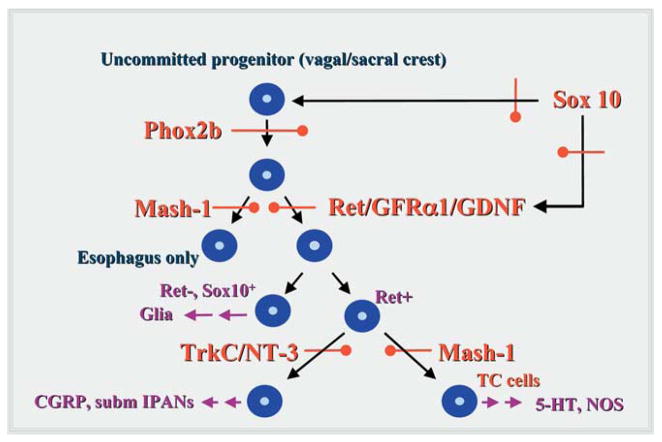
Little is currently known about genes or gene products that regulate the establishment of brain-gut interconnections or the development of interneurons. Disturbances in interconnections or in a minority population of neurons are not as easy to detect as Hirschsprung’s, but nevertheless are vital for normal ENS function. Defects in factors required early in ENS ontogeny tend to produce extensive abnormalities because they affect the development of multipotent precursors that are common to all, or to a large subset of the ENS.20 In contrast, factors that are required late in ENS development tend to produce smaller, much more restricted lesions, because they affect cells that have a limited developmental potential.20,65,66 A characteristic of all of the gene products implicated in the pathogenesis of Hirschsprung’s is that they are required early in ENS development (Figure 1). When knocked out in mice, all except those related to Edn3 signaling (EDNRB, EDN3, and ECE1 [endothelin converting enzyme1]), cause almost the whole bowel to be aganglionic. The knockout of edn3, ednrb, or ece1 leads only to aganglionosis of the terminal colon.67–69 In human patients, the loss of function of factors required for enteric neuronal development is usually not total; however, as a result of the defects, the proliferation of the colonizing pool of crest-derived precursors is insufficient to completely colonize the gut, leaving the terminal bowel aganglionic.1,2,5–7,70–74 In contrast to the other Hirschsprung’s-related genes, Edn3 is not required for the formation of enteric neurons, and in fact, even inhibits enteric neuronal differentiation in vitro.75,76 Two hypotheses have been advanced to account for the terminal bowel aganglionosis associated with the loss of Edn3 signaling. One is that Edn3 is required to prevent the premature differentiation of neuronal precursors and cessation of migration before the complete colonization of the bowel,75 and the other is that Edn3 first enhances the effects of GDNF/Ret and Sox10 on proliferation, and later prevents the trapping of crest-derived precursors in the cecum.62,76,77 GDNF exerts a chemoattractive effect on enteric crest-derived precursors, which migrate along a GDNF gradient.78–80 Because GDNF expression is maximal in the cecum, vagal crest-derived émigrés would not be expected to migrate distal to the cecum unless another factor were to intervene to enable them to escape the pull of cecal GDNF. Edn3 opposes the ability of GDNF to attract enteric crest-derived cells.62,77 This action may be related to the spatiotemporal enhancement of Ednrb expression by Sox10 as crest-derived precursors approach the cecum.63
Figure 1.
Specific transcription and growth factors define stages in ENS development. The earliest precursors of enteric neurons and glia are multipotent and dependent on the expression of the transcription factor, Phox2b. Derivatives of these cells that colonize all regions of the bowel, with the exception of the primordial esophagus and adjacent stomach, depend on the stimulation of Ret by GDNF in a complex with the Ret coreceptor, GFRα1. The ENS of the primordial esophagus and adjacent stomach is Ret-independent, and depends instead on the expression of the b-HLH transcription factor, Mash-1. Both the expression of Phox2b and Ret are promoted by the transcription factor, Sox10, the expression of which is essential for ENS development. Because they are required by multipotent precursors early in development, loss-of-function mutations in Phox2b, Sox10, or Ret (or its ligand or coreceptor) give rise to aganglionosis of the bowel. Only the esophagus and adjacent stomach become aganglionic due to loss-of-function mutations in Mash-1. Later stages in ENS ontogeny involve the development of committed lineages of enteric neuronal or glial precursors. Sets of enteric neurons that colonize the bowel distal to the esophagus and adjacent stomach are Mash-1-dependent or -independent. Loss-of function mutations in Mash-1 thus do not cause the gut to become aganglionic, but to lose only specific derivatives of Mash-1-dependent precursors, including transiently catecholaminergic (TC) precursors and the 5-HT- and NOS-containing neurons to which they give rise. A subset of Mash-1-independent precursors expresses TrkC (evidently in response to BMP-2 and/or -4 stimulation) and thus becomes NT-3-dependent. These cells include the submucosal IPANs that initiate peristaltic and secretory reflexes.
Defects in late-acting factors give rise to restricted lesions in the ENS that may be devastating
Identified factors that affect later stages of enteric neurons lead, as expected, to limited birth defects and not to aganglionosis. Even a restricted lesion, however, may be functionally catastrophic. The effects of deleting the neurotrophin, NT-3, or its high affinity receptor, TrkC, are illustrative65,81,82 (Figure 1). NT-3/TrkC deletions are associated with moderate decreases in the numbers of enteric neurons and the size of enteric ganglia.65 These changes initially went unnoticed;81,82 however, NT-3 was eventually found to be needed for the development and maintenance of submucosal IPANs and for myenteric interneurons.65 Submucosal IPANs are required for the initiation of peristaltic and secretory reflexes.24,83,84 These neurons are deficient when NT-3 or TrkC is deleted;65 moreover, the gut is hyperganglionic in transgenic mice that overexpress NT-3 directed to the developing ENS by the promoter for dopamine β-hydroxylase (DBH).65 NT-3/TrkC-deficient mice do not feed and die shortly after birth. NT-3 promotes the development of enteric neurons in vitro,85,86 but only if precursors are isolated from the gut after day E14.87 By this time, crest-derived cells have almost fully colonized the bowel2,88,89 and the ability of GDNF to stimulate their proliferation is lost.87 The early-developing neurons, thus are unaffected by NT-3 and the submucosal plexus, which develops later than the myenteric, is far more NT-3-dependent.65 The effects of GDNF/GFRα1/Ret, like those of NT-3, change as a function of developmental age. GDNF first promotes proliferation; however, after E14, GDNF enhances neuronal differentiation/survival.87
The basic helix-loop-helix transcription (b-HLH) factor, Mash-1 (mammalian homologue of Drosophila achaetescute) is another factor not required by all enteric neurons (Figure 1). Mash-1 expression is necessary earlier than NT-3/TrkC, but later than GDNF/GFRα1/Ret.66 Mash-1 dependence and independence thus distinguish two sublineages of enteric neurons. Because of its late requirement, the knockout of Mash-1 does not, like that of Phox2b, Sox10, or GDNF/GFRα1/Ret, result in aganglionosis of the intestine. Only the esophagus of mash-1 −/− mice is aganglionic.90–92 The remainder of the bowel lacks about a third of its normal complement of neurons.66 The deficiency, however, is not uniform among phenotypes of intestinal neurons. A specific subset is missing; this subset is born early in ENS ontogeny (E12–E15) from a transiently catecholaminergic (TC) population of crest-derived precursors.66 These TC precursors give rise to mature neurons, including those that contain serotonin or nitric oxide synthase (NOS), neither of which contain catecholamines; therefore, no TC cells appear in the gut during development of mash-1 −/− mice, and the resulting ENS is deficient in serotonin- and NOS-containing neurons.66,92 Curiously, the recently discovered enteric dopaminergic neurons develop normally in mash-1 −/− mice.93 Although dopaminergic neurons contain tyrosine hydroxylase and, obviously, dopamine (DA), which is a catecholamine, they are not derived from TC precursors and arise perinatally and postnatally, long after TC cells have disappeared (~E14). DA-containing neurons are mainly submucosal. Enteric neuronal development thus depends on growth and transcription factors, which are required in a regular temporal sequence. The extent of the defect that results from a loss of function of these factors depends, not only on how much of the function of the factors has been lost, but on when during development the factors are required. A mutation in an early-required factor such as GDNF or the receptors it stimulates, GFR1 and Ret, causes aganglionosis because all lineages of neurons in the intestines arise from precursors that require Ret stimulation. The length of the aganglionic region may reflect the degree to which function is lost as a result of the mutation. In contrast, subtle abnormalities in the ENS, which result from deficiencies in late-acting factors, such as NT-3, disturb relatively small subsets of neurons because only these subsets require TrkC stimulation. They do not lead to aganglionosis because most lineages of enteric neurons are TrkC-independent. A mutation that results in the loss of function of a factor like NT-3, however, can cause a lethal defect in intestinal motility because it affects the development of a critical type of neuron, the submucosal IPAN. Such a mutation might be anticipated to give rise to severe pediatric dysmotilities or CIP, where motility may be seriously abnormal in the face of an ENS diagnosed as “normal” by the conventional tools utilized to analyze pathological specimens. A similar mutation that disturbs the function of a regulatory interneuron, however, would be likely to give rise to a sublethal disturbance of motility. Such a defect might be anticipated in disorders, like the irritable bowel syndrome (IBS), in which anatomical/biochemical changes are neither obvious nor incompatible with life.
Functional gastrointestinal disease may have roots in ENS Ontogeny
Gastrointestinal (GI) disorders are classified as “functional” when potentially causative enteric anatomical or biochemical abnormalities are not apparent.94,95 The list of “functional” GI syndromes has been shrinking because disorders once thought to be “functional” have been re-classified as organic after enteric anatomical or biochemical abnormalities have been discovered. IBS, which occurs in up to 20% of Americans,96–98 is the most prevalent GI condition currently classified as “functional.” The pathogenesis of IBS, however, in at least a subset of patients, may be organic and enteric in origin. Mucosal expression of the serotonin transporter (SERT) and tryptophan hydroxylase-1 (TpH-1) have recently been discovered to be deficient in patients with either diarrhea- or constipation-predominant IBS.99 These abnormalities, which interfere with serotonin inactivation and biosynthesis, suggest that enteric serotonergic signaling is defective in IBS. The cause of IBS remains unknown; however, if enteric signaling pathways, such as those that involve serotonin, are abnormal in IBS, it is plausible that they are so because they have not developed normally. There are pediatric analogues of IBS100–103 and anecdotal evidence suggests that children with colic may become adults with IBS.
Serotonin may be a late acting growth factor promoting the development of subsets of enteric neurons and muscle/ICCs
Enteric serotonergic neurons are an example of neuron that might well cause a sublethal disorder of intestinal motility when they develop abnormally. Serotonergic neurons are among the first neurons to be born during ENS ontogeny.104 Mucosal enterochromaffin cells (EC) cells, which are the body’s largest depot of serotonin,105,106 also arise while neurons are being generated107; therefore, it is possible that the activity-dependent secretion of serotonin by early-developing serotonergic neurons and/or EC cells could affect the development/survival of subsets of later-developing neurons, particularly those neurons that are generated postnatally. Three serotonin receptor subtypes have been found to be expressed in the fetal ENS, 5-HT2A, 5-HT2B, and 5-HT4.108–111 Of these, the 5-HT2B receptor appears to be most important from a developmental point of view.108 Serotonin and a nonselective 5-HT2 agonist, (+/−)-2,5-dimethoxy-4-iodoamphetamine. HCl (DOI), enhance in vitro development of enteric neurons, both in dissociated cultures of mixed enteric cells and in cultures of E14 crest-derived cells isolated from the fetal mouse gut. The effects of each of these agonists are blocked by the 5-HT1/2 antagonist methysergide, the pan-5-HT2 antagonist ritanserin, and the 5-HT2B/2C-selective antagonist SB206553. In contrast, the 5-HT2A-selective antagonist, ketanserin, does not abolish the developmental effects of 5-HT. 5-HT induces the nuclear translocation of mitogen-activated protein kinase. This effect, like that on neuronal development, is blocked by ritanserin. Because serotonergic agonists promote neurogenesis/survival in cultures of isolated crest-derived precursors, the effects of serotonin are direct and not mediated by way of nonneuronal cells from the wall of the bowel. Transcripts encoding 5-HT2B receptors can be detected in the fetal bowel, but those encoding the 5-HT2C receptor cannot. Because the 5-HT2C receptor is not expressed in the gut, the observation that the effects of 5-HT and DOI are blocked by SB206553, strongly supports the conclusion that 5-HT-promoted neurogenesis is 5-HT2B-mediated. mRNA encoding the 5-HT2B receptor and 5-HT2B immunoreactivity are abundant in developing (E15–E16) but not in mature enteric ganglia where 5-HT2B receptors are present only in ~1/4 ganglia. 5-HT2B-immunoreactive cells all express the neuronal marker, PGP9.5 and thus are neurons. Because both 5-HT and a developmentally regulated 5-HT receptor, 5-HT2B, which promotes neuronal development, are simultaneously expressed in the fetal gut, it is possible that by stimulating 5-HT2B receptors, 5-HT affects the fates of some of the large number of enteric neurons that arise after the development of endogenous sources of 5-HT. The late action of serotonin is compatible with the possibility that it affects only subsets of enteric neurons, which express 5-HT2B receptors and give rise to the limited type of defect that could be associated with IBS or another subtle disorder of intestinal motility.
5-HT2B knockout animals survive poorly due to cardiac abnormalities.112 Defects in the gut of these animals are still being characterized; however the ENS appears to be deficient in serotonergic neurons. In contrast to 5-HT2B −/− mice, the 5-HT2A-deficient bowel appears to be normal at birth but enteric smooth muscle degenerates as the animals age109; the mice also exhibit smaller than normal enterocytes and a markedly reduced number of Paneth cells in the small intestine. A striking defect occurs in the colon of transgenic mice lacking 5-HT4 receptors.110,111 The wall of the bowel is smaller than normal, a defect mainly due to thinning of the muscle layers and the number of neurons is markedly reduced, suggesting that the 5-HT4, like the 5-HT2B receptor, may play a role the ontogeny of the enteric neuromuscular apparatus.
Future directions
There are several factors which are expressed and/or required in ENS development, but which have not yet been associated with dysmotilities. These molecules, which are undoubtedly subject to mutations, are likely to be linked to neuro-enteric diseases in the near future. They include bone morphogenetic factors (BMPs-2 and -4; their receptors, and antagonists),113,114 guidance molecules (netrins),115 the b-HLH transcription factor, Hand2,116–118 and synaptic cell adhesion proteins, β-neurexins (presynaptic receptors)/neuroligins (postsynaptic ligands).119
BMP Signaling
BMPs-2 and -4 have been demonstrated to play critical early roles in the epithelial-mesenchymal interactions that shape the morphogenesis and regionalization of the gut.120,121 BMPs also continue to be important in the mature bowel, where they are expressed in the mucosal connective tissue, and function in the formation of intestinal crypts122 and influence colonic epithelial cells.123 When regulated abnormally, mucosal BMPs have been linked to the development of juvenile polyposis, which is precancerous122 and to the formation of adenomas of the colon.123 In addition to their role in early development and in the regulation of mucosal growth, BMPs-2 and -4 have recently been found to act late in ontogeny to regulate ENS development.113 BMPs-2 and -4 (but not BMP-7) are expressed in the developing ENS as are the BMP receptors, BMPRs IA, IB, and II. In particular, the BMPs-2 and -4 act on crest-derived cells to promote neuronal differentiation, but with a bell-shaped concentration-effect relationship that suggests that the concentrations of BMPs in the immediate microenvironments of crest-derived neuronal precursors is critical. The simultaneous expression in the fetal bowel of BMP antagonists (noggin, gremlin, follistatin, chordin) with the BMPs and their receptors suggests that the effective enteric BMP concentration is controlled within strict limits. The BMPs act alone and also interact with GDNF/GFRα1/Ret, to enhance neuronal differentiation; however, by doing so, BMPs limit the extent of the GDNF-driven proliferation of crest-derived precursors. The BMPs, furthermore, specify the development of the lineage of enteric neurons that is characterized by TrkC expression and NT-3-dependence. The physiological significance of BMP signaling in ENS development was investigated in transgenic mice that overexpress the BMP antagonist, noggin, directed to the developing nervous system by the promoter for neuron specific enolase (NSE-noggin mice). It is impossible to study the effects of the knockout of BMPs on late events in ontogeny because of the critical early actions of these molecules. A conditional knockout or other means of inducing a time-dependent loss of BMP function is therefore required. Because NSE is not expressed by early precursors, but only by committed neurons,124 the overexpression of noggin in NSE-noggin mice does not occur in the bowel until the first enteric neurons arise. Enteric neurons are generated sequentially over a long period of time that begins before the earliest émigrés enters the bowel (at E8.5 in mice) and continues through at least the first 3 weeks of postnatal life.104 As a result, the function of BMPs is not impaired during the early development of the gut and noggin expression is directed to the ENS and not the mucosa during the process of neurogenesis. The NSE-noggin mice, therefore, provide a means of evaluating the development of the ENS in the absence of BMP stimulation. These studies have shown that by fostering differentiation in vivo BMPs-2 and -4 oppose the GDNF-driven expansion of the enteric neuronal precursor population and thus limit the ultimate size of the ENS.113 At the same time, the BMPs promote the development of the critical TrkC-expressing subset of neurons (that gives rise to submucosal IPANs). Actions of the BMPs in the bowel, moreover, are not ENS-restricted; they also promote the development of enteric smooth muscle, which, like crest-derived precursors, expresses BMPRs IA, IB, and II.
Netrin guidance
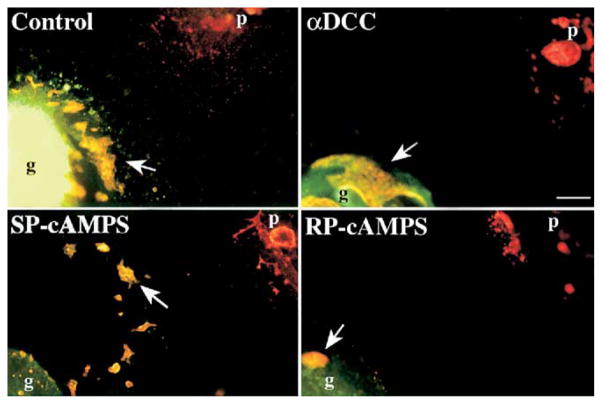
Vagal neural crest-derived precursors of the ENS colonize the bowel by descending within the enteric mesenchyme. Perpendicular secondary migrations, toward the mucosa115,125–127 and into the pancreas,128 result, respectively, in the formation of submucosal and pancreatic ganglia. This perpendicular migration is in part mediated by netrins, which are expressed in the mucosa of the fetal bowel and in the mesenchyme of the pancreas.115 Netrins are better known as guidance molecules in the CNS, where they attract and/or repel different populations of axons or neural precursors.129–131 A critical receptor for netrins is deleted in colorectal cancer (DCC).132,133 Crest-derived cells colonizing the bowel express DCC.115 A subset of enteric crest-derived cells migrates toward sources of netrin and antibodies to DCC block this chemoattraction. In vitro, crest-derived cells migrate from the outer gut mesenchyme toward the mucosa and others migrate out of the bowel toward a cocultured explant of pancreas (Figure 2); both of these migrations are blocked by antibodies to DCC or by the PKA antagonist, RP-cAMPS, which interferes with DCC signal transduction.134 Finally, the submucosal and pancreatic plexuses fail to develop in transgenic mice that lack DCC (Figure 3).115 The chemoattraction of DCC-expressing crest-derived cells is thus necessary for the formation of the submucosal plexus and for the colonization of the pancreas by neural precursors from gut.
Figure 2.
Anti-DCC and RP-cAMPS block the selective migration of crest-derived cells from gut to pancreas. Explants of fetal mouse stomach and duodenum were co-cultured with pancreatic rudiments. G, gut; p, pancreas; arrows, crest-derived cells. Scale bar, 100 μm. (From Jiang and coworkers.115)
Figure 3.
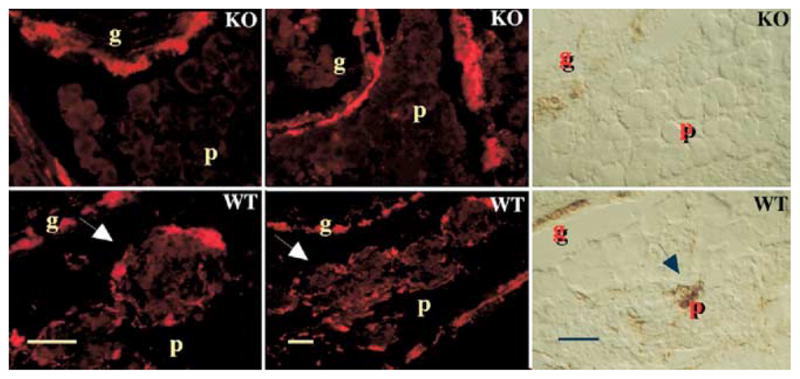
The submucosal and pancreatic plexuses are absent in transgenic mice that lack DCC. WT, wild-type; KO, DCC −/−; g, gut; p, pancreas; arrows, pancreatic ganglia. Neurons were shown by demonstrating PGP9.5 immunoreactivity (red) or acetylcholinesterase activity (brown). Scale bars, 100 μm. (From Jiang and coworkers.115)
Hand2 dependence
Hand2 (also called dHand) is expressed in the bowel exclusively by crest-derived cells118,135; only smooth muscle and interstitial cells of Cajal (ICCs) express Hand1 (also called eHand).135 Mice that lack Hand2 die of cardiovascular abnormalities at E10.5116; however, the gut is colonized at E9 to 9.5.1,2,88,89,136 For this reason, the bowel can be removed at E9.5 from Hand2 −/− mice and the effect of the gene can be studied on the in vitro development of neurons.135 E9.5 explants of bowel from both wild-type and Hand2 −/− mice contain cells that express Phox2b, Sox10, Ret, and p75NTR, confirming that crest-derived cells colonize the Hand2 −/− bowel as well as the wild-type gut; nevertheless, neurons arise only in cultured explants from wild-type mice and not in cultured explants from Hand2 −/− animals. Similarly, siRNA directed against Hand2 opposes Hand2 expression and prevents the in vitro development of neurons from crest-derived cells isolated from wild-type mice. Hand2 expression is thus necessary for the differentiation of enteric neurons (Figure 1). The effect is specific, because Hand2 knockout does not prevent the development of neurons from somite-derived crest-derived cells (predominantly DRG precursors) cocultured with gut.
Synapse formation in the ENS: β-neurexin and neuroligin expression
Although the abundance of synaptic connections in the ENS is far less than in the CNS, it is still large; moreover, it is necessary that the number, location, and function of these connections be tightly controlled. Synaptic circuits have to be formed in a very reproducible manner to give rise to a reproducible behavioral output in the form of peristaltic activity, segmentation, secretory reflexes, and neuro-immune interactions. This reproducibility implies that cellular processes must exist to determine the identity and connectivity of each enteric neuron. The usual pattern of synaptic development involves the initial formation of synapses, which may be temporary, followed by later processes that modify synaptic function, including synapse elimination, plasticity, and growth.137–139 Synapses are extremely asymmetrical structures; the cytosolic surfaces of both pre- and postsynaptic membranes are lined with scaffolds.140–142 Several of the proteins in these scaffolds are very important in the assembly and maturation of synapses. The synaptic cleft also contains an array of glycoproteins, comprised, in part, of the extracellular domains of receptor-ligand complexes that directly couple presynaptic active zones to postsynaptic densities. Adherence of pre- and postsynaptic elements is further enhanced by puncta adherens at sites lateral to active zones. During development, the cell-to-cell interactions responsible for synapse formation display considerable promiscuity; vertebrate neurons form synapses in vitro with other neurons, regardless of whether they normally do so in situ.137,138 This phenomenon suggests that there is a “basic machinery” of synapse formation. It has been proposed that pre- and postsynaptic partners respond to a combination of synapse-promoting and synapse-inhibiting signals, ultimately to form synapses at the sites with the best available combination.137 Signals appear to operate on 3 levels: (1) attraction and repulsion of growing axons by molecules such as netrins, semaphorins, or ephrins to bring the axons to the correct locations129; (2) formation of incipient synaptic connections through interactions of transmembrane pre- and postsynaptic molecules143–145; (3) activity-dependent synapse stabilization or elimination to refine mature circuits.138,146 After initial axon-target interactions bring axons to the correct vicinity, cell-surface receptor systems (adhesion and signaling) can mediate the coordination of the differentiation of pre- and postsynaptic membrane specializations. Trans-synaptic signaling must lead to the precisely aligned juxtaposition of pre- and postsynaptic membrane specializations that enable neurotransmission to be efficient; moreover, postsynaptic receptors must be matched to the transmitter released by the presynaptic axon. Directionality is established with fundamentally different structures assembled on the pre- and postsynaptic sides of the synapse. After initial synapses form, those made at the proper sites on appropriate cells must be stabilized, while synapses that are incorrect or redundant are eliminated. Homophilic interactions between matching cadherins or Ig-domain proteins appear to promote specific interactions between correct partners143,144; these specific interactions evidently act synergistically with a general synaptogenic mechanism common to all, or to most neurons.137 The general mechanism is heterophilic, which introduces directionality into the process of differentiation.
Neuroligins and neurexins have proven to be good candidates to be part of the heterophilic general mechanism of synapse formation in the CNS (Figure 4). Both of these protein families are widely distributed in the brain. Neurexins are transmembrane proteins of which the first to be discovered were β-neurexins, the receptors for α-latrotoxin (black widow spider venom).147 There are many neurexins. In mice, 3 genes encoding neurexins are each transcribed from 2 alternative promoters to give rise to 6 primary transcripts. From these transcripts, alternative splicing generates more than 103 α- and β-neurexin isoforms.148 The β-neurexin subset that lacks a splice insertion in splice site 4 binds to neuroligins, a family of transmembrane synaptic proteins149,150 with sequence homology to cholinesterases (but which function in protein-protein interactions and lack enzyme activity).151 Three genes in rodents and 5 genes in humans encode neuroligins. Neuroligin-1 has been localized to the postsynaptic membranes of excitatory synapses in the CNS. Neuroligins and β-neurexins function as Ca2+-dependent heterophilic adhesion molecules in cell aggregation assays, an observation that is consistent with the idea that they mediate synaptic adhesion.152,153 More significantly, overexpression of neuroligin in dissociated cultures of hippocampal or cerebellar neurons causes recruitment of β-neurexins to sites of cell-to-cell contact and a massive increase in the number of synapses.119,137 The formation of synapses in these cultures, furthermore, is inhibited by addition of recombinant soluble β-neurexin, which competes with endogenous β-neurexins for binding to neuroligins. The interaction of β-neurexins with neuroligins is sufficient to induce presynaptic differentiation because neuroligins can induce β-neurexin-expressing axons to form synapses even when expressed in nonneuronal cells.154 Neuroligins and β-neurexins both interact with cytosolic scaffolding proteins that are components of the transduction/effector machinery involved in synapse formation. Neuroligins bind to PSD-95,155 a component of postsynaptic densities that can recruit additional PSD proteins.142 Neuroligin activity depends on lateral clustering, which may cluster the molecules of β-neurexin to which they bind across the nascent synaptic gap. This clustering may recruit cytosolic scaffolding molecules, which interact directly with the cytosolic tail of β-neurexins.156–158
Figure 4.
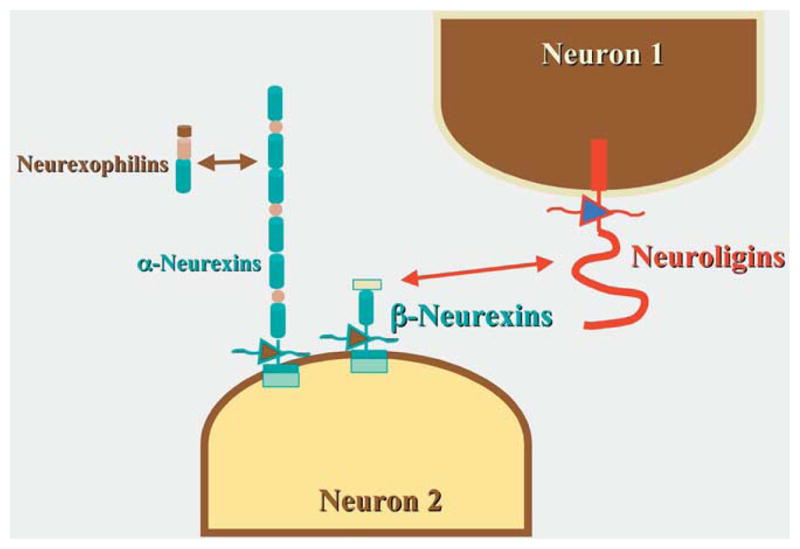
Interactions between neuroligins and β-neurexins are important general mechanisms in synapse formation. The neurexin family of proteins is a large one; however, only α-neurexins interact with neuroligins and play roles in synaptogenesis. β-Neurexins are important in calcium entry and interact with α-latrotoxin and neurexophilins, which are soluble.
Little is known about the mechanisms responsible for the formation of ENS synapses. Studies have just begun to be performed on the expression of β-neurexins and neuroligins in the bowel. The strong analogy between the CNS and ENS, however, provides a rationale for supposing that a general mechanism that functions in the formation of CNS synapses may function similarly in the formation of ENS synapses. This supposition is strongly supported by preliminary data (F. D’Autréaux, T.R. Gershon and M.D. Gershon, personal observations), which indicates that the splice variants of β-neurexins that can interact with neuroligins as well as all 3 rodent neuroligins are expressed in the bowel of fetal rat. The hypothesis that the interaction between β-neurexins and neuroligins is necessary and sufficient to trigger the formation of ENS synapses takes on added significance because of the observation that genes encoding neuroligins are mutated in cases of autism and that the autism-associated mutations lead to a loss of function in the molecules.159–162 GI dysfunction occurs frequently in children with autism spectrum disorders163–169; this association has led to the proposal that regressive autism is caused by the persistence of live measles virus in the bowel following vaccination against measles, mumps, and rubella (MMR).164,169–171 This hypothesis posits that viral persistence causes the gut to “leak,” leading to the absorption of peptides aberrantly formed in the intestinal lumen, which are postulated to disturb the forming brain, causing autism. Given the peptidases of enterocytes, the ability of the liver to take up and detoxify peptides, the blood-brain barrier, and the lack of known luminal peptides that cause an autism-like syndrome in animals, the “leaky gut” hypothesis lacks plausibility (M.D. Gershon, testimony US Congress Committee and Government Reform, 2002). There is also evidence against its core supposition172 and the relationship of autism to MMR vaccination.173 Still, even if unfounded, the frightening suggestion that vaccination might cause autism has adversely affected vaccine acceptance. It would thus be very important (and reassuring) to gain insight into a plausible alternative that might actually be the cause of the association of autism with GI dysfunction. If as is likely, mechanisms that govern the formation of synapses in the ENS and CNS are similar, then a neuroligin mutation that disturbs synapse formation and contributes to autism would also be expected to lead to GI malfunction.
Conclusions
The ENS is far more complex than extraenteric peripheral ganglia and quite different from them. There is a strong functional, ultrastructural, and chemical resemblance between the ENS and the CNS. It follows that the mechanisms that govern the development of the ENS are different from those responsible for the remainder of the PNS. Many of the genes, which when mutated, give rise to Hirschsprung’s disease have been identified. Unfortunately the list, while long, is still incomplete. One reason that many different mutations can result in the same disease, Hirschsprung’s, is that the bowel and the ENS are limited in their repertoire of responses to insults. Another is that all of the genes that are associated with the acquisition of Hirschsprung’s disease except those related to EDN3 signaling are required by early precursors of enteric neurons. These early cells are multipotent and give rise to all of the phenotypes of neurons that populate the bowel. A loss of the function of these early genes thus results in pan-neuronal lesions in which segments of bowel become aganglionic. It is plausible that the aganglionic regions are located in the terminal bowel because that is the last region of the gut to be colonized by émigrés from the neural crest. The length of the resulting defect presumably reflects the degree to which the function of the defective gene is compromised. Why EDN3 signaling gives rise to Hirschsprung’s disease is controversial. EDN3 and its related biosynthetic converting enzyme (ECE1) and receptor EDNRB may cause aganglionosis because EDN3 prevents premature differentiation of enteric neuronal precursors, because EDNRB interactions are required to enable Ret signaling to be sufficient, or because EDNRB stimulation enables crest-derived cells that are migrating along a GDNF gradient to escape becoming trapped by the high concentration of GDNF in the cecum. In any case, it is important to distinguish the effects of the early gene mutations that result in aganglionosis from the later mutations that affect development of specific subsets of enteric neurons, their guidance, or the synapses they form. These latter mutations do not cause the bowel to become aganglionic and their effects may be subtle and inapparent when the gut is examined by the routine methods of pathology. If the loss of function of a late-acting gene results in a defect in the development of a critical type of neuron, such as the submucosal IPAN that initiates peristaltic and secretory reflexes, which is NT-3/TrkC-dependent, then the resulting intestinal defect will be catastrophic. Such a defect, as yet unrecognized, could be the cause of CIP in which intestinal motility fails despite the presence of both ganglia and smooth muscle. Defects in neurons that modulate motility, but are not required for its manifestation, are compatible with life, but may give rise to dysmotility syndromes or IBS. Similarly, problems with synapse formation, common to the ENS and CNS, may enable both to function, but suboptimally resulting, for example in autism and autistic colitis. The rapid progress of current research on the ENS and its development, both molecular and physiological, promises that a subsequent review of this subject will be far more specific and full of treatments and schemes for disease prevention than can be managed right now.
Acknowledgments
Supported by grants from the National Institutes of Health, R01-NS15547 and R01-NS12969 (to M.D.G.) and K12-HD00850 (to E.M.R.).
References
- 1.Newgreen D, Young HM. Enteric nervous system: Development and developmental disturbances—part 2. Pediatr Dev Pathol. 2002;5:329–349. doi: 10.1007/s10024-002-0002-4. [DOI] [PubMed] [Google Scholar]
- 2.Newgreen D, Young HM. Enteric nervous system: Development and developmental disturbances—part 1. Pediatr Dev Pathol. 2002;5:224–247. doi: 10.1007/s10024-001-0142-y. [DOI] [PubMed] [Google Scholar]
- 3.Gariepy CE. Developmental disorders of the enteric nervous system: Genetic and molecular bases. J Pediatr Gastroenterol Nutr. 2004;39:5–11. doi: 10.1097/00005176-200407000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Gershon MD. Functional anatomy of the enteric nervous system: A developmental perspective relevant to the pathogenesis of Hirschsprung’s disease. In: Holschneider AM, Puri P, editors. Hirschsprung’s Disease and Allied Disorders. Amsterdam, The Netherlands: Harwood Academic Publishers; 2000. pp. 19–58. [Google Scholar]
- 5.Parisi MA, Kapur RP. Genetics of Hirschsprung disease. Curr Opin Pediatr. 2000;12:610–617. doi: 10.1097/00008480-200012000-00017. [DOI] [PubMed] [Google Scholar]
- 6.Amiel J, Lyonnet S. Hirschsprung disease, associated syndromes, and genetics: A review. J Med Genet. 2001;38:729–739. doi: 10.1136/jmg.38.11.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holschneider AM, Meier-Ruge W, Ure BM. Hirschsprung’s disease and allied disorders-a review. Eur J Pediatr Surg. 1994;4:260–266. doi: 10.1055/s-2008-1066115. [DOI] [PubMed] [Google Scholar]
- 8.Gershon MD. The Second Brain. New York, NY: Harper Collins; 1998. [Google Scholar]
- 9.Gershon MD, Kirchgessner AL, Wade PR. Functional anatomy of the enteric nervous system. In: Johnson LR, Alpers DH, Jacobson ED, Walsh JH, editors. Physiology of the Gastrointestinal Tract. 3. New York: Raven Press; 1994. pp. 381–422. [Google Scholar]
- 10.Gershon MD. Plasticity in serotonin control mechanisms in the gut. Curr Opin Pharmacol. 2003;3:600–607. doi: 10.1016/j.coph.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Furness JB. Types of neurons in the enteric nervous system. J Auton Nerv Syst. 2000;81:87–96. doi: 10.1016/s0165-1838(00)00127-2. [DOI] [PubMed] [Google Scholar]
- 12.Costa M, Brookes SJ, Hennig GW. Anatomy and physiology of the enteric nervous system. Gut. 2000;47(suppl 4):15–19. doi: 10.1136/gut.47.suppl_4.iv15. discussion 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Powley TL, Phillips RJ. Musings on the wanderer: What’s new in our understanding of vago-vagal reflexes? I. Morphology and topography of vagal afferents innervating the GI tract. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1217–G1225. doi: 10.1152/ajpgi.00249.2002. [DOI] [PubMed] [Google Scholar]
- 14.Van Laere K, Vonck K, Boon P, et al. Vagus nerve stimulation in refractory epilepsy: SPECT activation study. J Nucl Med. 2000;41:1145–1154. [PubMed] [Google Scholar]
- 15.Binnie CD. Vagus nerve stimulation for epilepsy: A review [editorial] Seizure. 2000;9:161–169. doi: 10.1053/seiz.1999.0354. [DOI] [PubMed] [Google Scholar]
- 16.George MS, Sackeim HA, Rush AJ, et al. Vagus nerve stimulation: A new tool for brain research and therapy [see comments] Biol Psychiatry. 2000;47:287–295. doi: 10.1016/s0006-3223(99)00308-x. [DOI] [PubMed] [Google Scholar]
- 17.Rush AJ, George MS, Sackeim HA, et al. Vagus nerve stimulation (VNS) for treatment-resistant depressions: A multicenter study [see comments] Biol Psychiatry. 2000;47:276–286. doi: 10.1016/s0006-3223(99)00304-2. [DOI] [PubMed] [Google Scholar]
- 18.Rosenbaum JF, Heninger G. Vagus nerve stimulation for treatment-resistant depression [editorial; comment] Biol Psychiatry. 2000;47:273–275. doi: 10.1016/s0006-3223(99)00318-2. [DOI] [PubMed] [Google Scholar]
- 19.Clark KB, Naritoku DK, Smith DC, et al. Enhanced recognition memory following vagus nerve stimulation in human subjects. Nat Neurosci. 1999;2:94–98. doi: 10.1038/4600. [DOI] [PubMed] [Google Scholar]
- 20.Gershon MD. Disorders of enteric neuronal development: insights from transgenic mice. Am J Physiol. 1999;40:G262–G267. doi: 10.1152/ajpgi.1999.277.2.G262. [DOI] [PubMed] [Google Scholar]
- 21.Bülbring E, Lin RCY, Schofield G. An investigation of the peristaltic reflex in relation to anatomical observations. Q J Exp Physiol. 1958;43:26–37. doi: 10.1113/expphysiol.1958.sp001305. [DOI] [PubMed] [Google Scholar]
- 22.Kirchgessner AL, Tamir H, Gershon MD. Identification and stimulation by serotonin of intrinsic sensory neurons of the submucosal plexus of the guinea pig gut: Activity-induced expression of Fos immunoreactivity. J Neurosci. 1992;12:235–249. doi: 10.1523/JNEUROSCI.12-01-00235.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirchgessner AL, Liu M-T, Gershon MD. In situ identification and visualization of neurons that mediate enteric and enteropancreatic reflexes. J Comp Neurol. 1996;371:270–286. doi: 10.1002/(SICI)1096-9861(19960722)371:2<270::AID-CNE7>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 24.Pan H, Gershon MD. Activation of intrinsic afferent pathways in submucosal ganglia of the guinea pig small intestine. J Neurosci. 2000;20:3295–3309. doi: 10.1523/JNEUROSCI.20-09-03295.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kunze WA, Bornstein JC, Furness JB. Identification of sensory nerve cells in a peripheral organ (the intestine) of a mammal. Neuroscience. 1995;66:1–4. doi: 10.1016/0306-4522(95)00067-s. [DOI] [PubMed] [Google Scholar]
- 26.Kuntz A. The Autonomic Nervous System. Philadelphia, PA: Lea and Febiger; 1953. [Google Scholar]
- 27.Kosterlitz HW, Lees GM. Pharmacological analysis of intrinsic intestinal reflexes. Pharmacol Rev. 1964;16:301–339. [PubMed] [Google Scholar]
- 28.Furness JB, Costa M. The Enteric Nervous System. New York: Churchill Livingstone; 1987. [Google Scholar]
- 29.Bayliss WM, Starling EH. The movements and innervation of the small intestine. J Physiol (Lond) 1899;24:99–143. doi: 10.1113/jphysiol.1899.sp000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bayliss WM, Starling EH. The movements and innervation of the small intestine. J Physiol (Lond) 1900;26:125–138. doi: 10.1113/jphysiol.1901.sp000827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bayliss WM, Starling EH. The movements and innervation of the large intestine. J Physiol (Lond) 1900;26:107–118. doi: 10.1113/jphysiol.1900.sp000825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Auerbach L. Vorläufige Mitteilung. Breslau: E. Morgenstern; 1862. Über einen Plexus myentericus, einen bisher unbekannten ganglio-nervösen Apparat im Darmkanal der Wirbeltiere. [Google Scholar]
- 33.Auerbach L. Fernere vorlaufige Mittielung über den Nervenapparat des Darmes. Arch Pathol Anat Physiol. 1864;30:457–460. [Google Scholar]
- 34.Meissner G. Über die Nerven der Darmwand. Z Ration Med. 8:1857. [Google Scholar]
- 35.Trendelenburg P. Physiologische und pharmakologische Versuche über die Dünndarm Peristaltick. Naunyn-Schmiedebergs Arch. Exp Pathol Pharmakol. 1917;81:55–129. doi: 10.1007/s00210-006-0052-7. [DOI] [PubMed] [Google Scholar]
- 36.Langley JN. The Autonomic Nervous System. Part 1. Cambridge: W. Heffer; 1921. [Google Scholar]
- 37.Gershon MD, Drakontides AB, Ross LL. Serotonin: Synthesis and release from the myenteric plexus of the mouse intestine. Science. 1965;149:197–199. doi: 10.1126/science.149.3680.197. [DOI] [PubMed] [Google Scholar]
- 38.Burnstock G, Gershon MD, Hokfelt T, et al. Non-adrenergic, non-cholinergic neurotransmission mechanisms. Neurosci Res Prog Bull. 1979;17:383–519. [PubMed] [Google Scholar]
- 39.Gabella G. Fine structure of the myenteric plexus of the guinea-pig ileum. J Anat (Lond) 1972;111:69–97. [PMC free article] [PubMed] [Google Scholar]
- 40.Gabella G. On the ultrastructure of the enteric nerve ganglia. Scand J Gastroenterol Suppl. 1982;71:15–25. [PubMed] [Google Scholar]
- 41.Gabella G. Structure of muscles and nerves in the gastrointestinal tract. In: Johnson LR, Alpers DH, Jacobson ED, Walsh JH, editors. Physiology of the Gastrointestinal Tract. 3. New York: Raven Press; 1994. pp. 751–793. [Google Scholar]
- 42.Yntema CL, Hammond WS. The origin of intrinsic ganglia of trunk viscera from vagal neural crest in the chick embryo. J Comp Neurol. 1954;101:515–542. doi: 10.1002/cne.901010212. [DOI] [PubMed] [Google Scholar]
- 43.Yntema CL, Hammond WS. Experiments on the origin and development of the sacral autonomic nerves in the chick embryo. J Exp Zool. 1955;129:375–414. [Google Scholar]
- 44.Le Douarin NM, Teillet MA. The migration of neural crest cells to the wall of the digestive tract in avian embryo. J Embryol Exp Morphol. 1973;30:31–48. [PubMed] [Google Scholar]
- 45.Le Douarin NM, Teillet MA. Experimental analysis of the migration and differentiation of neuroblasts of the autonomic nervous system and of neurectodermal mesenchymal derivatives, using a biological cell marking technique. Dev Biol. 1974;41:162–184. doi: 10.1016/0012-1606(74)90291-7. [DOI] [PubMed] [Google Scholar]
- 46.Le Douarin NM, Kalcheim C. The Neural Crest. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- 47.Pomeranz HD, Gershon MD. Colonization of the avian hindgut by cells derived from the sacral neural crest. Dev Biol. 1990;137:378–394. doi: 10.1016/0012-1606(90)90262-h. [DOI] [PubMed] [Google Scholar]
- 48.Pomeranz HD, Rothman TP, Gershon MD. Colonization of the post-umbilical bowel by cells derived from the sacral neural crest: Direct tracing of cell migration using an intercalating probe and a replication-deficient retrovirus. Development. 1991;111:647–655. doi: 10.1242/dev.111.3.647. [DOI] [PubMed] [Google Scholar]
- 49.Serbedzija GN, Burgan S, Fraser SE, et al. Vital dye labeling demonstrates a sacral neural crest contribution to the enteric nervous system of chick and mouse embryos. Development. 1991;111:857–866. doi: 10.1242/dev.111.4.857. [DOI] [PubMed] [Google Scholar]
- 50.Burns AJ, Le Douarin NM. The sacral crest contributes neurons and glia to the post-umbilical gut: spatiotemporal analysis of the development of the enteric nervous system. Development. 1998;125:4335–4347. doi: 10.1242/dev.125.21.4335. [DOI] [PubMed] [Google Scholar]
- 51.Hearn C, Newgreen D. Lumbo-sacral neural crest contributes to the avian enteric nervous system independently of vagal neural crest. Dev Dyn. 2000;218:525–530. doi: 10.1002/1097-0177(200007)218:3<525::AID-DVDY1003>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 52.Kapur RP. Colonization of the murine hindgut by sacral crest-derived neural precursors: Experimental support for an evolutionarily conserved model. Dev Biol. 2000;227:146–155. doi: 10.1006/dbio.2000.9886. [DOI] [PubMed] [Google Scholar]
- 53.Durbec PL, Larsson-Blomberg LB, Schuchardt A, et al. Common origin and developmental dependence on c-ret of subsets of enteric and sympathetic neuroblasts. Development. 1996;122:349–358. doi: 10.1242/dev.122.1.349. [DOI] [PubMed] [Google Scholar]
- 54.Weston JA. Sequential segregation and fate of developmentally restricted intermediate cell populations in the neural crest lineage. Curr Top Dev Biol. 1991;25:133–153. doi: 10.1016/s0070-2153(08)60414-7. [DOI] [PubMed] [Google Scholar]
- 55.Henion PD, Weston JA. Timing and pattern of cell fate restrictions in the neural crest lineage. Development. 1997;124:4351–4359. doi: 10.1242/dev.124.21.4351. [DOI] [PubMed] [Google Scholar]
- 56.Skinner MA. Hirschsprung’s disease. Curr Probl Surg. 1996;33:389–460. doi: 10.1016/s0011-3840(96)80009-8. [DOI] [PubMed] [Google Scholar]
- 57.Sullivan PB. Hirschsprung’s disease. Arch Dis Child. 1996;74:5–7. doi: 10.1136/adc.74.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Teitelbaum DH. Hirschsprung’s disease in children. Curr Opin Pediatr. 1995;7:316–322. doi: 10.1097/00008480-199506000-00014. [DOI] [PubMed] [Google Scholar]
- 59.McCallion AS, Stames E, Conlon RA, et al. Phenotype variation in two-locus mouse models of Hirschsprung disease: Tissue-specific interaction between Ret and Ednrb. Proc Natl Acad Sci U S A. 2003;100:1826–1831. doi: 10.1073/pnas.0337540100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lang D, Chen F, Milewski R, et al. Pax3 is required for enteric ganglia formation and functions with Sox10 to modulate expression of c-ret. J Clin Invest. 2000;106:963–971. doi: 10.1172/JCI10828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Garcia-Barcelo M, Sham MH, Lui VC, et al. Association study of PHOX2B as a candidate gene for Hirschsprung’s disease. Gut. 2003;52:563–567. doi: 10.1136/gut.52.4.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barlow A, de Graaff E, Pachnis V. Enteric nervous system progenitors are coordinately controlled by the G protein-coupled receptor EDNRB and the receptor tyrosine kinase RET. Neuron. 2003;40:905–916. doi: 10.1016/s0896-6273(03)00730-x. [DOI] [PubMed] [Google Scholar]
- 63.Zhu L, Lee HO, Jordan CS, et al. Spatiotemporal regulation of endothelin receptor-B by SOX10 in neural crest-derived enteric neuron precursors. Nat Genet. 2004;36:732–737. doi: 10.1038/ng1371. [DOI] [PubMed] [Google Scholar]
- 64.Kim J, Lo L, Dormand E, et al. SOX10 maintains multipotency and inhibits neuronal differentiation of neural crest stem cells. Neuron. 2003;38:17–31. doi: 10.1016/s0896-6273(03)00163-6. [DOI] [PubMed] [Google Scholar]
- 65.Chalazonitis A, Pham TD, Rothman TP, et al. Neurotrophin-3 is required for the survival-differentiation of subsets of developing enteric neurons. J Neurosci. 2001;21:5620–5636. doi: 10.1523/JNEUROSCI.21-15-05620.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Blaugrund E, Pham TD, Tennyson VM, et al. Distinct subpopulations of enteric neuronal progenitors defined by time of development, sympathoadrenal lineage markers, and Mash-1-dependence. Development. 1996;122:309–320. doi: 10.1242/dev.122.1.309. [DOI] [PubMed] [Google Scholar]
- 67.Baynash AG, Hosoda K, Giaid A, et al. Interaction of endothelin-3 with endothelin-B receptor is essential for development of epidermal melanocytes and enteric neurons. Cell. 1994;79:1277–1285. doi: 10.1016/0092-8674(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 68.Hosoda K, Hammer RE, Richardson JA, et al. Targeted and natural (piebald-lethal) mutation of endothelin-B receptor produce megacolon associated with spotted coat color in mice. Cell. 1994;79:1267–1276. doi: 10.1016/0092-8674(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 69.Puffenberger EG, Hosoda K, Washington SS, et al. A missense mutation of the endothelin-receptor gene in mutagenic Hirschsprung’s disease. Cell. 1994;79:1257–1266. doi: 10.1016/0092-8674(94)90016-7. [DOI] [PubMed] [Google Scholar]
- 70.Angrist M, Bolk S, Thiel B, et al. Mutation analysis of the RET receptor tyrosine kinase in Hirschsprung disease. Hum Mol Genet. 1995;4:821–830. doi: 10.1093/hmg/4.5.821. [DOI] [PubMed] [Google Scholar]
- 71.Kuhlbrodt K, Schmidt C, Sock E, et al. Functional analysis of Sox10 mutations found in human Waardenburg-Hirschsprung patients. J Biol Chem. 1998;273:23033–23038. doi: 10.1074/jbc.273.36.23033. [DOI] [PubMed] [Google Scholar]
- 72.Martucciello G, Ceccherini I, Lerone M, et al. Pathogenesis of Hirschsprung’s disease. J Pediatr Surg. 2000;35:1017–1025. doi: 10.1053/jpsu.2000.7763. [DOI] [PubMed] [Google Scholar]
- 73.Pingault V, Bondurand N, Kuhlbrodt K, et al. SOX10 mutations in patients with Waardenburg-Hirschsprung disease. Nat Genet. 1998;18:171–173. doi: 10.1038/ng0298-171. [DOI] [PubMed] [Google Scholar]
- 74.Southard-Smith EM, Angrist M, Ellison JS, et al. The Sox10(Dom) mouse: Modeling the genetic variation of Waardenburg-Shah (WS4) syndrome. Genome Res. 1999;9:215–225. [PubMed] [Google Scholar]
- 75.Wu JJ, Chen J-X, Rothman TP, et al. Inhibition of in vitro enteric neuronal development by endothelin-3: Mediation by endothelin B receptors. Development. 1999;126:1161–1173. doi: 10.1242/dev.126.6.1161. [DOI] [PubMed] [Google Scholar]
- 76.Kruger GM, Mosher JT, Tsai YH, et al. Temporally distinct requirements for endothelin receptor B in the generation and migration of gut neural crest stem cells. Neuron. 2003;40:917–929. doi: 10.1016/s0896-6273(03)00727-x. [DOI] [PubMed] [Google Scholar]
- 77.Heuckeroth RO. Finding your way to the end. A tale of GDNF and endothelin-3. Neuron. 2003;40:871–873. doi: 10.1016/s0896-6273(03)00763-3. [DOI] [PubMed] [Google Scholar]
- 78.Young HM, Hearn CJ, Farlie PG, et al. GDNF is a chemoattractant for enteric neural cells. Dev Biol. 2001;229:503–516. doi: 10.1006/dbio.2000.0100. [DOI] [PubMed] [Google Scholar]
- 79.Natarajan D, Marcos-Gutierrez C, Pachnis V, et al. Requirement of signalling by receptor tyrosine kinase RET for the directed migration of enteric nervous system progenitor cells during mammalian embryogenesis. Development. 2002;129:5151–5160. doi: 10.1242/dev.129.22.5151. [DOI] [PubMed] [Google Scholar]
- 80.Young HM, Bergner AJ, Anderson RB, et al. Dynamics of neural crest-derived cell migration in the embryonic mouse gut. Dev Biol. 2004;270:455–473. doi: 10.1016/j.ydbio.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 81.Fariñas I, Jones KR, Backus C, et al. Severe sensory and sympathetic deficits in mice lacking neurotrophin-3. Nature. 1994;369:658–661. doi: 10.1038/369658a0. [DOI] [PubMed] [Google Scholar]
- 82.Tessarollo L, Tsoulfas P, Donovan MJ, et al. Targeted deletion of all isoforms of the trkC gene suggests the use of alternate receptors by its ligand neurotrophin-3 in neuronal development and implicates trkC in normal cardiogenesis. Proc Natl Acad Sci U S A. 1997;94:14776–14781. doi: 10.1073/pnas.94.26.14776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Frieling T, Cooke HJ, Wood JD. Serotonin receptors on submucous neurons in guinea pig colon. Am J Physiol. 1991;261:G1017–G1023. doi: 10.1152/ajpgi.1991.261.6.G1017. [DOI] [PubMed] [Google Scholar]
- 84.Kim M, Javed NH, Yu JG, et al. Mechanical stimulation activates Galphaq signaling pathways and 5-hydroxytryptamine release from human carcinoid BON cells. J Clin Invest. 2001;108:1051–1059. doi: 10.1172/JCI12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chalazonitis A, Rothman TP, Chen J, et al. Neurotrophin-3 induces neural crest-derived cells from fetal rat gut to develop in vitro as neurons or glia. J Neurosci. 1994;14:6571–6584. doi: 10.1523/JNEUROSCI.14-11-06571.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chalazonitis A, Rothman TP, Chen J, et al. Promotion of the development of enteric neurons and glia by neuropoietic cytokines: Interactions with neurotrophin-3. Dev Biol. 1998;198:343–365. [PubMed] [Google Scholar]
- 87.Chalazonitis A, Rothman TP, Chen J-X, et al. Age-dependent differences in the effects of GDNF and NT-3 on the development of neurons and glia from neural crest-derived precursors immunoselected from the fetal rat gut: expression of GFRα-1 in vitro and in vivo. Dev Biol. 1998;204:385–406. doi: 10.1006/dbio.1998.9090. [DOI] [PubMed] [Google Scholar]
- 88.Young HM, Ciampoli D, Hsuan J, et al. Expression of Ret-, p75(NTR)-, Phox2a-, Phox2b-, and tyrosine hydroxylase-immunoreactivity by undifferentiated neural crest-derived cells and different classes of enteric neurons in the embryonic mouse gut. Dev Dyn. 1999;216:137–152. doi: 10.1002/(SICI)1097-0177(199910)216:2<137::AID-DVDY5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 89.Young HM, Bergner AJ, Muller T. Acquisition of neuronal and glial markers by neural crest-derived cells in the mouse intestine. J Comp Neurol. 2003;456:1–11. doi: 10.1002/cne.10448. [DOI] [PubMed] [Google Scholar]
- 90.Guillemot F, Joyner AL. Dynamic expression of the murine achaetescute homolog (MASH-1) in the developing nervous system. Mech Dev. 1993;42:171–185. doi: 10.1016/0925-4773(93)90006-j. [DOI] [PubMed] [Google Scholar]
- 91.Lo L, Guillemot F, Joyner AL, et al. MASH-1: A marker and a mutation for mammalian neural crest development. Persp Dev Neurobiol. 1994;2:191–201. [PubMed] [Google Scholar]
- 92.Sang Q, Ciampoli D, Greferath U, et al. Innervation of the esophagus in mice that lack MASH1. J Comp Neurol. 1999;408:1–10. doi: 10.1002/(sici)1096-9861(19990524)408:1<1::aid-cne1>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 93.Li ZS, Pham TD, Tamir H, et al. Enteric dopaminergic neurons: Definition, developmental lineage, and effects of extrinsic denervation. J Neurosci. 2004;24:1330–1339. doi: 10.1523/JNEUROSCI.3982-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Talley NJ. Irritable bowel syndrome: Disease definition and symptom description. Eur J Surg Suppl. 1998;583:24–28. doi: 10.1080/11024159850191193. [DOI] [PubMed] [Google Scholar]
- 95.Camilleri M. What’s in a name? Roll on Rome II [editorial] Gastroenterology. 1998;114:237. doi: 10.1016/s0016-5085(98)70470-6. [DOI] [PubMed] [Google Scholar]
- 96.Talley NJ. Scope of the problem of functional digestive disorders. Eur J Surg Suppl. 1998;582:35–41. doi: 10.1080/11024159850191427. [DOI] [PubMed] [Google Scholar]
- 97.Hamm LR, Sorrells SC, Harding JP, et al. Additional investigations fail to alter the diagnosis of irritable bowel syndrome in subjects fulfilling the Rome criteria. Am J Gastroenterol. 1999;94:1279–1282. doi: 10.1111/j.1572-0241.1999.01077.x. [DOI] [PubMed] [Google Scholar]
- 98.Locke G, Yawn B, Wollan P, et al. Incidence of a clinical diagnosis of the irritable bowel syndrome in a United States population. Aliment Pharmacol Ther. 2004;19:1025–1031. doi: 10.1111/j.1365-2036.2004.01938.x. [DOI] [PubMed] [Google Scholar]
- 99.Coates MD, Mahoney CR, Linden DR, et al. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and IBS. Gastroentrology. 2004;126:1657–1664. doi: 10.1053/j.gastro.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 100.Rasquin-Weber A, Hyman PE, Cucchiara S, et al. Childhood functional gastrointestinal disorders. Gut. 1999;45(suppl 2):60–68. doi: 10.1136/gut.45.2008.ii60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Staiano A, Andreotti MR, Greco L, et al. Long-term follow-up of children with chronic idiopathic constipation. Dig Dis Sci. 1994;39:561–564. doi: 10.1007/BF02088343. [DOI] [PubMed] [Google Scholar]
- 102.Milla PJ. Motility disorders in childhood. Baillieres Clin Gastroenterol. 1998;12:775–797. doi: 10.1016/s0950-3528(98)90007-0. [DOI] [PubMed] [Google Scholar]
- 103.Milla PJ. Acquired motility disorders in childhood. Can J Gastroenterol. 1999;13(suppl A):76A–84A. doi: 10.1155/1999/610486. [DOI] [PubMed] [Google Scholar]
- 104.Pham TD, Gershon MD, Rothman TP. Time of origin of neurons in the murine enteric nervous system. J Comp Neurol. 1991;314:789–798. doi: 10.1002/cne.903140411. [DOI] [PubMed] [Google Scholar]
- 105.Erspamer V. Occurrence of indolealkylamines in nature. In: Erspamer V, editor. Handbook of Experimental Pharmacology: 5-Hydroxytryptamine and Related Indolealkylamines. New York: Springer-Verlag; 1966. pp. 132–181. [Google Scholar]
- 106.Vialli M. Histology of the enterochromaffin cell system. In: Erspamer V, editor. Handbook of Experimental Pharmacology: 5-Hydroxytryptamine and Related Indolealkylamines. New York: Springer-Verlag; 1966. pp. 1–65. [Google Scholar]
- 107.Branchek TA, Gershon MD. Time course of expression of neuropeptide Y, calcitonin gene related peptide, and NADPH diaphorase activity in neurons of the developing murine bowel and the appearance of 5-hydroxytryptamine in mucosal enterochromaffin cells. J Comp Neurol. 1989;285:262–273. doi: 10.1002/cne.902850208. [DOI] [PubMed] [Google Scholar]
- 108.Fiorica-Howells E, Maroteaux L, Gershon MD. Serotonin and the 5-HT2B receptor in the development of enteric neurons. J Neurosci. 2000;20:294–305. doi: 10.1523/JNEUROSCI.20-01-00294.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fiorica-Howells E, Hen R, Gingrich J, et al. 5-HT2A receptors: location and functional analysis in the intestines of wild-type and 5-HT2A knockout mice. Am J Physiol. 2002;282:6877–6893. doi: 10.1152/ajpgi.00435.2001. [DOI] [PubMed] [Google Scholar]
- 110.Fiorica-Howells E, Liu M-T, Ponimaskin EG, et al. Distribution of 5-HT4 receptors in wild-type mice and anaylsis of intestinal molity in 5-HT4 knockout mice. Gastroenterology. 2003;124:A342. [Google Scholar]
- 111.Liu M-T, Fiorica-Howells E, Gershon MD. Alternative splicing of enteric 5-HT4 receptors and a patch-clamp analysis of function. Gastroenterology. 2003;124:A342. [Google Scholar]
- 112.Nebigil CG, Choi DS, Dierich A, et al. Serotonin 2B receptor is required for heart development. Proc Natl Acad Sci U S A. 2000;97:9508–9513. doi: 10.1073/pnas.97.17.9508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chalazonitis A, D’Autreaux F, Guha U, et al. Bone morphogenetic protein-2 and -4 limit the number of enteric neurons but promote development of a TrkC-expressing neurotrophin-3-dependent subset. J Neurosci. 2004;24:4266–4282. doi: 10.1523/JNEUROSCI.3688-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pisano JM, Colon-Hastings F, Birren SJ. Postmigratory enteric and sympathetic neural precursors share common, developmentally regulated, responses to BMP2. Dev Biol. 2000;227:1–11. doi: 10.1006/dbio.2000.9876. [DOI] [PubMed] [Google Scholar]
- 115.Jiang Y, Liu M, Gershon MD. Netrins and DCC in the guidance of migrating neural crest-derived cells in the developing bowel and pancreas. Dev Biol. 2003;258:364–384. doi: 10.1016/s0012-1606(03)00136-2. [DOI] [PubMed] [Google Scholar]
- 116.Srivastava D, Cserjesi P, Olson EN. A subclass of bHLH proteins required for cardiac morphogenesis. Science. 1995;270:1995–1999. doi: 10.1126/science.270.5244.1995. [DOI] [PubMed] [Google Scholar]
- 117.Cserjesi P, Brown D, Lyons GE, et al. Expression of the novel basic helix-loop-helix gene eHAND in neural crest derivatives and extraembryonic membranes during mouse development. Dev Biol. 1995;170:664–678. doi: 10.1006/dbio.1995.1245. [DOI] [PubMed] [Google Scholar]
- 118.Wu X, Howard MJ. Transcripts encoding HAND genes are differentially expressed and regulated by BMP4 and GDNF in developing avian gut. Gene Expr. 2002;10:279–293. doi: 10.3727/000000002783992361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dean C, Scholl FG, Choih J, et al. Neurexin mediates the assembly of presynaptic terminals. Nat Neurosci. 2003;6:708–716. doi: 10.1038/nn1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Roberts DJ, Johnson RL, Burke AC, et al. Sonic hedgehog is an endodermal signal inducing Bmp-4 and Hox genes during induction and regionalization of the chick hindgut. Development. 1995;121:3163–3174. doi: 10.1242/dev.121.10.3163. [DOI] [PubMed] [Google Scholar]
- 121.Roberts DJ, Smith DM, Goff DJ, et al. Epithelial–mesenchymal signaling during the regionalization of the chick gut. Development. 1998;125:2791–2801. doi: 10.1242/dev.125.15.2791. [DOI] [PubMed] [Google Scholar]
- 122.Haramis AP, Begthel H, van den Born M, et al. De novo crypt formation and juvenile polyposis on BMP inhibition in mouse intestine. Science. 2004;303:1684–1686. doi: 10.1126/science.1093587. [DOI] [PubMed] [Google Scholar]
- 123.Hardwick JC, Van Den Brink GR, Bleuming SA, et al. Bone morphogenetic protein 2 is expressed by, and acts upon, mature epithelial cells in the colon. Gastroenterology. 2004;126:111–121. doi: 10.1053/j.gastro.2003.10.067. [DOI] [PubMed] [Google Scholar]
- 124.Schmechel DE, Brightman MW, Marangos PJ. Neurons switch from non-neuronal enolase to neuron-specific enolase during differentiation. Brain Res. 1980;190:195–214. doi: 10.1016/0006-8993(80)91169-5. [DOI] [PubMed] [Google Scholar]
- 125.Gershon MD, Chalazonitis A, Rothman TP. From neural crest to bowel: Development of the enteric nervous system. J Neurobiol. 1993;24:199–214. doi: 10.1002/neu.480240207. [DOI] [PubMed] [Google Scholar]
- 126.McKeown SJ, Chow CW, Young HM. Development of the submucous plexus in the large intestine of the mouse. Cell Tissue Res. 2001;303:301–305. doi: 10.1007/s004410000303. [DOI] [PubMed] [Google Scholar]
- 127.Payette RF, Bennett GS, Gershon MD. Neurofilament expression in vagal neural crest-derived precursors of enteric neurons. Dev Biol. 1984;105:273–287. doi: 10.1016/0012-1606(84)90285-9. [DOI] [PubMed] [Google Scholar]
- 128.Kirchgessner AL, Adlersberg MA, Gershon MD. Colonization of the developing pancreas by neural precursors from the bowel. Dev Dyn. 1992;194:142–154. doi: 10.1002/aja.1001940207. [DOI] [PubMed] [Google Scholar]
- 129.Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- 130.Kennedy TE, Serafini T, de la Torre JR, et al. Netrins are diffusible chemotropic factors for commisural axons in the embryonic spinal cord. Cell. 1994;78:425–435. doi: 10.1016/0092-8674(94)90421-9. [DOI] [PubMed] [Google Scholar]
- 131.Serafini T, Colamarino SA, Leonardo ED, et al. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell. 1996;87:1001–1014. doi: 10.1016/s0092-8674(00)81795-x. [DOI] [PubMed] [Google Scholar]
- 132.Keino-Masu K, Masu M, Hinck L, et al. Deleted in colorectal cancer (DCC) encodes a netrin receptor. Cell. 1996;87:175–185. doi: 10.1016/s0092-8674(00)81336-7. [DOI] [PubMed] [Google Scholar]
- 133.de la Torre JR, Hopker VH, Ming GLP, et al. Turning of retinal growth cones in a netrin-1 gradient mediated by the netrin receptor DCC. Neuron. 1997;19:1211–1224. doi: 10.1016/s0896-6273(00)80413-4. [DOI] [PubMed] [Google Scholar]
- 134.Ming GL, Song HJ, Berninger B, et al. cAMP-dependent growth cone guidance by netrin-1. Neuron. 1997;19:1225–1235. doi: 10.1016/s0896-6273(00)80414-6. [DOI] [PubMed] [Google Scholar]
- 135.D’Autréaux F, Cserjesi P, Rothman TP, et al. Expression of the basic helix-loop-helix (b-HLH) transcription factor, Hand2 is essential for enteric neuronal development. Gastroenterology. 2004;126(suppl 2):119. [Google Scholar]
- 136.Rothman TP, Nilaver G, Gershon MD. Colonization of the developing murine enteric nervous system and subsequent phenotypic expression by the precursors of peptidergic neurons. J Comp Neurol. 1984;225:13–23. doi: 10.1002/cne.902250103. [DOI] [PubMed] [Google Scholar]
- 137.Scheiffele P. Cell–cell signaling during synapse formation in the CNS. Annu Rev Neurosci. 2003;26:485–508. doi: 10.1146/annurev.neuro.26.043002.094940. [DOI] [PubMed] [Google Scholar]
- 138.Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- 139.Turrigiano GG, Nelson SB. Hebb and homeostasis in neuronal plasticity. Curr Opin Neurobiol. 2000;10:358–364. doi: 10.1016/s0959-4388(00)00091-x. [DOI] [PubMed] [Google Scholar]
- 140.Garner CC, Nash J, Huganir RL. PDZ domains in synapse assembly and signalling. Trends Cell Biol. 2000;10:274–280. doi: 10.1016/s0962-8924(00)01783-9. [DOI] [PubMed] [Google Scholar]
- 141.Garner CC, Kindler S, Gundelfinger ED. Molecular determinants of presynaptic active zones. Curr Opin Neurobiol. 2000;10:321–327. doi: 10.1016/s0959-4388(00)00093-3. [DOI] [PubMed] [Google Scholar]
- 142.Sheng M, Kim MJ. Postsynaptic signaling and plasticity mechanisms. Science. 2002;298:776–780. doi: 10.1126/science.1075333. [DOI] [PubMed] [Google Scholar]
- 143.Benson DL, Colman DR, Huntley GW. Molecules, maps and synapse specificity. Nat Rev Neurosci. 2001;2:899–909. doi: 10.1038/35104078. [DOI] [PubMed] [Google Scholar]
- 144.Shapiro L, Colman DR. The diversity of cadherins and implications for a synaptic adhesive code in the CNS. Neuron. 1999;23:427–430. doi: 10.1016/s0896-6273(00)80796-5. [DOI] [PubMed] [Google Scholar]
- 145.Yamagata M, Weiner JA, Sanes JR. Sidekicks: synaptic adhesion molecules that promote lamina-specific connectivity in the retina. Cell. 2002;110:649–660. doi: 10.1016/s0092-8674(02)00910-8. [DOI] [PubMed] [Google Scholar]
- 146.Lichtman JW, Colman H. Synapse elimination and indelible memory. Neuron. 2000;25:269–278. doi: 10.1016/s0896-6273(00)80893-4. [DOI] [PubMed] [Google Scholar]
- 147.Ushkaryov YA, Petrenko AG, Geppert M, et al. Neurexins: Synaptic cell surface proteins related to the alpha-latrotoxin receptor and laminin. Science. 1992;257:50–56. doi: 10.1126/science.1621094. [DOI] [PubMed] [Google Scholar]
- 148.Missler M, Fernandez-Chacon R, Sudhof TC. The making of neurexins. J Neurochem. 1998;71:1339–1347. doi: 10.1046/j.1471-4159.1998.71041339.x. [DOI] [PubMed] [Google Scholar]
- 149.Ichtchenko K, Hata Y, Nguyen T, et al. Neuroligin 1: A splice site-specific ligand for beta-neurexins. Cell. 1995;81:435–443. doi: 10.1016/0092-8674(95)90396-8. [DOI] [PubMed] [Google Scholar]
- 150.Ichtchenko K, Nguyen T, Sudhof TC. Structures, alternative splicing, and neurexin binding of multiple neuroligins. J Biol Chem. 1996;271:2676–2682. doi: 10.1074/jbc.271.5.2676. [DOI] [PubMed] [Google Scholar]
- 151.Scholl FG, Scheiffele P. Making connections: Cholinesterase-domain proteins in the CNS. Trends Neurosci. 2003;26:618–624. doi: 10.1016/j.tins.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 152.Nguyen T, Sudhof TC. Binding properties of neuroligin 1 and neurexin 1beta reveal function as heterophilic cell adhesion molecules. J Biol Chem. 1997;272:26032–26039. doi: 10.1074/jbc.272.41.26032. [DOI] [PubMed] [Google Scholar]
- 153.Song JY, Ichtchenko K, Sudhof TC, et al. Neuroligin 1 is a postsynaptic cell-adhesion molecule of excitatory synapses. Proc Natl Acad Sci U S A. 1999;96:1100–1105. doi: 10.1073/pnas.96.3.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Scheiffele P, Fan J, Choih J, et al. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell. 2000;101:657–669. doi: 10.1016/s0092-8674(00)80877-6. [DOI] [PubMed] [Google Scholar]
- 155.Irie M, Hata Y, Takeuchi M, et al. Binding of neuroligins to PSD-95. Science. 1997;277:1511–1515. doi: 10.1126/science.277.5331.1511. [DOI] [PubMed] [Google Scholar]
- 156.Tabuchi K, Biederer T, Butz S, et al. CASK participates in alternative tripartite complexes in which Mint 1 competes for binding with caskin 1, a novel CASK-binding protein. J Neurosci. 2002;22:4264–4273. doi: 10.1523/JNEUROSCI.22-11-04264.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Biederer T, Sudhof TC. Mints as adaptors. Direct binding to neurexins and recruitment of munc 18. J Biol Chem. 2000;275:39803–39806. doi: 10.1074/jbc.C000656200. [DOI] [PubMed] [Google Scholar]
- 158.Kurschner C, Mermelstein PG, Holden WT, et al. CIPP, a novel multivalent PDZ domain protein, selectively interacts with Kir4.0 family members, NMDA receptor subunits, neurexins, and neuroligins. Mol Cell Neurosci. 1998;11:161–172. doi: 10.1006/mcne.1998.0679. [DOI] [PubMed] [Google Scholar]
- 159.Comoletti D, De Jaco A, Jennings LL, et al. The Arg451Cys-neuroligin-3 mutation associated with autism reveals a defect in protein processing. J Neurosci. 2004;24:4889–4893. doi: 10.1523/JNEUROSCI.0468-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Laumonnier F, Bonnet-Brilhault F, Gomot M, et al. X-linked mental retardation and autism are associated with a mutation in the NLGN4 gene, a member of the neuroligin family. Am J Hum Genet. 2004;74:552–557. doi: 10.1086/382137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jamain S, Quach H, Betancur C, et al. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet. 2003;34:27–29. doi: 10.1038/ng1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chih B, Afridi SK, Clark L, et al. Disorder-associated mutations lead to functional inactivation of neuroligins. Hum Mol Genet. 2004;13(14):1471–1477. doi: 10.1093/hmg/ddh158. [DOI] [PubMed] [Google Scholar]
- 163.Torrente F, Ashwood P, Day R, et al. Small intestinal enteropathy with epithelial IgG and complement deposition in children with regressive autism. Mol Psychiatry. 2002;7:375–382. doi: 10.1038/sj.mp.4001077. [DOI] [PubMed] [Google Scholar]
- 164.White JF. Intestinal pathophysiology in autism. Exp Biol Med (Maywood) 2003;228:639–649. doi: 10.1177/153537020322800601. [DOI] [PubMed] [Google Scholar]
- 165.Torrente F, Anthony A, Heuschkel RB, et al. Focal-enhanced gastritis in regressive autism with features distinct from Crohn’s and Helicobacter pylori gastritis. Am J Gastroenterol. 2004;99:598–605. doi: 10.1111/j.1572-0241.2004.04142.x. [DOI] [PubMed] [Google Scholar]
- 166.Afzal N, Murch S, Thirrupathy K, et al. Constipation with acquired megarectum in children with autism. Pediatrics. 2003;112:939–942. doi: 10.1542/peds.112.4.939. [DOI] [PubMed] [Google Scholar]
- 167.Furlano RI, Anthony A, Day R, et al. Colonic CD8 and gamma delta T-cell infiltration with epithelial damage in children with autism. J Pediatr. 2001;138:366–372. doi: 10.1067/mpd.2001.111323. [DOI] [PubMed] [Google Scholar]
- 168.Horvath K, Perman JA. Autistic disorder and gastrointestinal disease. Curr Opin Pediatr. 2002;14:583–587. doi: 10.1097/00008480-200210000-00004. [DOI] [PubMed] [Google Scholar]
- 169.Uhlmann V, Martin CM, Sheils O, et al. Potential viral pathogenic mechanism for new variant inflammatory bowel disease. Mol Pathol. 2002;55:84–90. doi: 10.1136/mp.55.2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wakefield AJ. Enterocolitis, autism and measles virus. Mol Psychiatry. 2002;7(suppl 2):S44–S46. doi: 10.1038/sj.mp.4001178. [DOI] [PubMed] [Google Scholar]
- 171.Wakefield AJ, Murch SH, Anthony A, et al. Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children [see comments] Lancet. 1998;351:637–641. doi: 10.1016/s0140-6736(97)11096-0. [DOI] [PubMed] [Google Scholar]
- 172.Thjodleifsson B, Davidsdottir K, Agnarsson U, et al. Effect of Pentavac and measles-mumps-rubella (MMR) vaccination on the intestine. Gut. 2002;51:816–817. doi: 10.1136/gut.51.6.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Miller E. Measles-mumps-rubella vaccine and the development of autism. Semin Pediatr Infect Dis. 2003;14:199–206. doi: 10.1016/s1045-1870(03)00034-7. [DOI] [PubMed] [Google Scholar]