Abstract
Neurogenesis occurs within the adult dentate gyrus of the hippocampal formation and it has been proposed that the newly born neurons, recruited into the preexistent neuronal circuits, might be involved in hippocampal-dependent learning processes. Age-dependent spatial memory impairments have been related to an alteration in hippocampal plasticity. The aim of the current study was to examine whether cognitive functions in aged rats are quantitatively correlated with hippocampal neurogenesis. To this end, we took advantage of the existence of spontaneous individual differences observed in aged subjects in a hippocampal-dependent task, the water maze. We expected that the spatial memory capabilities of aged rats would be related to the levels of hippocampal neurogenesis. Old rats were trained in the water maze, and, 3 weeks after training, rats were injected with 5-bromo-2′-deoxyuridine (BrdUrd, 50 or 150 mg/kg) to label dividing cells. Cell proliferation was examined one day after the last BrdUrd injection, whereas cell survival and differentiation were determined 3 weeks later. It is shown that a quantitative relationship exists between learning and the number of newly generated neurons. Animals with preserved spatial memory, i.e., the aged-unimpaired rats, exhibited a higher level of cell proliferation and a higher number of new neurons in comparison with rats with spatial memory impairments, i.e., the aged-impaired rats. In conclusion, the extent of memory dysfunction in aged rats is quantitatively related to the hippocampal neurogenesis. These data reinforce the assumption that neurogenesis is involved in memory processes and aged-related cognitive alterations.
In most mammalian brain regions, neurogenesis only occurs during development. However, within the hippocampal formation, the dentate gyrus continues to produce granule neurons throughout adulthood (1, 2). The majority of the newly born cells express neuronal markers, receive synaptic inputs, extend axons along the mossy-fibers tract, and exhibit electrophysiological properties similar to those of mature dentate granule neurons (3-6).
An increasing number of reports suggest that adult hippocampal neurogenesis is involved in hippocampal-mediated learning. Indeed, the hippocampus is implicated in various forms of memory, and it has been shown that conditions that increase memory performance, such as an enriched environment or running and physical exercise, also enhance neurogenesis (7-10). Conversely, situations that reduce neurogenesis, such as prenatal stress (11) or an antimitotic treatment (12), have been associated with cognitive impairments.
It has also been proposed that changes in neurogenesis may be involved in some of the alterations of cognitive function observed during aging (13-15) that are classically related to a decline in hippocampal plasticity (16-18). Thus, it has been shown that hippocampal neurogenesis declines with increasing age (11, 19-22), and this process is related to a decline in proliferative activity rather than to a general change in metabolic conditions or an alteration of the survival of the newly born neurons (8, 19-21, 23). However, whatever the role of these various relationships, a quantitative correlation between neurogenesis and performance still has not been demonstrated in a hippocampal-dependent test (24).
To address this issue, we took advantage of the well established presence of individual differences in spatial memory abilities within a population of old rats (25-27). Indeed, in the water maze, some old individuals show a clear impairment in spatial reference memory, whereas others are not impaired and exhibit cognitive capacities similar to those of younger individuals. The analysis of such individual differences is considered a valuable strategy for the study of the neurobiological substrates of cognitive aging. These investigations have successfully revealed the involvement of functional and structural modifications in the hippocampal formation in aging-related disorders (14, 28-35).
In this report, cell proliferation, cell survival, and the phenotype of the newly born cells in the dentate gyrus were correlated with the learning performances of old rats. The newly born cells were labeled with 5-bromo-2′-deoxyuridine (BrdUrd), a thymidine analogue incorporated into the genetic material during the synthetic DNA phase (S phase) of mitotic division or with an endogenous marker of the cell cycle, Ki67. The neuron-specific marker NeuN was used to phenotype the newly born neurons after longer survival times. Individual comparisons demonstrate that the extent of memory dysfunction in aged rats is quantitatively correlated with defects in hippocampal neurogenesis. These results provide new insights as to the possible neural mechanisms of the aging of cognitive functions and reinforce the hypothesis according to which neurogenesis is involved in memory function.
Materials and Methods
Animals. Sixty-one male Sprague-Dawley rats (Iffa Credo) between 10 and 11 months of age were purchased and maintained undisturbed until the behavioral testing. Four weeks before the start of the experiment, 2-month-old rats (n = 10) were obtained and added to the experiment. Animals were housed individually in plastic cages under a constant light-dark cycle (light on, 800-2000 h) with ad libitum access to food and water. Temperature (22°C) and humidity (60%) were kept constant. Animals with a bad general health status or tumors were excluded.
Behavioral Testing. Twenty- and 3-month-old rats were tested in a Morris water maze (180 cm diameter, 60 cm high; EIC, Bordeaux, France) filled with water (21°C) made opaque by addition of milk powder (36, 37). An escape platform was hidden 2 cm below the surface of the water in a fixed location in one of four quadrants halfway between the wall and the middle of the pool. Before the start of training, animals were habituated to the pool without a platform 1 min/day for 3 days. During training, animals were required to locate the submerged platform by using distal extramaze cues. They were tested for four trials per day (90 s with an intertrial interval of 30 s and beginning from three different start points that varied randomly each day). If an animal did not find the platform, it was set on it at the end of the trial. The time to reach the platform (latency in seconds) and the length of the swim path (distance in centimeters) were measured with a computerized tracking system (videotrack, Viewpoint, Lyon, France). To test the visual acuity and the motor functions of the aged rats, after the last day of training, the hidden platform was replaced by a visible platform located in the opposite quadrant, and the animals were tested for 2 additional days.
BrdUrd Injections. BrdUrd (Sigma), a thymidine analogue incorporated into genetic material during synthetic DNA phase (S phase) of mitotic division, was injected 3 weeks after the end of the behavioral testing. This protocol was chosen to avoid the confounding influence of behavioral training on neurogenesis. Thus, it has been shown that learning modifies the survival of the newly born cells that were labeled before the task (38). In contrast, the entire procedure of water maze training does not seem to modify cell proliferation (10). Furthermore, we have recently investigated the relationships between the number of new cells produced during learning and the performance of the animals. No correlations were found in either young (36) or aged rats (unpublished observation). Two different doses of BrdUrd were used. In the first and third experiments, rats received one daily i.p. injection of 50 mg/kg BrdUrd dissolved in phosphate buffer (0.1 M, pH 8.4) during 5 days. In the second experiment, rats received one daily injection of 150 mg/kg BrdUrd during 5 days.
BrdUrd and Ki67 Staining. Rats were perfused transcardiacally with paraformaldehyde 1 day (first and second experiments) or 3 weeks (third experiment) after the last BrdUrd injection. After a 24-h postfixation period, 50-μm frontal sections were cut on a vibratome. Free-floating sections were processed according to a standard immunohistochemical procedure (39). One in ten sections was treated for Ki67 immunoreactivity by using a mouse anti-KI67 monoclonal antibody (1:100, NovoCastra, Newcastle, U.K.). For BrdUrd labeling, adjacent sections were treated with 2 N HCl (30 min at 37°C), and then rinsed in borate buffer during 5 min (0.1 M, pH = 8.4). They were incubated with a mouse monoclonal anti-BrdUrd antibody (1/200, DAKO). Sections were processed in parallel and immunoreactivities were visualized by the biotin-streptavidin technique (ABC kit, DAKO) by using 3,3′-diaminobenzidine as chromogen.
Stereological Analysis. The number of X-immunoreactive (IR) cells in the left and right dentate gyrus was estimated by using a modified version of the optical fractionator method on a systematic random sampling of every tenth section along the rostrocaudal axis of the hippocampal formation. On each section, all X-IR cells were counted, with a ×100 microscope objective, in the granule and subgranular layers of the dentate gyrus and in the hilus excluding those in the outermost focal plane. Resulting numbers were tallied and multiplied by the inverse of the section-sampling fraction (1/ssf = 10). Then, the sections were counterstained and the surface of the granule cell layer was measured by using a samba 2640 system (Alcatel System, TITN Answare, Grenoble, France) and the granule cell layer sectional volume estimated by using the Cavalieri method: Vref = T × ΣA × 1/ssf, where T is the mean thickness of the vibratome section (50 μm) and A is the area of the granule and subgranular cell layers.
The number of granule cells, as assessed morphologically by hematoxylin staining, was determined by using the optical fractionator method (stereo investigator software, Micro-BrightField, Williston, VT). For each one-in-ten section, granule cells were counted at ×100, in 15 × 15 μm frames at evenly spaced x-y intervals of 330 × 330 μm (40).
Analysis of Phenotype. To examine the phenotype of BrdUrd-IR cells, one in ten sections obtained from the second experiment were incubated with the BrdUrd antibody (1/500, Accurate Scientific, Westbury, NY) which was revealed by using a CY3-labeled anti-rat IgG antibody (1/1,000, Jackson ImmunoResearch). Then sections were incubated with a mouse monoclonal anti-NeuN antibody (1/1,000, Chemicon, Euromedex, Souffel-weyersheim, France) and bound anti-NeuN monoclonal antibodies were visualized with an Alexa 488 goat anti-rabbit IgG (1/1,000, Jackson ImmunoResearch). The percentage of BrdUrd-labeled cells that expressed NeuN was determined throughout the dentate gyrus by using a confocal microscope with HeNe and argon lasers (Nikon PCM 2000). Confocal analysis was restricted to the top of the section where penetration of NeuN antibodies was reliable and all BrdUrd double-labeled cells were examined. Sections were optically sliced in the Z plane by using a 1-μm interval, and cells were rotated in orthogonal planes to verify double labeling.
Statistical Analysis. Relationships between behavioral scores and the number of BrdUrd-IR cells were evaluated by using the Pearson correlation test. Differences between the two groups of aged rats were analyzed with a Student t test or an ANOVA.
Results
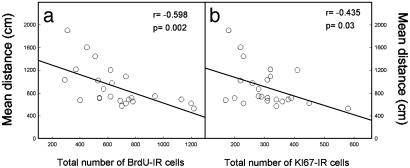
Behavioral Performances of Aged Rats Are Positively Correlated with Cell Proliferation in the Granule Cell Layer. Low BrdUrd dosage. In a first experiment we examined the existence of a correlation between behavioral performance tested in the Morris water maze and cell proliferation. Three weeks after the end of the behavioral testing, 24 aged rats were injected with BrdUrd. They received 1 daily injection of 50 mg/kg of BrdUrd during 5 days and were killed 1 day after the last BrdUrd injection. A negative correlation was found between the mean of the total distance covered to reach the hidden platform and the number of BrdUrd-IR cells in the granule cell layer (Fig. 1a, n = 24, r = -0.598, P = 0.002). Similar results were obtained by using the mean of the total latency to find the hidden platform (data not shown, n = 24, r = -0.584, P = 0.003). In fact, the best performances (shorter distance to reach the platform or lowest latency) were found in animals showing the highest number of BrdUrd-IR cells in the granule cell layer. In other words, animals with preserved spatial memory exhibited a higher level of cell proliferation in comparison with animals displaying spatial memory impairments.
Fig. 1.
Spatial memory performance (mean distance covered to find a hidden platform) in aged rats correlates with cell proliferation in the granule cell layer as measured by BrdUrd-IR (a) and Ki67-IR (b) cell number.
These effects were specific because no correlation occurred between cell proliferation and the distance covered to find a visible platform (data not shown, n = 24, r = -0.211, P = 0.321). Furthermore, no relationship occurred between learning performances and cell proliferation in the hilus (correlation between the distance covered to find a hidden platform and BrdUrd-IR cell number in the hilus, n = 24, r = -0.031, P = 0.136; correlation between the latency to find a hidden platform and BrdUrd-IR cell number in the hilus, n = 24, r = -0.028, P = 0.172). Finally, to confirm that the behavioral performance in the water maze was related to cell proliferation, we used another marker of cell birth, Ki67. We found a negative correlation between the mean of the total distance covered to reach the hidden platform and the number of Ki67-IR cells (Fig. 1b, n = 24, r = -0.435, P = 0.034). In contrast, no correlation occurred between the behavioral performances and the total number of granule cells (n = 24, r = -0.202, P = 0.343) or between the number of BrdUrd-IR cells in the granule cell layer and the total number of granule cells (n = 24, r = 0.243, P = 0.253).
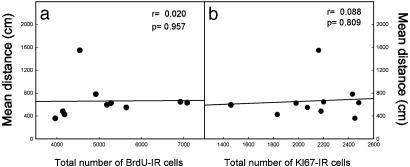
To verify that the relationship between cell proliferation and spatial memory performances was specifically observed in aged rats, 3-month-old rats were trained in the water maze and were injected with BrdUrd according to a protocol similar to that previously described. In this case, no correlation was found between the mean of the total distance covered to reach the hidden platform and the number of BrdUrd-IR cells (Fig. 2a, n = 10, r = 0.02, P = 0.957) or the number of Ki67-IR cells (Fig. 2b, n = 10, r = 0.088, P = 0.809).
Fig. 2.
Spatial memory performances (mean distance covered to find a hidden platform) in young rats do not correlate with cell proliferation in the granule cell layer as measured by BrdUrd-IR (a) and Ki67-IR (b) cell number.
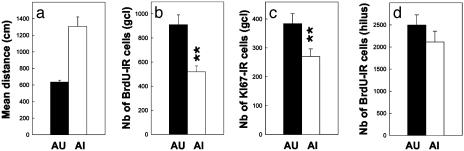
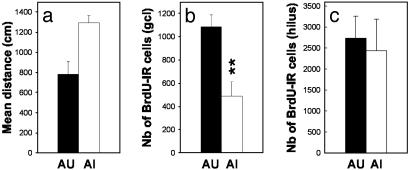
To further characterize the relationships between neurogenesis and learning, aged animals were ranked in terms of their performance in the water maze and the subjects with the eight highest (aged unimpaired, AU) and eight lowest values (aged impaired, AI), corresponding to the extremes (30% of the population; Fig. 3a), were compared for their BrdUrd-IR cell number (Fig. 3 a and b). As shown in Figs. 3 and 4, AU rats had the highest number of newly born cells when compared with the AI animals that displayed the lowest number (BrdUrd labeling, t14 = -4.119, P = 0.001; Ki67 staining, t14 = -2.562, P = 0.022). These differences in cell proliferation were not due to a difference in the reference volume (AU, 3.985 ± 0.090 mm3; AI, 4.121 ± 0.122 mm3, t14 = 0.896, P = 0.385). Differences in the ability of the AU and the AI animals to locate the submerged platform were not related either to any motor-performance or visual-acuity deficits, tigmotaxic behavior, or more general health status as the distance covered to find a visible platform was identical for the two subgroups (AU, 324.40 cm ± 63.36; AI, 337.65 cm ± 81.2, t14 = -0.128, P = 0.899). The relationship between cell proliferation and the cognitive status was specific for the granule cell layer of the dentate gyrus, because the number of BrdUrd-IR cells in the hilus did not differ between AU and AI animals (Fig. 3d, t14 =-1.13, P = 0.27). These results also indicate that differences in cell proliferation between the two groups do not result from nonspecific changes in BrdUrd bioavailability. Finally, no differences occurred in the total number of granule cells, as quantified on counterstained section, between the AI and AU groups (AU, 1,449,369 ± 63,373 cells; AI, 1,379,662 ± 91,044, t14 = -0.628, P = 0.539).
Fig. 3.
Cell proliferation within the dentate gyrus of AU and AI rats. (a) Behavioral scores, as measured by the mean distance covered to find a hidden platform, of the AU and AI groups. The number of BrdUrd-IR cells (b) and of Ki67-IR cells (c) in the granule cell layer of AU rats is greater than that measured in AI rats. (d) In contrast, cell proliferation within the hilus is not influenced by the cognitive status of the aged rats.
Fig. 4.
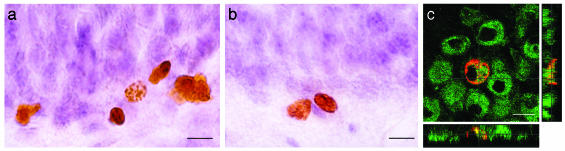
Illustration of BrdUrd-labeled cells in the granule cell layer of an AU rat (a) and an AI rat (b). (c) Optical section (of 0.7 μm) obtained by confocal microscopy showing that BrdUrd-labeled cells (red nuclear stain, Cy3) are double-stained with the neuronal marker NeuN (green stain, Alexa 488). (Scale bars: a and b, 10 μm; c, 8 μm.)
High BrdUrd dosage. Because it has been shown that low doses of BrdUrd label only some of the cells in S phase (41), we examined in a second experiment whether behavioral performances were still correlated with BrdUrd-IR cell number when animals received, 3 weeks after behavioral testing, one daily injection of 150 mg/kg of BrdUrd for 5 days. For this experiment, 17 rats were tested in the water maze; six were classified AI, and five of them were classified AU (Fig. 5a). Differences in the ability of the AU and the AI animals to locate the submerged platform were not related to motor or visual deficits, because the distance covered (T9 = -1.029, P = 0.330) or the latency (T9 = -1.665, P = 0.130) to find a visible platform were identical for the two subgroups. One day after the last injection of 150 mg/kg BrdUrd, we found that AU rats had significantly more BrdUrd-IR-labeled cells than AI animals (Fig. 5b; T9 = 3.336, P = 0.0052). These differences were not due to a difference in the reference volume (AU, 2.222 ± 0.244 mm3; AI, 1.847 ± 0.198 mm3; T9 = 1.208, P = 0.258) and were specific for the granule cell layer because no difference was observed in the hilus (Fig. 5c, T9 = 0.314, P = 0.761).
Fig. 5.
Cell proliferation as revealed by a high dose of BrdUrd (five injections of 150 mg/kg BrdUrd). (a) Behavioral scores, as measured by the mean distance covered to find a hidden platform, of the AU and AI groups. Cell proliferation within the granule cell layer (b) and not the hilus (c) depended on the cognitive status of the rats.
In conclusion, spatial memory performances measured in the water maze are correlated with cell proliferation in the granule cell layer of the dentate gyrus. Indeed, independently of the dosage of BrdUrd, we found that rats with preserved spatial memory exhibited a higher level of cell proliferation in comparison with rats with spatial memory impairments. However, it should be mentioned that the 150 mg/kg dose of BrdUrd induced body weight loss (62.4 ± 9.4 g lost in 5 days; t16 = 7.23, P < 0.001). Consequently the 50 mg/kg dose of BrdUrd was preferred for subsequent experiments.
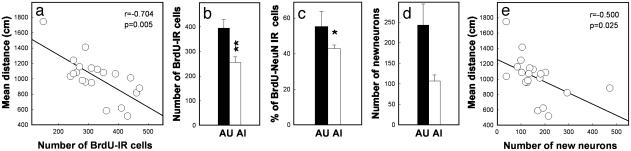
Behavioral Performances of Aged Rats Are Positively Correlated with Cell Survival and the Number of New Neurons in the Granule Cell Layer. In a third experiment, 20 additional rats were tested in the water maze and, 3 weeks after behavioral testing, the animals received injections BrdUrd (one daily injection of 50 mg/kg BrdUrd for 5 days). Three weeks after the last BrdUrd injection, the animals were killed for BrdUrd cell counting and phenotyping. A correlation was found between the mean distance covered to locate the hidden platform and the number of BrdUrd-IR cells that survived (Fig. 6a, n = 20, r = -0.704, P = 0.0005). These results indicate that animals with the best performances exhibited the highest numbers of surviving neurons when compared with rats with the worst scores.
Fig. 6.
Survival and differentiation of the newly generated cells in the granule cell layer. (a) Behavioral scores are correlated with the number of surviving BrdUrd-IR cells. (b) The number of BrdUrd-IR cells in AU rats is superior to that measured in AI rats. (c) More newly born cells differentiate into neurons in the AU rats. (d) The extrapolated number of newly born neurons is higher in the dentate gyrus of AU rats. (e) Behavioral scores are correlated with the number of newly born neurons in the dentate gyrus.
Of the 20 animals tested, six were designated AI and six were designated AU. As previously found, no difference occurred between AU and AI rats in the visible platform test (T10 = -1.590, P = 0.143). The number of BrdUrd-IR cells in the granular cell layer of the AU group was higher than that found in the AI group (Fig. 6b, T10 = 3.347, P = 0.0074). These differences were specific to the granular layer, because BrdUrd-IR cell number was similar in the hilus (AI, 671.7 ± 112.6 cells; AU, 628.3 ± 71.6 cells, T10 = -0.324, P = 0.752) and were not related to a difference in the reference volume (AI, 2.605 ± 0.186 mm3; AU, 2.878 ± 0.124 mm3, T10 = 1.191, P = 0.261).
We finally examined whether the cognitive status of the aged animals was related to the phenotype of the newly born cells. Differentiation of the BrdUrd-IR surviving cells was examined by determining the phenotype into which these cells had differentiated by means of immunofluorescent double-labeling for BrdUrd and a neuronal marker, NeuN (Fig. 4c). A confocal analysis revealed that in the AU group, 55.3 ± 5.2% of the BrdUrd-IR cells were NeuN-IR, indicating a neuronal differentiation to a granule cell phenotype, whereas in the AI group only 42.8 ± 2.1% of the BrdUrd-IR cells coexpressed NeuN (T10 = 2.221, P = 0.051). The ratio of BrdUrd-IR cells co-labeled with NeuN was multiplied by the total number of BrdUrd-labeled cells to give an estimate of the total number of BrdUrd-labeled neurons. The extrapolated total number of BrdUrd-labeled neurons of AU rats was higher than that of the AI rats (Fig. 6d, T10 = 2.515, P = 0.031). Moreover, when all animals were considered, the extrapolated total number of BrdUrd-labeled neurons was positively correlated with behavioral performances (Fig. 6e, n = 20, r = -0.514, P = 0.020).
Discussion
Using a naturalistic model of variation of cognitive abilities, we show that spatial memory performances of aged rats measured in a hippocampus-dependent task, the spatial water maze, are quantitatively correlated with the number of newly born neurons in the granule cell layer of the dentate gyrus. Thus, animals showing the best behavioral performances had the highest numbers of BrdUrd-IR cells, whereas rats with memory dysfunctions had the lowest BrdUrd-IR cell number.
Because it has been shown that low doses of BrdUrd label only some of the cells in S phase (41), we used two doses of BrdUrd (50 and 150 mg/kg) in our experiments. In both cases, we found that animals with the best behavioral performances had the highest number of BrdUrd-IR cells. The number of cells labeled by the two doses of BrdUrd was in the same range. This observation suggests that, at least in aged rats, all newly born cells were labeled after multiple injections of 50 mg/kg of BrdUrd.
To rule out that apoptotic cells or nonproliferating cells that synthesize DNA for repair be labeled with BrdUrd (42-45), we also used an endogenous cell cycle marker, Ki67 (46). We found that the memory performances were also positively correlated with the number of Ki-67-labeled cells. This result confirms that the cognitive status of aged rats is related to the level of cell proliferation in the granule cell layer. Differences in staining with Ki-67 also suggest that the observed variations in the number of BrdUrd-labeled cells are not due to differences among animals as to BrdUrd availability and metabolism (28). This potential bias also seems unlikely to occur because BrdUrd-labeled cell number in another area, the hilus, was similar in AI and AU animals.
Besides cell proliferation, we also examined whether the cognitive status of aged rats was predictive of the survival of newly born cells. We found that a positive correlation existed between memory performances and the number of BrdUrd-IR cells that survived 3 weeks after their birth. Thus, animals showing the best behavioral performance also had the highest numbers of BrdUrd-IR cells after this delay. Differentiation into a neuronal phenotype was also linked to the cognitive status of the aged rats. Indeed, more cells differentiated into neurons in aged rats without spatial learning impairment compared with rats with cognitive deficits.
Altogether, a higher neurogenesis was observed in animals with the best behavioral performances, indicating that a certain threshold of neurogenesis is required to maintain correct spatial memory. These results contrast with those obtained recently (47), which did not demonstrate a correlation between learning capabilities in aged rats and hippocampal neurogenesis. In the latest experiment, animals were given five injections of 50 mg/kg BrdUrd immediately after the completion of behavioral testing, and they were allowed to survive for 10 days after the final BrdUrd injection. The lack of relationship between behavioral performances and hippocampal neurogenesis may be due to the delay between training and BrdUrd injections. The discrepancy between the two studies may also be related to differences in strain (Fisher 344) and sex (female), parameters known to influence neurogenesis (48-52).
It could be questioned whether the behavioral deficit observed in AI animals specifically resulted from an alteration of cognitive performances or also depended on a nonspecific modification of the motivational status of the animal. Thus, several mood-related behaviors are known to be impaired by aging. In fact, it is unlikely that the differences observed in AI rats depend on mood alteration. First, swimming behavior during the first sessions of the water maze did not differ between AI and AU (four first trials: t14 = -1.43, P = 0.17) and was not correlated with neurogenesis (r = -0.23, P = 0.28). This observation is important because an alteration in the initial activity in the water maze is considered as an index of mood alterations. Second, in previous studies, we have shown that the existence of memory deficits in aged rats is not related to the mood and motivational status of the subject (53).
Although addition of neurons was different in AU and AI groups, the total number of granule neurons fails to account for age-related learning and memory impairments. This phenomenon has been reported previously for other strains of male or female rats (40, 47). Because the percentage of granule neurons produced in adulthood is relatively small compared with the 1 or 2 million mature granule neurons, it may be supposed that addition of newly born neurons is ”quantitatively” negligible in view of the total pool of granule neurons. This suggests that ”rejuvenilization” of granule cells rather than their number is the main factor sustaining memory formation. However, it remains to be determined how changes in hippocampal neurogenesis influence the granule cell/mossy fiber/Ammon's horn network and subsequently cognitive performance.
In line with previous reports (11, 19-22), hippocampal neurogenesis in young rats was greater than that observed in aged rats, confirming an age-related decline in hippocampal neurogenesis. All young rats rapidly learned the platform location and behavioral performances were higher than those observed in old animals. Furthermore, in young animals, no significant relationships were detected between cell proliferation measured 3 weeks after the completion of behavioral testing and learning performances in the water maze. This difference between young and old animals suggests that a critical number of new neurons is necessary for learning the spatial memory task and that cognitive deficits appear only beyond this threshold. This idea is consistent with recent data showing that administration of the antimitotic agent methylazoxymethanol did not reduce performances in the water maze (54). In fact, in this experiment, although neurogenesis was reduced, a large number of residual newly born cells were still generated (≈2,000 BrdUrd-labeled cells). Clearly, it is difficult to exactly compare the number of newly generated cells in the two studies because of differences in BrdUrd doses (three injections of 75 mg/kg of BrdUrd vs. five injections of 50 mg/kg in the present study). However, the hypothesis of Shors and coworkers (54), according to which ”spatial navigation learning can still occur with a very small percentage of new neurons,” is consistent with our observation that aged unimpaired rats can acquire hippocampal-dependent memories despite a low number (≈1,000 cells) of newly born cells.
The interindividual variations in hippocampal neurogenesis observed among a population of cognitively aged rats may be due to differences in inhibitory and/or stimulatory substances. One candidate could be the insulin-like growth factor-1 (IGF-1) because (i) it stimulates hippocampal neurogenesis in aged rats (20), (ii) its brain levels decrease with aging (55), (iii) the expression of its receptors is up-regulated as a function of aging and cognitive deficits (56), and (iv) IGF-1 treatment improves the cognitive status of aged rats (57). Another candidate is corticosterone, because variations in age-related memory dysfunction have been related to an up-regulation of the activity of the hypothalamic-pituitary-adrenal axis (58, 59), and corticosterone was shown to inhibit hippocampal neurogenesis in aged rats (60-62). Along these lines, we have very recently analyzed the effect of lowering corticosterone levels on neurogenesis and behavioral performance in the water maze. We found that lowering corticosterone levels in middle-age animals significantly increases hippocampal neurogenesis and spatial memory performances in old rats (M. F. Montaron, E.D., C.A., M.L.M., P.-V.P., D.N.A., unpublished data). The latest results confirm the relationships between corticosterone and age-related behavioral impairments. They also suggest that the decrease in neurogenesis and in behavioral performances could be causally related.
In conclusion, our results show, in rodents, a quantitative relationship between neurogenesis and memory capability by linking, for a given individual, levels of cognitive performances and levels of neurogenesis. Together with data accumulated during the past decade on birds, these results reinforce the assumption that neurogenesis is involved in memory storage (13, 63-67). The fact that the severity of cognitive impairments can predict the extent of alterations in hippocampal neurogenesis also probably mirrors a more general failure in the mechanisms underlying neuronal plasticity (16-18, 68).
Acknowledgments
This work was supported by Institut National de la Santé et de la Recherche Médicale, the European Community (QLK6 CT 2000-00179 and QRT 2002-02187), the Fondation pour la Recherche Médicale, and University of Bordeaux II, Institut Fédératif de Recherche no. 8.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: AU, aged unimpaired; AI, aged impaired; IR, immunoreactive.
References
- 1.Altman, J. (1962) Science 135, 1127-1128. [DOI] [PubMed] [Google Scholar]
- 2.Gross, C. G. (2000) Nat. Rev. Neurosci. 1, 67-73. [DOI] [PubMed] [Google Scholar]
- 3.Hastings, N. B. & Gould, E. (1999) J. Comp. Neurol. 413, 146-154. [DOI] [PubMed] [Google Scholar]
- 4.Markakis, E. A. & Gage, F. H. (1999) J. Comp. Neurol. 406, 449-460. [PubMed] [Google Scholar]
- 5.Stanfield, B. B. & Trice, J. E. (1988) Exp. Brain Res. 72, 399-406. [DOI] [PubMed] [Google Scholar]
- 6.van Praag, H., Schinder, A. F., Christie, B. R., Toni, N., Palmer, T. D. & Gage, F. H. (2002) Nature 415, 1030-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kempermann, G., Kuhn, H. G. & Gage, F. H. (1997) Nature 386, 493-495. [DOI] [PubMed] [Google Scholar]
- 8.Kempermann, G., Kuhn, H. G. & Gage, F. H. (1998) J. Neurosci. 18, 3206-3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Praag, H., Christie, B. R., Sejnowski, T. J. & Gage, F. H. (1999) Proc. Natl. Acad. Sci. USA 96, 13427-13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Praag, H., Kempermann, G. & Gage, F. H. (1999) Nat. Neurosci. 2, 266-270. [DOI] [PubMed] [Google Scholar]
- 11.Lemaire, V., Koehl, M., Le Moal, M. & Abrous, D. N. (2000) Proc. Natl. Acad. Sci. USA 97, 11032-11037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shors, T. J., Miesegaes, G., Beylin, A., Zhao, M., Rydel, T. & Gould, E. (2001) Nature 410, 372-376. [DOI] [PubMed] [Google Scholar]
- 13.Barnea, A. & Nottebohm, F. (1994) Proc. Natl. Acad. Sci. USA 91, 11217-11221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barnes, C. A. (1979) J. Comp. Physiol. Psychol. 93, 74-104. [DOI] [PubMed] [Google Scholar]
- 15.Barnes, C. A. (1988) Neurobiol. Aging 9, 563-568. [DOI] [PubMed] [Google Scholar]
- 16.Mesulam, M.-M. (1999) Neuron 24, 521-529. [DOI] [PubMed] [Google Scholar]
- 17.Mikkonen, M., Soininen, H., Alafuzof, I. & Miettinen, R. (2001) Rev. Neurosci. 12, 311-325. [DOI] [PubMed] [Google Scholar]
- 18.Petit, T. D. & Ivy, O. (1988) Neuroplasticity, Learning and Memory, Neurology and Neurobiology (Liss, New York).
- 19.Kuhn, H. G., Winkler, J., Kempermann, G., Thal, L. J. & Gage, F. H. (1997) J. Neurosci. 17, 5820-5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lichtenwalner, R. J., Forbes, M. E., Bennett, S. A., Lynch, C. D., Sonntag, W. E. & Riddle, D. R. (2001) Neuroscience 107, 603-613. [DOI] [PubMed] [Google Scholar]
- 21.Nacher, J., Alonso-Llosa, G., Rosell, D. R. & McEwen, B. S. (2003) Neurobiol. Aging 24, 273-284. [DOI] [PubMed] [Google Scholar]
- 22.Seki, T. & Arai, Y. (1995) NeuroReport 6, 2479-2482. [DOI] [PubMed] [Google Scholar]
- 23.Goldman, S. A., Kirschenbaum, B., Harrison-Restelli, C. & Thaler, H. T. (1997) J. Neurobiol. 32, 554-566. [DOI] [PubMed] [Google Scholar]
- 24.Kempermann, G. (2002) J. Neurosci. 22, 635-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gallagher, M. & Nicolle, M. M. (1993) Behav. Brain Res. 57, 155-162. [DOI] [PubMed] [Google Scholar]
- 26.Markowska, A. L., Stone, W. S., Ingram, D. K., Reynolds, J., Gold, P. E., Conti, L. H., Pontecorvo, M. J., Wenk, G. L. & Olton, D. S. (1989) Neurobiol. Aging 10, 31-43. [DOI] [PubMed] [Google Scholar]
- 27.Rapp, P. R. & Amaral, D. G. (1992) Trends Neurosci. 15, 340-345. [DOI] [PubMed] [Google Scholar]
- 28.Gage, F. H., Kelly, P. A. & Bjorklund, A. (1984) J. Neurosci. 4, 2856-2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gage, F. H., Chen, K. S., Buzsaki, G. & Armstrong, D. (1988) Neurobiol. Aging 9, 645-655. [DOI] [PubMed] [Google Scholar]
- 30.Gallagher, M., Burwell, R. D., Kodsi, M. H., McKinney, M., Southerland, S., Vella-Rountree, L. & Lewis, M. H. (1990) Neurobiol. Aging 11, 507-514. [DOI] [PubMed] [Google Scholar]
- 31.Geinisman, Y., Toledo-Morrell, L. & Morrell, F. (1986) Brain Res. 398, 266-275. [DOI] [PubMed] [Google Scholar]
- 32.Geinisman, Y., Toledo-Morrell, L. & Morrell, F. (1986) Proc. Natl. Acad. Sci. USA 83, 3027-3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kadar, T., Silbermann, M., Brandeis, R. & Levy, A. (1990) Brain Res. 512, 113-120. [DOI] [PubMed] [Google Scholar]
- 34.DeToledo-Morrell, L., Geinisman, Y. & Morrell, F. (1988) Neurobiol. Aging 9, 581-590. [DOI] [PubMed] [Google Scholar]
- 35.Matzel, L. D., Gandhi, C. C. & Muzzio, I. A. (2000) NeuroReport 11, 1253-1258. [DOI] [PubMed] [Google Scholar]
- 36.Döbrössy, M., Drapeau, E., Aurousseau, C., Le Moal, M., Piazza, P. V. & Abrous, D. N. Mol. Psychiatry, in press. [DOI] [PubMed]
- 37.Vallée, M., Mayo, W., Darnaudéry, M., Corpéchot, C., Young, J., Koehl, M., Le Moal, M., Baulieu, E. E., Robel, P. & Simon, H. (1997) Proc. Natl. Acad. Sci. USA 94, 14865-14870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gould, E., Beylin, A., Tanapat, P., Reeves, A. & Shors, T. J. (1999) Nat. Neurosci. 2, 260-265. [DOI] [PubMed] [Google Scholar]
- 39.Abrous, D. N., Adriani, W., Montaron, M. F., Aurousseau, C., Rougon, G., Le Moal, M. & Piazza, P. V. (2002) J. Neurosci. 22, 3656-3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rapp, P. R. & Gallagher, M. (1996) Proc. Natl. Acad. Sci. USA 93, 9926-9930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cameron, H. A. & McKay, R. D. (2001) J. Comp. Neurol. 435, 406-417. [DOI] [PubMed] [Google Scholar]
- 42.Nowakowski, R. S. & Hayes, N. L. (2000) Science 288, 771. [DOI] [PubMed] [Google Scholar]
- 43.Rakic, P. (2002) J. Neurosci. 22, 614-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rakic, P. (2002) Nat. Rev. Neurosci. 3, 65-71. [DOI] [PubMed] [Google Scholar]
- 45.Zucconi, G. G. & Giuditta, A. (2002) Rev. Neurosci. 13, 375-382. [DOI] [PubMed] [Google Scholar]
- 46.Endl, E., Hollmann, C. & Gerdes, J. (2001) in Methods in Cell Biology (Academic, San Diego), pp. 399-418. [DOI] [PubMed]
- 47.Merrill, D. A., Karim, R., Darraq, M., Chiba, A. A. & Tuszynski, M. H. (2003) J. Comp. Neurol. 459, 201-207. [DOI] [PubMed] [Google Scholar]
- 48.Banasr, M., Hery, M., Brezun, J. M. & Daszuta, A. (2001) Eur. J. Neurosci. 14, 1417-1424. [DOI] [PubMed] [Google Scholar]
- 49.Galea, L. A. & McEwen, B. S. (1999) Neuroscience 89, 955-964. [DOI] [PubMed] [Google Scholar]
- 50.Kempermann, G., Kuhn, H. G. & Gage, F. H. (1997) Proc. Natl. Acad. Sci. USA 94, 10409-10414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perfilieva, E., Risedal, A., Nyberg, J., Johansson, B. B. & Eriksson, P. S. (2001) J. Cereb. Blood Flow Metab. 21, 211-217. [DOI] [PubMed] [Google Scholar]
- 52.Tanapat, P., Hastings, N. B., Reeves, A. J. & Gould, E. (1999) J. Neurosci. 19, 5792-5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dellu, F., Mayo, W., Vallee, M., Le Moal, M. & Simon, H. (1994) Brain Res. 653, 51-56. [DOI] [PubMed] [Google Scholar]
- 54.Shors, T. J., Townsend, D. A., Zhao, M., Kozorovitskiy, Y. & Gould, E. (2002) Hippocampus 12, 578-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cohen, P., Ocrant, I., Fielder, P. J., Neely, E. K., Gargosky, S. E., Deal, C. I., Ceda, G. P., Youngman, O., Pham, H., Lamson, G., et al. (1992) Psychoneuroendocrinology 17, 335-342. [DOI] [PubMed] [Google Scholar]
- 56.Stenvers, K. L., Lund, P. K. & Gallagher, M. (1996) Neuroscience 72, 505-518. [DOI] [PubMed] [Google Scholar]
- 57.Markowska, A. L., Mooney, M. & Sonntag, W. E. (1998) Neuroscience 87, 559-569. [DOI] [PubMed] [Google Scholar]
- 58.Isaacson, R. L. (1976) The Limbic System (Plenum, New York).
- 59.Landfield, P. W., Waymire, J. C. & Lynch, G. (1978) Science 202, 1098-1102. [DOI] [PubMed] [Google Scholar]
- 60.Cameron, H. A. & Gould, E. (1994) Neuroscience 61, 203-209. [DOI] [PubMed] [Google Scholar]
- 61.Cameron, H. A. & McKay, R. D. (1999) Nat. Neurosci. 2, 894-897. [DOI] [PubMed] [Google Scholar]
- 62.Montaron, M. F., Petry, K. G., Rodriguez, J. J., Marinelli, M., Aurousseau, C., Rougon, G., Le Moal, M. & Abrous, D. N. (1999) Eur. J. Neurosci. 11, 1479-1485. [DOI] [PubMed] [Google Scholar]
- 63.Kirn, J., O'Loughlin, B., Kasparian, S. & Nottebohm, F. (1994) Proc. Natl. Acad. Sci. USA 91, 7844-7848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nottebohm, F. (1989) Sci. Am. 260 (2), 74-79. [DOI] [PubMed] [Google Scholar]
- 65.Nottebohm, F. (2002) J. Neurosci. 22, 624-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nottebohm, F. (2002) Brain Res. Bull. 57, 737-749. [DOI] [PubMed] [Google Scholar]
- 67.Scharff, C., Kirn, J. R., Grossman, M., Macklis, J. D. & Nottebohm, F. (2000) Neuron 25, 481-492. [DOI] [PubMed] [Google Scholar]
- 68.Agnati, L. F., Zoli, M., Biagini, G. & Fuxe, K. (1992) Acta Physiol. Scand. 145, 301-309. [DOI] [PubMed] [Google Scholar]