Abstract
Podocytes are specialized cells of the kidney that form the blood filtration barrier in the kidney glomerulus. The barrier function of podocytes depends upon the development of specialized cell–cell adhesion complexes called slit-diaphragms that form between podocyte foot processes surrounding glomerular blood vessels. Failure of the slit-diaphragm to form results in leakage of high molecular weight proteins into the blood filtrate and urine, a condition called proteinuria. In this work, we test whether the zebrafish pronephros can be used as an assay system for the development of glomerular function with the goal of identifying novel components of the slit-diaphragm. We first characterized the function of the zebrafish homolog of Nephrin, the disease gene associated with the congenital nephritic syndrome of the Finnish type, and Podocin, the gene mutated in autosomal recessive steroid-resistant nephrotic syndrome. Zebrafish nephrin and podocin were specifically expressed in pronephric podocytes and required for the development of pronephric podocyte cell structure. Ultrastructurally, disruption of nephrin or podocin expression resulted in a loss of slit-diaphragms at 72 and 96 h post-fertilization and failure to form normal podocyte foot processes. We also find that expression of the band 4.1/FERM domain gene mosaic eyes in podocytes is required for proper formation of slit-diaphragm cell–cell junctions. A functional assay of glomerular filtration barrier revealed that absence of normal nephrin, podocin or mosaic eyes expression results in loss of glomerular filtration discrimination and aberrant passage of high molecular weight substances into the glomerular filtrate.
Keywords: Kidney development, Podocyte, Pronephros, Slit diaphragm, Zebrafish
Introduction
The basis of kidney function is filtration of the blood and subsequent modification of the filtrate to regulate the salt and water content of body fluids. Podocytes are specialized kidney cells whose structure forms the basis of blood filtration in the glomerulus (Abrahamson and Wang, 2003). Podocyte foot processes are highly arborized cell extensions that form by extensive cell–cell interdigitation on the basement membrane surrounding the glomerular vascular capillary tuft (Pavenstadt et al., 2003). Filtration occurs as blood plasma flows out of the capillary and through “filtration-slits”, the space between podocyte foot processes (Pavenstadt et al., 2003). Specialized cell-junctions between podocyte foot processes, slit-diaphragms, are thought to maintain a barrier that discriminates against the diffusion of larger proteins out of the blood (Pavenstadt et al., 2003). Failure to form or loss of slit-diaphragms results in leakage of high molecular weight proteins and their appearance in urine, a condition called proteinuria (Pavenstadt et al., 2003). Proteinuria occurs in several human congenital nephropathies and is also a common complication of diabetes (Cooper et al., 2002).
The Nephrin gene is mutated in the condition congenital nephritic syndrome of the Finnish type (NPHS1) (Kestila et al., 1998). Nephrin is a transmembrane protein present in the slit-diaphragm itself and is thought to contribute to its zipper-like, extracellular structure (Ruotsalainen et al., 1999). The Nephrin cytoplasmic domain can be phosphorylated by the Src family kinase Fyn resulting in activation of signaling pathways in involving MAP kinase and AKT (Benzing, 2004; Huber et al., 2003a; Lahdenpera et al., 2003; Verma et al., 2003; Yu et al., 2001). In mice lacking Nephrin, podocyte foot processes form initially; however, slit-diaphragms are lacking and the animals exhibit massive proteinuria (Hamano et al., 2002; Putaala et al., 2001; Rantanen et al., 2002).
Podocin is an intracellular podocyte cell junction-associated protein (Roselli et al., 2002) that is mutated in autosomal recessive steroid-resistant nephrotic syndrome (NPHS2). Podocin is thought to play a role in the delivery of other slit-diaphragm proteins to the junction (Huber et al., 2003b; Roselli et al., 2004b). Other genes that are essential for assembly of the slit-diaphragm include the adaptor protein CD2-associated protein (CD2AP), the protocadherin FAT and the Nephrin related protein, Neph1. Mutations in CD2AP, FAT or Neph1, like Nephrin and Podocin mutations, also lead to retraction of podocyte foot processes and proteinuria (Ciani et al., 2003; Donoviel et al., 2001; Shih et al., 1999).
Despite their uncommon structure, slit-diaphragms are thought to be a specialized form of cell junction found in more traditional epithelial cells. The cadherins P-cadherin and FAT are present in slit-diaphragms (Ciani et al., 2003; Reiser et al., 2000; Tassin et al., 1994), suggesting that these junctions are modified adherens type junctions. Slit-diaphragms also contain the protein ZO-1, a protein found in both occludens type and adherens cell junctions (Schnabel et al., 1990). During early nephron differentiation, podocytes initially form a polarized epithelium with apical cell junctions containing ZO-1, p120 catenin and β-catenin (Ruotsalainen et al., 2000; Schnabel et al., 1990). Cell polarization is evident early on by the basal adhesion of podocytes to the glomerular basement membrane and the expression of cell surface proteins on basolateral and apical cell surfaces (Reeves et al., 1978). As podocyte differentiation proceeds, intercellular junctions migrate toward the basal cell surface such that by the capillary loop stage, the cell junctions are positioned at the lateral edge of the basal cell surface where they eventually go on to form the slit-diaphragm (Reeves et al., 1978; Ruotsalainen et al., 2000).
Overall podocyte cell shape and foot process formation is dependent on coordinated assembly of microtubule and actin components of the cytoskeleton and, as in other epithelial cells, podocyte cell junctions develop in concert with changes in cytoskeletal organization (Pavenstadt et al., 2003). Adapter proteins that link the cytoskeleton to transmembrane proteins, or junction proteins that regulate cytoskeletal assembly play important roles in this process. For example, CD2AP is thought to bind both nephrin and the actin cytoskeleton and thus stabilize slit-diaphragms (Wolf and Stahl, 2003). Cell junction migration and foot process formation may also be linked through the activity of proteins like p120 catenin which is expressed in migrating podocyte cell junctions (Usui et al., 2003) and may favor actin cytoskeletal rearrangement and basal foot process formation via inhibition of the small GTPase RhoA (Noren et al., 2000). It is clear however from the limited information on podocyte proteins linking cell-junctions to the cytoskeleton that our current description of slit-diaphragm junction formation is incomplete.
Protein 4.1 is a linker molecule originally identified in erythrocytes that interact with both transmembrane proteins and the actin cytoskeleton (Hoover and Bryant, 2000). The 4.1 gene family includes at least four other members, the 4.1-like proteins, which are broadly expressed in a variety of tissues. All 4.1 proteins are expressed as alternatively spliced transcripts encoding a diverse set of proteins which, in addition to stabilizing cytoskeleton-membrane interactions, may also play important roles in cell signaling (Gascard et al., 2004; Hoover and Bryant, 2000; Sun et al., 2002). Based on structural and functional homology, Protein 4.1 belongs to a larger class of molecules, the FERM domain containing proteins (Four point one/ezrin/radixin/moesin) which play key roles in integrating trans-membrane protein activity with changes in cytoskeletal organization (Chishti et al., 1998). In the mouse nephron, protein 4.1 family members 4.1R/EPB4.l, 4.1N/EPB41L1, 4.1G/EPB41L2 and 4.1B/EPB41L3 are expressed in distinct nephron cell types (Ramez et al., 2003); however, none have been shown to be expressed in podocytes. Of the proteins comprising the larger FERM domain containing superfamily, Radixin and Moesin are expressed in endothelial and supporting mesangial cells of the glomerulus but appear to be excluded from podocytes (Hugo et al., 1998). Ezrin is the one FERM domain protein known to be expressed in podocytes where it associates with the cytoplasmic tail of the apical transmembrane protein Podocalyxin and stabilizes its interaction with subapical actin filaments (Orlando et al., 2001). Recently, mosaic eyes (moe/EPB41L5), a novel gene encoding a FERM protein required for proper tissue organization in the eye, was shown to be expressed in zebrafish podocytes (Jensen et al., 2001; Jensen and Westerfield, 2004), suggesting that this FERM domain protein may also participate in linking transmembrane proteins to cytoskeletal elements in developing podocytes.
In the work presented here, we sought to determine whether the zebrafish pronephros could be used as a model of glomerular maturation and the development of filtration barrier function. We first determined the developmental stage when the glomerular filtration barrier was formed in the zebrafish pronephros, and then tested if proteins similar to those described in the mammalian kidney were employed in pronephric slit-diaphragm formation. We show here that the zebrafish slit-diaphragm proteins Nephrin and Podocin are required for foot process formation and slit-diaphragm assembly during podocyte terminal differentiation. Loss of either slit-diaphragm protein by morpholino knockdown results in altered podocyte morphology, plasma filtration and loss of podocyte barrier function in the mature pronephros. We also demonstrate that the FERM protein Mosaic eyes is required in podocytes to form or maintain slit-diaphragms and to maintain proper filtration discrimination. These results indicate that the zebrafish pronephros is a relevant model of glomerulus formation where the function of novel components of this process can be revealed.
Materials and methods
Zebrafish lines
Wild-type zebrafish were maintained and raised as described (Westerfield, 1995). The wild-type lines were of the TL or TÜAB background. The mosaic eyes mutant (moe) was originally isolated as a gamma irradiation deletion allele (b476) (Jensen et al., 2001). The b882 allele used in this work is an ENU allele that contains a splice donor site mutation that would be predicted to encode a transcript that retains intron 5 with a stop codon 84 base pairs into the intron. This would truncate the protein in the FERM domain (Jensen and Westerfield, 2004). Dechorionated embryos were kept in E3 solution with or without 0.003% PTU (1-Phenyl-2-thiourea, Sigma) to suppress pigmentation and staged according to hours post-fertilization (hpf) (Westerfield, 1995). Whole embryos were observed using a Nikon SMZ 645 or Leica MZ12 dissecting stereomicroscope, the latter with an attached digital camera (Spot Insight QE, USA) and processed with Adobe Photoshop software (Adobe, Inc.).
Cloning of nephrin and podocin
The zebrafish nephrin sequence was partially derived from sequence analysis by performing a tblastn (Basic Local Alignment Search Tool) search with the human and murine sequence against whole-genome-shotgun-reads and assembled contigs all provided by the zebrafish genome project (Sanger Institute, U.K.) (http://www.ensembl.org/Danio_rerio/), the result was used for 5′ and 3′ RACE reactions (Invitrogen). Zebrafish nephrin was cloned using nested PCR on single-stranded DNA obtained by reverse transcription of total RNA from wild-type fish, TL strain. Total RNA was obtained using the RNAqueous-4PCR kit (Ambion). The following primer-sets have been used: forward ATTTATTTTGACGCCGTGGACCAAT, reverse CTCCTAAACCTGACCTGAGCAGACC, nested forward CAATTGGCGGCGATGGATTC, nested reverse ACCTGAGCAGACCATATACGCCAGA. A 3775 bp product was subcloned in pCRII TOPO (Invitrogen) and sequenced.
Zebrafish podocin sequence was derived similarly and subcloned using the following primer sets: forward TGCAGAAAACCAAACACCAGAGGA, reverse gatgatgcgaaggaaatccgtcaa, nested forward CGGGATCCGCCACCATGCTTCCTGCGGGGACA, reverse CGGAATTCtgtctttacatcatgggtgagtctttgg. The 1225 bp fragment was subcloned into pCS2+ using the generated endonuclease restriction sites BamH1 and EcoR1 and sequenced.
Structural predictions for Nephrin and Podocin proteins were performed using the Simple Modular Architecture Research Tool (SMART; http://smart.embl-heidelberg.de/) using default search parameters (Schultz et al., 2000).
In situ hybridization
Whole-mount in situ hybridization was performed as previously described (Thisse and Thisse, 1998) with the exception of longer proteinaseK treatments (3 min for 24 hpf, 6 min for 36 hpf, 9 min for 48 hpf and 14 min for 72 hpf stage embryos). For the nephrin antisense probe, the template (pCRII-TOPO-nephrin 2029 bp, flanking primers forward CATCAACCAAGTTACCCGTTCACCA and reverse TGAAGGGTTAAGGCCTGTGACTGTA) was linearized with NotI and the antisense riboprobes were transcribed using SP6 RNA polymerase. The podocin antisense riboprobes were generated using a BamHI linearized template and T7 RNA polymerase. Embryos were hybridized with digoxigenin-labeled riboprobes. Anti-DIG AP (1:5000) and the NBT/BCIP substrate (Boehringer Mannheim) were used to detect the probe. After the color reaction was stopped, embryos were washed with methanol and equilibrated in clearing solution (1/3 benzoyl-alcohol and 2/3 benzoyl-benzoate) and photographed using the abovementioned dissecting microscope. For histological analysis, the stained embryos were equilibrated in PBS with 0.1% Tween-20 (PBT), dehydrated in series into ethanol, embedded in JB-4 (Polysciences) and sectioned.
Morpholino antisense oligonucleotides
Morpholino antisense oligonucleotides were designed to target an exon splice donor sites causing splicing defects of the mRNA. The morpholino oligonucleotides were obtained from GENE TOOLS, LLC, Philomath, OR. The following morpholinos were used: nephrinMO CGCTGTCCATTACCTTTCAGGCTCC, podocin MO TAGACTTACCTTCTCCAGGTCCCTC, Control, scrambledMO CCTCTTACCTCAGTTACAATTTATA. The morpholinos were diluted in injection solution containing: 100 mM KCl, 10 mM HEPES, 0.1% Phenol Red (Sigma) and titrated to determine the lowest concentration sufficient to consistently induce abnormal mRNA splicing. Morpholinos for both nephrin and podocin were injected at a final stock concentration of 0.1 mM into one-cell stage embryos. Injection volume was approximately 3 nl and final cytoplasmic morpholino concentration estimated to be approximately 1 μM. For each morpholino, more than 400 individual embryos were injected. Edema/kidney failure was observed in greater than 90% of the injected larvae at 96 hpf; control morpholino injections did not result in any overt phenotypic changes. Injections were performed using a microinjector PLI-90 (Harvard Apparatus, Cambridge, MA). The effect of the splice-morpholinos was verified by RT-PCR from single embryo total RNA with nested primers in flanking exons yielding a 300–500 bp amplicon.
Histochemistry
Embryos were fixed in BT-fix (4% paraformaldehyde, 0.1 M Na2HPO4 buffer pH 7.3, 3% sucrose, 0.12 mM CaCl2; Westerfield, 1995) at 4°C overnight. After being washed in PBS and taken through an ethanol dehydration series, they were embedded in JB-4 resin (Polysciences Inc.) and sectioned at 3–5 μm. Slides were stained in methylene blue/azure II (Humphrey and Pittman, 1974), mounted and examined using a Nikon microscope.
Transmission electron microscopy
Embryos were fixed for electron microscopy in 1.5% glutaraldehyde plus 1% paraformaldehyde in 70 mM Na2HPO4 buffer pH 7.2 plus 3% sucrose at 4°C overnight. They were then rinsed in 0.1 M cacodylate buffer and post-stained in 1% osmium tetroxide in 0.1 M sodium cacodylate pH 7.4 buffer for 1 h at room temperature. They were then rinsed in cacodylate buffer, followed by distilled water and placed in 2% aqueous uranyl acetate for en bloc staining for 1 h at room temperature. This was followed by dehydration through a graded series of ethanol and propylene oxide. Embryos were then infiltrated in a 1:1 solution of propylene oxide and EPON (Ted Pella, Inc.) overnight at room temperature. The following day, they were infiltrated in pure EPON and embedded at 60°C overnight. Thin sections (70–80 nm) were cut on a Reichert Ultracut E ultra-microtome and collected onto formvar-coated slot grids. The sections were stained with uranyl acetate and lead and examined in a Philips CM10 TEM at 80 kV.
Fluorescent dye injection
Lysine-fixable FITC-dextran (70KD, 500KD, Molecular Probes) or AlexaFluor488-BSA (Molecular Probes) was dissolved at 1% in phosphate buffer (1× PBS pH 7.4, 1.8 mM CaCl2, 0.1% Phenol red). Fluorescent tracers were injected into the common cardinal vein (CCV) or directly into the atrium of 80 hpf larvae anesthetized with 0.2 mg/ml tricaine (3-amino benzoic acid ethyl ester, Sigma) in egg water. Only embryos with good circulation, as judged by sufficiently moving blood cells, were used for filtration experiments. After overnight incubation, the embryos were fixed and sectioned. Uptake of filtered fluorescent dextran by duct cells was evaluated in serial sections using a Nikon fluorescent microscope.
Results
Development of the zebrafish glomerular filtration apparatus
In previous studies, we established that blood filtration by the zebrafish pronephric glomerulus begins as soon as capillary sprouts from the dorsal aorta invade clusters of forming podocytes at 40 hpf (Drummond et al., 1998; Majumdar and Drummond, 2000). However, the glomerulus at this stage does not display interdigitating fine foot processes and slit-diaphragms typical of a mature glomerulus (Fig. 1A). To determine when the filtration apparatus in the zebrafish pronephric glomerulus is established, we analyzed the structure of later stage day 3 and day 4 glomeruli. Histological sections (Fig. 1B) show the overall structure of the pronephros at day 3. Surprisingly, even though all glomerular cell types are present by 72 hpf and the pronephros is required to function as an osmoregulatory kidney, electron micrographs show that podocyte foot processes are still immature broad cell extensions, and slit-diaphragms between foot processes are rarely observed (Fig. 1C). In contrast, at 96 hpf, the filtration apparatus appears mature with podocyte foot processes present as fine, evenly spaced cell processes separated by slit-diaphragm cell–cell junctions (Fig. 1D). These observations show that the structure of the glomerulus matures relatively late in pronephric development, roughly 2 days after the onset of filtration at hatching.
Fig. 1.
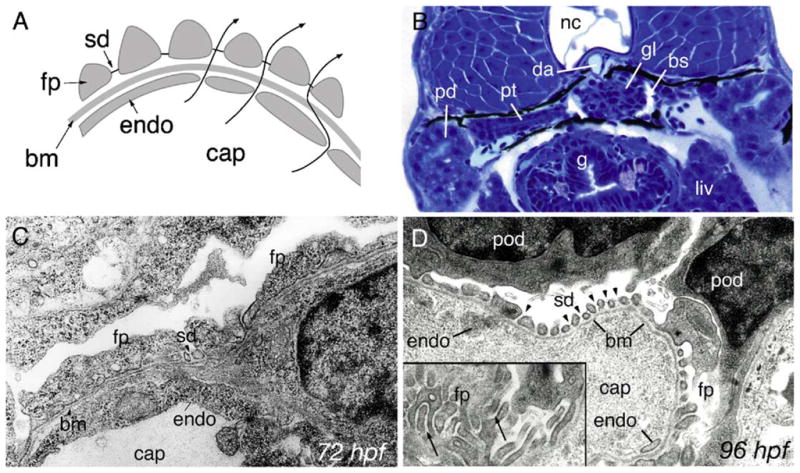
Development of pronephric glomerular filtration slits. (A) Diagram of the vertebrate glomerular filter. Glomerular capillaries (cap) are lined with fenestrated endothelial cells (endo) that rest on a basement membrane (bm). Apposed on the opposite face of the basement membrane, podocyte foot processes (fp) form filtration slits that contain the slit-diaphragm as a specialized cell–cell junction. Arrows represent the path of blood fluid filtration. (B) At 84 hpf, the glomerulus (gl), the pronephric tubule (pt) and the pronephric ducts (pd) are fully formed between the gut (g), liver (liv) and the notochord (nc). Bowman’s space (bs) is clearly evident around the glomerulus (gl) and is drained by the pronephric tubules. The glomerular capillary tuft originates from the dorsal aorta (da). (C) Electron micrograph of 72 hpf podoctye foot processes (fp) reveals broad spreading cell processes as opposed to the fine interdigitations present at later stages of development. Some slit-diaphragms (sd) are present. (D) Electron micrograph at 96 hpf shows a capillary lumen (cap) and endothelial cells (endo). Podocytes (pod) and their foot processes (fp) surround the capillary along the basement membrane (bm). Slit-diaphragms (sd, arrowheads) are clearly visible between the individual foot processes. On a tangential section of a capillary wall (inset), interdigitating foot processes (fp) can be seen (arrows).
Expression of nephrin and podocin in the pronephros
Nephrin and Podocin are highly expressed in mammalian podocytes where they function as essential components of the slit-diaphragm complex. To determine whether these two proteins functioned similarly in zebrafish, we cloned and analyzed the expression of zebrafish nephrin and podocin. Amino acid identity of zebrafish homologs of nephrin and podocin was low (36% and 45% identical to the human proteins, respectively; Supplementary Fig. 1); however, homology to human nephrin and podocin is more clearly evident when the predicted secondary structures are compared (Fig. 2). Expression of both nephrin and podocin is highly specific in podocytes (Fig. 3) and commenced by 24 hpf in podocyte precursor cells (Figs. 3A and C). Expression remained robust and podocyte-specific at later stages of glomerulus formation (Figs. 3B, D and E) and in the functioning glomerulus at 72 hpf (Fig. 3C) and at 120 hpf (Fig. 3F). No expression of nephrin or podocin was observed in endothelial cells in whole mounts (Figs. 3A, B, D and E) or in histological sections (Fig. 3F) of the glomerular capillary tuft.
Fig. 2.
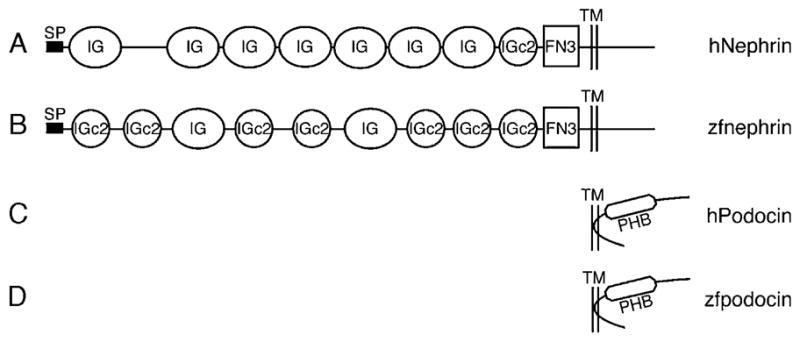
Structural homology between Nephrin and Podocin of man and zebrafish. SMART (Simple Modular Architecture Research Tool) was used for determination of structural similarity between the human and zebrafish homologs. Nephrin (A,B) is a type-1 integral membrane protein, with several immunoglobuline-like domains and fibronectin domain close to the transmembrane domain while podocin (C,D) has a hairpin shape with a prohibitin homology domain. FN3, Fibronectin type 3 domain; IG, Immunoglobulin-like domain; IGc2, Immunoglobulin C-2 type domain; PHB, prohibitin homology domain; SP, signal peptide; TM, transmembrane domain. Amino acid alignments for nephrin and podocin are shown in Supplementary Fig. 1.
Fig. 3.
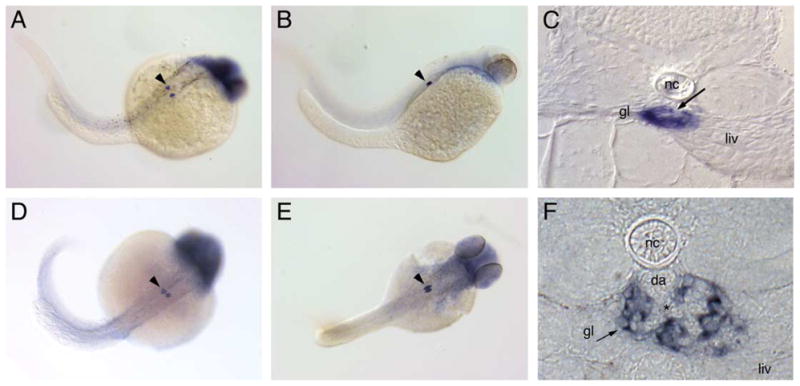
nephrin and podocin are exclusively expressed in the pronephric glomerulus. Nephrin is expressed in the forming glomerulus starting at 24 hpf (A) and continues to be expressed at 48 hpf (B) and 72 hpf (C). Expression of podocin at 36 hpf (D) marks the podocyte precursor cells; at 48 hpf (E), podocin positive nephron primordia lie adjacent to each other. Cross-sections at 72 hpf show the glomerular expression of nephrin (C) in a localization typical for podocytes surrounding the glomerular capillary tuft (C, arrow). At 120 hpf (F), nephrin expression is strong in podocytes adjacent to the capillary tuft (*) originating from the dorsal aorta (da). Endothelial cells in the aorta and the glomerular capillaries are negative for nephrin expression. Diffuse staining for both probes in the head was not strong or reproducible and most likely represents optical summation of background staining. Spots of dark pigment in the trunk in panel A are not nephrin expressing cells but rather pigment cells that escaped PTU treatment (gl, glomerulus; liv, liver; nc, notochord; da, dorsal aorta).
Altered kidney function and podocyte morphology in nephrin and podocin morpholino knockdown embryos
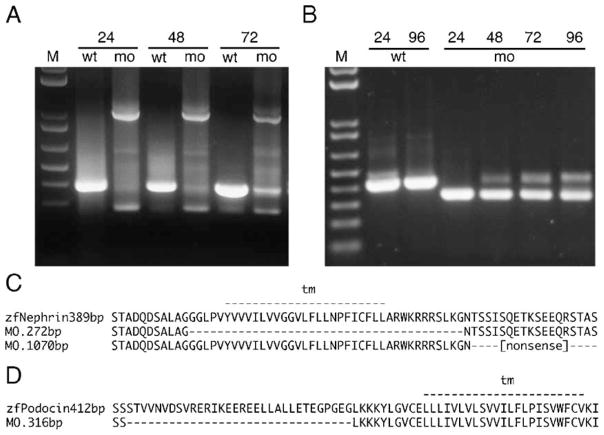
To determine the role of nephrin and podocin in zebrafish pronephric function, we targeted these two genes with antisense morpholino oligos. Morpholinos were designed against the splice donor site of the exon encoding the transmembrane domain in nephrin and the first coding exon in podocin. Morpholino injection resulted in mis-splicing mRNA for both genes as detected by sequencing altered RT-PCR products (Figs. 4A and B). The sequence of altered nephrin mRNAs predicts either an in-frame deletion of the transmembrane domain producing a protein that would be secreted and not anchored in the membrane, or a truncation of the cytosolic C-terminus after frame-shift and non-sense translation (Fig. 4C). The altered podocin mRNA is predicted to encode a protein lacking 32 amino acids in the N-terminus owing to an in-frame deletion 5′ to the membrane-associated domain (Fig. 4D). Normal nephrin mRNA was undetectable at 24 and 48 hpf while some recovery of wild-type message was seen at 72 hpf (Fig. 4A). Wild-type podocin mRNA was completely absent at 24 hpf; small amounts of normal message could however be detected at 48, 72 and 96 hpf (Fig. 4B).
Fig. 4.
nephrin and podocin mRNA splicing defects induced by morpholinos targeting exon donor sites. RT-PCR was performed from total RNA of single embryos at the indicated stage (24, 48, 72, 96 hpf) with flanking exon primers (M, 10 kb plus DNA marker, Invitrogen). (A) The nephrin morpholino targeting the splice donor of the transmembrane domain coding exon caused an in-frame deletion of this exon, visible as a decrease in amplicon size (272 bp versus 389 bp amino acid sequences are aligned in panel C), or a non-splicing of the adjacent intron, visible as an increase in amplicon size (1070 bp) leading to a truncation after a stretch of non-sense amino acids (C). Both forms of nephrin mRNA are predicted to encode a protein lacking the cytosolic C-terminus of the nephrin. The morpholino effects last for at least 72–96 hpf. (B) The podocin morpholino causes an in-frame deletion of part of the first coding exon (amplicon size 316 bp versus 412 bp leading to a shortened N-terminus of the Podocin molecule (D)).
Disruption of nephrin and podocin mRNA both resulted in pericardial edema at 96 hpf (Fig. 5A) and later caused general edema involving the entire larval body. The hydropic embryos eventually die at 1 week of age, suggesting a loss of pronephric osmoregulatory function. Embryos injected with control, scrambled sequence morpholino oligos did not exhibit edema and appeared wild-type (Kramer–Zucker; data not shown). By histology, the glomerulus in 72 hpf wild-type embryos appears well-developed (Fig. 5B) while in nephrin morphant larvae glomeruli appeared somewhat flattened (Fig. 5C). A significant proportion of morphant embryos (37% of the nephrinMO embryos and in 44% of the podocinMO embryos) exhibited cysts in the pronephric tubules with a stretched septal glomerulus in the midline (Fig. 5D). Since neither nephrin nor podocin is expressed in the cystic tubule epithelium, we examined morphant embryos for a possible indirect cause of cystic distension. Sections of the pronephric ducts distal to the cysts revealed the presence of large birefringent crystals completely obstructing the duct lumen (Fig. 5E) and in some podocin morphants, debris was observed in the duct lumen (Fig. 5F). These findings suggest that loss of podocin and nephrin function in glomerular podocytes allows for accumulation of debris and possibly protein in the tubules and ducts downstream, leading in some cases to occlusion of the duct lumen.
Fig. 5.
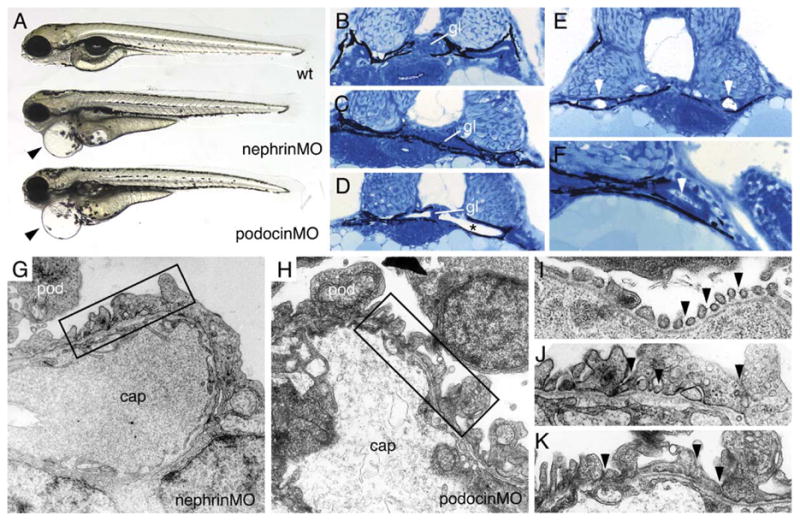
nephrin and podocin morphant embryos exhibit progressive edema, glomerular malformation and podocyte foot process effacement. (A) nephrin and podocin morphant larvae develop severe pericardial edema (arrowheads) at 96 hpf. (B–F) histological sections of 72 hpf embryos stained with methylene blue/azure II. (B) Normal wild-type glomerulus (gl). Glomeruli in nephrin morphant larva are somewhat flattened (C), and, in approximately 30% of morpholino injected embryos, the glomerulus appears as a midline septum with distended tubules (* in panel D). (E) In cystic nephrin morphants, evidence for obstruction of the pronephric duct could be seen in the presence of large, occluding crystaline deposits (white arrowheads) that completely fill the duct lumen. Particulate debris in the duct lumen was also observed in podocin morphants (F). In contrast to wild-type podocytes (see Fig. 1), nephrin morphant podocytes at 96 hpf show foot process effacement (G) and lack of fine interdigitation. Similarly, podocin morphant podocytes at 96 hpf exhibit irregular processes and foot process effacement (H). At 96 hpf, wild-type podocyte foot processes and slit-diaphragms appear as “beads on a string” (I; arrowheads) along the basement membrane of the capillary wall. nephrin and podocin foot processes at 96 hpf are broad and effaced and lack slit-diaphragms (panels J and K are higher magnifications of the boxed areas in panels G and H).
The effects of misplicing nephrin and podocin mRNA on foot process formation and barrier function in the glomerulus were further explored by electron microscopy. At 96 hpf, podocyte foot processes were flattened and effaced in morphant embryos (Figs. 5G and H) compared to control embryos at the same stage (96 hpf; Fig. 1D). At higher magnification, slit-diaphragms are clearly visible between well-spaced wild-type foot processes (Fig. 5I). Nephrin morphant embryos at 96 hpf rarely showed fine foot processes and, when present, slit-diaphragms were missing (Fig. 5J). Podocin morphant embryos also rarely showed fine foot processes or slit-diaphragms (Fig. 5K). The ultrastructure of nephrin and podocin morphant podocytes indicates that, as is the case in humans, these genes are essential to form the pronephric glomerular filtration barrier.
mosaic eyes is expressed in podocytes and is required for slit-diaphragm formation
The mosaic eyes (moe) gene product is a FERM domain containing protein (Jensen and Westerfield, 2004) that is expressed in podocytes (Fig. 6A). Similar to nephrin and podocin morphants, moe homozygous mutant embryos (Fig. 6C) exhibit severe pericardial edema at 80 hpf, suggesting that moe may be essential for proper osmoregulation and pronephric function. Histological sections indicated that the mutation in moe does not interfere with the overall formation of the glomerulus; despite some vascular edema, capillary tuft formation appeared relatively normal (compare Figs. 6D and E). To further investigate the role of moe in podocyte function, we examined podocyte ultrastructure in moe −/− embryos. moe −/− podocytes showed apical “microvillar” projections (Fig. 6F) that were not seen in wild-type sibling embryos (Drummond, data not shown; see also Fig. 1D). When examined at higher magnification, moe podocytes also appear abnormal in that slit-diaphragms appear to be absent between foot processes adherent to the basement membrane (Figs. 6G and H). Surprisingly, structures resembling slit-diaphragms were present in moe −/− podocytes between the cell processes projecting into Bowman’s space (Fig. 6I). These connections between cell projections appear as double cross-strands bridging adjacent cell membranes. These results suggest that moe is required for proper podocyte slit-diaphragm formation. Also apparent in electron micrographs of moe −/− glomeruli is precipitated material in Bowman’s space, suggesting substantial protein leakage past the glomerular basement membrane (asterisk in Fig. 6F).
Fig. 6.
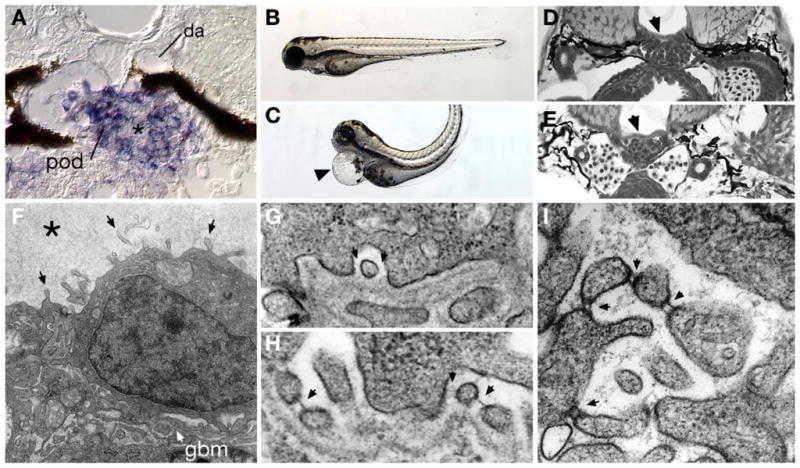
Expression of the FERM protein mosaic eyes (moe) in podocytes and cell-junction defects associated with moe loss of function. moe mRNA is highly expressed in 96 hpf wild-type podocytes (A) and not adjacent endothelial cells of the glomerular capillary (*) or the dorsal aorta (da). Homozygous moe mutants at 80 hpf show a dorsally bent body axis and pericardial edema (arrowhead in panel C) compared to sibling heterozygotes (B). Glomerular capillary formation in wild-type moe heterozygotes (D) and moe homozygotes (E) is similar, indicating that moe is not required for early glomerular morphogenesis. (F) In contrast to wild-type podocytes (see Fig. 1), moe mutant podocytes aberrantly extend cell processes from their apical surface (arrows; “microvillar” projections). Bowman’s space is also filled with an electron-dense precipitate (*) suggesting protein leakage. On moe podocyte foot processes that are adherent to the basement membrane (G), slit-diaphragms are not commonly observed (arrows denote missing slit-diaphragms); wild-type embryos commonly display slit-diaphragms (H) between similar types of foot processes (arrows). Podocyte cell processes in moe −/− embryos that project into Bowman’s space often show doublet cross-strands between nearby cell projections (arrows).
Loss of glomerular barrier function in slit-diaphragm morphant and moe mutant embryos
Failure to form proper podocyte foot processes and slit-diaphragms is associated with aberrant passage of high molecular weight proteins into the glomerular filtrate. To directly test the barrier function of the glomerulus in zebrafish pronephros, we injected different sized fluorescent molecules into the circulation and assayed their passage into the tubule and duct lumens by the appearance of fluorescent apical endosomes in pronephric duct cells (Drummond et al., 1998; Majumdar and Drummond, 2000). Initial attempts using 70 kDa rhodamine-dextran and 68 kDa Alexa-BSA revealed that the wild-type glomerulus at 72 hpf is relatively leaky; these tracers readily passed the glomerulus and appeared in the pronephric ducts (data not shown). We therefore tested a larger molecule, 500 kDa FITC-dextran, as a marker of glomerular leakage. 500 kDa FITC-dextran injected into the common cardinal vein of 84 hpf larvae was completely retained in the vascular system after a 1 to 2 h incubation and did not show any passage into the tubule and duct lumens as evidenced by the lack of fluorescent apical endosomes in pronephric duct epithelial cells (data not shown). Even after prolonged overnight incubation, only sporadic low levels of filtration and reuptake of dye were observed in the most proximal pronephric tubules and dye was not observed in distal, mid-trunk pronephric duct epithelial cells of wild-type embryos (Figs. 7A and D). This behavior of 500 kDa FITC-dextran demonstrated that it could be used as an indicator of pronephric glomerular filtration discrimination. When 500 kDa FITC-dextran was injected into nephrin and podocin morphants and moe −/− embryos, all embryos exhibited significant glomerular dye passage as evidenced by uptake into apical endosomes of cells in the distal midsection of the pronephric duct (Figs. 7B, C and E), suggesting a significantly increased rate of dye passage through an altered glomerular filter. moe heterozygote sibling embryos did not show dye uptake and appeared identical to wild-type embryos (Fig. 7D). The combined scores for lumenal dye uptake in the pronephric ducts at the level of the swim bladder are given in Fig. 7F. The results show that the zebrafish pronephros can be used as an assay for selective glomerular filtration and that disruption of slit-diaphragm structural or regulatory proteins can result in failed development of the pronephric glomerular filtration barrier.
Fig. 7.
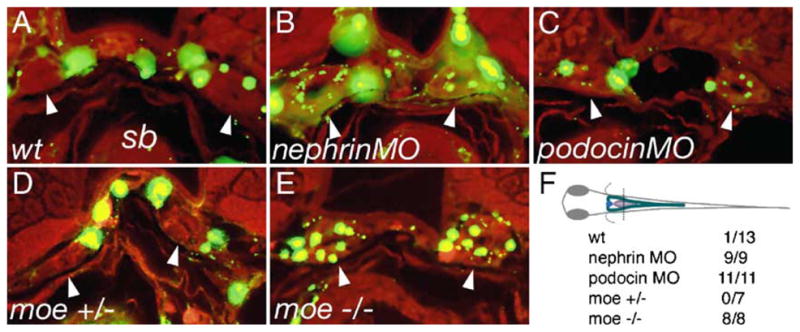
Failed slit-diaphragm formation correlates with loss of barrier function in the glomerulus. 500 kDa FITC-dextran was injected into the circulation of 80 hpf larvae and the larvae were fixed the next morning (96 hpf) and sectioned. Dye uptake by endocytosis from the pronephric duct lumen was assessed at the level of the swim bladder (sb). Arrowheads indicate the position of pronephric ducts in the cross-sections. In wild-type control larvae (A), FITC fluorescence is present in the vasculature surrounding the pronephric ducts; however, no endosomes containing filtered dye are visible (arrowheads) in the pronephric duct epithelial cells. In nephrin (B) and podocin (C) morpholino injected embryos, dye uptake is detectable in the form of small apical endosomes adjacent to the duct lumen. Wild-type moe heterozygous larvae (D) show no dye uptake in the pronephric ducts, while moe homozygous mutant larvae (E) exhibit abundant dye uptake (arrowheads) at the same position in the ducts. (F) The plane of section for cross-sections in panels A–E is shown. The table represents the number of larvae examined/number of larvae positive for endocytosed dye in the pronerphic duct. 500 kDa FITC-dextran (green), autofluorescence (red).
Discussion
The slit-diaphragm is a specialized cell–cell junction that is essential for maintaining the filtration barrier in the kidney glomerulus. Multiple interacting protein components that form the slit-diaphragm in mammalian podocytes have been defined both genetically and biochemically (Miner, 2003). In this study, we set out to determine whether the zebrafish pronephros could be used as an assay for slit-diaphragm formation and glomerular barrier function. We also sought to test the role of Moe, a FERM domain protein and potential link between the cytoskeleton and the cell membrane, in the assembly of slit-diaphragms. We established that the pronephros can be used as an assay of glomerular filtration by studying the function of known slit-diaphragm proteins Nephrin and Podocin. Zebrafish Nephrin and Podocin appear to function as true homologs: their expression was highly podocyte-specific and both were essential for pronephric kidney function. The FERM domain protein Moe appears to be important for proper slit-diaphragm formation in foot processes at the glomerular basement membrane. Our results implicate two known human disease genes in pronephric kidney development and establish Mosaic eyes/band 4.1-like 5 protein as a novel component in podocyte cell junction formation.
The zebrafish pronephric glomerulus as a model of selective plasma filtration
The establishment of an ultrafiltration barrier is a critical step in the development of the glomerulus and the onset of kidney function. The mature mammalian glomerular filter retains most of the protein larger than approximately 70 kDa in the vasculature (Pavenstadt et al., 2003). Small amounts of serum albumen and other proteins that do normally pass into the plasma filtrate are reabsorbed by endocytosis in proximal tubule epithelial cells (Russo et al., 2003). The stage of development when filtration discrimination is established in the mammalian fetal kidney has been difficult to determine. The presence of significant amounts of alpha-fetoprotein (AFP) in mammalian amniotic fluid, which is produced by the fetal kidney, suggests that forming glomeruli do not initially possess a tight filtration barrier and that the filtration discrimination seen in the adult kidney develops as the glomerulus matures (Burghard et al., 1987). Our observations on zebrafish pronephric glomerulus development indicate that slit-diaphragm formation occurs at a relatively late stage; slit-diaphragms are infrequently observed at 72 hpf but by 96 hpf most podocyte foot processes are fine, interdigitating structures linked by slit-diaphragm junctions. Consistent with the late development of slit-diaphragms, we find that zebrafish glomeruli are relatively leaky to plasma macromolecules prior to 72 hpf. Molecules such as serum albumin and 70 kDa dextran can pass into the tubule lumen via the glomerulus and are taken up in endocytic vesicles of the pronephric duct epithelium. Filtration discrimination was observed with the larger 500 kDa dextran; no glomerular passage of this tracer was observed after introducing it into the circulation for 2 h in 84 hpf larvae and little passage was seen even after an overnight incubation. Similar studies using vascular injection of fluorescent dyes in Xenopus larvae have shown that the forming pronephros in the frog is also relatively leaky. In these experiments, all tested tracers, including 500 kDa dextran, were seen to pass into urine in wild-type larvae (Zhou and Vize, 2004). The difference in these results may reflect the stage of glomerular maturation at which injections were performed; i.e. the frog pronephros at later stages of development may behave similarly to what we observe in the 84–96 hpf zebrafish pronephros. While it is possible that further filtration discrimination may develop as the zebrafish larva matures past 96 hpf, our experiments were constrained by the limited time window of morpholino efficacy (72–96 hpf) and the potential for recovery of wild-type nephrin or podocin function past this stage. Taken together with our previous results (Drummond et al., 1998; Majumdar and Drummond, 2000), this work shows that the zebrafish pronephros can be used as an assay for glomerular filtration. It might be anticipated that this assay could be further improved by testing additional tracers that vary in molecular size and/or charge.
The role of zebrafish nephrin and podocin in slit-diaphragm formation
The specialized cell–cell junctions that form the basis of filtration selectivity in the kidney have been the subject of intense study since the cloning of two human disease genes, nephrin and podocin, that regulate the formation or structure of slit-diaphragms (Boute et al., 2000; Kestila et al., 1998). Nephrin is a type-1 integral membrane protein and, as a member of the immunoglobulin family, consists of several extracellular immunoglobulin-like domains, which are thought to interact to form interdigitating polymers with other nephrin molecules and NEPH1 along the slit-diaphragm (Gerke et al., 2003; Liu et al., 2003; Wartiovaara et al., 2004). The cytosolic tail of Nephrin interacts with Podocin and CD2AP, also members of the slit-diaphragm protein complex (Huber et al., 2003a; Liu et al., 2005). The Nephrin cytosolic tail is also thought to play a role in junctional signaling. Nephrin is phosphorylated on tyrosine residues exposed on the cytosolic face of the slit-diaphragm by the Src family kinase Fyn (Lahdenpera et al., 2003; Verma et al., 2003). Loss of Nephrin phosphorylation in Fyn deficient mice is associated with proteinuria, implying that tyrosine phosphorylation of Nephrin is functionally significant (Yu et al., 2001). The zebrafish Nephrin cytosolic tail contains a putative SH3 site at P 1186 (CSPLPPAAH; Supplementary Fig. 1) and several conserved tyrosine residues including a predicted Fyn-SH2 site at Y1171 (VYEEVR; Supplementary Fig. 1). It is tempting to speculate that the zebrafish protein may also be tyrosine phosphorylated and serve as a nexus of signaling protein interactions. The predicted altered forms of Nephrin we induced in vivo using morpholino oligos would be expected either to possess complete extracellular and transmembrane domains with a truncated cytosolic tail or to lack an anchoring transmembrane domain and escape as a secreted protein. Either scenario would be expected to result in a loss of Nephrin signaling/protein interactions at the slit-diaphragm and could contribute to the overall loss of slit-diaphragms and podocyte morphology we observe. Alternatively, a secreted form of Nephrin could act as a dominant negative by interacting non-productively with other membrane anchored slit-diaphragm proteins. Further experiments will be required to fully examine potential mechanisms of Nephrin loss of function. The C-terminal truncated form of Nephrin we produced is similar to the Finminor mutation (R1109X), a non-sense mutation resulting in a truncated 1109 amino acid protein (Kestila et al., 1998) which gives rise to full-blown congenital nephrotic syndrome of the Finnish type (NPHS1). In addition to its potential role as a Fyn substrate, the cytoplasmic tail of nephrin is also necessary for Nephrin interactions with Podocin and its recruitment into lipid rafts (Huber et al., 2003b; Simons et al., 2001). Thus, the effects of deletions and truncations in zebrafish Nephrin we have induced using antisense morpholinos are consistent with previous nephrin loss of function studies in mammals and suggest that the foot process effacement, absence of slit-diaphragms and disruption of the glomerular filtration barrier (passage of 500 kDa dextran) we observe are specifically due to loss of Nephrin function in the pronephros.
Podocin is a slit-diaphragm-associated protein related to the band-7/stomatin protein family and to the Caenorhabditis elegans MEC-2 (Boute et al., 2000; Zhang et al., 2004). Podocin is proposed to take on a hairpin-like structure which allows for membrane association via a hydrophobic domain near the N-terminus (Boute et al., 2000). A PHB-(prohibitin homolog)-domain is present at the Podocin C-terminus and this is thought to be required for homo-oligomerization and interaction with Nephrin (Huber et al., 2003b). Human podocin mutations C-terminal to the membrane association domain result in loss of Podocin at the cell membrane and aberrant accumulation in ER secretory pathway vesicles (Huber et al., 2003b; Roselli et al., 2004b). C-terminal podocin mutants also result in failed delivery of Nephrin to the slit-diaphragm, indicating a role for podocin in Nephrin trafficking (Huber et al., 2003b; Roselli et al., 2004b). Mutations in the Podocin N-terminus do not cause a loss of Podocin delivery to the cell membrane but may prevent oligomerization of Podocin in lipid raft membrane domains (Huber et al., 2003b; Roselli et al., 2004b). Mice lacking a functional Podocin gene exhibit podocyte foot process fusion, develop proteinuria soon after birth and die of mesangial sclerosis and renal failure (Roselli et al., 2004a). In zebrafish, the disruption of podocyte foot process structure, absence of slit-diaphragms, passage of high molecular weight dextran and lethal edema we observe in Podocin morphant larvae point to a highly conserved role for Podocin in the zebrafish pronephric glomerulus.
In our podocin morpholino injection experiments, we observed some recovery of the wild-type, normally spliced podocin mRNA by 72–96 hpf, indicating that, at the dose of morpholino used, some mRNA was normally spliced at 72–96 hpf. Nonetheless, a clear loss of function phenotype was observed in pronephric glomerular podocytes and no recovery of the morphant phenotype, at least in terms of edema, was observed in 5–6 day larvae. Nephrotic syndrome in humans with podocin mutations is most commonly associated with homozygous loss and early onset of disease. However, patients with single, heterozygous podocin mutations also exhibit nephrotic syndrome as a milder, late-onset form of the disease (Weber et al., 2004). This suggests that a partial loss of podocin function may be sufficient to manifest as kidney disease and may help explain the phenotype we observed despite the presence of some wild-type podocin mRNA.
A significant proportion of both nephrin and podocin morphant embryos (37% of the nephrinMO embryos and in 44% of the podocinMO embryos) exhibited cysts in the pronephric tubules with a stretched septal glomerulus. This involvement of the pronephric tubule epithelium in the morphant phenotype was unexpected since neither nephrin nor podocin was expressed in the pronephric tubules. In human fetuses carrying the NPHS1 Finmajor mutation, the kidneys showed marked dilatation of proximal tubular lumens and Bowman’s space but no major changes in the glomeruli (Ruotsalainen et al., 2000). Similarly, in three different mouse nephrin knockout models, tubular dilatation was a consistent feature of the mutant kidneys (Hamano et al., 2002; Putaala et al., 2001; Rantanen et al., 2002). Tubular dilatation/cyst formation in the zebrafish pronephros is therefore consistent with the mammalian nephrin loss of function phenotype; however, the mechanism remains unexplained. Serial sectioning of the zebrafish nephrin morphant embryos revealed the presence of large, occluding crystalline deposits in the pronephric duct lumens distal to the tubular cysts. A simple explanation for cyst formation may therefore be that the increased protein or other particulate matter in the tubule filtrate of nephrin-deficient kidneys allows for the nucleation of larger precipitates which cause obstructions in the nephron. Continued delivery of fluid by glomerular filtration and/or trans-epithelial secretion may cause increased lumenal pressure and tubule distension.
Lethal edema was a consistent outcome of nephrin, podocin and moe loss of function. Edema first appeared as a pericardial swelling and progressed to involve the entire embryo. A similar progression from pericardial swelling to whole body edema is observed in zebrafish pronephric cyst mutants (Drummond et al., 1998) and in the no isthmus (pax2.1) mutant which lacks pronephric tubules (Majumdar et al., 2000). The generality of this response suggests that any loss of pronephric kidney function (osmoregulation) may result first in pericardial edema. It is not clear from our experiments why increased glomerular leakage should lead to failed osmoregulation (pronephric kidney failure). It is possible that decreased water elimination caused by partial pronephric obstruction (see above) could contribute to overall edema. Mammalian glomerulopathies are associated with tubule injury and fibrosis, and the degree of proteinuria is a predictor of the rate of disease progression. Excessive protein leakage could be a cause and not a consequence of disease although the underlying mechanisms remain unclear (Zandi-Nejad et al., 2004).
A role for the FERM protein mosaic eyes in formation of the slit-diaphragm
The structural integrity of slit-diaphragm junctions depends on their anchorage to the podocyte cytoskeleton. This was made clear in mice lacking the linker protein CD2AP. CD2AP interacts with nephrin and podocin in lipid rafts and anchors this complex to the podocyte actin cytoskeleton (Wolf and Stahl, 2003). Mice homozygous for CD2AP mutations develop congenital nephrotic syndrome and podocyte foot process effacement (Shih et al., 1999). FERM domain containing proteins represent another broad class of proteins that link transmembrane proteins to the cytoskeleton (Chishti et al., 1998). In podocytes, the FERM family protein ezrin has been shown to bind to podocalyxin and link it to the actin cytoskeleton on the apical cell surface (Orlando et al., 2001). However, neither ezrin nor the related proteins moesin and radixin are localized to basal slit-diaphragms (Hugo et al., 1998; Orlando et al., 2001). Band 4.1 proteins belong to the FERM superfamily and are known to affect the trafficking of ion channels and pumps and their association with the cell membrane (Sun et al., 2002). Several different protein 4.1-like genes are expressed in a segment-specific fashion in the mammalian nephron; however, none have so far been shown to be expressed in glomerular podocytes (Ramez et al., 2003). We find that the FERM domain/band 4.1-like protein Mosaic eyes/EPB41L5 is expressed specifically in pronephric podocytes and is required for proper formation of the slit-diaphragm. moe −/− podocytes lack slit-diaphragms on basement membrane-associated foot processes. Some double cross-strand structures spanning the space between processes extending into Bowman’s space are seen in moe −/− glomeruli, raising the possibility that slit-diaphragms may form at sites distant from the basement membrane in the mutant embryos. Slit-diaphragms have typically appeared in electron micrographs as a single cross-strands bridging the filtration slit. However, recent high-resolution studies of the mammalian slit-diaphragm show that it is a stratified, double-layered structure (Wartiovaara et al., 2004). Further experiments will be required to confirm that cross-strand structures in moe −/− embryos are indeed aberrantly formed slit-diaphragms. The apical “microvillar” projections we observed in moe −/− podocytes are also significant. In humans, microvillar projections from podocytes are observed in the early stages of focal segmental glomerular sclerosis and other glomerular pathologies. These projections are thought to lead to aberrant podocyte adhesions to the cells and basement membrane of Bowman’s capsule, leading to glomerular damage (Pavenstadt et al., 2003). We also observe similar apical “microvillar” projections in nephrin and podocin morphants. The human moe ortholog, EPB41L5, has been found to be expressed in human glomeruli (Chabardes-Garonne et al., 2003), suggesting that moe/EPB41L5 could be tested as a novel human disease gene associated with glomerular disease. Further studies of Moe localization and protein interactions will be required to fully characterize its role in foot process formation and potential roles in glomerular pathology.
Supplementary Material
Acknowledgments
The authors would like to thank Narendra Pahtak, Tomoko Obara and Jinhua Zhao for helpful discussions and support. We also thank Mary McKee and Dennis Brown for assistance with electron microscopy, Margaret Boulos, Humberto Urquiza, Amy Doherty, Marcellino Pina and Eric Stone for aquaculture support, and the other members of the Developmental Biology Lab at the Massachusetts General Hospital for critical input into this work. This work was supported by NIH RO1 HD/DK53093 to IAD.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.ydbio.2005. 06.038.
References
- Abrahamson DR, Wang R. Development of the glomerular capillary and its basement membrane. In: Vize P, Woolf A, Bard J, editors. The Kidney; From Normal Development to Congenital Disease. Academic Press; San Diego: 2003. pp. 221–243. [Google Scholar]
- Benzing T. Signaling at the slit diaphragm. J Am Soc Nephrol. 2004;15:1382–1391. doi: 10.1097/01.asn.0000130167.30769.55. [DOI] [PubMed] [Google Scholar]
- Boute N, Gribouval O, Roselli S, Benessy F, Lee H, Fuchshuber A, Dahan K, Gubler MC, Niaudet P, Antignac C. NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet. 2000;24:349–354. doi: 10.1038/74166. [DOI] [PubMed] [Google Scholar]
- Burghard R, Gordjani N, Leititis J, Bald R. Protein analysis in amniotic fluid and fetal urine for the assessment of fetal renal function and dysfunction. Fetal Ther. 1987;2:188–196. doi: 10.1159/000263316. [DOI] [PubMed] [Google Scholar]
- Chabardes-Garonne D, Mejean A, Aude JC, Cheval L, Di Stefano A, Gaillard MC, Imbert-Teboul M, Wittner M, Balian C, Anthouard V, Robert C, Segurens B, Wincker P, Weissenbach J, Doucet A, Elalouf JM. A panoramic view of gene expression in the human kidney. Proc Natl Acad Sci U S A. 2003;100:13710–13715. doi: 10.1073/pnas.2234604100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chishti AH, Kim AC, Marfatia SM, Lutchman M, Hanspal M, Jindal H, Liu SC, Low PS, Rouleau GA, Mohandas N, Chasis JA, Conboy JG, Gascard P, Takakuwa Y, Huang SC, Benz EJ, Jr, Bretscher A, Fehon RG, Gusella JF, Ramesh V, Solomon F, Marchesi VT, Tsukita S, Hoover KB, et al. The FERM domain: a unique module involved in the linkage of cytoplasmic proteins to the membrane. Trends Biochem Sci. 1998;23:281–282. doi: 10.1016/s0968-0004(98)01237-7. [DOI] [PubMed] [Google Scholar]
- Ciani L, Patel A, Allen ND, ffrench-Constant C. Mice lacking the giant protocadherin mFAT1 exhibit renal slit junction abnormalities and a partially penetrant cyclopia and anophthalmia phenotype. Mol Cell Biol. 2003;23:3575–3582. doi: 10.1128/MCB.23.10.3575-3582.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ME, Mundel P, Boner G. Role of nephrin in renal disease including diabetic nephropathy. Semin Nephrol. 2002;22:393–398. doi: 10.1053/snep.2002.34724. [DOI] [PubMed] [Google Scholar]
- Donoviel DB, Freed DD, Vogel H, Potter DG, Hawkins E, Barrish JP, Mathur BN, Turner CA, Geske R, Montgomery CA, Starbuck M, Brandt M, Gupta A, Ramirez-Solis R, Zambrowicz BP, Powell DR. Proteinuria and perinatal lethality in mice lacking NEPH1, a novel protein with homology to NEPHRIN. Mol Cell Biol. 2001;21:4829–4836. doi: 10.1128/MCB.21.14.4829-4836.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond IA, Majumdar A, Hentschel H, Elger M, Solnica-Krezel L, Schier AF, Neuhauss SC, Stemple DL, Zwartkruis F, Rangini Z, Driever W, Fishman MC. Early development of the zebrafish pronephros and analysis of mutations affecting pronephric function. Development. 1998;125:4655–4667. doi: 10.1242/dev.125.23.4655. [DOI] [PubMed] [Google Scholar]
- Gascard P, Parra MK, Zhao Z, Calinisan VR, Nunomura W, Rivkees SA, Mohandas N, Conboy JG. Putative tumor suppressor protein 4.1B is differentially expressed in kidney and brain via alternative promoters and 5′ alternative splicing. Biochim Biophys Acta. 2004;1680:71–82. doi: 10.1016/j.bbaexp.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Gerke P, Huber TB, Sellin L, Benzing T, Walz G. Homodimerization and heterodimerization of the glomerular podocyte proteins nephrin and NEPH1. J Am Soc Nephrol. 2003;14:918–926. doi: 10.1097/01.asn.0000057853.05686.89. [DOI] [PubMed] [Google Scholar]
- Hamano Y, Grunkemeyer JA, Sudhakar A, Zeisberg M, Cosgrove D, Morello R, Lee B, Sugimoto H, Kalluri R. Determinants of vascular permeability in the kidney glomerulus. J Biol Chem. 2002;277:31154–31162. doi: 10.1074/jbc.M204806200. [DOI] [PubMed] [Google Scholar]
- Hoover KB, Bryant PJ. The genetics of the protein 4.1 family: organizers of the membrane and cytoskeleton. Curr Opin Cell Biol. 2000;12:229–234. doi: 10.1016/s0955-0674(99)00080-0. [DOI] [PubMed] [Google Scholar]
- Huber TB, Hartleben B, Kim J, Schmidts M, Schermer B, Keil A, Egger L, Lecha RL, Borner C, Pavenstadt H, Shaw AS, Walz G, Benzing T. Nephrin and CD2AP associate with phospho-inositide 3-OH kinase and stimulate AKT-dependent signaling. Mol Cell Biol. 2003a;23:4917–4928. doi: 10.1128/MCB.23.14.4917-4928.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber TB, Simons M, Hartleben B, Sernetz L, Schmidts M, Gundlach E, Saleem MA, Walz G, Benzing T. Molecular basis of the functional podocin–nephrin complex: mutations in the NPHS2 gene disrupt nephrin targeting to lipid raft microdomains. Hum Mol Genet. 2003b;12:3397–3405. doi: 10.1093/hmg/ddg360. [DOI] [PubMed] [Google Scholar]
- Hugo C, Nangaku M, Shankland SJ, Pichler R, Gordon K, Amieva MR, Couser WG, Furthmayr H, Johnson RJ. The plasma membrane-actin linking protein, ezrin, is a glomerular epithelial cell marker in glomerulogenesis, in the adult kidney and in glomerular injury. Kidney Int. 1998;54:1934–1944. doi: 10.1046/j.1523-1755.1998.00195.x. [DOI] [PubMed] [Google Scholar]
- Humphrey C, Pittman F. A simple methylene blue-azure II-basic fuchsin stain for epoxy-embedded tissue sections. Stain Technol. 1974;49:9–14. doi: 10.3109/10520297409116929. [DOI] [PubMed] [Google Scholar]
- Jensen AM, Westerfield M. Zebrafish mosaic eyes is a novel FERM protein required for retinal lamination and retinal pigmented epithelial tight junction formation. Curr Biol. 2004;14:711–717. doi: 10.1016/j.cub.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Jensen AM, Walker C, Westerfield M. Mosaic eyes: a zebrafish gene required in pigmented epithelium for apical localization of retinal cell division and lamination. Development. 2001;128:95–105. doi: 10.1242/dev.128.1.95. [DOI] [PubMed] [Google Scholar]
- Kestila M, Lenkkeri U, Mannikko M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K. Positionally cloned gene for a novel glomerular protein–nephrin–is mutated in congenital nephrotic syndrome. Mol Cell. 1998;1:575–582. doi: 10.1016/s1097-2765(00)80057-x. [DOI] [PubMed] [Google Scholar]
- Lahdenpera J, Kilpelainen P, Liu XL, Pikkarainen T, Reponen P, Ruotsalainen V, Tryggvason K. Clustering-induced tyrosine phosphorylation of nephrin by Src family kinases. Kidney Int. 2003;64:404–413. doi: 10.1046/j.1523-1755.2003.00097.x. [DOI] [PubMed] [Google Scholar]
- Liu G, Kaw B, Kurfis J, Rahmanuddin S, Kanwar YS, Chugh SS. Neph1 and nephrin interaction in the slit diaphragm is an important determinant of glomerular permeability. J Clin Invest. 2003;112:209–221. doi: 10.1172/JCI18242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XL, Kilpelainen P, Hellman U, Sun Y, Wartiovaara J, Morgunova E, Pikkarainen T, Yan K, Jonsson AP, Tryggvason K. Characterization of the interactions of the nephrin intracellular domain. FEBS J. 2005;272:228–243. doi: 10.1111/j.1432-1033.2004.04408.x. [DOI] [PubMed] [Google Scholar]
- Majumdar A, Drummond IA. The zebrafish floating head mutant demonstrates podocytes play an important role in directing glomerular differentiation. Dev Biol. 2000;222:147–157. doi: 10.1006/dbio.2000.9642. [DOI] [PubMed] [Google Scholar]
- Majumdar A, Lun K, Brand M, Drummond IA. Zebrafish no isthmus reveals a role for pax2.1 in tubule differentiation and patterning events in the pronephric primordia. Development. 2000;127:2089–2098. doi: 10.1242/dev.127.10.2089. [DOI] [PubMed] [Google Scholar]
- Miner JH. A molecular look at the glomerular barrier. Nephron Exp Nephrol. 2003;94:e119–e122. doi: 10.1159/000072495. [DOI] [PubMed] [Google Scholar]
- Noren NK, Liu BP, Burridge K, Kreft B. p120 catenin regulates the actin cytoskeleton via Rho family GTPases. J Cell Biol. 2000;150:567–580. doi: 10.1083/jcb.150.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlando RA, Takeda T, Zak B, Schmieder S, Benoit VM, McQuistan T, Furthmayr H, Farquhar MG. The glomerular epithelial cell anti-adhesin podocalyxin associates with the actin cytoskeleton through interactions with ezrin. J Am Soc Nephrol. 2001;12:1589–1598. doi: 10.1681/ASN.V1281589. [DOI] [PubMed] [Google Scholar]
- Pavenstadt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev. 2003;83:253–307. doi: 10.1152/physrev.00020.2002. [DOI] [PubMed] [Google Scholar]
- Putaala H, Soininen R, Kilpelainen P, Wartiovaara J, Tryggvason K. The murine nephrin gene is specifically expressed in kidney, brain and pancreas: inactivation of the gene leads to massive proteinuria and neonatal death. Hum Mol Genet. 2001;10:1–8. doi: 10.1093/hmg/10.1.1. [DOI] [PubMed] [Google Scholar]
- Ramez M, Blot-Chabaud M, Cluzeaud F, Chanan S, Patterson M, Walensky LD, Marfatia S, Baines AJ, Chasis JA, Conboy JG, Mohandas N, Gascard P. Distinct distribution of specific members of protein 4.1 gene family in the mouse nephron. Kidney Int. 2003;63:1321–1337. doi: 10.1046/j.1523-1755.2003.00870.x. [DOI] [PubMed] [Google Scholar]
- Rantanen M, Palmen T, Patari A, Ahola H, Lehtonen S, Astrom E, Floss T, Vauti F, Wurst W, Ruiz P, Kerjaschki D, Holthofer H. Nephrin TRAP mice lack slit diaphragms and show fibrotic glomeruli and cystic tubular lesions. J Am Soc Nephrol. 2002;13:1586–1594. doi: 10.1097/01.asn.0000016142.29721.22. [DOI] [PubMed] [Google Scholar]
- Reeves W, Caulfield JP, Farquhar MG. Differentiation of epithelial foot processes and filtration slits: sequential appearance of occluding junctions, epithelial polyanion, and slit membranes in developing glomeruli. Lab Invest. 1978;39:90–100. [PubMed] [Google Scholar]
- Reiser J, Kriz W, Kretzler M, Mundel P. The glomerular slit diaphragm is a modified adherens junction. J Am Soc Nephrol. 2000;11:1–8. doi: 10.1681/ASN.V1111. [DOI] [PubMed] [Google Scholar]
- Roselli S, Gribouval O, Boute N, Sich M, Benessy F, Attie T, Gubler MC, Antignac C. Podocin localizes in the kidney to the slit diaphragm area. Am J Pathol. 2002;160:131–139. doi: 10.1016/S0002-9440(10)64357-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roselli S, Heidet L, Sich M, Henger A, Kretzler M, Gubler MC, Antignac C. Early glomerular filtration defect and severe renal disease in podocin-deficient mice. Mol Cell Biol. 2004a;24:550–560. doi: 10.1128/MCB.24.2.550-560.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roselli S, Moutkine I, Gribouval O, Benmerah A, Antignac C. Plasma membrane targeting of podocin through the classical exocytic pathway: effect of NPHS2 mutations. Traffic. 2004b;5:37–44. doi: 10.1046/j.1600-0854.2003.00148.x. [DOI] [PubMed] [Google Scholar]
- Ruotsalainen V, Ljungberg P, Wartiovaara J, Lenkkeri U, Kestila M, Jalanko H, Holmberg C, Tryggvason K. Nephrin is specifically located at the slit diaphragm of glomerular podocytes. Proc Natl Acad Sci U S A. 1999;96:7962–7967. doi: 10.1073/pnas.96.14.7962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruotsalainen V, Patrakka J, Tissari P, Reponen P, Hess M, Kestila M, Holmberg C, Salonen R, Heikinheimo M, Wartiovaara J, Tryggvason K, Jalanko H. Role of nephrin in cell junction formation in human nephrogenesis. Am J Pathol. 2000;157:1905–1916. doi: 10.1016/S0002-9440(10)64829-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo LM, Osicka TM, Brammar GC, Candido R, Jerums G, Comper WD. Renal processing of albumin in diabetes and hypertension in rats: possible role of TGF-beta1. Am J Nephrol. 2003;23:61–70. doi: 10.1159/000068039. [DOI] [PubMed] [Google Scholar]
- Schnabel E, Anderson JM, Farquhar MG. The tight junction protein ZO-1 is concentrated along slit diaphragms of the glomerular epithelium. J Cell Biol. 1990;111:1255–1263. doi: 10.1083/jcb.111.3.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz J, Copley RR, Doerks T, Ponting CP, Bork P. SMART: a web-based tool for the study of genetically mobile domains. Nucleic Acids Res. 2000;28:231–234. doi: 10.1093/nar/28.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih NY, Li J, Karpitskii V, Nguyen A, Dustin ML, Kanagawa O, Miner JH, Shaw AS. Congenital nephrotic syndrome in mice lacking CD2-associated protein. Science. 1999;286:312–315. doi: 10.1126/science.286.5438.312. [DOI] [PubMed] [Google Scholar]
- Simons M, Schwarz K, Kriz W, Miettinen A, Reiser J, Mundel P, Holthofer H. Involvement of lipid rafts in nephrin phosphorylation and organization of the glomerular slit diaphragm. Am J Pathol. 2001;159:1069–1077. doi: 10.1016/S0002-9440(10)61782-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun CX, Robb VA, Gutmann DH. Protein 4.1 tumor suppressors: getting a FERM grip on growth regulation. J Cell Sci. 2002;115:3991–4000. doi: 10.1242/jcs.00094. [DOI] [PubMed] [Google Scholar]
- Tassin MT, Beziau A, Gubler MC, Boyer B. Spatiotemporal expression of molecules associated with junctional complexes during the in vivo maturation of renal podocytes. Int J Dev Biol. 1994;38:45–54. [PubMed] [Google Scholar]
- Thisse C, Thisse B. The Zebrafish Science Monitor, vol. 5. ZFIN. Zebrafish International Resource Center, University of Oregon; Eugene, OR 97403-5274: 1998. High resolution whole-mount in situ hybridization; pp. 8–9. http://zfin.org) [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usui J, Kurihara H, Shu Y, Tomari S, Kanemoto K, Koyama A, Sakai T, Takahashi T, Nagata M. Localization of intercellular adherens junction protein p120 catenin during podocyte differentiation. Anat Embryol (Berl) 2003;206:175–184. doi: 10.1007/s00429-002-0298-x. [DOI] [PubMed] [Google Scholar]
- Verma R, Wharram B, Kovari I, Kunkel R, Nihalani D, Wary KK, Wiggins RC, Killen P, Holzman LB. Fyn binds to and phosphorylates the kidney slit diaphragm component Nephrin. J Biol Chem. 2003;278:20716–20723. doi: 10.1074/jbc.M301689200. [DOI] [PubMed] [Google Scholar]
- Wartiovaara J, Ofverstedt LG, Khoshnoodi J, Zhang J, Makela E, Sandin S, Ruotsalainen V, Cheng RH, Jalanko H, Skoglund U, Tryggvason K. Nephrin strands contribute to a porous slit diaphragm scaffold as revealed by electron tomography. J Clin Invest. 2004;114:1475–1483. doi: 10.1172/JCI22562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber S, Gribouval O, Esquivel EL, Moriniere V, Tete MJ, Legendre C, Niaudet P, Antignac C. NPHS2 mutation analysis shows genetic heterogeneity of steroid-resistant nephrotic syndrome and low post-transplant recurrence. Kidney Int. 2004;66:571–579. doi: 10.1111/j.1523-1755.2004.00776.x. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book. University of Oregon Press; Eugene: 1995. [Google Scholar]
- Wolf G, Stahl RA. CD2-associated protein and glomerular disease. Lancet. 2003;362:1746–1748. doi: 10.1016/S0140-6736(03)14856-8. [DOI] [PubMed] [Google Scholar]
- Yu CC, Yen TS, Lowell CA, DeFranco AL. Lupus-like kidney disease in mice deficient in the Src family tyrosine kinases Lyn and Fyn. Curr Biol. 2001;11:34–38. doi: 10.1016/s0960-9822(00)00024-5. [DOI] [PubMed] [Google Scholar]
- Zandi-Nejad K, Eddy AA, Glassock RJ, Brenner BM. Why is proteinuria an ominous biomarker of progressive kidney disease? Kidney Inter. 2004;(Suppl):S76–S89. doi: 10.1111/j.1523-1755.2004.09220.x. [DOI] [PubMed] [Google Scholar]
- Zhang S, Arnadottir J, Keller C, Caldwell GA, Yao CA, Chalfie M. MEC-2 is recruited to the putative mechanosensory complex in C. elegans touch receptor neurons through its stomatin-like domain. Curr Biol. 2004;14:1888–1896. doi: 10.1016/j.cub.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Zhou X, Vize PD. Proximo-distal specialization of epithelial transport processes within the Xenopus pronephric kidney tubules. Dev Biol. 2004;271:322–338. doi: 10.1016/j.ydbio.2004.03.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.