Structured Abstract
Objective
To determine the event-free survival (EFS) and overall survival (OS) of children with very low risk Wilms tumor (VLRWT) treated with surgery only.
Background
Previous studies suggested that post-operative chemotherapy had not improved the prognosis of children with VLRWT. Seventy-seven children < 24 months of age with small (<550 gm) Stage I favorable histology Wilms tumors were treated with surgery only. This study was closed based on stopping rules to insure the 2-year EFS was ≥ 90%.
Methods
Seventy-seven children were assessed for EFS and OS. Twenty-one of these patients enrolled at the time of closure were recalled, treated with dactinomycin and vincristine (regimen EE4A), and censored for analysis thereafter. One hundred and eleven children subsequently treated with EE4A were available for comparison.
Results
Median follow-up of surviving patients was 8.2 years for surgery only (range 1.9 to 11.8 years) and 5.2 years for the EE4A group (range 1.6 to 8.9 years). The estimated 5-year EFS for surgery only was 84% (95% confidence interval [CI]: 73%, 91%); for the EE4A patients it was 97% (95% CI: 92%, 99%, p=0.002). One death was observed in each treatment group. The estimated 5-year OS was 98% (95% CI: 87%, 99%) for surgery only and 99% (95% CI: 94%, 99%) for EE4A (p=0.70).
Conclusion
The surgery-only EFS was lower than anticipated but, coupled with a much higher than anticipated salvage rate of the chemotherapy naïve patients whose disease recurred, led to an observed long-term overall survival equivalent to that seen with 2-drug chemotherapy. This approach to the treatment of patients with very low risk Wilms tumor eliminates the toxic side-effects of chemotherapy for a large majority of patients. A follow-up study is underway to confirm these findings.
Introduction
Analysis of both single institution studies and those of the National Wilms Tumor Study Group (NWTSG) suggested that a cohort of children under 24 months of age with a stage I favorable histology Wilms tumor with a specimen weighting less than 550 gm may not benefit from adjuvant chemotherapy. These findings led the investigators of the NWTSG to test the hypothesis, in NWTS – 5, that the omission of adjuvant chemotherapy would not compromise long-term survival in children with very low risk Wilms tumors (VLRWT). Stringent stopping rules were designed to ensure closure of the study if the 2-year relapse-free survival rate was 90% or lower. The expectation was that approximately 50% of the surgery only children would be salvaged after recurrence thus attaining the 95% predicted survival of children with VLRWT treated with standard chemotherapy according to regimen EE4A (vincristine and dactinomycin). This study was stopped on June 14, 1998 when the predicted 2-year event-free survival fell below 90%.1 This report provides an analysis of the long-term survival of this cohort of children and compares them with a subsequent near concurrent cohort. The latter was enrolled in the NWTS-5 study after the “surgery only” arm was terminated and received standard EE4A chemotherapy.
Materials and Methods
Patients available for analysis included those with VLRWT enrolled on NWTS-5 which was initiated on August 1, 1995. They include those assigned to initial therapy with either “surgery only” (N=80) or “EE4A” (vincristine and dactinomycin, N=112). The NWTS-5 protocol and an informed consent document were approved by the Institutional Review Board of each institution registering patients, and informed consent was obtained from the parents of all patients before participation in this study of nephrectomy-only treatment. The data here reported were current as of May, 2008.
Exclusions from the “surgery only” cohort
Three “surgery only” patients were upstaged on central pathology review to stage 2 and the patients' therapy was changed to EE4A. These infants were not part of the N=75 cohort included in the previous report of this study by Green et al. 1
The “surgery only” cohort analyzed here includes 77 patients. There are four (4) “surgery only” patients in this analysis that were not included in the Green et al. report. All four patients were initially assigned to “surgery only”, but had treatment with EE4A initiated as part of the recall during June-September 1998. Time from enrollment to start of EE4A for these four patients was 0.04, 0.11, 0.19 and 0.23 years. There are two patients included in the prior report that are excluded here. One had EE4A mistakenly administered within the first 10 days following surgery in violation of the protocol. The second was “registered too late for study” and thus was ineligible.
Exclusions from the “EE4A” cohort
One EE4A patient upstaged to Stage III on central pathologic review and switched to DD4A (doxorubicin, dactinomycin, and vincristine) was excluded from the control group. Thus, this analysis contained a total of 188 patients, 77 “surgery only” and 111 “EE4A” patients.
Statistical considerations
Event-free survival (EFS) was defined as the time from enrollment to the first evidence of recurrent or metachronous disease or death as a first event. Overall survival (OS) was defined as the time from enrollment to death. Patients without an event were censored at their time of last follow-up. However, we have attempted to gather as much information as possible regarding the event-free and overall survival associated with the strategy of “surgery only”. Accordingly, data concerning the 21 “surgery only” patients were gathered until they were recalled to receive EE4A therapy during the period June-September 1998. The information was censored after they began EE4A treatment.
Results
Seventy-seven (77) children with very low risk Wilms tumor (VLRWT) were initially assigned to “surgery only” treatment. As noted above, twenty-one of the 77 patients were recalled at a median of 0.54 years from enrollment (range, 0.04-0.87 years) to receive EE4A after the closure of the experimental arm of the study. One hundred and eleven infants were initially assigned to EE4A chemotherapy. Median follow-up of patients alive and without censoring for recall was 8.2 years for “surgery only” (range 1.9-11.8 years) and 5.2 years for “EE4A” (range 1.6-8.9 years).
Event-free survival
Eleven treatment failures were observed among the 77 patients assigned to “surgery only”. One of these failures, however, occurred after the patient was recalled to receive EE4A (recalled at 0.85 years; failure [contralateral kidney] at 2.26 years) and is not included as a “surgery only” failure in this analysis. Three of the 111 patients initially assigned to EE4A chemotherapy were treatment failures. The analysis failures were distributed as follows for “surgery only”: lung only (5); operative bed (3); contralateral kidney (2). For the “EE4A” cohort the failures were: lung only (2) and contralateral kidney (1). Although the total number of relapses is higher in the surgery only group, the patterns of failure seem similar.
The estimated EFS for the 77 patients in the “surgery only” cohort (with censoring at EE4A recall therapy) was 84% at 5 years (95% confidence interval [CI]: 73%, 91%), with no failures seen after 1.42 years. In contrast, the estimated 5-year EFS for the 111 patients who were assigned to chemotherapy with EE4A was 97% (95% CI: 92%, 99%), with no failures observed after 0.55 years. This difference in EFS was statistically significant (p=0.002) (Fig. 1). Patients on the “surgery only” study who relapsed were treated as follows. Four of the five patients with pulmonary metastases, received therapy according to the recommendations of the NWTSG protocol for patients with relapsed Wilms tumor (Pediatric Oncology Group 9444/Children's Cancer Group 4942) and included the child who did not survive.2 That regimen calls for doxorubicin, dactinomycin, vincristine and pulmonary radiation. The fifth child was managed with carboplatin/etoposide alternating with ifosfamide/doxorubicin and bilateral pulmonary radiation of 1200 cGy according to a local institutional protocol (Children's Hospital of Los Angeles 91LA-2).3 The two patients with operative bed recurrence were managed using the same NWTSG relapse protocol including doxorubicin, dactinomycin, vincristine and abdominal radiation. The three patients with metachronous contralateral tumors were treated with the same relapsed tumor protocol, two with vincristine and dactinomycin after having microscopically complete resection of the tumor and the third child was treated with vincristine, dactinomycin, and doxorubicin and local radiation therapy for gross residual disease. Of the three relapsed patients in the EE4A cohort, one of the two survivors received vincristine, cyclophosphamide, doxorubicin and whole lung radiation to 1200 cGy. The second received vincristine, doxorubicin and etoposide but no radiation therapy (ultimately succumbed). The third child received vincristine, dactinomycin, doxorubicin and no radiation.
Figure 1.
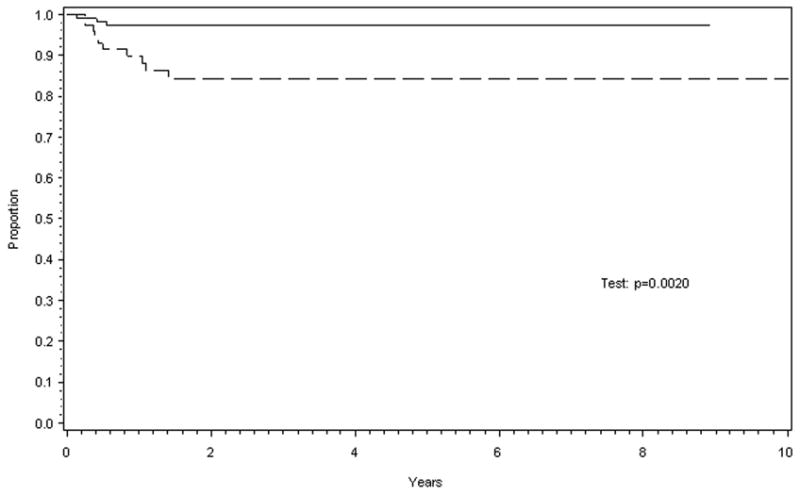
Estimated event-free survival (EFS) for the two treatment groups was statistically different. Solid line for patients receiving dactinomycin and vincristine after surgery and dashed line those receiving no adjuvant therapy after resection.
Survival
Only one death was observed in each of the two treatment groups (1 in “surgery only” at 4.28 years [censoring follow-up for recall therapy, but no deaths censored] and 1 in EE4A at 1.15 years. Estimated overall survival (OS) at five years is 98% (95% CI: 87%, 99%) for “surgery only” and 99% (95% CI: 94%, 99%) for EE4A (p=0.70).
Discussion
In the 1950's, Dr. Sidney Farber et al. at Children's Hospital Boston demonstrated the efficacy of dactinomycin in the treatment of children with Wilms tumor. 4 Dr. Farber advocated the use of adjuvant therapy for children with this tumor stating its use is “based upon the supposition that in children with Wilms' tumor who died, the tumor must have metastasized already at the time of discovery of the primary tumor”, although no evidence of spread by the means then available was recognized. 5 He went on to state that “The assumption was made that the clinical agent carried throughout the body by the blood stream might destroy small foci of tumor before solid implantation and further growth could take place.” Since that time, essentially all children with Wilms tumor have received adjuvant chemotherapy.
The initial identification of a favorable cohort of children with Wilms tumor characterized by age less than 24 months of age, stage I favorable histology and specimen/tumor weight less than 550 grams was made by Garcia and colleagues and confirmed by a review of patients treated at Children's Hospital Medical Center in Boston by Cassady and associates. 6, 7 Green and Jaffe in a subsequent review of patients treated at the Dana-Farber Cancer Institute and Children's Hospital Medical Center demonstrated the favorable outcome of these children whether they were treated with nephrectomy alone, nephrectomy and abdominal radiation, or nephrectomy, abdominal radiation, and chemotherapy. 8 Analysis of comparable infants treated during NWTS-1 to -3 demonstrated EFS of 89.1%, 96.0% and 93.2% respectively, outcomes that were not significantly different (p=.99).9 From these data, it was hypothesized that nephrectomy alone was adequate therapy in this select group. Children with these favorable tumors were treated with nephrectomy alone in a small prospective single institution pilot study, ironically at Dr. Farber's own institution. Only one child with hypospadias out of the initially reported eight and subsequently reported eleven total children developed a metachronous lesion three months after resection in the opposite kidney.10, 11
Pathologic examination of tumors from the cohort of children with stage I favorable histology Wilms tumor treated in the NWTSG studies revealed important data. Their tumors had a very low incidence of those microscopic findings that Weeks and Beckwith reported were associated with a higher risk of recurrence. 12, 13
All of this information led the investigators in NWTS-5 to address the hypothesis that “surgery alone” would not compromise survival expectancy for children with VLRWT. The current analyses update information previously presented by Green and colleagues and provides a “near concurrent” control group treated with EE4A for comparison.1 The long-term, EFS for the “surgery only” infants is estimated to be 84% compared to 97% with EE4A. However, nearly all of the patients who recurred after “surgery only” were successfully treated, yielding five-year OS of about 98% for both treatment groups. Based on prior reports of children who relapsed after chemotherapy, an estimate of 50% salvage was used when determining the “stopping rule” for the protocol. Fortunately, the chemotherapy naïve infants fared much better than predicted. This outcome underscores the importance of weighing both OS and EFS when assessing the results of a trial. The present results demonstrate that EFS is not a surrogate marker for OS in VLRWT patients. This has been shown in other studies of Wilms tumor patients. An analysis of the role of radiotherapy in prevention of flank recurrence in patients with favorable histology Wilms tumor demonstrated that while higher doses up to 20 Gy decreased the incidence of flank recurrence, overall survival was not improved demonstrating the need to evaluate entire treatment policies with regard to long-term outcomes.14
The morbidity of therapy must be considered in all treatment decisions and balanced against the documented benefits. Life-threatening sinusoidal obstruction syndrome occurs during the first 10 weeks of treatment in approximately 3.5% of unirradiated children with Wilms tumor.15,16 There are also inherent risks to catheter placement including bacteremia and thrombosis as well as the direct and indirect costs for chemotherapy administration and monitoring of the children.17 Recent results from the Childhood Cancer Survivor Study have shown that 44% of survivors report long-term adverse effects in their health forcing us to increasingly assess the risks of therapy, particularly in cohorts with favorable survival.18 While the incidence of relapse is higher in the “surgery only” cohort, the long-term survival in both treatment groups is similar. The balance then becomes the consideration of more intensive therapy and its potential long-term sequelae required for the 16% of children who relapse versus the avoidance of any postoperative chemotherapy in 84% of the children with VLRWT. Continued evaluation of these children treated on cooperative group clinical trials with “surgery only” will be required to better answer these questions and establish the optimal therapy.
Acknowledgments
We thank the investigators of the Children's Oncology Group (COG) and the many pathologists, surgeons, pediatricians, radiation oncologists, and other health professionals who managed the children entered on the National Wilms Tumor Studies.
Supported in part by: USPHS; Grant number: CA-42326 and 5 U10-CA098413
References
- 1.Green DM, Breslow N, Beckwith JB, et al. Treatment with nephrectomy only for small, stage I/Favorable histology Wilms' tumor: A report from the National Wilms' Tumor Study Group. J Clin Oncol. 2001;19(17):3719–3724. doi: 10.1200/JCO.2001.19.17.3719. [DOI] [PubMed] [Google Scholar]
- 2.Green D, Cotton C, Malogolowkin M, et al. Treatment of Wilms tumor relapsing after initial treatment with Vincristine and Actinomycin D: A Report from the National Wilms Tumor Study Group. Pediatr Blood Cancer. 2007;48:493–499. doi: 10.1002/pbc.20822. [DOI] [PubMed] [Google Scholar]
- 3.Malogolowkin MH, Feusner J, Steele DA, et al. Carboplatin (CBDCA)/Etoposide (VP-16) for the treatment of children with high-risk (HR) or recurrent Wilms' tumor (RWT) ASCO. 1994;13:424. [Google Scholar]
- 4.Farber S, D'Angio GJ, Evans A, Mitus A. Clinical studies of actinomycin D with special reference to Wilms' Tumor in children. Ann NY Acad Sci. 1960;89:421–425. doi: 10.1111/j.1749-6632.1960.tb20165.x. [DOI] [PubMed] [Google Scholar]
- 5.Farber S. Chemotherapy in the treatment of leukemia and Wilms' Tumor. JAMA. 1966;198(8):826–836. [PubMed] [Google Scholar]
- 6.Garcia M, Douglass EC, Schlosser JV. Classification and prognosis in Wilms' tumor. Radiology. 1963;80:574–580. doi: 10.1148/80.4.574. [DOI] [PubMed] [Google Scholar]
- 7.Cassady JR, Tefft M, Filler RM, et al. Considerations in the radiation therapy of Wilms' tumor. Cancer. 1973;32:598–608. doi: 10.1002/1097-0142(197309)32:3<598::aid-cncr2820320312>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 8.Green DM, Jaffe N. The role of chemotherapy in the treatment of Wilms' tumor. Cancer. 1979;44:52–57. doi: 10.1002/1097-0142(197907)44:1<52::aid-cncr2820440110>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 9.Green DM, Breslow NE, Beckwith JB, et al. Treatment outcomes in patients less than 2 years of age with small, Stage I, favorable-histology Wilms' tumors: A report from the National Wilms' Tumor Study. J Clin Oncol. 1993;11:91–95. doi: 10.1200/JCO.1993.11.1.91. [DOI] [PubMed] [Google Scholar]
- 10.Larsen E, Perez-Atayde AR, Green DMea. Surgery only for the treatment of patients with Stage I (Cassady) Wilms' Tumor. Cancer. 1990;66:264–266. doi: 10.1002/1097-0142(19900715)66:2<264::aid-cncr2820660212>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 11.Shamberger RC, Macklis RM, Sallan SE. Recent experience with Wilms' Tumor: 1978-1991. Ann Surg Onc. 1994;1:59–65. doi: 10.1007/BF02303542. [DOI] [PubMed] [Google Scholar]
- 12.Weeks DA, Beckwith B, Luckey DW. Relapse-associated variables in Stage I favorable histology Wilms' tumor: A report of the National Wilms' Tumor Study. Cancer. 1987;60:1204–1212. doi: 10.1002/1097-0142(19870915)60:6<1204::aid-cncr2820600608>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 13.Green DM, Beckwith JB, Weeks DA, et al. The relationship between microsubstaging variables, age at diagnosis, and tumor weight of children with Stage I/favorable histology Wilms' tumor: A report from the National Wilms' tumor study. Cancer. 1994;74:1817–1820. doi: 10.1002/1097-0142(19940915)74:6<1817::aid-cncr2820740626>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 14.Breslow NE, Beckwith JB, Haase GM, et al. Radiation therapy for favorable histology Wilms tumor: Prevention of flank recurrence did not improve survival on National Wilms Tumor Studies 3 and 4. Int J Radiation Oncol Bio Phy. 2006;65:203–209. doi: 10.1016/j.ijrobp.2005.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green DM, Finklestein JZ, Norkool P, et al. Severe hepatic toxicity after treatment with single dose dactinomycin and vincristine. Cancer. 1988;62:270–273. doi: 10.1002/1097-0142(19880715)62:2<270::aid-cncr2820620208>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 16.Green DM, Norkool P, Breslow N, et al. Severe hepatic toxicity after treatment with vincristine and actinomycin D using single-dose or divided dose schedule. J Clin Oncol. 1990;8:1525–1530. doi: 10.1200/JCO.1990.8.9.1525. [DOI] [PubMed] [Google Scholar]
- 17.Green DM, Breslow NE, Beckwith JB, et al. Effect of duration of treatment on treatment outcome and cost of treatment for Wilms' tumor: a report from the National Wilms' Tumor Study Group. J Clin Oncol. 1998;16(12):3744–51. doi: 10.1200/JCO.1998.16.12.3744. [DOI] [PubMed] [Google Scholar]
- 18.Hudson MM, Mertens AC, Yasui Y, et al. Health status of adult long-term survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. JAMA. 2009;290:1583–1592. doi: 10.1001/jama.290.12.1583. [DOI] [PubMed] [Google Scholar]


