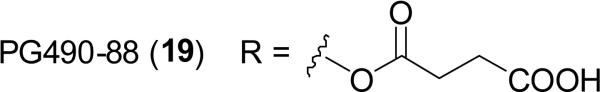Abstract
Plant secondary metabolites and their semi-synthetic derivatives continue to play an important role in anticancer drug therapy. In this short review, selected single chemical entity antineoplastic agents from higher plants that are currently in clinical trials as cancer chemotherapy drug candidates are described. These compounds are representative of a wide structural diversity. In addition, the approaches taken toward the discovery of anticancer agents from tropical plants in the laboratory of the authors are summarized. The successful clinical utilization of cancer chemotherapeutic agents from higher plants has been evident for about half a century, and, when considered with the promising pipeline of new plant-derived compounds now in clinical trials, this augurs well for the continuation of drug discovery research efforts to elucidate additional candidate substances of this type.
Keywords: Higher plants, secondary metabolites, anticancer agents, compounds in clinical trials, antineoplastic drug discovery
1. Introduction
Cancer is one of the leading causes of death in both developed countries and developing countries and is therefore of worldwide concern. According to global cancer statistics released by the American Cancer Society, the total number of deaths from cancer in 2007 was 7.6 million, or about 20,000 deaths each day, with 38% in developed countries and 62% in developing countries. By 2050, 27 million new cancer cases and 17.5 million cancer deaths are projected to occur in the world (American Cancer Society, 2007). Accordingly, much effort has been made to develop various approaches to reduce the threat caused by cancer. Chemotherapy is an important option in modern cancer treatment, and many clinically available anticancer drugs, of synthetic or natural product origin, are currently used to treat some types of leukemias, lymphomas and solid tumors (Chabner et al., 2005; DeVita et al., 2008). A recent analysis of the anticancer drug market in North America, Europe, and Japan during the period 1981 to 2006 revealed that 47.1% of a total of 155 clinically approved anticancer drugs were either unmodified natural products or their semi-synthetic derivatives, or synthesized molecules based on natural product compound pharmacophores (Newman and Cragg, 2007). Natural products, characterized as small-molecule secondary metabolites that originate from terrestrial and marine plants, microorganisms and animals, tend to present more structurally diverse “drug-like” and “biologically friendly” molecular qualities than pure synthetic compounds at random, and are an important source of novel lead structures for the synthetic and combinatorial chemistry aspects of anticancer drug discovery (Bindseil et al., 2001; Firn and Jones, 2003; Vuorelaa et al., 2004). Despite certain technical limitations inherent in the investigation of the small-molecule organic constituents of organisms using modern drug discovery platforms, improvements in automated high-throughput bioactivity screening techniques and technologies applied in the processes of compound analysis, purification, and structural identification have significantly speeded up the natural product bioassay-guided fractionation procedure (Koehn and Carter, 2005; Potterat and Hamburger, 2006; Anonymous, 2007). The application of biotechnological methods has allowed selected natural product metabolites to be produced in a relatively controlled manner, and to be less limited by sourcing problems caused by environmental, seasonal and geographical effects (Fischbach and Walsh, 2006). It has been concluded recently that natural products from all types or organisms offer an “unlimited” resource for future drug discovery (Li and Vederas, 2009).
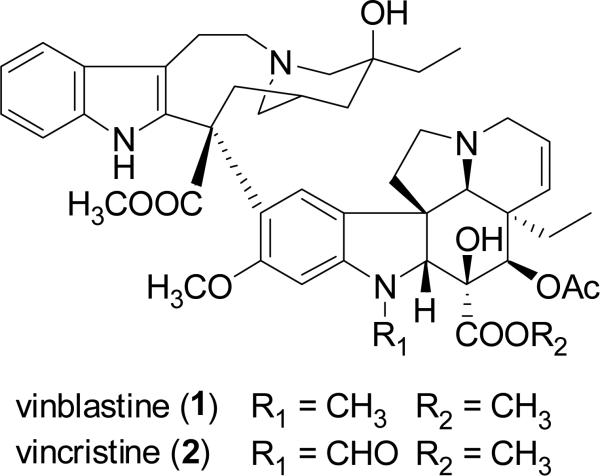
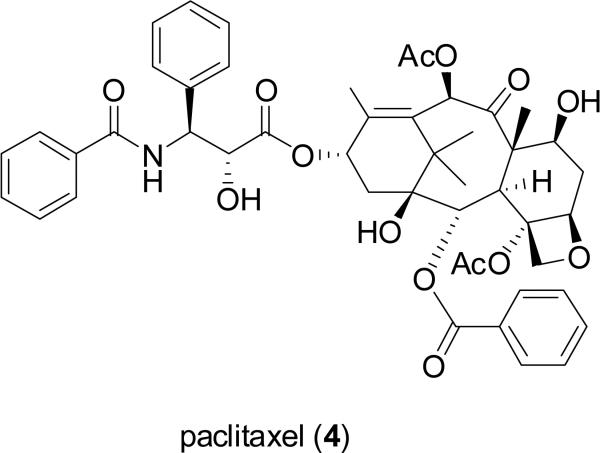
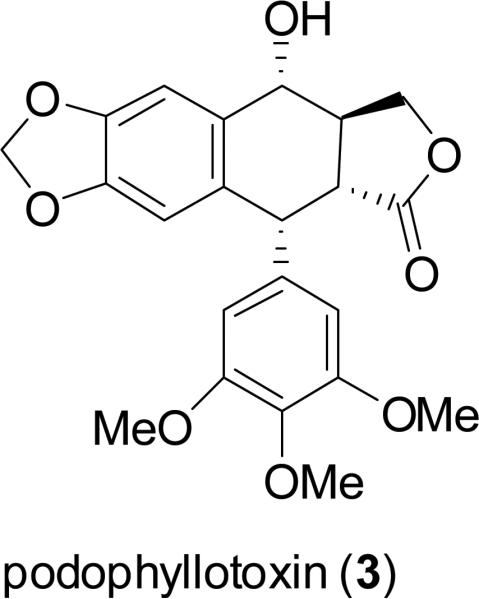
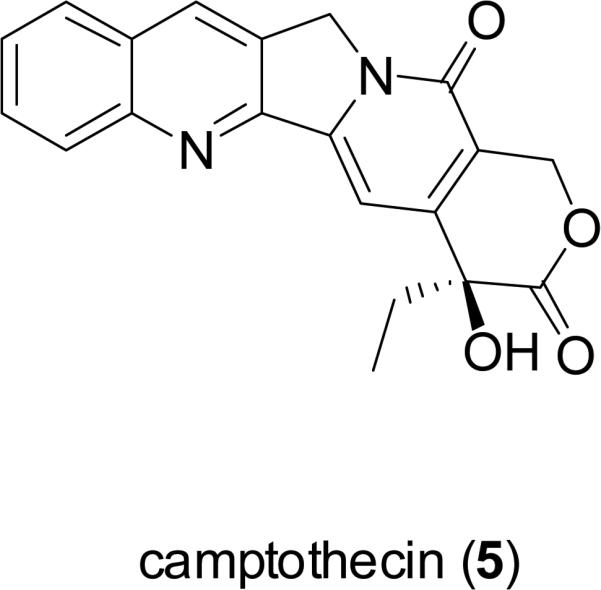
For millennia, terrestrial plants have been used as a prominent source of medicines, and their early use in this regard in Ancient Egypt, India, China, and the Arab world has been documented (Sneader, 2005). In a volume summarizing a series of review articles that appeared previously in the journal Lloydia by the late Dr. Jonathan L. Hartwell, then at the U.S. National Cancer Institute, more than 3000 plant species were described with evidence of previous use for treating cancer (Hartwell, 1982). However, it must be pointed out that cancer is not easy to be diagnosed in a reliable manner like certain other diseases such as skin infections and gastrointestinal disturbances, so claims of efficacy in treating cancer by plants used in traditional medicine should be treated with some skepticism (Cragg et al., 2009). Despite this reservation, there are now four major structural classes of plant-derived compounds used in western medicine as single chemical entity compounds, namely, the vinca alkaloids (vinblastine, vincristine, vinorelbine), the epipodophyllotoxin lignans (etoposide, teniposide, etoposide phosphate), the taxane diterpenoids (paclitaxel, docetaxel), and the camptothecin quinoline alkaloid derivatives (toptotecan, irinotecan), as listed in order of their introduction to established oncology therapy in the United States (Chabner et al., 2005; DeVita et al., 2008). Since a detailed treatment of these four classes of plant-derived agents has appeared in the literature recently (Cragg et al., 2005), these compounds will not be further discussed in the present review. However, it should be noted that the contributions of pioneering natural product chemists in North American academic, governmental, industrial, and private research institutions were instrumental in the isolation and/or structure elucidation of the key lead compounds vinblastine (1) (Noble et al., 1958; Svoboda et al., 1959; Neuss et al., 1962), vincristine (2) (Svoboda, 1961; Neuss et al., 1962), podophyllotoxin (3) (Hartwell and Schecker, 1951), paclitaxel (originally taxol, 4) (Wani et al., 1971), and camptothecin (5) (Wall et al., 1966). The antineoplastic activities of these five lead compounds were discovered through systematic laboratory studies, rather than relying on ethnomedical observations on their respective plant of origin (Fabricant and Farnsworth, 2001).
In the following sections of this short review, we will provide a brief overview of a number of plant-derived substances currently under clinical trial as antineoplastic drug candidates. Approaches taken towards the discovery of anticancer agents from tropical plants, as carried out in the laboratory of the authors, will be described, prior to some concluding remarks. It must be considered that, in the last fifty years, most new drugs of natural origin have been obtained from soil microorganisms, terrestrial fungi, and higher plants (Kinghorn, 2008). However, there is now a sizeable pipeline of about 25 potential anticancer drugs of marine origin presently in clinical trials, and the first of these, ecteinascidin (trabectidin) is used clinically to treat soft tissue sarcoma in Europe (Sashidhara et al., 2009). Therefore, a central question to be posed is whether or not it is desirable to continue to search for potential new anticancer compounds from terrestrial higher plants specifically, or if it would be preferable to de-emphasize this work in order to concentrate available research resources on natural products derived from other types of organisms for this purpose, such as those of marine origin.
2. Selected plant-derived antineoplastic agents in clinical trials
Natural products continue to be an invaluable resource of anticancer drug discovery in recent years, by considering the comparatively large number of chemical entities of natural origin currently under clinical trials (Corson and Crews, 2007; Butler, 2008; Harvey, 2008; Saklani and Kutty, 2008; Sashidhara et al., 2009). In the paragraphs below, plant-derived oncology drug candidates, now in clinical trials, will be considered specifically. Presently, a large number of derivatives of paclitaxel and camptothecin are in clinical trials to treat various types of cancer, with, among these, several taxanes [ABI-007 (suspension), DHA-paclitaxel, paclitaxel poliglumex, RPR-116278A, XRP-9881 (RPR109881A)] and camptothecin derivatives [9-aminocamptothecin, exatecin mesylate, oral topotecan, rubitecan (9-nitrocamptothecin)] being the most advanced, and in Phase III clinical trials (Saklani and Kutty, 2008). Two additional vinca alkaloid derivatives are also in phase clinical trials (vinflunine ditartrate and anhydrovinblastine) as well as two epipodophyllotoxin derivatives (NK-611 and tafluposide) (Saklani and Kutty, 2008). In the paragraphs following, selected plant-derived compounds of structural types other than of the vinca alkaloid, epipopodophyllotoxin, taxane, and camptothecin classes will be highlighted in alphabetical order, in order to further emphasize the structural diversity of promising candidate drug molecules from plants as potential cancer chemotherapeutic agents. Several of the compounds to be mentioned are obtained from traditional Chinese medicine (TCM).
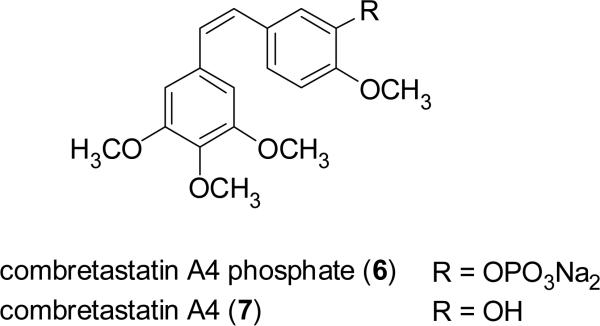
In the 1980s, Pettit and colleagues at Arizona State University isolated several groups of structurally simple stilbenoid compounds from the South African tree, Combretum caffrum Kuntze (Combretaceae), called the combretastatins (Pettit et al., 1982; 1987). The combretastatins comprise several series of related compounds, with the A series being cis-stilbenes active in vitro against the leukemic P388 and L1210 cell lines (Pinney et al., 2005). Combretastatin A4 phosphate (6, CA4P) is a water-soluble prodrug of the natural stilbenoid, combretastatin A4 (7) (Young and Chaplin, 2004; Pinney et al., 2005). Compound 6 is the prototype compound of a new class of antineoplastic agents being developed as tubulin-binding vascular targeting agents (VTAs), which can induce morphological changes within endothelial cells and lead to rapid and selective vascular dysfunction in tumors (Young and Chaplin, 2004; Delmonte and Sessa, 2009). CA4P (6), both alone, and in combination with established chemotherapeutic agents, is being investigated in phase II clinical trials for the treatment of anaplastic thyroid carcinoma and other forms of cancer in the United States (Pinney et al., 2005; ClinicalTrials.gov, 2009a,b).
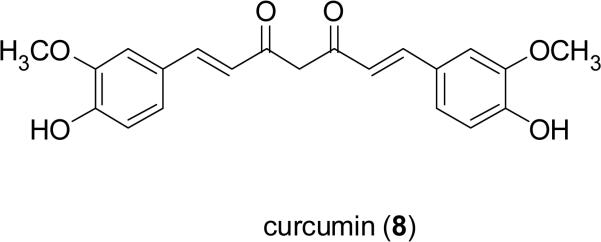
Curcumin (8), a yellow polyphenol pigment isolated from Curcuma longa L. (Zingiberaceae) also known as turmeric, has a long history of utility in South Asia not only as a dietary supplement but also for healthcare (Singh, 2007). A broad spectrum of activities has been attributed to curcumin including antioxidant, antiinflammatory, antimicrobial, and immunomodulatory effects as well as potential antitumor effects. Curcumin inhibits proliferation of a wide variety of malignant cells through regulating numerous intracellular signaling pathways, by acting on various targets, including transcription factors, growth factor receptors, cell surface adhesion molecules, and protein kinases (Aggarwal et al., 2007). A phase II clinical trial of curcumin (8) in patients with advanced pancreatic cancer has been launched at the M. D. Anderson Cancer Center in Houston, Texas (Dhillon et al., 2008).
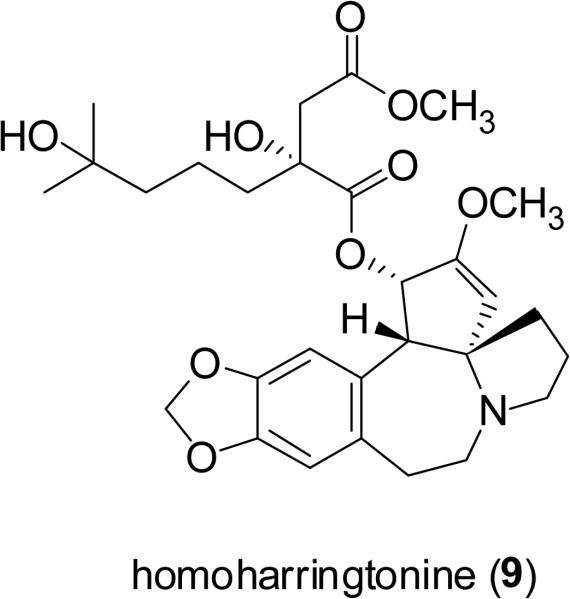
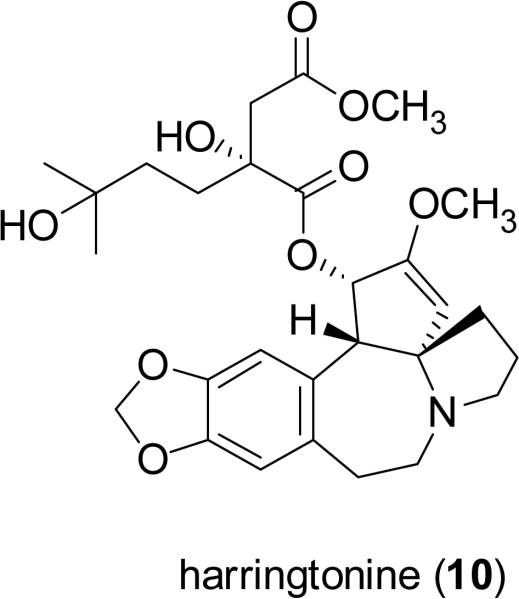
Homoharringtonine (9, HHT, Ceflatonin®) is a Cephalotaxus alkaloid isolated from several Cephalotaxus species indigenous to eastern Asia. Compound 9 was isolated in the early 1970s from Cephalotaxus harringtonia (Knight ex J. Forbes) K. Koch (Cephalotaxaceae) by Powell and colleagues, at the United States Department of Agriculture facility at Peoria, Illinois, along with three structurally similar, naturally occurring cephalotaxine esters, inclusive of harringtonine (10) (Powell et al., 1972). Compound 9 remains the most promising antileukemic agent of numerous structurally related compounds, including many semisynthetic derivatives, due to its natural abundance and potent biological activity. This alkaloid induces the differentiation and apoptosis of cancer cells by inhibiting protein synthesis at the ribosome level (Luo et al., 2004, Meng et al., 2008). Homoharringtonine (9) as a single chemical entity is currently under phase II/III clinical trials for the treatment of patients with chronic myeloid leukemia (CML) in the United States and Europe (Itokawa et al., 2005; Quintas-Cardama et al., 2007; The Rocky Mountain Blood and Marrow Transplant Program, 2009). A mixture of homoharringtonine (9) and harringtonine (10) has been used clinically for the treatment of acute myeloid leukemia (AML) in the People's Republic of China for some time (Kantarjian, 2001; Itokawa et al., 2005). Homoharringtonine (9) has also been evaluated in clinical trials for additional forms of leukemia and for some solid tumors (Itokawa et al., 2005).
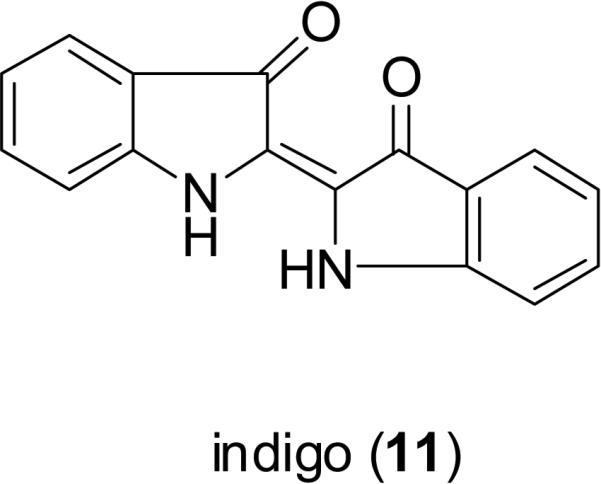
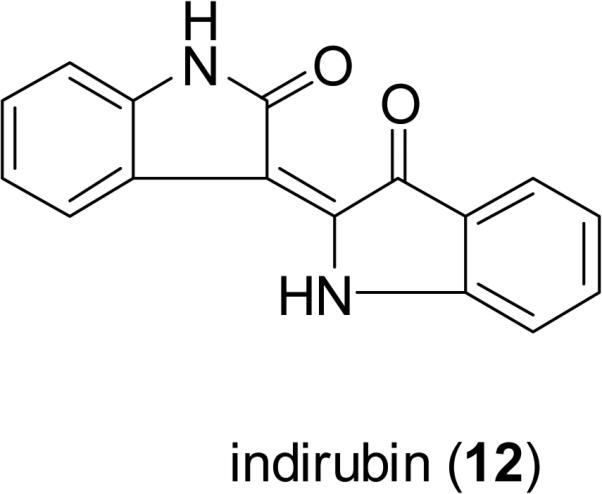
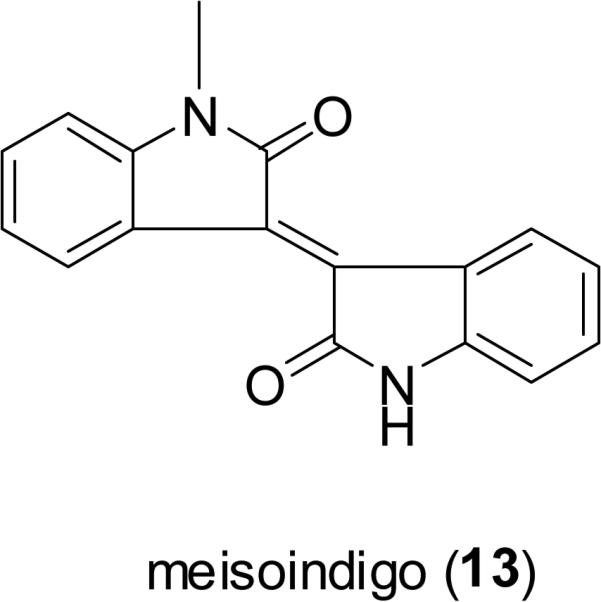
Indigo (11) and its isomer, indirubin (12), are two bis-indole alkaloids found in a traditional Chinese medicine called “Qingdai” (Indigo Naturalis), which has been used in mainland China for the treatment of eczema, hematemesis, tumefaction, and other conditions (Zhongyaoshijia, 2009). “Qingdai” is composed of several plant species containing a dark blue dye, including the leaves and/or stems of Baphicacanthus cusia (Nees) Bremek. (Acanthaceae), Indigofera tinctoria L. (Fabaceae), Indigofera suffruticosa Mill. (Fabaceae), Isatis tinctoria L. (Brassicaceae), and Polygonum tinctorium Ait. (Polygonaceae) (Deng, 1986). Indirubin (12) has been reported to possess cyclin-dependent kinase (CDK) inhibitory potential, and broad-spectrum antitumor activity (Eisenbrand et al., 2004). Meisoindigo (13) is an indirubin derivative, developed to increase the solubility in water and reduce the side effects of indirubin. This compound showed significant activity against cancer cells through an ability to inhibit cyclin-dependent kinases, induce cell differentiation, promote apoptosis, and arrest cells in the G0/G1 phase of the cell cycle, and it also inhibited tumor growth in HT-29 colon cancer xenografts (100 mg/kg, i.p.) (Zuo et al., 2008). Meisoindigo is undergoing a phase III clinical trial in the People's Republic of China for the treatment of chronic myelogenous leukemia (CML) (Cooperative Study Group of Phase III Clinical Trial on Meisoindigo, 1997).
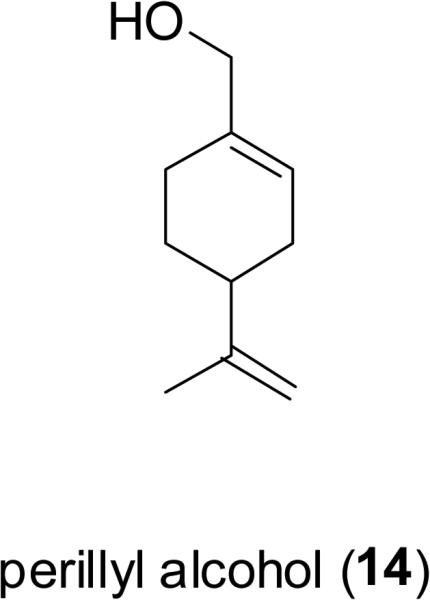
Perillyl alcohol (14), a widely distributed limonene-type monoterpenoid, is found from the essential oils of some aromatic herbs, such as lavender (Lavandula x intermedia, Lamiaceae), peppermint (Mentha x piperita L., Lamiaceae), spearmint (Mentha spicata L., Lamiaceae), and celery seeds (Apium graveolens L. Apiaceae) (Belanger, 1998). The observed effects of perillyl alcohol include the induction of apoptosis, differentiation, and cell cycle arrest in the G1 phase, through the regulation various cellular substances that are involved in cell growth and differentiation (Peffley et al., 2007; Yeruva et al., 2007). A phase II clinical trial sponsored by the U.S. National Cancer Institute (NCI) to study the effectiveness of perillyl alcohol in treating patients with metastatic breast cancer refractory to previous chemotherapy has been conducted (Bailey et al., 2008).
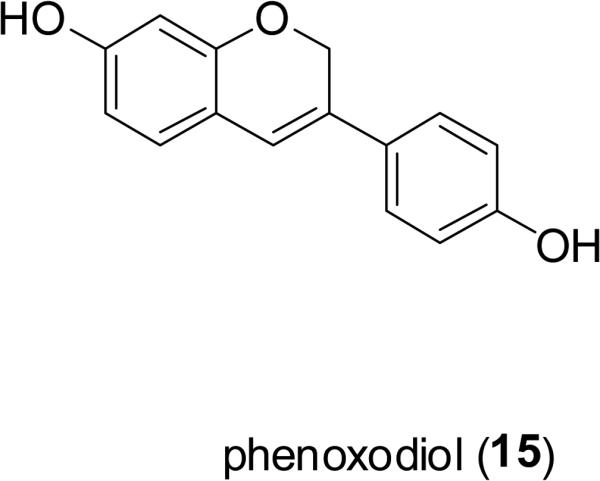
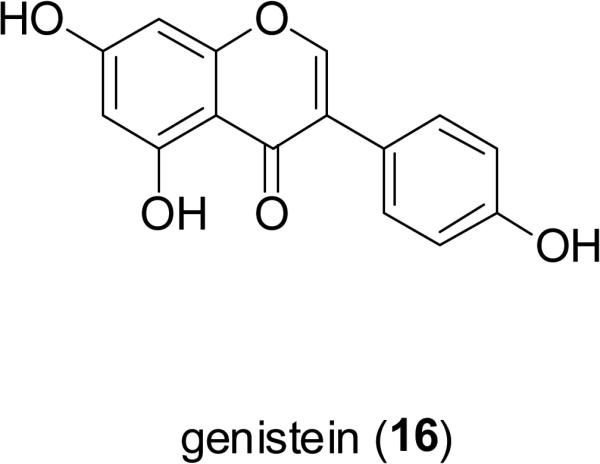
Phenoxodiol (15), derived from genistein (16), a natural isoflavone constituent of the soybean [Glycine max (L.) Merr.; Fabaceae]. Phenoxodiol has been promoted as a new promising drug candidate in oncology for its broad anticancer spectrum and minimal toxicity (Choueri et al., 2006; Butler, 2008). As a multiple signal transduction regulator, phenoxodiol leads to mitotic arrest and apoptosis of tumor cells through multiple mechanisms such as decreasing the level of antiapoptotic proteins and inhibiting the activity of DNA topoisomerase (topo) II (Constantinou and Husband, 2002; Choueiri et al., 2006). Compound 15 is under advanced clinical trial for development as a “chemosensitizer” when combined with platinum drugs for the treatment of ovarian cancer, and as a monotherapy for cervical and prostate cancers (Mor et al., 2006; Silasi et al., 2009). There is also an interest in developing genistein (16) as an antileukemic agent (Raynal et al., 2008).
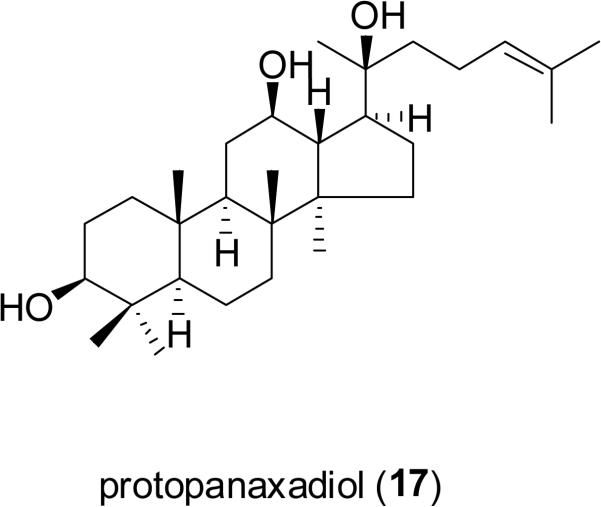
Protopanaxadiol [17; 20S-protopanaxadiol (PPD)] is a dammarane-type triterpene aglycone derived by hydrolysis of certain saponins from ginseng [Panax ginseng C.A. Mey.; Araliaceae. Protopanaxadiol has demonstrated effects on apoptosis induction in cancer cells and cell cycle arrest through various signaling pathways including caspases, and has also been found to show cytotoxic activity against multidrug-resistant tumors by acting as an effective P-glycoprotein blocker (P-gp, MDR1) (Popovich and Kitts, 2002; Li et al., 2006; Liu et al., 2007). Protopanaxadiol (17, under the trade name Pandimex™), is now in a Phase I clinical trial launched by PanaGin in the United States for the treatment of lung cancer and other solid tumors (Saklani and Kutty, 2008; Panagin Pharmaceuticals, Inc., 2009). In addition, it has been approved conditionally in mainland China for the treatment of advanced cancers of the breast, colon-rectum, lung, and pancreas (PanaGin Pharmaceuticals, Inc., 2009).
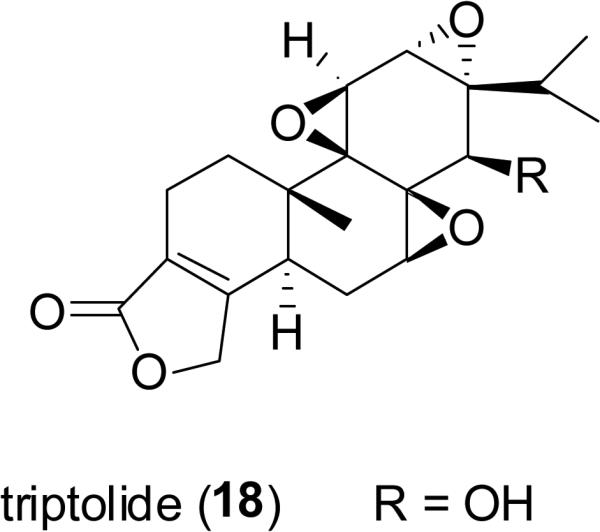
Triptolide (18; PG490), is a diterpenoid triepoxide isolated from Tripterygium wilfordii Hook.f. (Celastraceae), which is a perennial woody vine distributed in eastern Asia, with a long history of use in traditional Chinese medicine for the treatment of immune-related disorders and inflammation (Ramgolam et al., 2000). Besides the anti-inflammatory and immunosuppressive properties ascribed to triptolide, it is also reported that this compound exhibits antitumor effects and induces apoptosis by modulating p53 and related proteins in a broad range of malignant cell lines (Kiviharju et al., 2002). PG490-88Na (19; 14-succinyl triptolide sodium salt), a water-soluble prodrug of PG490, which is metabolized into triptolide in vivo after intravenous administration, has entered Phase I clinical trials for patients with solid tumors (Kiviharju et al., 2002; Fidler et al., 2003; Saklani and Kutty, 2008).
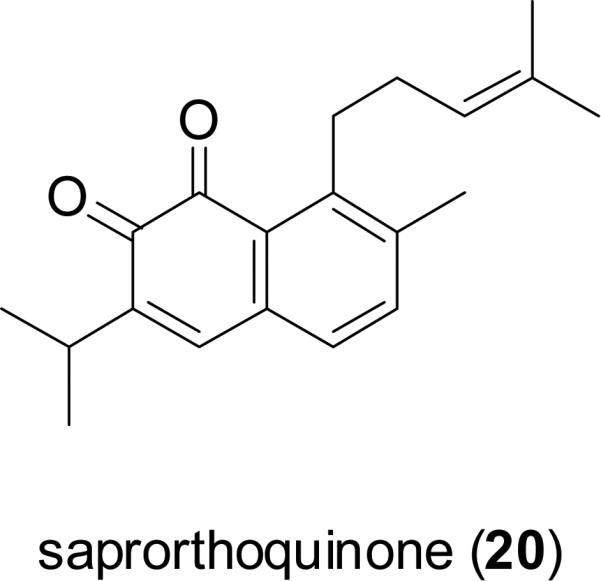
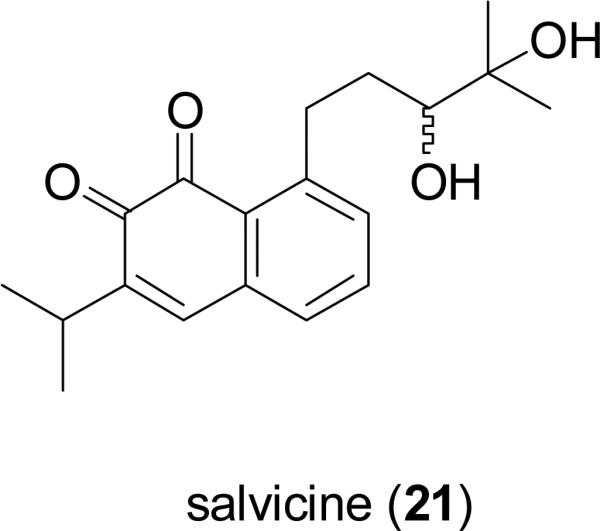
Saprorthoquinone (20), a diterpenoid quinone isolated from Salvia prionitis Hance (Lamiaceae), a native herb in the southern part of mainland China, is used as therapy in TCM for the treatment of fever, tonsillitis, pneumonia, and diarrhea (Xu, 1990). Salvicine (21), a semi-synthetic analog of compound 20, was found to exhibit significant growth inhibitory activity against a wide spectrum of human cancer cells in vitro, and showed effects in in vivo xenograft models bearing S-180 sarcoma, Lewis lung, A-549 lung, and LAX-83 lung adenocarcinoma cells (Zhang et al., 1999). Salvicine also demonstrated a potent cytotoxic effect on multidrug-resisitant (MDR) tumor cells (Miao et al., 2003). Salvicine exerts its antineoplastic effects by acting as a non-intercalative topoisomerase II inhibitor that induces apoptosis. Salvicine (21) has entered phase II clinical trials for the treatment of solid tumors in mainland China (Cai et al., 2008).
3. Approach to the Discovery of Anticancer Agents from Plants
While the pharmaceutical industry has had a long track-record of success in developing natural product drugs for the oncology market, for more than half a century there has also been an active interest in the systematic screening of extracts from plants and other organisms for their potential anticancer activity by the U.S. National Cancer Institute (NCI) (Suffness and Douros, 1982; Cragg et al., 1999; 2006). In initial work during the period 1960-1982, over 114,000 plant-derived extracts were screened through a combination of prescreens, cell-based (cytotoxicity) screens, and in vivo testing in mice implanted with tumors (Suffness and Douros, 1982). During the period 1986-2004, the Natural Products Branch of the Developmental Therapeutics Program (DTP) at NCI organized the collection of some 60,000 higher plant samples in various targeted tropical locations of the world. Extracts of these plant samples were evaluated first against a pre-screen, with active samples then tested against a 60-cell line tumor panel derived from nine cancer types, followed by further in vivo evaluation when merited (Cragg et al., 1999; 2006). Since 1999, the prescreen was modified to screening through a three-cell panel, constituting MCF-7 breast, NCI-H460 lung, and SF-268 CNS cancer cells (Cragg et al., 1999; 2006). Although taxol (now known as paclitaxel) was discovered in the laboratory of the late Dr. Monroe Wall and Dr. Mansukh Wani at Research Triangle Institute, North Carolina (Wani et al., 1971), the plant of origin of this compound (the bark of Taxus brevifolia Nutt.) was collected in Washington State initially as part of a plant collection program organized by the USDA for the NCI (Cragg et al., 1999).
In the 1980s, the U.S. National Cancer Institute set up a mechanism for funding collaborative teams from academia and industry via the “National Cooperative Drug Discovery Groups” (NCDDG) mechanism, directed toward the discovery of novel anticancer agents of synthetic and natural origin (Hallock and Cragg, 2003). While at the University of Illinois at Chicago, the senior author of this review served as a Principal Investigator for a NCDDG project (1995-2005) aimed at investigating new cancer chemotherapeutic agents from mainly tropical plants, with the other partners being Research Triangle Institute, Research Triangle Park, North Carolina and Bristol-Myers Squibb, Princeton, New Jersey. For an initial five-year period of this NCDDG funding award (1990-1995), the industrial partner was Glaxo-Wellcome Medicines Research Center, Greenford, Middlesex, U.K. In previous reviews, our group has described the general technical approaches taken towards plant collections (including intellectual property agreement development), phytochemical procedures, known compound dereplication methods, in vitro cell-based and mechanism-based bioassay systems, and in vivo assays that were used in our NCDDG project (Kinghorn et al., 1999; Kinghorn et al., 2003; Balunas and Kinghorn, 2005), as well as those used in a follow-up program project, also funded by the U.S. NCI (Kinghorn et al., 2009). We have found the in vivo hollow fiber assay useful as a discriminatory procedure in helping to prioritize compounds active in preliminary in vitro biological test systems for evaluation in mouse xenograft systems (Mi et al., 2009). The in vivo hollow fiber assay was originally developed at the U.S. NCI (Hollingshead et al., 1995). While a large number of compounds were obtained from tropical plants in our work that showed activity in one or more in vitro or in vivo bioassays (Kinghorn et al., 1999; 2003; 2009), two examples of promising antineoplastic compounds that were obtained will be featured in the following paragraphs.
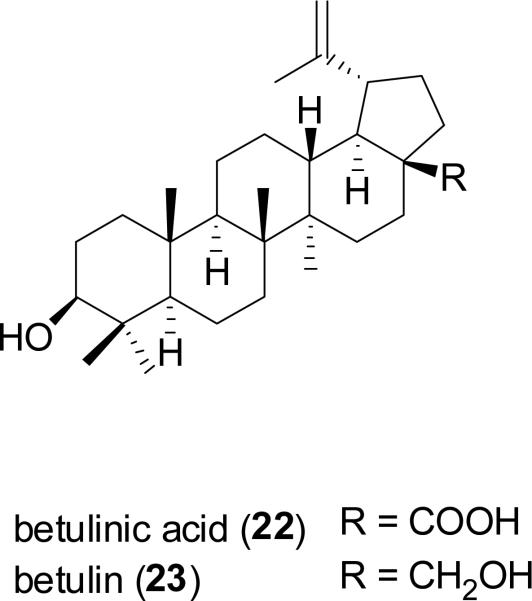
The lupane-type triterpenoid, betulinic acid (22) was isolated in our NCDDG project from Ziziphus mauritiana Lam. (Rhamnaceae), collected in Zimbabwe. Although known already as a phytochemical of widespread distribution in the plant kingdom, betulinic acid was found to selectively inhibit the growth of human melanoma cell lines by causing apoptosis, and also exhibited activity in vivo for athymic mice bearing human melanoma cells (Pisha et al., 1995). Betulinic acid has also been shown to be cytotoxic to neuroectodermal and malignant brain tumor cell lines (Cichewicz and Kouzi, 2004). The compound can be produced by semi-synthesis from its abundant naturally occurring analog, betulin (23) (Alakurtii et al., 2006). An ointment containing 20% of betulinic acid is currently under phase I/II clinical trials launched by NCI as a portential therapy for the treatment of dysplastic melanocytic nevi (U.S. National Institutes of Health, 2009).
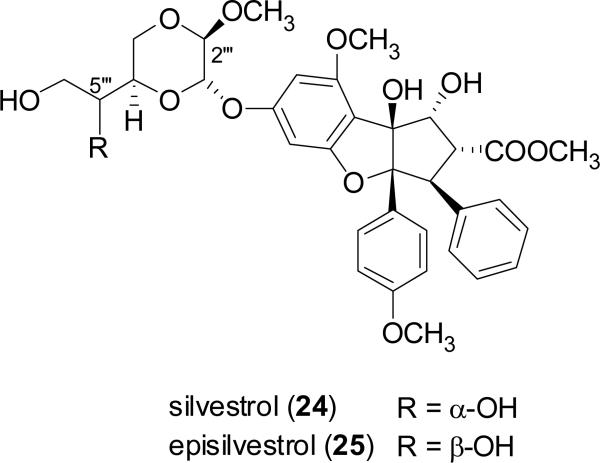
Silvestrol (24), a rocaglate derivative, was first characterized structurally and sterochemically from the tropical plant Aglaia foveolata Pannell (Meliaceae) in 2004, along with its 5″′S epimer, episilvestrol (25), using a combination of spectroscopic data interpretation and X-ray crystallography (Hwang et al., 2004). Compounds 24 and 25 may be distinguished with other rocaglates in having a unique 1,4-dioxanyloxy group affixed to the cyclopenta[b]benzofuran moiety (Kim et al., 2006). The first rocaglate, rocaglamide, was reported in the literature less than 30 years ago, so this is a relatively recently discovered class of plant secondary metabolites (King et al., 1982). Silvestrol was found to possess comparable cytotoxic activity against several human cancer cell lines to those of the well-known anticancer compounds, paclitaxel (Taxol) and campotothecin, and was further found to be active in the in vivo hollow fiber assay and the P-388 lymphocytic leukemia test system in vivo, when administered both ip and sc (Hwang et al., 2004). Furthermore, this promising anticancer candidate was found to exhibit B-cell selectivity in both chronic lymphocytic leukemia in vitro and acute lymphocytic leukemia in vivo models (Lucas et al., 2009). The antineoplastic activity of silvestrol is associated with increased apoptosis, decreased proliferation, and inhibition of angiogenesis, and it acts as a strong translation inhibitor of malignancy-related mRNA by modulating the activity of initiation factor elF4A (Bordeleau et al., 2008; Cencic et al., 2009). Silvestrol has also been reported as an antineoplastic constituent of Aglaia leptantha Miq. collected in Sarawak, Malaysia, although its stereochemistry was not established in this work (Meurer-Grimes et al., 2004). Silvestrol (24) has been totally synthesized by two independent groups (Gerard et al., 2007; El Sous et al., 2007; Adams et al., 2009). Further preclinical testing of silvestrol (24) as a potential antileukemic agent is currently underway.
4. Future Prospects
There is an impressive number of higher plant-derived anticancer drugs of diverse structural types both in present clinical use and as antineoplastic candidates undergoing clinical trials (Cragg et al., 2005; Butler, 2008; Saklani and Kutty, 2008). This suggests that higher plants will continue to be an important and valuable resource for anticancer drug discovery, especially since 300,000-500,000 such species are known, and these represent about 15% of the taxonomically authenticated global organism biodiversity (Tan et al., 2006). Moreover, probably less than 20% of the known plants on earth have ever been subjected to laboratory investigation for potential therapeutic effects, and a smaller percentage still for possible anticancer activity (Cragg et al., 2009). In recent years, an intriguing observation has been that fungal endophytes occur on host plants that produce anticancer lead compounds, and themselves biosynthesize the same substances. Recent examples of this phenomenon are podophyllotoxin (3) (from Phialocephala fortunii growing on Podophyllum peltatum L.) (Eyberger et al., 2006) and camptothecin (5) [from a fungus in the family Phycomycetes growing on Nothapodytes foetida (Wight) Sleumer] (Puri et al., 2005). Laboratory manipulation of endophytic fungi that produce important biological molecules may lead to more reliable supplies of rare anticancer agent lead compounds of plant origin in the future.
While the pipeline of potential anticancer agents from plants is promising, the same can be said for interesting new natural product leads to treat cancer from terrestrial microbes and marine organisms (Cragg et al., 2005; 2009; Butler, 2008; Sashidhara et al., 2009). Moreover, from a structural point of view, the lead compounds of this type from higher plants perhaps do not intrigue the scientific community so much, because fewer prototype compounds based on new carbon skeleta are represented. Newman and Cragg commented on this matter in 2005: “On the basis of recent experience using the National Cancer Institute (NCI) 60-cell line screen, the potential for the discovery of a new chemotype from plants, comparable to the taxanes or camptothecins, appears to be relatively low. In spite of work covering vascular plants from extensive collections in the tropics over the last 18+ years, no novel chemotypes have, as yet, been reported.” (Newman and Cragg, 2005). Arguably, either the taxanes or the camptothecin derivatives may be seen to rival any example of a microbial- or marine-derived lead natural product in terms of the number of new analogs that have entered clinical trials recently (Butler, 2008). However, it is to be hoped that future investigators working on plant-derived anticancer agents will eagerly take on the challenge of discovering new lead compounds that may become of comparable interest to the biomedical community as camptothecin (5) and paclitaxel (4).
Acknowlegement
In regard to the studies on betulinic acid and silvestrol mentioned in this review that were carried out in our laboratory, we wish to acknowledge support of grants from the National Cancer Institute (U19 CA52956 and P01 CA125066), awarded to A.D. Kinghorn.
References
- Adams TE, El Sous M, Hawkins BC, Hirner S, Holloway G, Khoo ML, Owen DJ, Savage GP, Scammells PJ, Rizzacasa MA. Total synthesis of the potent anticancer Aglaia metabolites (-)-silvestrol and (-)-episilvestrol and the active analogue (-)-4′-desmethoxyepisilvestrol. J. Am. Chem. Soc. 2009;131:1607–1616. doi: 10.1021/ja808402e. [DOI] [PubMed] [Google Scholar]
- Aggarwal BB, Sundaram C, Malani N, Ichikawa H. Curcumin: the Indian solid gold. Adv. Exp. Med. Biol. 2007;595:1–75. doi: 10.1007/978-0-387-46401-5_1. [DOI] [PubMed] [Google Scholar]
- Alakurtti S, Mäkelä T, Koskimies S, Yli-Kauhaluoma J. Pharmacological properties of the ubiquitous natural product betulin. Eur. J. Pharm. Sci. 2006;29:1–13. doi: 10.1016/j.ejps.2006.04.006. [DOI] [PubMed] [Google Scholar]
- American Cancer Society . Global Cancer Facts and Figures 2007. American Cancer Society; Atlanta: 2007. [Google Scholar]
- Anonymous Assay development. Nat. Rev. Drug. Disc. 2007;6:681. [Google Scholar]
- Bailey HH, Attia S, Love RR, Fass T, Chappell R, Tutsch K, Harris L, Jumonville A, Hansen R, Shapiro GR, Stewart JA. Phase II trial of daily oral perillyl alcohol (NSC 641066) in treatment-refractory metastatic breast cancer. Cancer Chemother. Pharmacol. 2008;62:149–157. doi: 10.1007/s00280-007-0585-6. [DOI] [PubMed] [Google Scholar]
- Balunas MJ, Kinghorn AD. Drug discovery from medicinal plants. Life. Sci. 2005;78:431–441. doi: 10.1016/j.lfs.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Belanger JT. Perillyl alcohol : applications in oncology. Altern. Med. Rev. 1998;3:448–457. [PubMed] [Google Scholar]
- Bindseil KU, Jakupovic J, Wolf D, Lavayre J, Leboul J, van der Pyl D. Pure compound libraries: a new perspective for natural product based drug discovery. Drug. Discov. Today. 2001;6:840–847. doi: 10.1016/s1359-6446(01)01856-6. [DOI] [PubMed] [Google Scholar]
- Bordeleau ME, Robert F, Gerard B, Lindqvist L, Chen SMH, Wendel HG, Brem B, Greger H, Lowe SW, Porco JA, Jr., Pelletier JJ. Therapeutic suppression of translation initiation modulates chemosensitivity in a mouse lymphoma model. Clin. Inv. 2008;118:1–11. doi: 10.1172/JCI34753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MS. Natural products to drugs: natural product-derived compounds in clinical trials. Nat. Prod. Rep. 2008;25:475–516. doi: 10.1039/b514294f. [DOI] [PubMed] [Google Scholar]
- Cai Y-J, Lu JJ, Zhu H, Xie H, Huang M, Lin LP, Zhang X-W, Ding J. Salvicine triggers DNA double-strand breaks and apoptosis by GSH-depletion-driven H2O2 generation and topoisomerase II inhibition. Free Radic. Biol. Med. 2008;45:627–635. doi: 10.1016/j.freeradbiomed.2008.05.017. [DOI] [PubMed] [Google Scholar]
- Cencic R, Carrier M, Galicia-Vázquez G, Bordeleau M-E, Sukarieh R, Bourdeau A, Brem B, Teodoro JG, Greger H, Tremblay ML, Porco JA, Jr., Pelletier J. Antitumor activity and mechanism of action of the cyclopenta[b]benzofuran, silvestrol. PLoS ONE. 2009;4(4):e5223. doi: 10.1371/journal.pone.0005223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabner BA, Amrein PC, Druker BJ, Michaelson MD, Mitsiades CS, Gross PE, Ryan DP, Ramachandra S, Richardson PG, Supko JG, Wilson WH. In: Goodman & Gilman's The Pharmacological Basis of Therapeutics. 11th ed. Brunton LL, Laso JS, Parker KL, editors. McGraw-Hill; New York: 2005. pp. 1315–1403. [Google Scholar]
- Chichewicz RH, Kouzi SA. Chemical, biological activity, and chemotherapeutic potential of betulinic acid for the prevention and treatment of cancer and HIV infection. Med. Res. Rev. 2004;24:90–114. doi: 10.1002/med.10053. [DOI] [PubMed] [Google Scholar]
- Choueiri TK, Wesolowski R, Mekhail TM. Phenoxodiol: isoflavone analog with antineoplastic activity. Curr. Oncol. Rep. 2006;8:104–107. doi: 10.1007/s11912-006-0044-2. [DOI] [PubMed] [Google Scholar]
- ClinicalTrials.gov [October 2009];Study of Combretastatin and Paclitaxel/Carboplatin in the Treatment of Anaplastic Thyroid Cancer. 2009a Further information available at: http://clinicaltrials.gov/ct2/show/NCT00507429.
- ClinicalTrials.gov [October 2009];A Study to Assess the Effectiveness of the Combination of Carboplatin, Paclitaxel, Bevacizumab and Combretastatin (CA4P) in Patients With Chemotherapy Naïve Lung Cancer. 2009b Further information available at: http://clinicaltrials.gov/ct2/show/NCT00653939.
- Constantinou AI, Husband A. Phenoxodiol [2H-1-benzopyran-7-ol, 3-(4-hydroxyphenyl)], a novel isoflavone derivative, inhibits DNA topoisomerase II by stabilizing the cleavable complex. Anticancer Res. 2002;22:2581–2585. [PubMed] [Google Scholar]
- Cooperative Study Group of Phase III Clinical Trial on Meisoindigo Phase II clinical trial on meisoindigo in the treatment of chronic myelogenous leukemia. Zhonghua Xueyexue Zazhi. 1997;18:69–72. [PubMed] [Google Scholar]
- Corson TW, Crews CM. Molecular understanding and modern application of traditional medicines: Triumphs and trials. Cell. 2007;130:769–774. doi: 10.1016/j.cell.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg GM, Boyd MR, Khanna R, Kneller R, Mays TD, Mazan KD, Newman DJ, Sausville EA. International collaboration in drug discovery and development: the NCI experience. Pure Appl. Chem. 1999;71:1619–1633. [Google Scholar]
- Cragg GM, Kingston DGI, Newman DJ, editors. Anticancer Agents from Natural Products. CRC/Taylor & Francis; Boca Raton, FL: 2005. [Google Scholar]
- Cragg GM, Newman DJ, Yang SS. Natural product extracts of plant and marine origin having antileukemia potential. The NCI experience. J. Nat. Prod. 2006;69:488–498. doi: 10.1021/np0581216. [DOI] [PubMed] [Google Scholar]
- Cragg GM, Grothaus PG, Newman DJ. Impact of natural products on developing new anti-cancer agents. Chem. Rev. 2009;109:3012–3043. doi: 10.1021/cr900019j. [DOI] [PubMed] [Google Scholar]
- Delmonte A, Sessa C. AVE8062: a new combretastatin derivative vascular disrupting agent. Expert. Opin. Investig. Drugs. 2009;18:1541–1548. doi: 10.1517/13543780903213697. [DOI] [PubMed] [Google Scholar]
- Deng B. Direct colorimetric method for determination of indigo and indirubin in Qingdai. Zhong Cao Yao. 1986;17:163–164. [Google Scholar]
- DeVita VT, Hellman S, Rosenberg SA, editors. Cancer: Principles and Practice of Oncology. 8th ed. Lippincott-Williams & Wilkins; Philadelphia: 2008. [Google Scholar]
- Dhillon N, Aggarwal BB, Newman RA, Wolff RA, Kunnumakkara AB, Abbruzzese JL, Ng CS, Badmaev V, Kurzrock R. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin. Cancer Res. 2008;14:4491–4499. doi: 10.1158/1078-0432.CCR-08-0024. [DOI] [PubMed] [Google Scholar]
- Eisenbrand G, Hippe F, Jakobs S, Muehlbeyer S. Molecular mechanisms of indirubin and its derivatives: novel anticancer molecules with their origin in traditional Chinese phytomedicine. J. Cancer Res. Clin. Oncol. 2004;130:627–635. doi: 10.1007/s00432-004-0579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Sous M, Khoo ML, Holloway G, Owen D, Scammells PJ, Rizzacasa MA. Total synthesis of (-)-silvestrol and (-)-episilvestrol. Angew. Chem. Int. Ed. 2007;46:7835–7838. doi: 10.1002/anie.200702700. [DOI] [PubMed] [Google Scholar]
- Eyberger AL, Dondapati R, Porter JR. Endophytic fungal isolates from Podophyllum peltatum produce podophyllotoxin. J. Nat. Prod. 2006;69:1121–1124. doi: 10.1021/np060174f. [DOI] [PubMed] [Google Scholar]
- Fabricant DS, Farnsworth NR. The value of plants used in traditional medicine for drug discovery. Environ. Health Perspec. 2001;109:69–75. doi: 10.1289/ehp.01109s169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler JM, Li K, Chung C, Wei K, Ross JA, Gao M, Rosen GD. PG490-88, a derivative of triptolide, causes tumor regression and sensitizes tumors to chemotherapy. Mol. Cancer Ther. 2003;2:855–862. [PubMed] [Google Scholar]
- Fischbach MA, Walsh CT. Biochemistry: Directing biosynthesis. Science. 2006;314:603–605. doi: 10.1126/science.1132692. [DOI] [PubMed] [Google Scholar]
- Firn RD, Jones CG. Natural products-a simple model to explain chemical diversity. Nat. Prod. Rep. 2003;20:382–391. doi: 10.1039/b208815k. [DOI] [PubMed] [Google Scholar]
- Gerard B, Cencic R, Pelletier J, Porco JA., Jr. Enantioselective synthesis of the complex rocaglate (-)-silvestrol. Angew. Chem. Int. Ed. 2007;46:7831–7835. doi: 10.1002/anie.200702707. [DOI] [PubMed] [Google Scholar]
- Hallock YF, Cragg GM. National Cooperative Drug Discovery Groups (NCDDGs): a successful model for public private partnerships in cancer drug discovery. Pharm. Biol. 2003;41(Suppl):78–91. [Google Scholar]
- Hartwell JL. Plants Used against Cancer: a Survey. Quarterman; Lawrence, MA: 1982. [Google Scholar]
- Hartwell JL, Schrecker AW. Components of podophyllin. V. The constitution of podophyllotoxin. J. Am. Chem. Soc. 1951;73:2909–2916. [Google Scholar]
- Harvey AL. Natural products in drug discovery. Drug Discov. Today. 2008;13:894–901. doi: 10.1016/j.drudis.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Hollingshead MG, Alley MC, Camalier RF, Abbott BJ, Mayo JG, Malspeis L, Grever MR. Life Sci. 1995;57:131–141. doi: 10.1016/0024-3205(95)00254-4. [DOI] [PubMed] [Google Scholar]
- Hwang BY, Su BN, Chai H-B, Mi Q, Kardono LBS, Afriastini JJ, Riswan S, Santarsiero BD, Mesecar AD, Wild R, Fairchild CR, Vite GD, Rose WC, Farnsworth NR, Cordell GA, Pezzuto JM, Swanson SM, Kinghorn AD. Silvestrol and episilvestrol, potential anticancer rocaglate derivatives from Aglaia silvestris. J. Org. Chem. 2004;69:3350–3358. doi: 10.1021/jo040120f. ibid. 6156. [DOI] [PubMed] [Google Scholar]
- Itokawa H, Wang X, Lee K-H. Homoharringtonine and related compounds. In: Cragg GM, Kingston DGI, Newman DJ, editors. Anticancer Agents from Natural Products. CRC/Taylor & Francis; Boca Raton, FL: 2005. pp. 47–70. [Google Scholar]
- Kantarjian HM, Talpaz M, Santini V, Murgo A, Cheson B, O'Brien SM. Homoharringtonine: history, current research, and future direction. Cancer. 2001;92:1591–605. doi: 10.1002/1097-0142(20010915)92:6<1591::aid-cncr1485>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Kim S, Salim AA, Swanson SM, Kinghorn AD. Potential of cyclopenta[b]benzofurans from Aglaia species in cancer chemotherapy. Anti-cancer Agents Med. Chem. 2006;6:319–345. doi: 10.2174/187152006777698123. [DOI] [PubMed] [Google Scholar]
- King ML, Chiang CC, Ling HC, Fukita E, Ochiai M, McPhail AT. X-ray crystal structure of rocaglamide, a novel antileukemic 1H-cyclopenta[b]benzofuran from Aglaia elliptifolia. J. Chem. Soc., Chem. Commun. 1982:1150–1151. [Google Scholar]
- Kinghorn AD. Drug discovery from natural products. In: Lemke R, Williams DA, editors. Foye's Principles of Medicinal Chemistry. 6th Edn. Walters Kluwer/Lippincott Williams & Wilkins; Baltimore: 2008. pp. 12–25. [Google Scholar]
- Kinghorn AD, Farnsworth NR, Soejarto DD, Cordell GA, Pezzuto JM, Udeani GO, Wani MC, Wall ME, Navarro HA, Kramer RA, Menendez AT, Fairchild CR, Lane KE, Forenza S, Vyas DM, Lam KS, Shu Y-Z. Novel strategies for the discovery of plant-derived anticancer agents. Pure Appl. Chem. 1999;71:611–1618. [Google Scholar]
- Kinghorn AD, Farnsworth NR, Soejarto DD, Cordell GA, Swanson SM, Pezzuto JM, Wani MC, Wall ME, Oberlies NH, Kroll DJ, Kramer RA, Rose WC, Vite GD, Fairchild CR, Peterson RW, Wild R. Novel strategies for the discovery of plant-derived anticancer agents. Pharm. Biol. 2003;41(Suppl):53–67. [Google Scholar]
- Kinghorn AD, Carcache-Blanco EJ, Chai H-B, Orjala J, Farnsworth NR, Soejarto DD, Oberlies NH, Wani MC, Kroll DJ, Pearce CJ, Swanson SM, Kramer RA, Rose WC, Fairchild CR, Emanuel S, Vite GD, Jarjoura D, Cope FO. Discovery of anticancer agents of diverse natural origin. Pure Appl. Chem. 2009;81:1051–1063. doi: 10.1351/PAC-CON-08-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiviharju TM, Lecane PS, Sellers RG, Peehl DM. Antiproliferative and proapoptotic activities of triptolide (PG490), a natural product entering clinical trials, on primary cultures of human prostatic epithelial cells. Clin. Cancer Res. 2002;8:2666–2674. [PubMed] [Google Scholar]
- Koehn FE, Carter GT. The evolving role of natural products in drug discovery. Nature Rev. Drug Discov. 2005;4:206–220. doi: 10.1038/nrd1657. [DOI] [PubMed] [Google Scholar]
- Li G, Wang ZH, Sun YX, Liu K, Wang Z. Ginsenoside 20(S)-protopanaxadiol inhibits the proliferation and invasion of human fibrosarcoma HT1080 cells. Basic Clin. Pharmacol. Toxicol. 2006;98:588–592. doi: 10.1111/j.1742-7843.2006.pto_415.x. [DOI] [PubMed] [Google Scholar]
- Li JH-W, Vederas JG. Drug discovery and natural products: end of an era or an endless frontier? Science. 2009;325:161–165. doi: 10.1126/science.1168243. [DOI] [PubMed] [Google Scholar]
- Liu GY, Bu X, Yan H, Jia WWG. 20S-Protopanaxadiol-induced programmed cell death in glioma cells through caspase-dependent and -independent pathways. J. Nat. Prod. 2007;70:259–265. doi: 10.1021/np060313t. [DOI] [PubMed] [Google Scholar]
- Lucas DM, Edwards RB, Lozanski G, West DA, Shin JD, Vargo MA, Davis ME, Rozewski DM, Johnson AJ, Su BN, Goettl VM, Heerema NA, Lin TS, Lehman A, Zhang XL, Jarjoura D, Newman DJ, Byrd JC, Kinghorn AD, Grever MR. The novel plant-derived agent silvestrol has B-cell selective activity in chronic lymphocytic leukemia and acute lymphoblastic leukemia in vitro and in vivo. Blood. 2009;113:4656–4666. doi: 10.1182/blood-2008-09-175430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo CY, Tang JY, Wang YP. Homoharringtonine: a new treatment option for myeloid leukemia. Hematology. 2004;9:259–270. doi: 10.1080/10245330410001714194. [DOI] [PubMed] [Google Scholar]
- Meng H, Yang C, Jin J, Zhou Y, Qian W. Homoharringtonine inhibits the AKT pathway and induces in vitro and in vivo cytotoxicity in human multiple myeloma cells. Leuk. Lymphoma. 2008;49:1954–1962. doi: 10.1080/10428190802320368. [DOI] [PubMed] [Google Scholar]
- Meurer-Grimes BM, Yu J, Vairo GL. Therapeutic compounds and methods. 2004 U.S. Patent 6710075.
- Mi Q, Pezzuto JM, Farnsworth NR, Wani MC, Kinghorn AD, Swanson SM. Use of the in vivo hollow fiber assay in natural products drug discovery. J. Nat. Prod. 2009;72:573–580. doi: 10.1021/np800767a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao ZH, Tang T, Zhang YX, Zhang JS, Ding J. Cytotoxicity, apoptosis induction and downregulation of MDR-1 expression by the anti-topoisomerase II agent, salvicine, in multidrug-resistant tumor cells. Int. J. Cancer. 2003;106:108–115. doi: 10.1002/ijc.11174. [DOI] [PubMed] [Google Scholar]
- Mor G, Fu HH, Alvero AB. Phenoxodiol, a novel approach for the treatment of ovarian cancer. Curr. Opin. Investig. Drugs. 2006;7:542–548. [PubMed] [Google Scholar]
- Newman DJ, Cragg GM. Developments and future trends in anticancer natural products drug discovery. In: Cragg GM, Kingston DGI, Newman DJ, editors. Anticancer Agents from Natural Products. CRC Taylor & Francis; Boca Raton, FL: 2005. pp. 553–571. [Google Scholar]
- Newman DJ, Cragg GM. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 2007;70:461–77. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- Neuss N, Gorman M, Boaz HW, Cone NJ. Vinca alkaloids. XI. Structures of leurocristine and vincaleukoblastine. J. Am. Chem. Soc. 1962;84:1509–1510. [Google Scholar]
- Noble RH, Cutts JH, Beer CH. Further biological activities of vincaleukoblastine – an alkaloid isolated from Vinca rosea (L.). Biochem. Pharmacol. 1958;1:347–348. [Google Scholar]
- PanaGin Pharmaceuticals, Inc. [October 2009];2009 Further information available at http://www.panagin.com.
- Peffley DM, Sharma C, Hentosh P, Buechler RD. Perillyl alcohol and genistein differentially regulate PKB/Akt and 4E-BP1 phosphorylation as well as eIF4E/eIF4G interactions in human tumor cells. Arch. Biochem. Biophys. 2007;465:266–273. doi: 10.1016/j.abb.2007.05.022. [DOI] [PubMed] [Google Scholar]
- Pettit GR, Cragg GM, Herald DL, Schmidt JM, Lohavanijaya P. Isolation and structure of combretastatin. Can. J. Chem. 1982;60:1374–1376. [Google Scholar]
- Pettit GR, Singh SB, Niven ML, Hamel E, Schmidt JM. Isolation, structure, and synthesis of combretastatins A-1 and B-1, potent new inhibitors of tubulin assembly, derived from Combretum caffrum. J. Nat. Prod. 1987;50:119–131. doi: 10.1021/np50049a016. [DOI] [PubMed] [Google Scholar]
- Pinney KG, Jelinek C, Edvardsen K, Chaplin DJ, Pettit GR. The discovery and development of the combretastatins. In: Cragg GM, Kingston DGI, Newman DJ, editors. Anticancer Agents from Natural Products. CRC Taylor & Francis; Boca Raton, FL: 2005. pp. 23–46. [Google Scholar]
- Pisha E, Chai H, Lee I-S, Chagwedera TE, Farnsworth NR, Cordell GA, Beecher CWW, Fong HHS, Kinghorn AD, Brown DM, Wani MC, Wall ME, Heijken TE, Das Gupta TK, Pezzuto JM. Discovery of betulinic acid as a selective inhibitor of human melanoma that functions by induction of apoptosis. Nature (Med.) 1995;1:1046–1051. doi: 10.1038/nm1095-1046. [DOI] [PubMed] [Google Scholar]
- Popovich DG, Kitts DD. Structure-function relationship exists for ginsenosides in reducing cell proliferation and inducing apoptosis in the human leukemia (THP-1) cell line. Arch. Biochem. Biophys. 2002;406:1–8. doi: 10.1016/s0003-9861(02)00398-3. [DOI] [PubMed] [Google Scholar]
- Potterat O, Hamburger M. Natural products in drug discovery – concepts and approaches for tracking bioactivity. Curr. Org. Chem. 2006;10:899–920. [Google Scholar]
- Powell RG, Weisleder D, Smith CR. Antitumor alkaloids from Cephalotaxus harringtonia: structure and activity. J. Pharm. Sci. 1972;61:1227–1230. doi: 10.1002/jps.2600610812. [DOI] [PubMed] [Google Scholar]
- Puri SC, Verma V, Amna T, Qazi GN, Spiteller M. An endophytic fungus from Nothapodytes foetida that produces camptothecin. J. Nat. Prod. 2005;68:1717–1719. doi: 10.1021/np0502802. [DOI] [PubMed] [Google Scholar]
- Quintas-Cardama A, Kantarjian H, Garcia-Manero G, O'Brien S, Faderl S, Estrov Z, Giles F, Murgo A, Ladie N, Verstovsek S, Cortes J. Phase I/II study of subcutaneous homoharringtonine in patients with chronic myeloid leukemia who have failed prior therapy. Cancer. 2007;109:248–255. doi: 10.1002/cncr.22398. [DOI] [PubMed] [Google Scholar]
- Ramgolam V, Ang SG, Lai YH, Loh CS, Yap HK. Traditional Chinese medicines as immunosuppressive agents. Ann. Acad. Med. Singapore. 2000;29:11–16. [PubMed] [Google Scholar]
- Raynal NJ-M, Momparler L, Charbonneau M, Momparler RL. Antileukemic activity of genistein, a major isoflavone present in soy products. J. Nat. Prod. 2008;71:3–7. doi: 10.1021/np070230s. [DOI] [PubMed] [Google Scholar]
- Sashidhara KV, White KN, Crews P. A selective account of effective paradigms and significant outcomes in the discovery of inspirational marine natural products. J. Nat. Prod. 2009;71:588–603. doi: 10.1021/np800817y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saklani A, Kutty SK. Plant-derived compounds in clinical trials. Drug Disc. Today. 2008;13:161–171. doi: 10.1016/j.drudis.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Silasi D-A, Alvero AB, Rutherford TJ, Brown D, Mor G. Phenoxodiol: pharmacology and clinical experience in cancer monotherapy and in combination with chemotherapeutic drugs. Exp. Opin. Pharmacother. 2009;10:1059–1067. doi: 10.1517/14656560902837980. [DOI] [PubMed] [Google Scholar]
- Singh S. From exotic spice to modern drug? Cell. 2007;130:765–768. doi: 10.1016/j.cell.2007.08.024. [DOI] [PubMed] [Google Scholar]
- Sneader W. Drug Discovery: a History. John Wiley & Sons, Ltd.; Chichester, UK: 2005. [Google Scholar]
- Suffness M, Douros J. Current status of the NCI plant and animal program. J. Nat. Prod. 1982;45:1–14. doi: 10.1021/np50019a001. [DOI] [PubMed] [Google Scholar]
- Svoboda GH, Neuss N, Gorman M. Alkaloids of Vinca rosea (Catharanthus roseus). V. Preparation and characterization of alkaloids. J. Am. Pharm. Soc., Sci. Ed. 1959;48:659–666. doi: 10.1002/jps.3030481115. [DOI] [PubMed] [Google Scholar]
- Svoboda GH. Alkaloids of Vinca rosea (Catharanthus roseus). IX. Extraction and characterization of leurosidine and leurocristine. Lloydia. 1961;24:173–178. [Google Scholar]
- Tan GT, Gylenhaal C, Soejarto DD. Biodiversity as a source of anticancer drugs. Curr. Drug Targ. 2006;7:265–277. doi: 10.2174/138945006776054942. [DOI] [PubMed] [Google Scholar]
- The Rocky Mountain Blood and Marrow Transplant Program (RMBMTP) [October 2009];Initial patients treated in multinational phase 2/3 study of ceflatonin 09-20-2006. 2009 Further information available at : http://www.rockymountainbmt.com/news/Initial-Patients-Treated-in-Multinational-Phase-23-Study-of-Ceflatonin-4682.html.
- U.S. National Institutes of Health [October 2009];Evaluation of 20% betulinic acid ointment for treatment of dysplastic nevi (moderate to severe dysplasia) 2009 Further information available at: http://www.cancer.gov/search/ViewClinicalTrials.aspx?cdrid=494341&version=HealthProfessional&protocolsearchid=3664931.
- Vuorelaa P, Leinonenb M, Saikkuc P, Tammelaa P, Rauhad JP, Wennberge T, Vuorela H. Natural products in the process of finding new drug candidates. Curr. Med. Chem. 2004;11:1375–1389. doi: 10.2174/0929867043365116. [DOI] [PubMed] [Google Scholar]
- Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. Plant antitumor agents. VI. Isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J. Am. Chem. Soc. 1971;93:2325–2327. doi: 10.1021/ja00738a045. [DOI] [PubMed] [Google Scholar]
- Wall ME, Wani MC, Cook CE, Palmer KH, McPhail AI, Sim GA. Plant antitumor agents. I. The isolation and structure of camptothecin, a novel alkaloidal leukemia and tumor inhibitor from Camptotheca acuminata. J. Am. Chem. Soc. 1966;88:3888–3890. [Google Scholar]
- Xu RS. Dan-Shen (Salvia miltiorrhiza) – its Biology and Applications. Science Press; Beijing: 1990. [Google Scholar]
- Yeruva L, Pierre KJ, Elegbede A, Wang RC, Carper SW. Perillyl alcohol and perillic acid induced cell cycle arrest and apoptosis in non small cell lung cancer cells. Cancer Lett. 2007;257:216–226. doi: 10.1016/j.canlet.2007.07.020. [DOI] [PubMed] [Google Scholar]
- Young SL, Chaplin DJ. Combretastatin A4 phosphate: background and current clinical status. Expert. Opin. Invest. Drugs. 2004;13:171–1182. doi: 10.1517/13543784.13.9.1171. [DOI] [PubMed] [Google Scholar]
- Zhang J-S, Ding J, Tang Q-M, Li M, Zhao M, Lu L-J, Chen L-J, Yuan S-T. Synthesis and antitumor activity of novel diterpene quinone salvicine and the analogs. Bioorg. Med. Chem. Lett. 1999;9:2731–2736. doi: 10.1016/s0960-894x(99)00472-2. [DOI] [PubMed] [Google Scholar]
- Zhongyaoshijia [October 2009];2009 Further information available at http://www.zysj.com.cn/zhongyaocai/yaocai_q/qingdai.html#399.
- Zuo M, Li Y, Wang H, Zhou J, Li H, Liu H, Liu H, Xin H, Zhang S, Chen X. The antitumor activity of meisoindigo against human colorectal cancer HT-29 cells in vitro and in vivo. J. Chemother. 2008;20:728–733. doi: 10.1179/joc.2008.20.6.728. [DOI] [PubMed] [Google Scholar]