Abstract
Exogenous or endogenous β2-adrenergic receptor agonists enhance alveolar epithelial fluid transport via a cAMP-dependent mechanism that protects the lungs from alveolar flooding in acute lung injury. However, impaired alveolar fluid clearance is present in most of the patients with acute lung injury and is associated with increased mortality, although the mechanisms responsible for this inhibition of the alveolar epithelial fluid transport are not completely understood. Here, we found that transforming growth factor β1 (TGF-β1), a critical mediator of acute lung injury, inhibits β2-adrenergic receptor agonist-stimulated vectorial fluid and Cl− transport across primary rat and human alveolar epithelial type II cell monolayers. This inhibition is due to a reduction in the cystic fibrosis transmembrane conductance regulator activity and biosynthesis mediated by a phosphatidylinositol 3-kinase (PI3K)-dependent heterologous desensitization and down-regulation of the β2-adrenergic receptors. Consistent with these in vitro results, inhibition of the PI3K pathway or pretreatment with soluble chimeric TGF-β type II receptor restored β2-adrenergic receptor agonist-stimulated alveolar epithelial fluid transport in an in vivo model of acute lung injury induced by hemorrhagic shock in rats. The results demonstrate a novel role for TGF-β1 in impairing the β- adrenergic agonist-stimulated alveolar fluid clearance in acute lung injury, an effect that could be corrected by using PI3K inhibitors that are safe to use in humans.
Keywords: Channels/Chloride, Cytokines/TGF-β, G Proteins/Coupled Receptors (GPCR), Signal Transduction/Protein Kinases, Tissue/Organ Systems/Lung, Alveolar Fluid Clearance, β-adrenergic receptor
Introduction
Acute lung injury (ALI)3 is a clinical syndrome manifested by a rapid onset of respiratory failure associated with high mortality (1). ALI is characterized by increased permeability of the alveolar-capillary barrier, decreased surfactant function, and impaired alveolar fluid clearance (2). Importantly, a small subset of patients with ALI who retain maximal alveolar fluid clearance show better clinical outcome (3). Although significant efforts have been made to pharmacologically up-regulate alveolar fluid clearance to reverse the progression of lung injury, these approaches have not been successful (4). β2-Adrenergic receptor (β2AR) agonists have been shown to enhance alveolar epithelial fluid transport via a cAMP-dependent mechanism under physiological conditions (5–11) and in various experimental models of ALI (12–14) as well as in one small prospective study in ALI patients (15). However, one of the main limitations of this therapeutic approach is that β-adrenergic agonist hyporesponsiveness has been reported in some experimental models of ALI (16, 17), although none of the critical mediators of ALI have yet been shown to inhibit the β-adrenergic-stimulated fluid transport of the distal lung epithelium.
There is a growing body of experimental and clinical evidence that transforming growth factor β1 (TGF-β1) plays a central role in the early phase of ALI. First, the concentration of TGF-β1 is elevated in edema fluid from patients with acute lung injury (18), and active TGF-β1 is present early in the syndrome, detectable within 24 h of initial diagnosis (19). TGF-β1 has been associated with increased endothelial and epithelial permeability, leading to pulmonary edema (20, 21). We have also shown that increased TGF-β1 activity in the distal airspaces during ALI promotes alveolar edema by reducing distal airspace epithelial sodium and fluid clearance. This reduction occurred mainly by a loss of apical membrane α-ENaC expression in lung epithelial cells mediated through an ERK1/2-dependent inhibition of the α-ENaC promoter activity (22). Although we have shown that TGF-β1 inhibits basal and corticosteroid-stimulated alveolar fluid transport (22), the potential effect of TGF-β1 on the β-adrenergic-stimulated alveolar fluid transport is still unknown. Recent experimental evidence convincingly demonstrated in mice and humans a role for the cystic fibrosis transmembrane conductance regulator (CFTR) in the β2AR agonist-mediated stimulation of the alveolar epithelial fluid transport (23–29). Thus, the objective of this study was to examine the mechanisms by which TGF-β1 would inhibit the β2AR agonist-stimulated CFTR-dependent alveolar epithelial fluid transport.
EXPERIMENTAL PROCEDURES
In Vitro Studies
Reagents
All of the cell culture media were prepared by the University of California, San Francisco Cell Culture Facility using deionized water and analytical grade reagents. Epinephrine, terbutaline, ICI-118,551, nystatin, protease inhibitors, and IBMX were obtained from Sigma. (−)-[125I]iodocyanopindolol ([125I]ICYP) was purchased from PerkinElmer Life Sciences. 8-CPT-cAMP was purchased from Calbiochem. The CFTR inhibitor, CFTRinh-172, was a kind gift from Alan S. Verkman (30). The PI3K inhibitor PIK-90 was provided by Kevan M. Shokat (31). The GRK2 inhibitor was purchased from EMD Biosciences (San Diego, CA). PD 98059, an inhibitor of the kinase upstream of ERK1/2; SB203580, an inhibitor of the α- and β-isoforms of p38 MAPK; and SP600125, a reversible inhibitor of the c-Jun N-terminal kinase, were obtained from Calbiochem. Human recombinant TGF-β1 was obtained from R & D Systems (Minneapolis, MN). Soluble chimeric TGF-β type II receptor (TGF-β-scRII) was a generous gift from Gerald Horan (Biogen Idec, Cambridge, MA). Antibodies and phosphoantibodies for the β2AR were purchased from Santa Cruz Biotechnology (StressGen, Ann Arbor, MI). Antibodies and phosphoantibodies for Akt were purchased from Calbiochem. Antibodies for PI3Kα and GRK2 were purchased from Calbiochem. Antibody 217 for CFTR was purchased from the University of North Carolina Department of Biochemistry/Biophysics and Cystic Fibrosis Center (Chapel Hill, NC). Goat anti-mouse and goat anti-rabbit IRDye®-conjugated secondary antibodies were purchased from LI-COR Biosciences (Lincoln, NE). Cationic liposomes (FuGENE 6) were obtained from Roche Applied Science. The CFTR-luc plasmids were a kind gift from G. Stanley McKnight (University of Washington, Seattle, WA). The protein concentrations of cell lysates were determined using a Bio-Rad protein assay kit.
Cell Culture
Primary cultures of rat and human alveolar epithelial cells were used for the in vitro studies. Rat alveolar epithelial type II (ATII) cells were isolated as previously described (32, 33) with slight modifications. Briefly, the cells were isolated by elastase digestion followed by negative selection using four monoclonal antibodies against cell surface molecules expressed on rat macrophages (CD4/CD32/CD45/RMA) purchased from BD Biosciences-Pharmingen (San Diego, CA). These monoclonal antibodies were preincubated with Dynabeads M-450 (magnetic beads with sheep anti-mouse IgG; Dynal ASA, Oslo, Norway) in 0.1% bovine serum albumin/PBS. After removing unbound monoclonal antibodies, rat ATII cells were mixed with the bead suspension and rocked gently for 30 min at 4 °C. Unbound cells were isolated and plated on polycarbonate ‘s (Corning Costar Co., Cambridge, MA) with a 0.4-μm pore size. The cells were seeded at a concentration of 1.5 × 106 cells/cm2 in Dulbecco's modified Eagle's medium/H21 medium containing 10% low endotoxin fetal bovine serum, 1% penicillin and streptomycin and kept at 37 °C in a humidified 95% air, 5% CO2 environment. Twenty-four hours later, nonadherent epithelial cells were removed by washing with PBS and fresh medium added to the lower compartments of the Transwells, thus maintaining the ATII cell monolayers with an air-liquid interface on their apical side. After 72–96 h, the cells that formed confluent monolayers reaching a transepithelial electrical resistance greater than 1500 ohms·cm2 were used for experimentation.
Following approval of the University of California, San Francisco Committee on Human Research, human alveolar epithelial type II cells were isolated using a modification of methods previously described (34). Briefly, alveolar type II cells were isolated from human lungs that were not used by the Northern California Transplant Donor Network. Our studies indicate that these lungs were in good condition, both physiologically and pathologically (35). Cells were isolated after the lungs have been preserved for 4–8 h at 4 °C. A lobe of the human lung was selected that had no evidence of injury on the preharvest chest radiograph, could be normally inflated, and had no area of consolidation or hemorrhage. The pulmonary artery for this segment was perfused with 37 °C PBS solution, and the distal airspaces of a segmental bronchus was lavaged 10 times with 37 °C Ca2+- and Mg2+-free PBS solution containing 0.5 mm EGTA and EDTA. 60–90 ml of pancreatic porcine elastase (8 units/ml) diluted in a Ca2+- and Mg2+-free HBSS solution was instilled into the airspaces of 50 g of the chosen segment of lung tissue. The lung was incubated in a water bath for 30 min at 37 °C and minced finely in the presence of fetal bovine serum and DNase I (500 μg/ml). The cell-rich fraction was filtered sequentially through one-layer gauze, two-layer gauze, 150- and 30-μm nylon meshes. The cell suspension was then layered onto a discontinuous Percoll density gradient of 1.04–1.09 g/ml solution and centrifuged at 400 × g for 20 min to remove red blood cells. The cells that accumulated at the interface of the solution and the Percoll were a mixture of type II pneumocytes and alveolar macrophages. These cells were recovered by centrifugation at 200 × g for 10 min at 4 °C. The pellet was resuspended in Dulbecco's modified Eagle's medium containing 10% fetal calf serum. The cells were incubated in Dulbecco's modified Eagle's medium containing magnetic beads coated with an anti-CD-14 antibody (Dynabeads M/450 CD14; Dynal, Oslo, Norway) at 4 °C for 40 min under constant mixing to eliminate macrophages. The cell viability was assessed by trypan blue exclusion. The purity of isolated human alveolar type II cells was checked by Papanicolaou staining or by staining with anti-human type II cell antibody (obtained from Leland Dobbs, University of California, San Francisco), and the purity has consistently been more than 90%. Human alveolar type II cells were seeded on collagen I-coated Transwells at a density of 1 × 106 cells/cm2. The cells were grown in an air-liquid interface 72 h after seeding. Five days after the cells are seeded, the monolayer developed a transepithelial electrical resistance greater than 1500 ohms·cm2, as reported for rat ATII cell monolayers.
Cell Viability
The cell viability after exposure to different experimental conditions was measured by the Alamar Blue assay (36). The cell media were replaced by medium containing 10% Alamar Blue and placed at 37 °C in the cell incubator for 2–3 h. The medium was then collected and read on a plate reader at 570 nm.
Short Circuit Current Studies of Rat and Human ATII Cell Monolayers
Freshly isolated rat or human ATII cells (0.75 × 106) were seeded on polycarbonate Snapwell membranes (pore size, 0.4 μm; surface area, 1.13 cm2). The culture medium was changed daily. Hydrocortisone (10−7 m) and insulin-transferrin-Sel-G were added to the culture medium for human cells. The cells were grown in an air-liquid interface 48–72 h after seeding. At 120–144 h, the Snapwell inserts were mounted in an Ussing chamber system (Physiologic Instruments Inc., San Diego, CA). The bathing solution in the apical chamber contained 130 mm NaCl, 2.7 mm KCl, 1.5 mm KH2PO4, 1 mm CaCl2, 0.5 mm MgCl2, 10 mm Na-HEPES, and 10 mm glucose, pH 7.3. The bathing solution in the basolateral chamber was modified by substituting all of the NaCl with sodium gluconate and increasing the CaCl2 concentration to 2 mm to compensate for calcium buffering caused by gluconate. The basolateral membrane of the cells was permeabilized with 40 μm nystatin. Complete permeabilization was verified by adding ouabain (1 mm), a specific Na+/K+ ATPase blocker, which did not reduce the Isc level in the permeablized monolayer compared with intact monolayers. The measurements were performed at 37 °C. The hemichambers were connected to a VCC MC6 voltage clamp (Physiologic Instruments Inc., San Diego, CA) via Ag/AgCl electrodes and 1 m KCl agar bridges. Short circuit current (Isc) data were acquired by PowerLab (ADInstruments Inc., Colorado Springs, CO) and recorded in Chart software (ADInstruments Inc.).
Measurement of Net Fluid Transport across Rat ATII Cell Monolayers
Fluid transport across rat primary ATII cell monolayers was measured as described previously (22). Briefly, after freshly isolated rat ATII cells were plated on Transwells and grown in an air-liquid interface for 24 h, 150 μl of medium containing 0.5 μCi/ml [125I]albumin was added to the apical side of the monolayers, and the Transwells were placed in a humidified tent. Five minutes later, 20 μl of medium containing [125I]albumin were sampled from the apical side (initial sample). The Transwells were then incubated for 24 h at 37 °C with 100% humidity in a CO2 incubator. The medium in the upper and lower compartments of the Transwell were kept at the same level to avoid any effect of the hydrostatic pressure. Twenty-four hours later, a second 20-μl sample was removed from the apical side of the monolayers (final sample). The collected samples were weighed and counted in a γ-counter (Beckman). Fluid absorption = (1 − radioactivity in the initial sample/radioactivity in the final sample) × 100%. In the pilot experiments, we were able to detect a 2-fold increase in the fluid transport across these ATII cell monolayers after c-AMP stimulation (10−5 m forskolin + 4 × 10−4 m IBMX) and a 50% inhibition of the fluid transport after exposure to amiloride (10−4 m).
Measurement of cAMP
cAMP levels were measured in triplicate by a commercially available enzyme-linked immunosorbent assay (ELISA; from R & D Systems, Minneapolis, MN). The assays were performed according to the manufacturer's protocol in cell lysates. The sensitivity of the assay was 1.43 pmol/ml. There was no significant cross-reactivity or interference observed with the cAMP assay.
PKA Activity Assay
Active PKA was measured in triplicate by a commercially available solid phase ELISA from Assays Designs (Ann Arbor, MI). The assays were performed according to the manufacturer's protocol; with cell lysates. Relative kinase activity in the cell lysate = (average absorbance at 450 nm (sample) − average absorbance (blank))/quantity of crude protein used per assay.
Isolation of Plasma Membrane-enriched Fraction
The cells were scraped into with a hypotonic buffer (10 mm HEPES, 10 mm NaCl, 5 mm MgCl2, 1 mm dithiothreitol, protease inhibitors) and allowed to swell for 30 min at 4 °C with rotation. The cells were broken open with a Dounce apparatus using a loose fitting pestle and 20 strokes. The nuclei were pelleted by centrifugation at 1000 × g for 10 min at 4 °C. The supernatant was centrifuge at 15,000 × g for 15 min at 4 °C to pellet the enriched membrane fraction. This fraction was resuspended in Laemmli sample buffer and further analyzed by SDS-PAGE and Western blot. Western blot for calreticulin (an endoplasmic reticulum protein marker) was performed to ensure the purity of plasma membrane proteins (supplemental Fig. S1).
Western Blot Analyses
Western blot analyses from frozen lungs and cells homogenates were performed as described previously (37). After equal amounts of protein were loaded in each lane and separated by 10% SDS-PAGE, the proteins were transferred to Invitrogen iBlotTM polyvinylidene difluoride membranes (Invitrogen). The membranes were blocked for 1 h with Odyssey blocking buffer (LI-COR Biosciences), which was also used as primary and secondary antibodies incubation buffer. The primary antibodies were used at dilutions of 1:500 and 1:1000, incubated overnight at 4 °C. Near-infrared detection was used with the IRDye®-conjugated secondary antibodies (LI-COR Biosciences), which were either goat anti-mouse IRDye® 800CW or goat anti-rabbit IRDye® 680, used at 1:10,000 dilution and imaged at 84-μm resolution with the Odyssey infrared imaging system (LI-COR Biosciences). Quantification was performed with the LI-COR Biosciences analysis software.
Saturation Binding Experiments
The plasma membrane-enriched fractions (1 μg of membrane protein) from primary rat alveolar type II cells were incubated with different concentrations (0–160 pm) of the β-AR antagonist [125I]ICYP for 90 min at 37 °C in the following binding buffer: 50 mm Tris·HCl, 100 mm KCl, and 5 mm MgCl2, pH 7.4. Nonspecific binding was determined by displacement of ICYP binding with the specific β2-AR antagonist ICI-118,551 (100 μm). The reactions were stopped by dilution with 4 ml of ice-cold washing buffer (50 mm Tris·HCl, 154 mm NaCl, pH 7.4), followed by rapid filtration through presoaked Whatman GF/C glass fiber filters in a Millipore cell harvester (Millipore, Billerica, MA). The filters were washed three times with 4 ml of washing buffer. The radioactivity on the filters was counted with a γ-counter (PerkinElmer Life Sciences). We found that specific [125I]ICYP binding was saturable and of high affinity.
CFTR Promoter Reporter Cells and Luciferase Assay
Rat ATII cells were transiently transfected with a CFTR-promoter reporter vector (38), containing the luciferase gene subcloned downstream of the CFTR promoter (CFTR(wt)-luc). After rat ATII cells were plated on Transwells (0.5 × 106 cells/Transwell) and grown in an air-liquid interface for 24 h, the cells were transfected in triplicate by incubation with cationic liposomes (6 μl of FuGENE 6, 1 μg of DNA/Transwell). Forty-eight hours later, the cells were treated as indicated in the text. The cell extracts were prepared and analyzed for luciferase activity according to the manufacturer's instructions (Promega, Madison, WI) using a luminometer. Luciferase activity was corrected for total cellular protein and reported as a percentage of the control cells (cells that were transfected and treated with corresponding vehicles). The base-line luciferase activity values obtained after transfection with the empty vector were within the range of the background values measured in cells that were not transfected. The cells were also co-transfected with a β- galactosidase plasmid, and β-galactosidase activity was determined to verify that the efficiency of transfection was comparable within and between experiments.
Primers and Probes
Real time RT-PCR primers and probes (Table 1) were designed using Primer Express software (PE-Applied Biosystems, Warrington, UK). The TaqMan probes were labeled with a fluorophore reporter dye (6-carboxy-fluorescein, FAM) at the 5′ end and a quencher dye (black hole quencher; Biosearch Technologies, Inc.) at the 3′ end.
TABLE 1.
Real time RT-PCR primers and probes
| Designation | Sequence | GenBankTM accession number |
|---|---|---|
| Rat GAPDH | AB017801 | |
| Forward | 5′-CTGCCAAGTATGATGACATCAAGAA-3′ | |
| Reverse | 5′-AGCCCAGGATGCCCTTTAGT-3′ | |
| Probe | 5′-TCGGCCGCCTGCTTCACCA-3′ | |
| Rat CFTR | M89906 | |
| Forward | 5′-GCTCTGCCTCGCAGCAAC-3′ | |
| Reverse | 5′-CAGACTGTCTTCTTCTGCCTGGA-3′ | |
| Probe | 5′-TGATCACCGCCGGGCCCA-3′ | |
| Rat β2-AR | BC086538 | |
| Forward | 5′-TTCCAGGTGGCCAAAAGG-3′ | |
| Reverse | 5′-GCTGAGGTTTTGGGCATGAA-3′ | |
| Probe | 5′-TGCAGAAGATAGACAAATCCGAGGGCA-3′ | |
Quantitative Real Time RT-PCR
Quantitative real time RT-PCR was performed as described previously (39). Total RNA was extracted from rat or human ATII cells cultured after 4 days at the air-liquid interface, using the RNeasy Mini kit (Qiagen). 1 μg of total RNA was reverse transcribed using the Superscript first strand synthesis system (Invitrogen). The RT-PCRs were performed and analyzed using the ABI PRISM 7700 sequence detection system (PE-Applied Biosystems). Briefly, the RT-PCR was carried out in a 25-μl reaction mixture containing 1× TaqMan Universal PCR Master Mix (PE-Biosystems, Foster City, CA), 10 pmol of primers, 5 pmol of TaqMan probe, and an equivalent of 100 ng of total RNA for 40 cycles at 95 °C for 15 s and 60 °C for 1 min. The number of cycles to threshold (CT) of fluorescence detection was normalized to the CT of glyceraldehyde-3-phosphate dehydrogenase for each sample tested. The results are expressed as percentages in complementary DNA abundance compared with control. The percentage values were used for all statistical comparisons.
In Vivo Studies
Measurement of Alveolar Fluid Clearance after Hemorrhagic Shock
This protocol was approved by the University of California, San Francisco Committee on Animal Research and was performed as previously described (37) with the following modifications. Briefly, male Sprague-Dawley rats weighing 300–350 g were anesthetized with pentobarbital (60 mg/kg intraperitoneally), and anesthesia was maintained with 30 mg/kg of pentobarbital sodium every hour. An endotracheal tube (14 G) was inserted through a tracheotomy. Catheters (PE50) were inserted into both carotid arteries to monitor systemic arterial pressure, obtain blood samples, and withdraw blood for induction of prolonged hemorrhagic shock. The rats were ventilated with a constant volume pump (Harvard Apparatus, Holliston, MA) with an inspired oxygen fraction of 1.0, peak airway pressures of 8–12 cm H2O, supplemented with positive end expiratory pressure of 3 cm H2O. The respiratory rate was adjusted to maintain the PaCO2 between 35 and 40 mm Hg during the base-line period. After the surgery, the heart rate and systemic blood pressure were allowed to stabilize for 60 min. Hemorrhagic shock was induced by withdrawing blood from the carotid artery to maintain a mean systemic arterial pressure of 40–45 mm Hg for 60 min. This corresponded to the removal of 9–12 ml of blood. After 1 h of hemorrhagic shock, the rats were resuscitated with intravascular 4% albumin solution in 0.9% NaCl over 30 min to maintain a central venous pressure <8 mm Hg, as we have done before (16, 40). The volume of 4% albumin solution administered was twice the amount of blood withdrawn. Five hours after the onset of the hemorrhagic shock, alveolar fluid clearance (AFC) was determined in the absence of ventilation or blood flow by measuring the increase in protein tracer concentration (125I-labeled albumin, 1 μCi) in the lungs over a 30-min period using our previously described in situ model (22). For these experiments, we instilled 12 ml/kg of warmed, radioactive 5% albumin in 0.9% NaCl solution intratracheally, aspirated and reinstilled the solution three times, applied continuous positive airway pressure (8 cm H2O, 100% FIO2) to prevent alveolar collapse, and kept the animals at 37 °C body core temperature. The instillate, an initial sample (after aspiration and reinstillation), and a sample after 30 min were obtained and analyzed. The increase in protein concentration over 30 min has been shown to be a good estimate of the liquid volume removed from the distal air spaces of the lungs (22). In some experiments, the rats were pretreated with a soluble chimeric TGF-β type II receptor (2 mg/kg) or its vehicle that was injected once intraperitoneally 30 min before the hemorrhage. In other experiments, 5 mg/kg of PIK-90 or its vehicle was injected intraperitoneally 30 min before the hemorrhage and every 1.5 h, as well as added in the instillate (0.5 mg/kg) with or without epinephrine (10 μm).
Statistics
All of the data are summarized as the means ± S.E. One-way analysis of variance and the Fisher's exact t test were used to compare experimental with control groups. A p value of <0.05 was considered statistically significant. Saturation binding experiments were analyzed by nonlinear regression. The maximal number of ICYP binding sites (Bmax) and the equilibrium dissociation constant (KD) were calculated from saturation binding curves by nonlinear least squares curve fittings for one binding site. The goodness of the fit was determined by the F-ratio test. All of the fits were calculated with GraphPad Prism (La Jolla, CA).
RESULTS
TGF-β1 Inhibits β2AR Agonist-stimulated Vectorial Alveolar Epithelial Fluid Transport
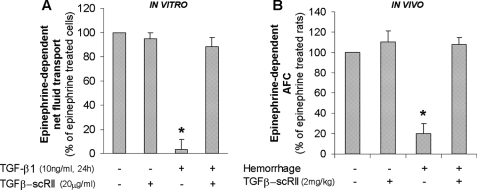
The first series of experiments was to determine whether TGF-β1 would decrease the β2AR-stimulated net vectorial fluid transport across primary rat alveolar epithelial ATII monolayers, using a recently described method to measure net fluid transport across these monolayers (34). Active TGF-β1 (10 ng/ml) significantly inhibited the epinephrine-dependent fluid absorption across primary rat alveolar epithelial ATII monolayers (Fig. 1A). We then verified our in vitro findings using an in vivo model of ALI induced by hemorrhagic shock that we have shown to be associated with hyporesponsiveness to β2AR agonist stimulation of the AFC (16). We found that the inhibition of TGF-β1 signaling by pretreatment with a TGF-β1-soluble receptor II (TGF-β-scRII) restored the epinephrine-dependent increase in AFC after hemorrhagic shock in rat (Fig. 1B). Taken together, this first series of experiments demonstrates that TGF-β1 inhibits the β2AR agonist-stimulated vectorial fluid transport across the distal lung epithelium.
FIGURE 1.
TGF-β1 decreases β2AR agonist-stimulated alveolar fluid transport across primary rat ATII cell monolayers and in an in vivo model of hemorrhagic shock in rat. A, active TGF-β1 decreases epinephrine-dependent net fluid transport across polarized rat ATII cell monolayers. Rat ATII cell monolayers cultured at an air-liquid interface for 4 days were exposed to TGF-β1 (10 ng/ml) for 24 h; fluid absorption was calculated by the concentration of [125I]albumin (control 4.1% ± 0.9 of instilled, epinephrine (20 μm)-treated 7.3% ±1.1). The results are the means ± S.E. of at least nine monolayers from three experiments. *, p < 0.05 from cell monolayers exposed to TGF-β1 vehicle. B, TGF-β1 blockade restores the epinephrine-stimulated AFC in rats after hemorrhagic shock. The rats were pretreated intraperitoneally with TGF-β-soluble receptor (TGF-β-scRII; 2 mg/kg) or with its vehicle 30 min prior to hemorrhage or sham surgery and fluid resuscitation (AFC control 7.6% ± 1.4/30 min, epinephrine-treated 14.2% ± 2/30 min). The results are the means ± S.E. of six experiments for each animal group. *, p < 0.05 from control group.
TGF-β1 Decreases β2AR Agonist-stimulated CFTR-specific Cl− Transport across Rat and Human Alveolar Epithelial Type II Cell Monolayers
We and others have previously reported a critical role for CFTR in the β2-adrenergic-mediated stimulation of alveolar epithelial fluid transport (23, 27). Thus, we first measured the epinephrine-stimulated vectorial Cl− transport across the apical membrane of rat ATII cells in Ussing chambers in the presence of a Cl− gradient after permeabilization of the basolateral membrane with nystatin (40 μm). Complete permeabilization was verified by adding ouabain (1 mm), a specific Na+/K+ ATPase blocker, which did not reduce the Isc level in the permeabilized monolayer compared with intact monolayers (data not shown). The threshold (0.5 μm) and the optimal dose of epinephrine (20 μm) were determined by dose-response experiments (supplemental Fig. S2). The results showed that the epinephrine-stimulated vectorial Cl− transport across the apical membrane was completely inhibited in the presence of a specific CFTR inhibitor, CFTRinh-172 (Fig. 2A). The inhibitory effect of TGF-β1 on Cl− transport was detected as early as a 5-min exposure to TGF-β1 (Fig. 2B) and was observed at a concentration as low as 3 ng/ml (Fig. 2C). Exposure to TGF-β1 (10 ng/ml) for 6 h resulted in a 50% decrease of the epinephrine-stimulated vectorial Cl− transport (Fig. 2C). A cell viability assay after TGF-β1 treatment (10–100 ng/ml) did not demonstrate an increase in cell death (data not shown). Because epinephrine stimulates both β1- and β2-adrenergic receptors, the experiments were repeated with terbutaline, a specific β2-adrenergic agonist. The results showed that the TGF-β1-mediated inhibition of the terbutaline-dependent CFTR specific Cl− transport was comparable with that reported for epinephrine-stimulated cell monolayers (Fig. 2, D and E). We validated our observation in rat alveolar epithelial type II cells by demonstrating that exposure to TGF-β1 (10 ng/ml) from 5 min to 6 h also inhibited up to 80% of the epinephrine-stimulated CFTR-specific apical Cl− transport in primary human ATII cell monolayers (Fig. 3A). This TGF-β1-dependent inhibition was observed at a concentration as low as 1 ng/ml (Fig. 3B). Taken together, this second series of experiments demonstrates that TGF-β1 inhibits the β2AR agonist-stimulated CFTR-specific Cl− transport across rat and human alveolar epithelia.
FIGURE 2.
TGF-β1 decreases β2AR agonist-stimulated CFTR-specific Cl− transport across primary rat ATII cell monolayers. Short circuit current was measured in Ussing chambers in the presence of a Cl− gradient after permeabilization of the basolateral membrane with nystatin (see “Experimental Procedures”). The total fraction of vectorial Cl− transport through the apical membrane of rat ATII cells stimulated with epinephrine (20 μm) or terbutaline (20 μm) was inhibited by the CFTR inhibitor, CFTRinh-172 (10 μm). A, TGF-β1 decreases the epinephrine-stimulated CFTR-specific Cl− absorption across the apical membrane of polarized rat ATII cells. A representative Ussing chamber recording (Isc) of polarized rat ATII cells treated or untreated with TGF-β1 is shown. B, TGF-β1 causes a time-dependent inhibition of the epinephrine-stimulated CFTR-specific Cl− absorption across the apical membrane of polarized rat primary ATII cells. Pretreatment with a TGF-β soluble receptor II (TGF-β-scRII) specifically blocks TGF-β1 inhibition. C, TGF-β1 causes a dose-dependent inhibition of the epinephrine-stimulated CFTR-specific Cl− absorption across the apical membrane of polarized rat ATII cells. D, TGF-β1 decreases the terbutaline-stimulated CFTR-specific Cl− absorption across the apical membrane of polarized rat ATII cells. A representative Ussing chamber recording (Isc) of polarized rat ATII cells treated or untreated with TGF-β1 is shown. E, TGF-β1 causes a time-dependent inhibition of the terbutaline-stimulated CFTR-specific Cl− absorption across the apical membrane of polarized rat primary ATII cells. Pretreatment with a TGF-β soluble receptor II (TGF-β-scRII) specifically blocks TGF-β1 inhibition. For all experiments, mean basal Isc was −11 ± 1.7 μA, mean epinephrine-treated Isc was −31 ± 2.1 μA, and mean terbutaline-treated Isc was −35 ± 1.8 μA in Cl− gradient conditions; the results are the means ± S.E. of at least 12 monolayers from four experiments. *, p < 0.05 from monolayers exposed to TGF-β1 vehicle.
FIGURE 3.
TGF-β1 decreases β2AR agonist-stimulated CFTR-specific Cl− transport across primary human ATII cell monolayers. The short circuit current was measured in Ussing chambers in the presence of a Cl− gradient after permeabilization of the basolateral membrane with nystatin (see “Experimental Procedures”). The total fraction of vectorial Cl− transport through the apical membrane of human ATII cells stimulated with epinephrine (20 μm) was inhibited by the CFTR inhibitor, CFTRinh-172 (10 μm) (not shown). A, TGF-β1 causes a time-dependent inhibition of the epinephrine-stimulated CFTR-specific Cl− absorption across the apical membrane of polarized human primary ATII cells. TGF-β-soluble receptor II (TGF-β-scRII) blocks specifically TGF-β1 inhibition. B, TGF-β1 causes a dose-dependent inhibition of the epinephrine-stimulated CFTR-specific Cl− absorption across the apical membrane of polarized human ATII cells. For the two series of experiments, the mean basal Isc was −17 ± 2 μA, and the mean epinephrine-treated Isc was −34 ± 2.2 μA; the results are the means ± S.E. of at least 12 monolayers from four experiments. *, p < 0.05 from monolayers exposed to the TGF-β1 vehicle.
Short Time Exposure (5–30 min) to TGF-β1 Induces a Heterologous Desensitization of the β2AR via a PI3K-dependent Mechanism
Several studies have shown that β2AR signaling in the distal lung epithelium is mediated by a cAMP- and PKA-mediated mechanism (41). We found that exposure to TGF-β1 significantly inhibited epinephrine-induced cAMP accumulation and PKA activity in rat ATII cell monolayers (Fig. 4, A and B). Interestingly, the inhibitory effect on PKA activity and on the epinephrine-stimulated chloride transport across the apical membrane of ATII cell monolayers was restored when cell monolayers were exposed to a cell-permeable cAMP analog (CPT-cAMP) (Fig. 4, C and D). TGF-β1 has been shown to activate multiple cell signaling pathways including but not limited to PI3K, MAPKs, and Smads. Because the activation of PI3K has been shown to play an important role in homologous desensitization and down-regulation of β2AR in cardiomyocytes (42) and TGF-β1 directly activates PI3K pathway in ATII cells (43), we pretreated ATII cell monolayers with PIK-90, a specific inhibitor of the PI3K. We first found that TGF-β1 (10 ng/ml for 30 min) induced the phosphorylation of the downstream kinase, Akt on serine 473 in rat ATII cell monolayers (Fig. 5A) and a translocation of PI3K (PI3Kα isoform) at the cell membrane (Fig. 5B). Furthermore, pretreatment with PIK-90 prevented the inhibitory effect of TGF-β1 on β2AR agonist-stimulated cAMP accumulation (Fig. 5C) and CFTR-specific alveolar Cl− transport across the apical membrane of rat alveolar type II cell monolayers (Fig. 5D), although TGF-β1 did not cause a decrease of the β2AR density at the cell membrane of ATII cells measured by saturation binding experiments (Fig. 5E). We next examined the effect of TGF-β1 on GRK2 activity, because PI3K activity has been shown to be required for the insulin-induced translocation of GRK2 at the plasma membrane of opossum kidney cells (44). First, we found that TGF-β1 induced the translocation of GRK2 to the cell membrane of ATII cell monolayers (Fig. 5F) and the subsequent phosphorylation of the β2AR on the serine residue in position 355 without the presence of a β-adrenergic agonist (Fig. 5G). We further validated the role of GRK2 in the TGF-β1-dependent decrease of β2AR agonist-stimulated CFTR-specific apical Cl− transport by inhibiting GRK2. In these experiments, inhibition of GRK2 preserved the β2AR-dependent Cl− transport through the apical membrane of rat ATII cells in the presence of TGF-β1 (Fig. 5H). Finally, previous studies have shown that PI3K recruits the clathrin adaptor protein AP-2 to β-arrestin that itself recruits phosphodiestesterase to the protein complex (42). Thus, in the last series of experiments, we examined whether IBMX, an inhibitor of the phosphodiesterases would inhibit the effect of TGF-β1 on the epinephrine-stimulated chloride transport across alveolar type II cell monolayers. We found that pretreatment with IBMX prevented the TGF-β1-mediated inhibition of the epinephrine-stimulated chloride transport (Fig. 5I). Taken together, this third series of experiments demonstrate that short exposure to TGF-β1 causes the desensitization of β2AR on the alveolar epithelium via a PI3K-dependent mechanism.
FIGURE 4.
Short exposure (30 min) to TGF-β1 induces a heterologous desensitization of the β2AR and inhibits the β2AR-dependent activation of the cAMP/PKA pathway in primary rat ATII cell monolayers. A and B, short exposure (30 min) to TGF-β1 significantly decreases epinephrine (20 μm)-dependent cAMP and PKA activation in polarized rat primary ATII cells. cAMP content (control 4.1 ± 1.3 pmol/ml, epinephrine-treated 12.7 ± 2.4 pmol/ml) and PKA activity were measured in cell lysates by ELISA (control 1.9 ± 0.5 ng of active PKA/μg of protein, epinephrine-treated 4 ± 0.9). C, short exposure (30 min) to TGF-β1 does not affect the CPT-cAMP-dependent PKA activation at any concentration studied in polarized rat primary ATII cells. PKA activity was measured in cell lysates by ELISA (control 1.8 ± 0.4 ng active PKA/μg of protein). D, pretreatment with CPT-cAMP prevents TGF-β1-dependent decrease in β2AR agonist-stimulated CFTR-specific alveolar Cl− transport across polarized rat primary ATII cell monolayers. Short circuit current was measured in Ussing chambers in the presence of a Cl− gradient after permeabilization of the basolateral membrane with nystatin (see “Experimental Procedures”). The mean basal Isc was −10 ± 1.9 μA, and the mean epinephrine-treated Isc was −29 ± 2.6 μA; CPT-cAMP-treated Isc was −26 ± 1.7 μA in Cl− gradient conditions. The results are the means ± S.E. of at least 12 monolayers from four experiments. *, p < 0.05 from monolayers exposed to TGF-β1 vehicle.
FIGURE 5.
Short exposure (30 min) to TGF-β1 decreases β2AR agonist-stimulated Cl− transport across primary rat ATII cell monolayers via a PI3K-dependent mechanism. A, short exposure (30 min) to TGF-β1 induces phosphorylation of Akt on serine 473 in polarized rat primary ATII cells. Phospho-Akt (Ser473) and total Akt protein expressions were measured by Western blot. B, short exposure (30 min) to TGF-β1 induces PI3Kα translocation to the plasma membrane in polarized rat primary ATII cells. Membrane PI3Kα and total PI3Kα protein expressions were measured by Western blot. C, pretreatment with PIK-90, a PI3K inhibitor, prevents the TGF-β1-mediated decrease in epinephrine-dependent cAMP in polarized rat primary ATII cells. cAMP content was measured in cell lysates by ELISA (control, 3.8 ± 1.2 pmol/ml; epinephrine-treated 11.9 ± 2.1, pmol/ml). D, PI3K inhibition (PIK-90, a specific PI3K inhibitor) prevents TGF-β1-dependent decrease in β2AR agonist-stimulated CFTR-specific alveolar Cl− transport across polarized rat primary ATII cell monolayers. Isc was measured in Ussing chambers. E, short exposure (30 min) to TGF-β1 does not affect β2AR density at the cell membrane measured by saturation binding experiments ([125I]ICYP) (control Bmax = 533.5 ± 19.08 fmol/mg). ATII cell membranes were incubated with increasing concentrations of ICYP for 90 min at 37 °C. Nonspecific binding was determined in the presence of ICI-118,551 (100 μm). The values are representative of three independent experiments each performed in duplicate. F, short exposure (5 min) to TGF-β1 induces GRK2 protein translocation to the plasma membrane in polarized rat primary ATII cells without exposure to a β2-adrenergic agonist. GRK2 membrane and total protein expressions were measured by Western blot. G, short exposure (5 min) to TGF-β1 induces phosphorylation of the β2AR on its serine residue in position 355 in polarized rat primary ATII cells. Total and phospho-β2AR (Ser355), protein expressions were measured by Western blot. H, GRK2 inhibitor prevents the TGF-β1 short exposure-mediated inhibition of the epinephrine-stimulated CFTR-specific Cl− absorption across the apical membrane of polarized rat primary ATII cells. Isc was measured in Ussing chambers. I, pretreatment with IBMX, a phosphodiesterase inhibitor (0.5 mm), prevents the TGF-β1 short exposure-induced decrease of the epinephrine-stimulated CFTR-specific Cl− absorption across the apical membrane of polarized rat primary ATII cells. Short circuit current was measured in Ussing chambers in the presence of a Cl− gradient after permeabilization of the basolateral membrane with nystatin (see “Experimental Procedures”). The mean basal Isc was −9 ± 2.2 μA, and the mean epinephrine-treated Isc was −29 ± 2.3 μA, in Cl− gradient conditions. For all experiments, the results are the means ± S.E. of at least 12 monolayers from four experiments. *, p < 0.05 from monolayers exposed to TGF-β1 vehicle. For Western blot experiments, one representative experiment is shown, and three additional experiments gave comparable results; densitometry analysis results are the means ± S.E. of four experiments. *, p < 0.05 from monolayers exposed to TGF-β1 vehicle.
Prolonged Exposure (6 h) to TGF-β1 Induces a Heterologous Down-regulation of the β2AR from the Cell Membrane via a PI3K-dependent Mechanism
Endocytosis of the β2AR, causing inhibition of its cell signaling, has been described after prolonged exposure to β-adrenergic agonists (42). Thus, we determined whether prolonged exposure to TGF-β1 would decrease β2AR expression in the plasma membrane by performing saturation binding experiments. The dissociation constant of [125I]ICYP (KD) was not affected after treatment with TGF-β1 (10 ng/ml, 6 h) (legend to Fig. 6A). However, we found that maximal binding (Bmax) was significantly decreased in TGF-β1 treated cells (Fig. 6A), indicating that 6 h of exposure to TGF-β1 (10 ng/ml) causes a decrease of β2AR expression at the cell membrane of ATII cell monolayers, an effect that was blocked by PIK-90, a specific blocker of PI3K (Fig. 6A). In contrast, exposure to TGF-β1 for 6 h at a concentration of 10 ng/ml had no effect on β2AR total protein expression nor on β2AR mRNA expression (supplemental Fig. S3). Furthermore, this down-regulation of the β2AR expression at the cell membrane of ATII cell monolayers was associated with an inhibition of the epinephrine-stimulated PKA activity (Fig. 6B) that was restored by treating these cell monolayers with CPT-cAMP (Fig. 6C). In addition, the prolonged inhibitory effect of TGF-β1 on the epinephrine-stimulated Cl− transport across the apical membrane of ATII cell monolayers was prevented by pretreatment with a PIK-90, a specific PI3K inhibitor (Fig. 6D). Prolonged exposure to TGF-β1 had no effect on CPT-cAMP-stimulated Cl− transport across the apical membrane of ATII cell monolayers (data not shown), indicating that the TGF-β1 prolonged inhibitory effect on Cl− transport is not downstream of cAMP generation.
FIGURE 6.
Prolonged exposure (6 h) to TGF-β1 inhibits the β2AR-dependent activation of the PKA and CFTR promoter activity, gene expression, and function via a PI3K-mediated down-regulation of the β2-adrenergic receptor at the cell membrane. A, prolonged exposure (6 h) to TGF-β1 decreases β2AR cell membrane density, measured by saturation binding experiments ([125I]ICYP). PI3K inhibition (PIK-90, a specific PI3K inhibitor) prevents TGF-β1-dependent decrease in β2AR plasma membrane density (control Bmax = 512.3 ± 17.28 fmol/mg; KD = 25.37 ± 3.087 pm; TGF-β1-treated Bmax = 305.9 ± 17.6 fmol/mg, KD = 25.23 ± 5.249 pm). ATII cell membranes were incubated with increasing concentrations of ICYP for 90 min at 37 °C. Nonspecific binding was determined in the presence of ICI-118,551 (100 μm). The values are representative of three independent experiments, each performed in duplicate. B, prolonged exposure (6 h) to TGF-β1 inhibits the epinephrine-dependent PKA activation in polarized rat primary ATII cells. PKA activity was measured in cell lysates by ELISA (control 2.1 ± 0.6 ng of active PKA/μg of protein, epinephrine-treated 4.7 ± 1.1). C, prolonged exposure (6 h) to TGF-β1 does not affect the CPT-cAMP-dependent PKA activation in polarized rat primary ATII cells. PKA activity was measured in cell lysates by ELISA (control 2.3 ± 0.7 ng of active PKA/μg of protein). D, PI3K inhibition (PIK-90, a specific PI3K inhibitor) prevents the TGF-β1-induced decrease in epinephrine-stimulated CFTR-specific Cl− absorption across the apical membrane of polarized rat primary ATII cells. Short circuit current was measured in Ussing chambers in the presence of a Cl− gradient after permeabilization of the basolateral membrane with nystatin (see “Experimental Procedures”). The mean basal Isc was −10 ± 2.6 μA, and the mean epinephrine-treated Isc was −30 ± 2.9 μA, in Cl− gradient conditions. E, prolonged exposure (6 h) to TGF-β1 inhibits the epinephrine-stimulated but not the CPT-cAMP-stimulated CFTR promoter activity in rat ATII cells. CFTR promoter activity was measured in rat ATII cells transiently transfected with a CFTR-promoter reporter vector (38), containing the luciferase gene subcloned downstream of the CFTR promoter (CFTR(wt)-luc). Control luciferase activity = 88.7 ± 4.9 (RLU/μg protein). F, prolonged exposure (6 h) to TGF-β1 decreases the epinephrine-stimulated but not the CPT-cAMP-stimulated CFTR mRNA expression in polarized rat primary ATII cells. PI3K inhibition (PIK-90, a specific PI3K inhibitor) also prevents the TGF-β1-mediated decrease in the epinephrine-stimulated CFTR mRNA expression. CFTR mRNA levels were measured by real time RT-PCR normalized with glyceraldehyde-3-phosphate dehydrogenase mRNA levels. G, prolonged exposures (6 and 24 h) to TGF-β1 decrease the epinephrine-dependent CFTR protein expression at the plasma membrane of polarized rat primary ATII cells. The results are the means ± S.E. of at least 12 monolayers from four experiments. *, p < 0.05 from monolayers exposed to TGF-β1 vehicle; **, p < 0.05 from monolayers exposed to TGF-β1. For Western blot experiments, one representative experiment is shown, three additional experiments gave comparable results; densitometry analysis results are the means ± S.E. of four experiments. *, p < 0.05 from monolayers exposed to TGF-β1 vehicle.
Previously published work has shown that cAMP plays a critical role in activating CFTR promoter and mRNA expression in lung epithelial cells (41). Thus, we also hypothesized that TGF-β1 prolonged exposure to TGF-β1 would result in a reduction in the PKA-dependent CFTR promoter activity and mRNA expression. The results showed that a 6-h exposure to TGF-β1 significantly inhibited CFTR promoter activity and mRNA expression, an effect prevented by pretreatment with CPT-cAMP or PIK-90, a specific PI3K inhibitor (Fig. 6, E and F), but not by pretreatment with MAPK inhibitors (supplemental Fig. S4). Importantly, none of the MAPK inhibitors affected the base-line CFTR mRNA expression (data not shown). CFTR protein levels at the plasma membrane were also decreased after 6-h exposures to TGF-β1, with a greater effect observed after 24-h exposure to TGF-β1 (Fig. 6G). In the last series of experiments, we determined whether PI3K signaling would mediate the TGF-β1-dependent inhibition of epinephrine-stimulated alveolar fluid clearance observed after severe hemorrhagic shock in rats (Fig. 1B). We found that there was an activation of PI3K signaling in the whole lungs of rats that underwent hemorrhagic shock (Fig. 7A). Furthermore, the inhibition of the PI3K pathway with PIK-90 restored a physiologic β2AR agonist-stimulated alveolar fluid clearance after severe hemorrhagic shock in rats (Fig. 7B). Taken together, this fourth series of experiments demonstrates that prolonged exposure to TGF-β1 caused endocytosis of β2AR and inhibition of cAMP generation in response to β-adrenergic agonist via the activation of the PI3K pathway in alveolar type II cell monolayers. In addition, these results also demonstrate a new role for TGF-β1 in inhibiting β2AR agonist-stimulated alveolar fluid transport via the activation of the PI3K pathway after severe hemorrhagic shock, a clinically relevant model of acute lung injury.
FIGURE 7.
PI3K inhibition prevents the TGF-β1-mediated decrease of the β2AR agonist-stimulated alveolar fluid transport in a rat model of hemorrhagic shock. A, hemorrhagic shock induces phosphorylation of Akt on serine 473 in rat lungs. Phospho-Akt (Ser473) and total Akt protein expressions were measured by Western blot. One representative experiment is shown, and three additional experiments gave comparable results; densitometry analysis results are the means ± S.E. of four experiments. *, p < 0.05 from control group. B, a specific PI3K inhibitor, PIK-90, restores the epinephrine stimulated AFC in rats after hemorrhagic shock. The rats were pretreated intraperitoneally with PIK-90 (5 mg/kg) or with its vehicle 30 min prior to hemorrhage or sham surgery and fluid resuscitation (AFC: control, 6.9% ±1.5/30 min; epinephrine-treated, 15.2% ±1.7/30 min). The results are the means ± S.E. of six experiments for each animal group. *, p < 0.05 from control group.
DISCUSSION
Experimental and clinical studies have shown that the cAMP-mediated stimulation of CFTR-dependent alveolar fluid clearance by endogenous or exogenous β2AR agonists is one of the major mechanisms that prevent the flooding of the airspaces after onset of ALI (5–15, 23). However, despite the massive release of catecholamines associated with several conditions that predispose to ALI, such as septic or hemorrhagic shock, impairment of alveolar fluid clearance is present in 80% of the patients with ALI. This impairment is associated with increased morbidity and mortality, although the mechanisms of the inhibition of the alveolar epithelial fluid transport observed in these patients are not completely understood (3). We have previously reported that TGF-β1, one of the critical mediators of ALI, inhibits basal vectorial fluid transport across the distal lung epithelium (22). We thus examined the effect of TGF-β1 on the β2AR agonist-stimulated alveolar epithelial fluid transport. We found that TGF-β1 inhibits vectorial fluid and Cl− transport across rat and human alveolar epithelial cell monolayers in response to an β-adrenergic agonist (epinephrine or terbutaline). TGF-β1 caused the inhibition of the β-adrenergic agonist-mediated cAMP generation and PKA activity that was corrected with a cell-permeable cAMP analog. However, only prolonged exposure to TGF-β1 (6 h) decreased the expression level of β2ARs at the cell membrane of alveolar epithelial cells but without affecting the total mRNA and protein expression of this receptor. Furthermore, down-regulation of the β2AR caused a significant inhibition of CFTR promoter activity and mRNA expression. Finally, consistent with our in vitro results, we found that TGF-β1 inhibited β2AR agonist-stimulated alveolar fluid clearance in an experimental model of ALI induced by hemorrhagic shock in rats.
Previous studies have reported that long term exposure to TGF-β1 inhibits cAMP-stimulated vectorial fluid transport and CFTR expression, although none of them has examined the effect of TGF-β1 on the β2AR-stimulated ion and fluid transport across the alveolar epithelium. First, for example, TGF-β1 down-regulated CFTR expression and cAMP-dependent current across epithelial cell monolayers from nasal polyps (45). Second, TGF-β1 decreased cAMP-dependent current across colonic epithelia via a decrease in CFTR mRNA and protein expression (46, 47). Third, TGF-β1 decreased adenylylcyclase mRNA expression in pulmonary artery smooth muscle cells (48). Finally, TGF-β1 caused an inhibition of β2AR transcription in human embryonic lung fibroblast cell line (49) and in human tracheal smooth muscle cells (50). In contrast, our results show for the first time that TGF-β1 causes a rapid inhibition of the β2AR agonist-stimulated CFTR-dependent Cl− and fluid transport via a heterologous desensitization followed by a down-regulation of the β2AR from the cell membrane in rat and human primary cultures of alveolar epithelial type II cells. These results are of importance because the chloride channel, CFTR, has been shown to play an important role in the β2AR agonist-dependent stimulation of alveolar epithelial fluid transport (23–29). Interestingly, results from our in vitro experiments measuring the transepithelial fluid transport across ATII cell monolayers for 24 h as well as from our in vivo experimental model of hemorrhagic shock in rats (6 h) demonstrated that TGF-β1 inhibited more than 80–90% of the β2AR agonist-dependent stimulation of transepithelial fluid transport (Fig. 1), a value much greater than the TGF-β1-mediated inhibition of chloride transport in ATII cell monolayers (40–50%) (Fig. 2). Previous studies have shown that both the chloride channel CFTR and the sodium channel ENaC present in the alveolar epithelium are under β-adrenergic control (41). Thus, although we found that short exposure to TGF-β1 (5–30 min) does not inhibit the epinephrine-stimulated amiloride-sensitive Isc (data not shown), our results suggest that long term (6–24 h) exposure to TGF-β1 likely inhibits both the chloride channel CFTR and the sodium channel ENaC, explaining why we observed a near complete inhibition of β2AR agonist-dependent stimulation of alveolar epithelial fluid transport in these experiments.
By which mechanism(s) does exposure to TGF-β1 affect β2AR agonist-stimulated CFTR-dependent Cl− and fluid transport across the distal lung epithelium? TGF-β1 is known to activate multiple cell signaling pathways including, but not limited to Smads, MAPKs, and PI3K. In the present study, we found that the PI3K pathway was implicated in the TGF-β1-mediated heterologous desensitization and later down-regulation of the β2AR in alveolar epithelial cells. First, TGF-β1 inhibited cAMP generation in response to β-adrenergic agonist via the activation of the PI3K. Second, long term exposure to TGF-β1 (6 h) caused the down-regulation of the β2AR from the cell membrane of ATII cells without affecting the expression of total β2AR protein, an effect inhibited by a specific PI3K inhibitor. Previous studies have shown that the PI3K pathway is implicated in the homologous desensitization of the β2AR in cardiomyocytes. In particular, investigators have reported that the PI3K interacts with GRK2 via its phosphoinositide kinase (PIK) homology domain and enhances the translocation of GRK2 at the cell membrane (42), an effect that we observed in the present study (Fig. 5F). Furthermore, lipid and protein kinase activities of PI3K are involved in the β-arrestin-mediated endocytic process of the β2AR that is associated with a homologous down-regulation of this receptor from the cell membrane (51). Third, we found that the inhibition of the β-adrenergic agonist stimulation of Cl− transport by short term exposure to TGF-β1 was reversed by a pretreatment with IBMX, a phosphodiesterase inhibitor. This result is in accordance with those of a previous study indicating that PI3K recruits the clathrin adaptor protein AP-2 to β-arrestin as well as phosphodiestesterase to the β2AR protein complex (42). Furthermore, phosphodiesterase activity has been shown to play a major role in controlling cAMP expression in response to the activation of β2AR signaling (52). Fourth, long term exposure (6 h) to TGF-β1 caused an inhibition of the β2AR-dependent cAMP-mediated stimulation of CFTR transcription that was reversed by pretreatment with a specific PI3K inhibitor. Fifth, PI3K pathway blockade restored a physiologic β2AR agonist-stimulated alveolar fluid clearance (driven by both ENaC and CFTR on the apical membrane) that was inhibited (80–90%) by TGF-β1 in an experimental model of ALI induced by hemorrhagic shock in rats. Finally, TGF-β1 triggers distinct signaling cascades including the Smad signaling and also Smad-independent pathways, in particular the MAPK pathways, the PI3K/Akt pathway, and the NF-κB pathway (53). Although we have shown that TGF-β1 activates ERK1/2, p38, and c-Jun N-terminal kinase (JNK) MAPK in rat ATII cell monolayers (22) and that TGF-β1-dependent activation of ERK1/2 inhibited basal alveolar epithelial Na+ transport through ENaC, we did not find here any significant implication of the MAPKs in the TGF-β1-dependent inhibition of the β2AR agonist-stimulated alveolar epithelial fluid transport. Taken together, our results indicate for the first time that the PI3K pathway plays a critical role in mediating TGF-β1-dependent inhibition of the β2AR agonist-stimulated alveolar epithelial fluid transport via heterologous desensitization and followed by down-regulation of the β2AR in alveolar epithelium.
In summary, we found that TGF-β1, a critical mediator of ALI, inhibits β2AR agonist-stimulated fluid transport across rat and human alveolar epithelia via a reduction of CFTR activity and biosynthesis. This reduction is mediated by a PI3K-dependent desensitization and later down-regulation of the β2AR from the cell membrane associated with an inhibition of cAMP generation normally observed in response to β2AR agonist stimulation. Finally, consistent with our in vitro results, we found that the PI3K pathway blockade restored a physiologic β2AR agonist-stimulated alveolar fluid clearance that was inhibited by TGF-β1 in an experimental model of ALI induced by hemorrhagic shock in rats.
The results presented here may have clinical significance for patients with ALI. Indeed, a recent phase III multicenter trial conducted by the acute respiratory distress syndrome network group and sponsored by the National Institutes of Health that aimed to study the effect of a β-adrenergic agonist on lung function and outcome in patients with ALI was suspended for futility.4 Our results provide a first possible explanation for the lack of success of this clinical trial and suggest a potential combined therapy including β-adrenergic agonist and specific PI3K inhibitors. Indeed, this study reveals a novel role for TGF-β1 in promoting alveolar flooding in ALI, an effect that could be antagonized by PI3K inhibitors that have already entered clinical trials for cancer therapy, inflammation, and coronary heart diseases (54).
Supplementary Material
Acknowledgments
We thank G. Stanley McKnight (CFTR promoter luciferase constructs) and Alan S. Verkman (CFTR inhibitor, CFTRinh-172) for the generous gifts.
This work was supported, in whole or in part, by National Institutes of Health Grants P50HL074005, Project 4 (to J.-F. P.), HL-51854 and HL-51856 (to M. A. M.), and T32 GM008440 (to J. R.). This work was also supported by a American Lung Association Senior Research Training Fellowship (to J. R.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S4.
M. A. Matthay, personal communication.
- ALI
- acute lung injury
- TGF
- transforming growth factor
- CFTR
- cystic fibrosis transmembrane conductance regulator
- PI3K
- phosphatidylinositol 3-kinase
- β2AR
- β2-adrenergic receptor
- ERK
- extracellular signal-regulated kinase
- IBMX
- isobutylmethylxanthine
- ICYP
- iodocyanopindolol
- MAPK
- mitogen-activated protein kinase
- ATII
- alveolar epithelial type II
- PBS
- phosphate-buffered saline
- ELISA
- enzyme-linked immunosorbent assay
- PKA
- cAMP-dependent protein kinase
- RT
- reverse transcription
- AFC
- alveolar fluid clearance
- ENaC
- epithelial sodium channel
- GRK
- G-protein-coupled receptor kinase.
REFERENCES
- 1.Rubenfeld G. D., Caldwell E., Peabody E., Weaver J., Martin D. P., Neff M., Stern E. J., Hudson L. D. (2005) N. Engl. J. Med. 353, 1685–1693 [DOI] [PubMed] [Google Scholar]
- 2.Ware L. B., Matthay M. A. (2000) N. Engl. J. Med. 342, 1334–1349 [DOI] [PubMed] [Google Scholar]
- 3.Ware L. B., Matthay M. A. (2001) Am. J. Respir. Crit. Care Med. 163, 1376–1383 [DOI] [PubMed] [Google Scholar]
- 4.Calfee C. S., Matthay M. A. (2007) Chest 131, 913–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berthiaume Y. (1991) J. Appl. Physiol. 70, 2490–2497 [DOI] [PubMed] [Google Scholar]
- 6.Berthiaume Y., Staub N. C., Matthay M. A. (1987) J. Clin. Invest. 79, 335–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Norlin A., Finley N., Abedinpour P., Folkesson H. G. (1998) Am. J. Physiol. 274, L235–L243 [DOI] [PubMed] [Google Scholar]
- 8.Icard P., Saumon G. (1999) Am. J. Physiol. 277, L1232–L1238 [DOI] [PubMed] [Google Scholar]
- 9.Fukuda N., Folkesson H. G., Matthay M. A. (2000) J. Appl. Physiol. 89, 672–679 [DOI] [PubMed] [Google Scholar]
- 10.Sakuma T., Folkesson H. G., Suzuki S., Okaniwa G., Fujimura S., Matthay M. A. (1997) Am. J. Respir Crit. Care Med. 155, 506–512 [DOI] [PubMed] [Google Scholar]
- 11.Sakuma T., Tuchihara C., Ishigaki M., Osanai K., Nambu Y., Toga H., Takahashi K., Ohya N., Kurihara T., Matthay M. A. (2001) J. Appl. Physiol. 90, 10–16 [DOI] [PubMed] [Google Scholar]
- 12.Su X., Robriquet L., Folkesson H. G., Matthay M. A. (2006) Am. J. Physiol. Lung. Cell Mol. Physiol. 290, L769–L776 [DOI] [PubMed] [Google Scholar]
- 13.McAuley D. F., Frank J. A., Fang X., Matthay M. A. (2004) Crit. Care Med. 32, 1470–1476 [DOI] [PubMed] [Google Scholar]
- 14.Pittet J. F., Wiener-Kronish J. P., McElroy M. C., Folkesson H. G., Matthay M. A. (1994) J. Clin. Invest. 94, 663–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perkins G. D., McAuley D. F., Thickett D. R., Gao F. (2006) Am. J. Respir Crit Care Med. 173, 281–287 [DOI] [PubMed] [Google Scholar]
- 16.Modelska K., Matthay M. A., Brown L. A., Deutch E., Lu L. N., Pittet J. F. (1999) Am. J. Physiol. 276, L844–L857 [DOI] [PubMed] [Google Scholar]
- 17.Lee H., Pespeni M., Roux J., Dennery P. A., Matthay M. A., Pittet J. F. (2005) FASEB J. 19, 287–289 [DOI] [PubMed] [Google Scholar]
- 18.Hamacher J., Lucas R., Lijnen H. R., Buschke S., Dunant Y., Wendel A., Grau G. E., Suter P. M., Ricou B. (2002) Am. J. Respir. Crit. Care Med. 166, 651–656 [DOI] [PubMed] [Google Scholar]
- 19.Fahy R. J., Lichtenberger F., McKeegan C. B., Nuovo G. J., Marsh C. B., Wewers M. D. (2003) Am. J. Respir. Cell Mol. Biol. 28, 499–503 [DOI] [PubMed] [Google Scholar]
- 20.Pittet J. F., Griffiths M. J., Geiser T., Kaminski N., Dalton S. L., Huang X., Brown L. A., Gotwals P. J., Koteliansky V. E., Matthay M. A., Sheppard D. (2001) J. Clin. Invest. 107, 1537–1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hurst V., IV, Goldberg P. L., Minnear F. L., Heimark R. L., Vincent P. A. (1999) Am. J. Physiol. 276, L582–L595 [DOI] [PubMed] [Google Scholar]
- 22.Frank J., Roux J., Kawakatsu H., Su G., Dagenais A., Berthiaume Y., Howard M., Canessa C. M., Fang X., Sheppard D., Matthay M. A., Pittet J. F. (2003) J. Biol. Chem. 278, 43939–43950 [DOI] [PubMed] [Google Scholar]
- 23.Mutlu G. M., Adir Y., Jameel M., Akhmedov A. T., Welch L., Dumasius V., Meng F. J., Zabner J., Koenig C., Lewis E. R., Balagani R., Traver G., Sznajder J. I., Factor P. (2005) Circ. Res. 96, 999–1005 [DOI] [PubMed] [Google Scholar]
- 24.Jiang X., Ingbar D. H., O'Grady S. M. (1998) Am. J. Physiol. 275, C1610–C1620 [DOI] [PubMed] [Google Scholar]
- 25.Nielsen V. G., Duvall M. D., Baird M. S., Matalon S. (1998) Am. J. Physiol. 275, L1127–L1133 [DOI] [PubMed] [Google Scholar]
- 26.Jiang X., Ingbar D. H., O'Grady S. M. (2001) J. Membr. Biol. 181, 195–204 [DOI] [PubMed] [Google Scholar]
- 27.Fang X., Fukuda N., Barbry P., Sartori C., Verkman A. S., Matthay M. A. (2002) J. Gen. Physiol. 119, 199–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fang X., Song Y., Hirsch J., Galietta L. J., Pedemonte N., Zemans R. L., Dolganov G., Verkman A. S., Matthay M. A. (2006) Am. J. Physiol. Lung. Cell Mol. Physiol. 290, L242–L249 [DOI] [PubMed] [Google Scholar]
- 29.Leroy C., Privé A., Bourret J. C., Berthiaume Y., Ferraro P., Brochiero E. (2006) Am. J. Physiol. Lung. Cell Mol. Physiol. 291, L1207–L1219 [DOI] [PubMed] [Google Scholar]
- 30.Ma T., Thiagarajah J. R., Yang H., Sonawane N. D., Folli C., Galietta L. J., Verkman A. S. (2002) J. Clin. Invest. 110, 1651–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knight Z. A., Gonzalez B., Feldman M. E., Zunder E. R., Goldenberg D. D., Williams O., Loewith R., Stokoe D., Balla A., Toth B., Balla T., Weiss W. A., Williams R. L., Shokat K. M. (2006) Cell 125, 733–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dobbs L. G. (1990) Am. J. Physiol. 258, L134–L147 [DOI] [PubMed] [Google Scholar]
- 33.Dobbs L. G., Gonzalez R., Williams M. C. (1986) Am. Rev. Respir Dis. 134, 141–145 [DOI] [PubMed] [Google Scholar]
- 34.Fang X., Song Y., Zemans R., Hirsch J., Matthay M. A. (2004) Am. J. Physiol. Lung. Cell Mol. Physiol. 287, L104–L110 [DOI] [PubMed] [Google Scholar]
- 35.Ware L. B., Wang Y., Fang X., Warnock M., Sakuma T., Hall T. S., Matthay M. (2002) Lancet 360, 619–620 [DOI] [PubMed] [Google Scholar]
- 36.Tiballi R. N., He X., Zarins L. T., Revankar S. G., Kauffman C. A. (1995) J. Clin. Microbiol. 33, 915–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pittet J. F., Lu L. N., Morris D. G., Modelska K., Welch W. J., Carey H. V., Roux J., Matthay M. A. (2001) J. Immunol. 166, 6301–6310 [DOI] [PubMed] [Google Scholar]
- 38.McDonald R. A., Matthews R. P., Idzerda R. L., McKnight G. S. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 7560–7564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roux J., Kawakatsu H., Gartland B., Pespeni M., Sheppard D., Matthay M. A., Canessa C. M., Pittet J. F. (2005) J. Biol. Chem. 280, 18579–18589 [DOI] [PubMed] [Google Scholar]
- 40.Modelska K., Matthay M. A., McElroy M. C., Pittet J. F. (1997) Am. J. Physiol. 273, L305–L314 [DOI] [PubMed] [Google Scholar]
- 41.Mutlu G. M., Factor P. (2008) Am. J. Respir. Cell Mol. Biol. 38, 127–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naga Prasad S. V., Laporte S. A., Chamberlain D., Caron M. G., Barak L., Rockman H. A. (2002) J. Cell Biol. 158, 563–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bakin A. V., Tomlinson A. K., Bhowmick N. A., Moses H. L., Arteaga C. L. (2000) J. Biol. Chem. 275, 36803–36810 [DOI] [PubMed] [Google Scholar]
- 44.Banday A. A., Fazili F. R., Lokhandwala M. F. (2007) Am. J. Physiol. Renal Physiol. 293, F877–F884 [DOI] [PubMed] [Google Scholar]
- 45.Prulière-Escabasse V., Fanen P., Dazy A. C., Lechapt-Zalcman E., Rideau D., Edelman A., Escudier E., Coste A. (2005) Am. J. Physiol. Lung Cell Mol. Physiol. 288, L77–L83 [DOI] [PubMed] [Google Scholar]
- 46.Howe K., Gauldie J., McKay D. M. (2002) Am. J. Physiol. Cell Physiol. 283, C1667–C1674 [DOI] [PubMed] [Google Scholar]
- 47.Howe K. L., Wang A., Hunter M. M., Stanton B. A., McKay D. M. (2004) Exp. Cell Res. 298, 473–484 [DOI] [PubMed] [Google Scholar]
- 48.El-Haroun H., Bradbury D., Clayton A., Knox A. J. (2004) Circ. Res. 94, 353–361 [DOI] [PubMed] [Google Scholar]
- 49.Mak J. C., Rousell J., Haddad E. B., Barnes P. J. (2000) Naunyn-Schmiedebergs Arch. Pharmacol. 362, 520–525 [DOI] [PubMed] [Google Scholar]
- 50.Nogami M., Romberger D. J., Rennard S. I., Toews M. L. (1994) Am. J. Physiol. 266, L187–L191 [DOI] [PubMed] [Google Scholar]
- 51.Perrino C., Naga Prasad S. V., Schroder J. N., Hata J. A., Milano C., Rockman H. A. (2005) Circulation 111, 2579–2587 [DOI] [PubMed] [Google Scholar]
- 52.Bruss M. D., Richter W., Horner K., Jin S. L., Conti M. (2008) J. Biol. Chem. 283, 22430–22442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gordon K. J., Blobe G. C. (2008) Biochim. Biophys. Acta 1782, 197–228 [DOI] [PubMed] [Google Scholar]
- 54.Marone R., Cmiljanovic V., Giese B., Wymann M. P. (2008) Biochim. Biophys. Acta 1784, 159–185 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.