Abstract
In many cell types, translation can be regulated by a redistribution of translation initiation factors to actin-based cytoskeletal compartments that contain bound mRNAs. This process is evoked by extracellular signals and is regulated by determinants of cytoskeletal organization, such as integrins. In the present experiments, we provide evidence that similar mechanisms regulate local translation in dendrites during synaptic plasticity. Treatment of various neuronal preparations with the brain-derived neurotrophic factor (BDNF) resulted in redistribution of the critical eukaryotic initiation factor 4E (eIF4E) to an mRNA granule-rich cytoskeletal fraction isolated from detergent-solubilized samples. eIF4E linkage to cap structures mediates the recruitment of other translation factors in the initiation of translation events. Immunoprecipitation studies confirmed that eIF4E associates with mRNA granules and that BDNF increased this association. BDNF-induced redistribution of eIF4E was blocked by preincubation with either a peptide (GRGDSP) that inhibits integrin-matrix interactions or by a high concentration (20 μM) of the F-actin depolymerizing agent latrunculin A. Immunohistochemical studies in cultured neurons demonstrated that BDNF facilitated translocation of eIF4E into dendritic spines. Together, the findings suggest that BDNF regulates translation in dendrites by altering the localization of eIF4E relative to cytoskeletally bound mRNA granules. Integrins, which are known to be essential for stabilizing certain forms of synaptic plasticity, may be critical regulators of local translation events at synapses.
Many observations suggest that reciprocal interactions between synaptic structure and local translation in dendrites may be important for stabilizing long-term changes in synaptic efficacy. Primary among these is the observation that translation of dendritically localized mRNAs is required for the stable expression of at least three forms of plasticity at glutamatergic synapses: long-term potentiation (LTP, refs. 1-3), long-term depression (LTD, ref. 4), and synaptic enhancement induced by brain-derived neurotrophic factor (BDNF, refs. 5 and 6). Local translation may stabilize efficacy states by providing proteins involved in creating persistent changes in the morphology of dendritic spines (1, 7-11). Such changes are a major correlate of LTP expression (12) and may also occur in unique ways with LTD (11, 13, 14) and signaling through BDNF receptors (15-17).
Observations in nonneuronal systems suggest that structural changes may reciprocally regulate local translation through specific effects on the activity and distribution of components of the translation machinery. Translation factors, ribosomal subunits, and mRNAs are associated with F-actin (18-20), and it has been proposed that their ordered distribution facilitates translation initiation and elongation (21, 22). This ordered distribution of translation factors suggests that changes in the state of the actin-based cytoskeleton can affect translation. Consistent with this idea, cellular events that involve reorganization of microfilaments result in changes in the distribution of translation factors and mRNAs, as well as in the rates and profiles of protein synthesis (23-26). It follows that molecules governing the organization of the actin cytoskeleton may also regulate translation. In particular, integrins, which provide highly modifiable linkages between the extracellular matrix and the actin-based cytoskeleton, have been shown to regulate translation by controlling redistribution of ribosomes, mRNA, and eIF4E (23, 24). Redistribution of eIF4E may be particularly important because this factor affects the translation of many mRNAs by binding to the 5′ cap structure and mediating the recruitment of other factors and 40S ribosomal subunits.
Local translation in neurons during synaptic plasticity may involve similar mechanisms. Dendritic spines contain high concentrations of F-actin (27-29), and pharmacological perturbation of actin dynamics impairs LTP (30, 31), causing it to decay at a rate that closely matches the effects of translation inhibitors (32). Recent data suggest that dendritically targeted mRNAs, transported in the form of granules along microtubules, may dock to the actin-based cytoskeleton in dendrites (19, 33-35) and move into spines during intense synaptic activity (36). Translation factors are present in dendrites (37-40), and polysomes, often observed at the base of spines (41), seem to move into spine heads during LTP (8). Given evidence that mRNA granules are translationally silent during transport (33, 42) and gain essential factors on binding to F-actin (33), synaptic microfilaments could mediate site-specific associations of granules with translation factors and ribosomes. Axospinous glutamatergic synapses also contain several integrin subunits (43-46) that may regulate F-actin organization. Antagonism of integrins during or shortly after LTP induction reveals that they also play a necessary role in its stabilization (43, 47). The hypothesis thus arises that integrin-dependent rearrangements of the actin cytoskeleton may regulate translation during plasticity by controlling the association of initiation factors with mRNA granules.
In the present study, we monitored the distribution of eIF4E within hippocampal and cortical neurons that had been treated with BDNF. BDNF signaling is required for the production of stable LTP (48, 49), and application of the neurotrophin alone induces a form of synaptic potentiation in hippocampus that depends on local protein synthesis (6, 50). It has been directly demonstrated that BDNF enhances eIF4E activity (51) and induces local translation of plasticity-related mRNAs in cultured neurons (5) and synaptoneurosomes (SNS, ref. 52), two of the preparations used in the present study. We observed that BDNF treatment induces translocation of eIF4E to a compartment that is rich in mRNA granules. This effect depended both on the integrity of actin filaments and on the status of integrins. Further experiments suggested that eIF4E was redistributed to mRNA granules that were bound to synaptic actin filaments. The data are discussed in terms of their relevance to processes underlying synaptic plasticity.
Methods
Cultures and SNS. Hippocampal slice cultures (11) and dissociated hippocampal neurons (53) were prepared as previously described and maintained in culture for at least 10 days and 3 weeks, respectively, before use. Cortical neurons were plated as mixed cultures onto poly-l-lysine 10-cm dishes in 10% FCS plus DMEM and were then maintained in neurobasal medium with B27 and 2 mM glutamine. SNS were prepared according to the methods of Hollingsworth (54) as described (52). The resulting particulates were resuspended in 5-10 ml of an oxygenated medium (35°C) containing 127 mM NaCl, 3 mM KCl, 1.25 mM KH2PO4, 10 mM glucose, 26 mM NaHCO3, 2.5 mM MgCl2, 2 mM CaCl2, and 0.5 mM ascorbate.
Treatments. Pharmacological treatments of slice cultures and dissociated neurons were initiated by addition of fresh, preequilibrated media containing the following reagents as described in the text (final concentration): BDNF (50 ng/ml, Sigma), Lat A (0.2, 2, and 20 μM, Calbiochem), GRGDSP (250 μM, Calbiochem), Mn2+/EGTA (100 μM/5 mM). Treatments of SNS were initiated by addition of the above reagents from stock solutions directly into 500-μl aliquots of SNS. All treatments lasted 30 min.
Cytoskeletal Fractionation. Methods for the crude fractionation of the actin-based cytoskeleton were adapted from Fox (55). Cortical microfilaments were obtained by low-speed centrifugation (15,000 × g for 4 min), and membrane microfilaments were obtained by high-speed centrifugation (100,000 × g for 2.5 h) of the low-speed supernatant. After treatments, cultures were harvested on ice in 0.3 M sucrose, 20 mM Hepes, 2 mM EGTA, 20 mM NaF, 200 μM Na3VO4, and complete protease inhibitor mixture (Roche Biochemicals), pH 7.4. Cultures were then pelleted, resuspended in harvest buffer, and homogenized by brief tip sonication. Homogenates were combined with an equal volume of 2 × solubilization buffer (100 mM Tris/10 mM EGTA/2% Triton X-100/2 mM Na3VO4/protease inhibitors, pH 7.4), vortexed briefly, and placed on ice for 20 min. For SNS, an equal volume of ice-cold solubilization buffer was added directly to the treatment aliquot. Lysates were fractionated, and the low and high-speed pellets and supernatant were saved for Western blot analyses. Slice and neuron lysate protein concentrations were equilibrated before centrifugation, or values were normalized to the levels of β-actin in the starting homogenates.
Analysis of Membrane Attachments of mRNA-Binding Proteins. Cortices from five P10 rats were homogenized in 5 ml of harvest buffer with 5 mM vanadyl ribonucleoside complex (VRC) and protease inhibitors. The homogenate was centrifuged at 500 × g for 5 min to remove nuclei; the supernatant was layered onto 7.5 ml of 55% sucrose and centrifuged at 100,000 × g for 4 h at 4°C. The membranous float was washed in harvest buffer, then resuspended in 3 ml of this buffer and split into two samples: one as a vehicle control and one treated with 20 μM Lat A, 4 mM Ca2+, and 130 mM KCl for 30 min at 37°C. Both samples were then layered onto 4.5 ml of 55% sucrose and centrifuged at 100,000 × g at 4°C overnight; the resulting pellets were saved for Western blot analysis.
Immunoprecipitation. Neurons were collected on ice in harvest buffer, pelleted, and then solubilized by brief tip sonication in 50 mM Tris/150 mM NaCl/0.5% Nonidet P-40/5 mM VRC/ protease inhibitors/20 mM NaF/pH 7.4. Lysates were precleared by centrifugation at 1,000 × g and incubation with protein A-conjugated CL4B Sepharose beads. Preincubated complexes of anti-eIF4E antibody (1-5 μg) and beads were added to lysates overnight at 4°C. The beads were then washed in lysis buffer six times, and proteins were eluted with SDS/ PAGE buffer for Western blot analysis.
Western Blots and Quantification. Samples were boiled in NuPage sample buffer (Invitrogen) and resolved via SDS/PAGE minigels. Proteins were transferred to poly(vinylidene difluoride) membranes, which were then blocked, probed with antibodies, and developed by using the Western breeze kit (Invitrogen). The following antibodies were used at ≥1:1,000 dilutions: eIF4E (Cell Signaling, Beverly, MA; mAb), β-actin (Sigma, mAb), Hu C/D (Molecular Probes), Staufen 1 (CeMines, Evergreen, CO), and FXR2 (BD Transduction Laboratories, San Diego); anti-40S ribosomal antibodies were produced to the purified whole subunit in rabbits. Developed blots were scanned, and band intensities were quantified by using SCION IMAGE freeware. Plotting and statistical comparisons were made by using GRAPH PAD PRISM software.
Immunohistochemistry, Image Acquisition, and Analysis. Dissociated hippocampal neurons were fixed and processed for staining as described (56). The anti-eIF4E primary and the anti-mouse FITC-conjugated secondary (Molecular Probes) antibodies were used at 1:1,000. Phalloidin-Texas red (Molecular Probes) was added at 1:1,000. After staining, neurons were mounted onto slides by using Prolong Anti-Fade (Molecular Probes).
Digital images were acquired on a Zeiss Axioplan 2 fluorescent microscope by using a 63× oil immersion objective with the appropriate filter cubes as described (11). Fluorescent intensity measurements of eIF4E were made with the SLIDEBOOK software package (Intelligent Imaging Innovations, Denver). Spines were masked based on threshold segmentation of the phalloidin staining intensity histogram, then gated by size. Mean fluorescent intensities of eIF4E within masked spines were compared by using graph pad prism software.
Results
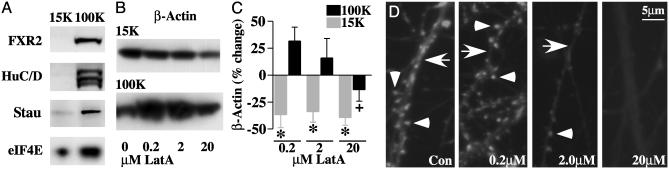
Characterization of Two Distinct Subcellular F-Actin-Containing Fractions. To fractionate distinct pools of F-actin from hippocampal and cortical neurons, we followed a methodology developed initially by Fox et al. (55) for the centrifugal separation of membrane and cortical actin filaments from platelets extracted with nonionic detergents. Application of this methodology to neuronal preparations resolves actin-based cytoskeletal fractions that are distinct in several respects. First, as shown in Fig. 1A, pellet fractions obtained by centrifugation of lysates at 100,000 × g (100K pellet) appeared to contain more mRNA granules than structures pelleted at 15,000 × g (15K pellet), inasmuch as they had much higher levels of three mRNA-binding proteins, FXR2, Hu C/D, and Staufen 1, known to be found in granules. Second, β-actin concentrations in the 15K and 100K pellets were differentially susceptible to the actin depolymerizing agent Lat A. Prior incubation of hippocampal slices with low concentrations of Lat A (0.2 and 2.0 μM) significantly reduced subsequent recovery of β-actin in the 15K pellet (Fig. 1B), shifting some of the β-actin immunoreactivity to the 100K pellet. A considerably higher Lat A concentration (20 μM) was required to reduce β-actin levels in the 100K pellet. These effects are summarized for the entire experimental group (expressed as percent change from control) in Fig. 1C. Similar results were obtained in SNS and dissociated cortical neurons.
Fig. 1.
Characterization of two distinct F-actin containing cytoskeletal fractions. (A) Western blots showing relative levels of FXR2, Staufen 1 (Stau), and Hu isoforms C and D (Hu C/D) in the 15K and 100K pellet fractions isolated from Triton X-100 lysates of hippocampal slice cultures. (B) Western blots illustrating the effects of varying concentrations of Lat A on the content of β-actin in the 15K and 100K fractions. (C) Bar graph of the average levels of β-actin in the 15K and 100K pellets after treatment of slices with the indicated concentrations of Lat A (percent change from control, mean ± SEM). Decreases in 15K β-actin levels were significant (*, one-sample t test) at each Lat A concentration tested (0.2 μM, P < 0.05, n = 6; 2.0 μM, P < 0.01, n = 10; 20 μM, P < 0.005, n = 8). Reductions in 100K β-actin by 20 μM Lat A were significant with respect to 0.2 μMLatA(+ P < 0.05, two-tailed t test). (D) Images of dissociated hippocampal neurons stained with Texas red phalloidin to visualize actin filaments. Dendritic shaft F-actin (arrow) is sensitive to disruption by 0.2 μM Lat A, whereas spine actin filaments (arrow heads) are disrupted only when 20 μM Lat A is added.
Visualization of F-actin in cultured hippocampal neurons by staining with Texas red phalloidin suggested that the 15K and 100K pellet fractions contain F-actin from dendritic shafts and spines, respectively (Fig. 1D). At the concentration of Lat A that disrupted microfilaments in the 15K pellet (0.2 μM), we observed a loss of F-actin in dendritic shafts but not in dendritic spine heads. A much higher concentration of Lat A (20 μM) (the only concentration effective in reducing F-actin in the granule-rich 100K pellet) was required to eliminate phalloidin staining in spine heads. These observations accord well with other reports on the relative sensitivities of dendritic shaft and spine microfilaments to actin-depolymerizing agents (56) and suggest that a pool of F-actin within the synaptic compartment is represented in the 100K-pellet fraction.
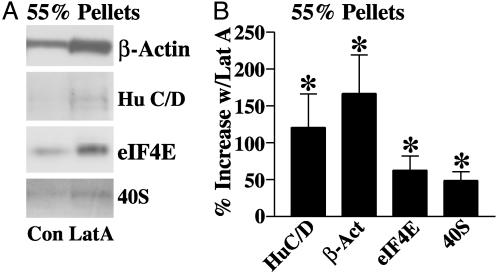
To determine whether mRNA granules were associated with membrane-bound F-actin (which includes synaptic F-actin), homogenates of hippocampal and cortical tissue were layered onto a 55% sucrose cushion and centrifuged to isolate membrane-bound material. The material at the 55% interface was then treated with a mixture containing 20 μM Lat A to destabilize the actin-based cytoskeleton. The amounts of Hu C/D, eIF4E, β-actin, and 40S ribosomal subunit were then analyzed in pellets resulting from a second centrifugation of the treated membranes on a 55% sucrose cushion. Western blots from one such experiment are presented in Fig. 2A. Relative to control samples, Hu C/D immunoreactivity in the 55% sucrose pellet was increased following treatment with the mixture, suggesting that mRNA granules are associated with the membrane cytoskeleton and released by disruption of F-actin. β-Actin, eIF4E, and 40S ribosomes were also found in greater amounts in the 55% sucrose pellet after treatment. The data from six identical experiments are summarized in Fig. 2B. Statistically significant increases were observed for each of the above proteins. We also observed that Lat A increased the amounts of Staufen 1 and FXR2 in the 55% pellet, but changes in these proteins did not meet statistical significance. It is likely, then, that a portion of the mRNA granules found in the 100K pellet associates with microfilaments.
Fig. 2.
Granule mRNA-binding proteins and translation machinery associate with actin-based cytoskeletal structures of brain membranes. (A) Western blot showing the levels of the granule mRNA-binding protein Hu C/D, β-actin, eIF4E, and 40S ribosomal protein in 55% sucrose pellet fractions derived from untreated brain membranes (Con) or those treated with 20 μM Lat A (Lat A) to disrupt the microfilaments. (B) Bar graph summarizing the average changes in Hu C/D, β-actin, eIF4E, and 40S in the 55% pellet (percent increase, mean ± SEM) for six experiments (*, P < 0.05, one-sample t test).
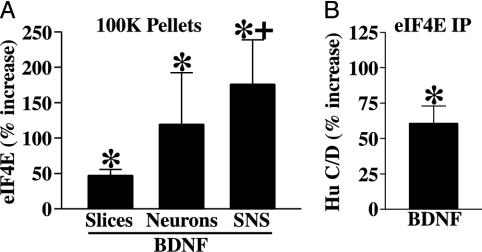
BDNF Induces Translocation of eIF4E to the Granule-Rich 100K Actin Fraction. We tested the effects of BDNF on the levels of eIF4E in the granule-rich cytoskeletal fraction by using three neuronal preparations: slice cultures, dissociated neurons, and SNS. Relative to slice cultures, the latter two preparations are enriched in neurons and synaptodendritic compartments, respectively. Fig. 3A shows that in each preparation studied, brief treatment with BDNF resulted in a significant increase in the amount of eIF4E in the 100K pellet compared with control. The effect was significantly larger in SNS than in slices, consistent with the idea that BDNF is regulating the distribution of eIF4E selectively in neuronal dendrites.
Fig. 3.
BDNF induces translocation of eIF4E to a granule-rich cytoskeletal fraction. (A) Bar graph summarizing the effects of BDNF on the levels of eIF4E in 100K pellet fractions from three experimental preparations (mean percent increase ± SEM; *, one-sample t test): slices (47.2 ± 8.5; P < 0.01, n = 8), dissociated cortical neurons (119.3 ± 72.9; P < 0.05, n = 7), and SNS (175.8 ± 62.9; P < 0.05, n = 7). Compared with slices, BDNF-induced eIF4E translocation was significantly higher in SNS (+, P < 0.05, one-tailed t test), which are relatively enriched in synaptic processes. (B) Bar graph summarizing the effects of BDNF on the association of Hu C/D with eIF4E; significantly larger amounts (60.8 ± 12.2%) of Hu C/D coimmunoprecipitate with eIF4E from samples treated with BDNF (*, P < 0.05, one-sample t test; n = 4).
Immunoprecipitation of eIF4E was used to determine whether translocation of the initiation factor actually increased its association with mRNA granules. Treatment of slices and cortical neurons with BDNF resulted in a significant increase in the amount of Hu C/D coprecipitating with eIF4E (Fig. 3B). We also observed increased coprecipitation of FXR2 and Staufen 1, but changes in the levels of these proteins were highly variable.
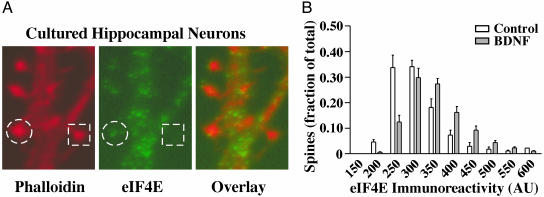
BDNF Increases the Amount of eIF4E in Dendritic Spines. If the redistribution of eIF4E to cytoskeletal assemblies retrieved in the 100K pellet represents translocation to the synaptic compartment, then BDNF should increase the amount of eIF4E within synapses. This prediction was confirmed by using immunofluorescent staining of eIF4E in dissociated hippocampal neurons. Fig. 4A shows a dendritic branch in which eIF4E staining (green) is evident in some, but not all, spines labeled with phalloidin (red). Analysis of the amount of eIF4E immunofluorescent labeling in dendritic spines delineated with phalloidin revealed that (relative to untreated cultures) BDNF induced significant increases in eIF4E localized within spines (n = three experiments). Data from a typical experiment are presented in Fig. 4B as frequency distributions of eIF4E immunoreactivity levels across all delineated spines in control and BDNF-treated cultures. As can be seen, the proportion of spines having higher eIF4E immunofluorescence is increased by BDNF.
Fig. 4.
BDNF increases the concentration of eIF4E in dendritic spines. (A) Images of dissociated hippocampal neurons immunolabeled with anti-eIF4E and counter stained with Texas red phalloidin. Bright phalloidin staining (red) is evident in spine heads. Punctate eIF4E staining (green) was prominent in dendritic shafts, but in only a subset of spines. A spine containing eIF4E is circled and one without eIF4E is indicated with a square. (B) Frequency distribution showing the effects of BDNF on eIF4E immunoreactivity within phalloidin-stained dendritic spines. The incidence and intensity of eIF4E labeling in spines was significantly higher after treatment with BDNF (P < 0.05; n = three experiments, 17,889 spines).
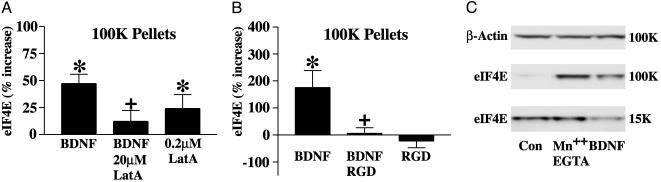
Regulation of eIF4E Translocation by Microfilaments and Integrins. In a number of nonneuronal cell types, translocation of eIF4E depends on the integrity of microfilaments and on the status of integrins. In cultured slices, the BDNF-induced increase in eIF4E in the 100K pellet fraction was prevented by coincubation with 20 μM Lat A (Fig. 5A). This concentration of Lat A, as noted above, depolymerizes both dendritic shaft and spine head actin. However, low concentrations of Lat A preferentially depolymerize dendritic shaft F-actin and filaments that sediment at 15K, leaving intact the β-actin content in spines and the 100K pellet. As eIF4E was found in both fractions (see Fig. 1 A), it was of interest to test the effects of low Lat A concentrations on the levels of the initiation factor in the 100K pellet. Addition of 0.2 μM Lat A alone to slices significantly increased the amount of eIF4E detected in the 100K pellet (Fig. 5A). Comparable effects were obtained in SNS (data not shown). Thus, whereas high Lat A abrogates BDNF-induced translocation of eIF4E, selective depolymerization of one population of actin filaments (presumably nonsynaptic cortical actin filaments of the dendritic shaft) with low Lat A has an effect similar to BDNF on the distribution of eIF4E.
Fig. 5.
F-actin and integrins regulate BDNF-induced translocation of eIF4E. (A) Bar graph summarizing the levels of eIF4E in 100K pellet fractions from slices that were treated with BDNF or 0.2 μM LatA alone, BDNF plus 20 μM Lat A. Lat A at a high concentration (20 μM) prevented BDNF-induced translocation of eIF4E to the 100K pellet (+, P < 0.05, two-tailed t test; n = 6); when applied alone at a low concentration (0.2 μM), Lat A significantly increased levels of eIF4E in the 100K pellet (*, P < 0.05, one-sample t test; n = 14). (B) Bar graph summarizing the effects of integrin perturbation on the levels of eIF4E in the 100K pellets. Coincubation of SNS with GRGDSP blocked BDNF-induced translocation of eIF4E in SNS (+, P < 0.01, two-tailed t test; n = 5). (C) Western blots showing that activation of integrins in cortical neurons with Mn2+/EGTA increases the level of eIF4E in the 100K pellet (112 ± 70.1% increase; P < 0.05, one-sample t test; n = 10), similar to the effect of BDNF. β-actin levels in the 100K pellets remained relatively constant (bars are mean percent increase ± SEM).
We next tested whether the manipulation of integrins affects the redistribution of eIF4E. Incubation of SNS with BDNF induced the translocation of eIF4E to the 100K pellet, and this effect was blocked by coincubation with a peptide, GRGDSP (250 μM), that inhibits integrin-matrix interactions (Fig. 5B). The effects of activating integrins were also tested. Addition of Mn2+, in conjunction with the calcium chelator EGTA, to cultured cells has been shown to activate many integrins (e.g., ref. 57), including α/β heterodimers likely to be present in synapses (43-46, 58). Application of Mn2+/EGTA to cortical neurons resulted in increased translocation of eIF4E to the 100K pellet. A Western blot illustrating this effect is presented in Fig. 5C. Samples from BDNF-treated neurons are also shown for comparison; note that the increase of eIF4E in the 100K pellet is accompanied by a decrease in the 15K pellet with BDNF but not with Mn2+/EGTA.
Discussion
We observed that BDNF induces translocation of the translation initiation factor eIF4E to a distinct actin-based cytoskeletal fraction, one enriched in mRNA granules. Several lines of evidence suggested that this effect represents a BDNF-induced association of eIF4E with mRNA granules via a mechanism involving synaptic microfilaments and integrins. The results constitute the first evidence that BDNF can facilitate the association of a translation factor with mRNA granules, and they support the hypothesis that changes in synaptic structure can regulate local translation during plasticity.
In our studies, the relative effects of Lat A on microfilaments suggested that the 15K pellet contains cortical F-actin of the dendritic shaft, whereas the 100K pellet contains spine head F-actin. If this is the case, then eIF4E associates with two distinct actin networks in dendrites. Dendritic spine heads contain a dense, highly branched network of short actin filaments (28, 29, 59) that is very dynamic (27), yet relatively resistant to disruption by drugs that depolymerize F-actin (56); larger spine heads seem to be unique in having a high proportion of filaments that are orthogonally arranged in the cytoplasm. In contrast, microfilaments in dendritic shafts and spine necks are less dense and arranged into long fascicles (28, 59, 60); these filaments are readily depolymerized by low concentrations of Lat A. eIF4E may be sequestered along actin filaments in the dendritic shaft or spine neck and translocated to the specialized arrangements of F-actin in the spine head on stimulation of translation with BDNF. Such a model is supported by our finding that BDNF increases immunolabeling of eIF4E in dendritic spine heads, and it is consistent with the idea that eIF4E moves into spines with other actin-associated components of the translation machinery that may bind these arrangements (8, 36).
In conjunction with recent reports, our data suggest that changes in dendritic microfilaments regulate local translation from mRNA granules by mediating their association with translation machinery. It has been shown, for example, that the formation of translational complexes between ribosomes and Hu B granules is blocked by depolymerization of F-actin (33). In addition, mRNA granules containing the zipcode-binding protein have been observed to move into spines during KCl-induced depolarization (36). This relocalization is likely to depend on interactions between actin-binding proteins present in granules, such as myosin Va and elongation factor 1α, and synaptic microfilaments (19, 34). Thus, it seems that translocation of eIF4E could reflect its association with granules as they dock to synaptic microfilaments to initiate local translation. Accordingly, we observed that granule proteins and translation machinery are associated with the membrane actin-based cytoskeleton and that BDNF increases the association of eIF4E with granule proteins in immunoprecipitation assays.
Is the BDNF-induced relocalization of eIF4E to granule-rich fractions relevant to translation events that accompany other forms of synaptic plasticity? Inhibitors of cap-dependent translation completely block BDNF-induced potentiation and LTD and substantially reduce LTP expression, indicating that eIF4E is required for each of these forms of plasticity (4, 39). Moreover, various data suggest that these events involve translation from mRNA granules (5, 61). Reorganization of F-actin likely underlies shape changes correlated with LTP (12, 27), and it may also occur with LTD (11, 13, 14), leaving open the possibility that structure regulates translation during their consolidation. Disruption of F-actin dynamics with high Lat A causes LTP to decay at a rate that is virtually identical to that caused by the translation inhibitor cyclohexamide (32). This correlation and the fact that BDNF signaling is essential for LTP stabilization suggest that the effects we observed by using BDNF may also occur when LTP is elicited.
Our observation that integrins modulate eIF4E-actin associations in neurons suggests that their functions described in the regulation of translation in other cell types may also apply to the synapse. During platelet aggregation, which is characterized by a profound reorganization of F-actin structures, αIIbβ3 integrins regulate translocation of eIF4E between membrane-associated and mRNA-rich cortical actin filaments (23). This process triggers a wave of translation. In fibroblasts, mechanical tension communicated through integrins to the actin-based cytoskeleton induces recruitment of ribosomes and poly A mRNA to focal adhesion complexes (24). Spines and their interface with parent dendrites make up a compartment that has both of the above features: a heterogeneous pool of actin filaments and an integrin-containing structure that undergoes morphological transitions capable of producing tension in the actin network. Integrins are involved in various types of synaptic plasticity (46, 58, 62), and LTP in particular can be disrupted by integrin antagonists, translation inhibitors, and perturbants of F-actin shortly after induction. It is thus reasonable to propose that integrins contribute to synaptic plasticity by reorganizing the local actin network into a configuration that mediates interactions among mRNA granules, eIF4E, and components of the translation machinery, in essence providing a tag in the structure of the local cytoskeleton that directs translation of dendritic mRNAs. Consistent with this model, BDNF (52) and integrins (63) have been shown to regulate local translation of the activity-regulated cytoskeletal protein (Arc), an immediate-early gene product required for LTP stabilization (1). In addition, the time frame over which LTP is labile to translation inhibitors is comparable to that required for maximal ribosome recruitment in response to mechanical tension transduced through integrins. It will be of interest to determine whether integrin activation during LTP favors the production of special F-actin arrangements, such as orthogonal networks, that may bind mRNAs or granules.
Protein synthesis resulting from localized assembly of the translation machinery on synaptic microfilaments may contribute to further changes in spine morphology. BDNF signaling regulates spine and PSD structure (15-17) and the local synthesis of CaMKIIα (5), a major PSD component. There is some evidence that during LTP, polysomes redistributed into spine heads contribute to synaptic remodeling (8). Arc, which exhibits a robust transcriptional response to LTP-inducing stimuli (64), binds actin, suggesting that its local synthesis in particular may alter synaptic structure. Local translation has also been implicated in the lengthening of dendritic spines following stimulation of group I metabotropic glutamate receptors (11), a manipulation that induces translation-dependent LTD (65), and in the overabundance of long, thin spines observed in fragile X syndrome (9-11, 61, 66).
The results of our studies and a variety of other data thus suggest that there are reciprocal regulatory influences between synaptic structure and local translation that are integral to the stabilization of plasticity. We propose that an early role of spine shape change during plasticity is to regulate local translation. Altered F-actin arrangements produced by the disassembly of existing structures and elaboration of new integrin-mediated contacts may lead to docking of mRNA granules and serve as a platform for the assembly of translation machinery. Local protein synthesis resulting from this process may then contribute to further remodeling of synaptic contacts by providing proteins with structural functions. Synthesis of glutamate receptor subunits or proteins involved in their cycling to and from the synaptic membrane may occur in parallel with structural changes which, in turn, could organize and limit receptors at the synaptic interface. These events may differ for distinct forms of plasticity in terms of the mRNAs that are recruited and their rate of translation. Particular spine morphologies and efficacy states may then persist by mechanisms of normal protein replacement, or via lasting influences of structure on the profile of mRNAs locally translated.
Acknowledgments
We thank Joanne Gabot for excellent technical assistance, Dr. Bruce A. Cunningham for providing antibodies to the 40S ribosome, and Drs. Vince Mauro and Cunningham for valuable criticisms. This work was supported by National Institute of Mental Health Grant MH64036 (to P.W.V.) and National Institutes of Health Grant HD09635 (to G.M.E.), as well as a grant from the G. Harold and Leila Mathers Foundation.
Abbreviations: BDNF, brain-derived neurotrophic factor; LTP, long-term potentiation; LTD, long-term depression; 15K, 15,000 × g; 100K, 100,000 × g; SNS, synaptoneurosomes.
References
- 1.Guzowski, J. F., Lyford, G. L., Stevenson, G. D., Houston, F. P., McGaugh, J. L., Worley, P. F. & Barnes, C. A. (2000) J. Neurosci. 20, 3993-4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller, S., Yasuda, M., Coats, J. K., Jones, Y., Martone, M. E. & Mayford, M. (2002) Neuron 36, 507-519. [DOI] [PubMed] [Google Scholar]
- 3.Frey, U. & Morris, R. G. (1997) Nature 385, 533-536. [DOI] [PubMed] [Google Scholar]
- 4.Huber, K. M., Kayser, M. S. & Bear, M. F. (2000) Science 288, 1254-1257. [DOI] [PubMed] [Google Scholar]
- 5.Aakalu, G., Smith, W. B., Nguyen, N., Jiang, C. & Schuman, E. M. (2001) Neuron 30, 489-502. [DOI] [PubMed] [Google Scholar]
- 6.Kang, H. & Schuman, E. M. (1996) Science 273, 1402-1406. [DOI] [PubMed] [Google Scholar]
- 7.Fifkova, E., Anderson, C. L., Young, S. J. & Van Harreveld, A. (1982) J. Neurocytol. 11, 183-210. [DOI] [PubMed] [Google Scholar]
- 8.Ostroff, L. E., Fiala, J. C., Allwardt, B. & Harris, K. M. (2002) Neuron 35, 535-545. [DOI] [PubMed] [Google Scholar]
- 9.Greenough, W. T., Klintsova, A. Y., Irwin, S. A., Galvez, R., Bates, K. E. & Weiler, I. J. (2001) Proc. Natl. Acad. Sci. USA 98, 7101-7106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Irwin, S. A., Galvez, R. & Greenough, W. T. (2000) Cereb. Cortex 10, 1038-1044. [DOI] [PubMed] [Google Scholar]
- 11.Vanderklish, P. W. & Edelman, G. M. (2002) Proc. Natl. Acad. Sci. USA 99, 1639-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuste, R. & Bonhoeffer, T. (2001) Annu. Rev. Neurosci. 24, 1071-1089. [DOI] [PubMed] [Google Scholar]
- 13.Snyder, E. M., Philpot, B. D., Huber, K. M., Dong, X., Fallon, J. R. & Bear, M. F. (2001) Nat. Neurosci. 4, 1079-1085. [DOI] [PubMed] [Google Scholar]
- 14.Matsuzaki, M., Ellis-Davies, G. C., Nemoto, T., Miyashita, Y., Iino, M. & Kasai, H. (2001) Nat. Neurosci. 4, 1086-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinez, A., Alcantara, S., Borrell, V., Del Rio, J. A., Blasi, J., Otal, R., Campos, N., Boronat, A., Barbacid, M., Silos-Santiago, I. & Soriano, E. (1998) J. Neurosci. 18, 7336-7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shimada, A., Mason, C. A. & Morrison, M. E. (1998) J. Neurosci. 18, 8559-8570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horch, H. W., Kruttgen, A., Portbury, S. D. & Katz, L. C. (1999) Neuron 23, 353-364. [DOI] [PubMed] [Google Scholar]
- 18.Hesketh, J. E. & Pryme, I. F. (1991) Biochem. J. 277, 1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bassell, G. & Singer, R. H. (1997) Curr. Opin. Cell Biol. 9, 109-115. [DOI] [PubMed] [Google Scholar]
- 20.Shestakova, E. A., Motuz, L. P., Minin, A. A. & Gavrilova, L. P. (1993) Cell Biol. Int. 17, 409-416. [DOI] [PubMed] [Google Scholar]
- 21.Negrutskii, B. S., Stapulionis, R. & Deutscher, M. P. (1994) Proc. Natl. Acad. Sci. USA 91, 964-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stapulionis, R., Kolli, S. & Deutscher, M. P. (1997) J. Biol. Chem. 272, 24980-24986. [DOI] [PubMed] [Google Scholar]
- 23.Lindemann, S., Tolley, N. D., Eyre, J. R., Kraiss, L. W., Mahoney, T. M. & Weyrich, A. S. (2001) J. Biol. Chem. 276, 33947-33951. [DOI] [PubMed] [Google Scholar]
- 24.Chicurel, M. E., Singer, R. H., Meyer, C. J. & Ingber, D. E. (1998) Nature 392, 730-733. [DOI] [PubMed] [Google Scholar]
- 25.Wada, H., Ivester, C. T., Carabello, B. A., Cooper, G. T. & McDermott, P. J. (1996) J. Biol. Chem. 271, 8359-8364. [DOI] [PubMed] [Google Scholar]
- 26.Farmer, S. R., Ben-Ze'av, A., Benecke, B. J. & Penman, S. (1978) Cell 15, 627-637. [DOI] [PubMed] [Google Scholar]
- 27.Matus, A. (2000) Science 290, 754-758. [DOI] [PubMed] [Google Scholar]
- 28.Fifkova, E. & Morales, M. (1992) Int. Rev. Cytol. 139, 267-307. [DOI] [PubMed] [Google Scholar]
- 29.Matus, A., Ackermann, M., Pehling, G., Byers, H. R. & Fujiwara, K. (1982) Proc. Natl. Acad. Sci. USA 79, 7590-7594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krucker, T., Siggins, G. R. & Halpain, S. (2000) Proc. Natl. Acad. Sci. USA 97, 6856-6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim, C. H. & Lisman, J. E. (1999) J. Neurosci. 19, 4314-4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fukazawa, Y., Saitoh, Y., Ozawa, F., Ohta, Y., Mizuno, K. & Inokuchi, K. (2003) Neuron 38, 447-460. [DOI] [PubMed] [Google Scholar]
- 33.Antic, D. & Keene, J. D. (1998) J. Cell Sci. 111, 183-197. [DOI] [PubMed] [Google Scholar]
- 34.Ohashi, S., Koike, K., Omori, A., Ichinose, S., Ohara, S., Kobayashi, S., Sato, T. A. & Anzai, K. (2002) J. Biol. Chem. 277, 37804-37810. [DOI] [PubMed] [Google Scholar]
- 35.Knowles, R. B., Sabry, J. H., Martone, M. E., Deerinck, T. J., Ellisman, M. H., Bassell, G. J. & Kosik, K. S. (1996) J. Neurosci. 16, 7812-7820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tiruchinapalli, D. M., Oleynikov, Y., Kelic, S., Shenoy, S. M., Hartley, A., Stanton, P. K., Singer, R. H. & Bassell, G. J. (2003) J. Neurosci. 23, 3251-3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Asaki, C., Usuda, N., Nakazawa, A., Kametani, K. & Suzuki, T. (2003) Brain Res. 972, 168-176. [DOI] [PubMed] [Google Scholar]
- 38.Inamura, N., Hoshino, S., Uchiumi, T., Nawa, H. & Takei, N. (2003) Brain Res. 111, 165-174. [DOI] [PubMed] [Google Scholar]
- 39.Tang, S. J., Reis, G., Kang, H., Gingras, A. C., Sonenberg, N. & Schuman, E. M. (2002) Proc. Natl. Acad. Sci. USA 99, 467-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tiedge, H. & Brosius, J. (1996) J. Neurosci. 16, 7171-7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steward, O. & Levy, W. B. (1982) J. Neurosci. 2, 284-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krichevsky, A. M. & Kosik, K. S. (2001) Neuron 32, 683-696. [DOI] [PubMed] [Google Scholar]
- 43.Kramar, E. A. & Lynch, G. (2003) Neuroscience 118, 387-398. [DOI] [PubMed] [Google Scholar]
- 44.Nishimura, S. L., Boylen, K. P., Einheber, S., Milner, T. A., Ramos, D. M. & Pytela, R. (1998) Brain Res. 791, 271-282. [DOI] [PubMed] [Google Scholar]
- 45.Einheber, S., Schnapp, L. M., Salzer, J. L., Cappiello, Z. B. & Milner, T. A. (1996) J. Comp. Neurol. 370, 105-134. [DOI] [PubMed] [Google Scholar]
- 46.Chan, C. S., Weeber, E. J., Kurup, S., Sweatt, J. D. & Davis, R. L. (2003) J. Neurosci. 23, 7107-7116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Staubli, U., Chun, D. & Lynch, G. (1998) J. Neurosci. 18, 3460-3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Minichiello, L., Korte, M., Wolfer, D., Kuhn, R., Unsicker, K., Cestari, V., Rossi-Arnaud, C., Lipp, H. P., Bonhoeffer, T. & Klein, R. (1999) Neuron 24, 401-414. [DOI] [PubMed] [Google Scholar]
- 49.Korte, M., Kang, H., Bonhoeffer, T. & Schuman, E. (1998) Neuropharmacology 37, 553-559. [DOI] [PubMed] [Google Scholar]
- 50.Ying, S. W., Futter, M., Rosenblum, K., Webber, M. J., Hunt, S. P., Bliss, T. V. & Bramham, C. R. (2002) J. Neurosci. 22, 1532-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takei, N., Kawamura, M., Hara, K., Yonezawa, K. & Nawa, H. (2001) J. Biol. Chem. 276, 42818-42825. [DOI] [PubMed] [Google Scholar]
- 52.Yin, Y., Edelman, G. M. & Vanderklish, P. W. (2002) Proc. Natl. Acad. Sci. USA 99, 2368-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Banker, G. & Goslin, K. (1991) in Cellular and Molecular Neuroscience Series, ed. Stevens, C. F. (MIT Press, Cambridge, MA).
- 54.Hollingsworth, E. B., McNeal, E. T., Burton, J. L., Williams, R. J., Daly, J. W. & Creveling, C. R. (1985) J. Neurosci. 5, 2240-2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fox, J. E. B., Lipfert, L., Chalrk, E. A., Reynolds, C. C., Austin, C. D. & Brugge, J. S. (1993) J. Biol. Chem. 268, 25973-25984. [PubMed] [Google Scholar]
- 56.Allison, D. W., Gelfand, V. I., Spector, I. & Craig, A. M. (1998) J. Neurosci. 18, 2423-2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shimizu, Y. & Mobley, J. L. (1993) J. Immunol. 151, 4106-4115. [PubMed] [Google Scholar]
- 58.Chavis, P. & Westbrook, G. (2001) Nature 411, 317-321. [DOI] [PubMed] [Google Scholar]
- 59.Fifkova, E. & Delay, R. J. (1982) J. Cell Biol. 95, 345-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hirokawa, N. (1989) Neurosci. Res. 6, 269-275. [DOI] [PubMed] [Google Scholar]
- 61.Huber, K. M., Gallagher, S. M., Warren, S. T. & Bear, M. F. (2002) Proc. Natl. Acad. Sci. USA 99, 7746-7750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rohrbough, J., Grotewiel, M. S., Davis, R. L. & Broadie, K. (2000) J. Neurosci. 20, 6868-6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dong, E., Caruncho, H., Liu, W. S., Smalheiser, N. R., Grayson, D. R., Costa, E. & Guidotti, A. (2003) Proc. Natl. Acad. Sci. USA 100, 5479-5484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Steward, O. & Worley, P. F. (2001) Neuron 30, 227-240. [DOI] [PubMed] [Google Scholar]
- 65.Huber, K. M., Roder, J. C. & Bear, M. F. (2001) J. Neurophysiol. 86, 321-325. [DOI] [PubMed] [Google Scholar]
- 66.Zhang, Y. Q., Bailey, A. M., Matthies, H. J., Renden, R. B., Smith, M. A., Speese, S. D., Rubin, G. M. & Broadie, K. (2001) Cell 107, 591-603. [DOI] [PubMed] [Google Scholar]