Abstract
The upstream signaling pathway leading to the activation of AMP-activated protein kinase (AMPK) by high density lipoprotein (HDL) and the role of AMPK in HDL-induced antiatherogenic actions were investigated. Experiments using genetic and pharmacological tools showed that HDL-induced activation of AMPK is dependent on both sphingosine 1-phosphate receptors and scavenger receptor class B type I through calcium/calmodulin-dependent protein kinase kinase and, for scavenger receptor class B type I system, additionally serine-threonine kinase LKB1 in human umbilical vein endothelial cells. HDL-induced activation of Akt and endothelial NO synthase, stimulation of migration, and inhibition of monocyte adhesion and adhesion molecule expression were dependent on AMPK activation. The inhibitory role of AMPK in the adhesion molecule expression and monocyte adhesion on endothelium of mouse aorta was confirmed in vivo and ex vivo. On the other hand, stimulation of ERK and proliferation were hardly affected by AMPK knockdown but completely inhibited by an N17Ras, whereas the dominant-negative Ras was ineffective for AMPK activation. In conclusion, dual HDL receptor systems differentially regulate AMPK activity through calcium/calmodulin-dependent protein kinase kinase and/or LKB1. Several HDL-induced antiatherogenic actions are regulated by AMPK, but proliferation-related actions are regulated by Ras rather than AMPK.
Keywords: Cytokines/Tumor Necrosis Factor, Diseases/Atherosclerosis, G Proteins/Coupled Receptors (GPCR), Lipid/Phospholipid, Lipoprotein/HDL, Signal Transduction/Protein Kinases
Introduction
Circulating levels of high density lipoprotein (HDL)2 are inversely correlated to the risk of atherosclerosis and associated cardiovascular disease (1, 2). HDL promotes the process of cholesterol transport from arterial and other peripheral cells to the liver and excretes it as bile acids. The so-called reverse cholesterol transport is thought to be important for antiatherogenic properties of HDL (1, 2). HDL also exerts a variety of actions that are independent of reverse cholesterol transport; for example, HDL protects endothelium from its dysfunction, which is composed of several responses in endothelial cells (ECs), including proliferation, migration, nitric oxide (NO) production, and inhibition of adhesion molecule expression (3, 4). The adhesion of monocytes and leukocytes on endothelium is thought to be an early event of atherogenic or inflammatory responses (4). AMP-activated protein kinase (AMPK) has been shown to be involved in energy homeostasis and the regulation of a variety of cell functions (5–7). In ECs, AMPK has been shown to regulate the activity of endothelial NO synthase (eNOS) and NO synthesis evoked by a variety of extracellular stimuli, including HDL (8), sphingosine 1-phosphate (S1P) (9), thrombin (10), vascular endothelial growth factor (9, 11), and adiponectin (12). AMPK has also been suggested to be involved in the inhibition of monocyte adhesion and adhesion molecule expression in ECs, although the role of eNOS in the regulation of cell adhesion is controversial (13–16).
We and others have shown that HDL is a carrier of potent bioactive lipid mediators, including S1P, in addition to apolipoproteins (4). Recent studies have shown that HDL induced eNOS activation through lipoprotein-associated apoA-I and/or S1P, although the roles of dual receptors, i.e. scavenger receptor class B type I (SR-BI) and S1P receptors, are still controversial (4, 17–20). Thus, several independent reports have shown that HDL and S1P stimulate AMPK and eNOS in ECs. However, roles of SR-BI and S1P receptors and their signaling mechanism in HDL-induced AMPK activation have not been fully characterized. Moreover, roles of AMPK in HDL-regulated functions related to the protection of endothelial dysfunctions other than eNOS activation remain poorly understood. In this study, we examined these unanswered questions in human umbilical vein endothelial cells (HUVECs) in vitro and mouse aorta in vivo.
EXPERIMENTAL PROCEDURES
Materials
S1P was purchased from Cayman Chemical Co.; wortmannin and STO-609 were from Calbiochem-Novabiochem; AICAR was from Biomol; antibodies for AMPKα, phospho-Thr-172 AMPKα, eNOS, phospho-Ser-1179 eNOS, Akt, phospho-Ser-473 Akt, ERK, phospho-Thr-202/Tyr-204 ERK1/2, LKB1, phospho-Ser-428 LKB1, Ras, and β-actin were from Cell Signaling Technology Inc.; antibody for VCAM-1 was from Chemicon International; and antibodies for S1P1 receptor, CaMKKβ, SR-BI, and PDZK1 were from Santa Cruz Biotechnology, Inc. Plasma HDL (1.063–1.21 g/ml) was separated from freshly isolated plasma of healthy volunteers by sequential ultracentrifugation (21). HDL was delipidated, and the lipid-free apolipoprotein A (apoA) mixture was then dialyzed against 5× 1 liter of Tris-buffered saline (0.01 m Tris buffer, pH 7.4, containing 0.15 m NaCl, 0.01% (w/v) EDTA, and 0.02% (w/v) NaN3). The discoidal lipoprotein particle (termed rHDL in this study) was prepared by the sodium cholate dialysis method as described previously (19), using apoA and palmitoyloleoylphosphatidylcholine at a molar ratio of 1:80 (18). In experiments shown in Fig. 1A, we also prepared cholesterol-loaded rHDL, in which apoA, palmitoyloleoylphosphatidylcholine, and cholesterol were included during sodium cholate dialysis in a molar ratio of 1:100:5. The S1P content in the rHDL and cholesterol-loaded rHDL was undetectable by our S1P assay method (22). The sources of all other reagents were described previously (19, 23, 24). Unless otherwise stated, S1P (1 μm), rHDL (500 μg protein/ml), and native HDL (500 μg protein/ml) were used in this study.
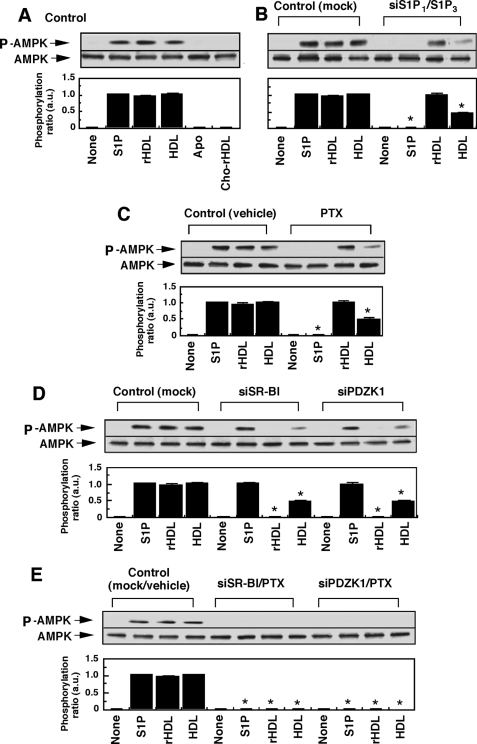
FIGURE 1.
Both SR-BI/PDZK1 and S1P receptor/Gi protein systems contribute to HDL-induced AMPK phosphorylation in HUVECs. A, HUVECs were incubated for 10 min with S1P (1 μm), rHDL (500 μg of protein/ml), native HDL (500 μg of protein/ml), apoA mixture (Apo, 500 μg of protein/ml), cholesterol-loaded rHDL (Cho-rHDL, 500 μg of protein/ml), or PBS (None) to measure AMP phosphorylation activity. B–E, cells were incubated with the indicated agents after the following treatments to analyze the roles of S1P receptors, Gi protein, SR-BI, and PDZK1 in AMPK phosphorylation: siRNAs for S1P1 and S1P3 receptors (siS1P1/siS1P3) (B); PTX (C); siRNA for either SR-BI (siSR-BI) or PDZK1 (siRDZK1) (D); and siRNA for either SR-BI or PDZK1, together with PTX (E). As control, cells were treated with nonsilencing RNA and/or PBS. Other experimental conditions are the same as those for A. Upper column shows a typical Western blotting of phosphorylated AMPK (P-AMPK) and total AMPK of three separate experiments, and lower column shows the results of densitometer analysis from pooled data, in which the ratio (a.u., arbitrary units) of phosphorylated AMPK to total AMPK was determined. The results are expressed as fold of the value obtained by S1P (1 μm) in each control experiment. *, effects of the indicated treatments on the responses to each agents compared with those in control cells were significant.
Cell Culture and Transfection of siRNA
HUVECs (passage number 3) were purchased from Whittaker BioProducts. The cells were cultured in RPMI 1640 medium supplemented with 15% (v/v) fetal bovine serum (FBS) and several growth factors as described previously (21, 23, 25). We usually used 5–8 passages of the cells and checked the cobblestone-like cell shape before experiments. Where indicated, pertussis toxin (PTX, 100 ng/ml) or its vehicle (PBS) was added to the culture medium 12 h before experiments, unless otherwise stated. STO-609, a specific CaMKK inhibitor, at 10 μm or its vehicle was added to the culture medium 20 min before the experiments. As for the transfection of siRNAs specific to SR-BI, PDZK1, S1P1, S1P3, AMPKα1, CaMKKβ, and LKB1, HUVECs were seeded at a density of 5.0 × 104 cells/cm2 on 96-well plates for adhesion molecule expression and on 6-well plates for Western blotting and NOS activation. Sixteen h later, siRNA (100 nm) was introduced into cells using RNAiFect reagent (Qiagen K.K., Tokyo, Japan) according to the manufacturer's instructions. The cells were further cultured for 48 h. The nonsilencing siRNA (D-001206-13) and siRNAs targeted for SR-BI (M-010592-00), PDZK1 (M-010615-01), S1P1 (M-003655-01), S1P3 (M-005208-01), AMPKα1 (L-005027-00), CaMKKβ (L-004842-00), and LKB1 (L-005035-00) were obtained from Dharmacon Inc. (Lafayette, CO). THP-1 monocytic cells were cultured in RPMI 1640 medium containing 10% FBS. WEHI-274.1 mouse monocytes were purchased from ATCC and cultured with Dulbecco's modified Eagle's medium with 4.5 mg/ml glucose, 10% FBS, and 0.05 mm 2-mercaptoethanol (24).
Quantitative Reverse Transcription-PCR Analysis
Total RNA was isolated using TRI Reagent® (Sigma) according to the instructions from the manufacturer. After DNase I (Promega, Madison, WI) treatment to remove possible traces of genomic DNA contaminating in the RNA preparations, 5 μg of the total RNA was reverse-transcribed using the high capacity cDNA archive kit according to the instructions from the manufacturer (Applied Biosystems, Foster City, CA). To evaluate the expression level of mRNAs for S1P1 (Hs00173499), S1P3 (Hs00245464), AMPKα1 (Hs00178893), and AMPKα2 (Hs00178903), quantitative reverse transcription-PCR was performed using real time TaqMan technology with a sequence detection system model 7700 (Applied Biosystems). The expression level of the target mRNA was normalized to the relative ratio of the expression of glyceraldehyde-3-phosphate dehydrogenase mRNA. Each reverse transcription-PCR assay was performed at least three times, and the results are expressed as means ± S.E.
Construction of Adenoviral Vector and Infection of Recombinant Adenovirus
The recombinant adenovirus for a dominant-negative form of human H-Ras, N17Ras, was constructed (26) and infected (23) as described previously. In brief, 80% confluent HUVECs were infected with recombinant at a multiplicity of infection of 30 for 2 h at 37 °C in RPMI 1640 medium containing 5% FBS. Cells were then cultured for an additional 48 h with RPMI 1640 medium containing 15% FBS and other supplements before treatment. Under these conditions, infection with adenovirus coding green fluorescent protein resulted in almost 100% cells positive to green fluorescent protein.
Cell Surface Expression of Adhesion Molecules
HUVECs were plated on 96-well plates and transfected with siRNA as described above. Cells were then washed twice and incubated in RPMI 1640 medium containing 0.1% bovine serum albumin (BSA) with test agents for 8 h. Thereafter, cells were washed with PBS twice and fixed with PBS containing 1% paraformaldehyde under 4 °C. The plates were blocked at 4 °C overnight with 5% skim milk powder in PBS. Cell surface expression of adhesion molecules was determined by primary binding with specific mouse antibody for VCAM-1, followed by secondary binding with a horseradish peroxidase-conjugated goat anti-mouse IgG antibody as described previously (19, 23). Quantification was performed by determination of colorimetric conversion at A450 nm of 3,3′,5,5′-tetramethylbenzidine using the TMB peroxidase enzyme immunoassay substrate kit (Bio-Rad).
Western Blotting
HUVECs were cultured and pretreated with several reagents as described above and then incubated for the indicated times with test agents. For detection of eNOS phosphorylation (23), the reaction was terminated by washing twice with ice-cold PBS and adding 0.1 ml of lysis buffer containing 1% Triton X-100, 50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 2 mm EDTA, 8 mm EGTA, 25 mm NaF, 10 mm Na4P2O7, 1 mm Na3VO4, 5 μg/ml leupeptin, 5 μg/ml pepstatin, 5 μg/ml aprotinin, and 0.5 mm phenylmethylsulfonyl fluoride. The lysate was separated by 6% SDS-PAGE and analyzed by Western blotting with eNOS- and phospho-eNOS-specific antibody. As for the detection of Akt, phospho-Akt, ERK, phospho-ERK, Ras (which recognizes also dominant-negative N17Ras), S1P1 receptor, SR-BI, PDZK1, AMPKα, phospho-AMPKα, CaMKKβ, LKB1, phospho-LKB1, and β-actin, the procedures were essentially the same as those for eNOS phosphorylation except for the primary antibody and separation by 10% SDS-PAGE.
NOS Enzymatic Activity in Cell Lysate
NOS enzymatic activity was measured according to the method described previously (27). Briefly, HUVECs were grown to 80% confluence, and the cells were rinsed twice in ice-cold PBS, harvested from the dishes, and resuspended in ice-cold lysis buffer (27). The cells were disrupted by sonication (Branson Ultrasonics, Chicago) three times for 10 s each. NOS enzymatic activity in the resulting cell lysates was determined by measuring the conversion of l-[3H]arginine to l-[3H]citrulline. Fifty μl of cell lysate were added to 50 μl of reaction mixtures containing 2 μm cold l-arginine and 2 μCi/ml l-[3H]arginine. After incubation at 37 °C for 1 h, the assay was terminated by the addition of 400 μl of 40 mm HEPES buffer, pH 5.5, with 2 mm EDTA and 2 mm EGTA. The terminated reactions were applied to 1-ml columns of Dowex AG-50W-X8 (Tris form) and eluted with 1 ml of 40 mm HEPES buffer. The l-[3H]citrulline generated was collected into scintillation vials and quantified by liquid scintillation spectroscopy.
Distribution of LKB1 in Cytosol and Nuclear Fraction
The ReadyPrep protein extraction kit (Bio-Rad) was used to prepare nuclear and cytoplasmic fractions from HUVECs according to the manufacturer's instructions. Protein samples (15 μg per lane) in each fraction were subjected to SDS-PAGE and transferred to polyvinylidene fluoride membranes, and Western blotting was performed with the LKB1 antibody.
THP-1 Cell Adhesion Assay
THP-1 monocytic cells were washed twice and resuspended in RPMI 1640 medium containing 0.1% BSA. The cell suspensions were overlaid (7.5 × 105 cells in 500 μl) on the confluent monolayers of HUVECs that had been grown in 12-well plates and treated with various reagents for 8 h. After incubation for 15 min at 37 °C, nonadherent THP-1 monocytic cells were removed by washing four times with prewarmed RPMI 1640 medium containing 0.1% BSA. The number of THP-1 monocytic cells on the HUVECs was counted in four places under microscopy at ×400 magnification as adhering cells (19, 23).
Migration Assay
The migration experiment was performed using a blind Boyden chamber apparatus as described previously (25, 28). In brief, HUVECs were cultured for 4 h with fresh RPMI 1640 medium containing 0.1% BSA. The cells were then washed twice at 37 °C in a HEPES-buffered medium containing 0.1% BSA, pH 7.5. These cells were loaded on the upper chamber, and test agents were placed in the lower chamber. The number of cells that had migrated during 4 h to the lower surface was determined by counting the cells in four places under microscopy at ×400 magnification.
Proliferation
A cell proliferation assay was performed as described previously (29). Briefly, HUVECs (5 × 103 cells per well) were plated onto collagen-coated 96-well plates in culture medium. The next day, the cells were treated with or without the indicated agents in RPMI 1640 medium containing 5% FBS to avoid cell detachment. After 48 h, cells were fixed by the addition of 10 μl of an 11% glutaraldehyde solution to 100 μl of medium and shaken for 15 min. After being washed three times with deionized water, the cells on the plates were air-dried and stained for 20 min with 0.1% crystal violet solution in 200 mm MES, pH 6.0. The cells were again washed with deionized water three times and air-dried. The dye bound on the cells was solubilized with 10% acetic acid, and the absorbance of dye extracts was measured at 600 nm by using a microplate reader. In each experiment, proliferation activity was assessed in three wells under a specified condition. Similar experiments were done with three batches of cells. The cell number was evaluated from the absorbance obtained with known number of cells.
Isolation of Mouse Aorta, Western Blotting, and ex Vivo Monocyte Adhesion Assay
Male C57BL/6J mice were purchased from Japan SLC, Inc. Mice were fed rodent chow and housed in micro-isolator cages in a pathogen-free facility. All experiments followed guidelines from the Association for Assessment of Laboratory Animal Care guidelines, and approval for use of rodents was obtained from the Gunma University. The mice were intraperitoneally injected with AICAR (500 mg/kg) or 0.9% physiological saline 3 days before the experiments and then injected intravenously with recombinant murine TNF-α (20 μg/kg) or PBS at 2 h before experiments. Aortas were harvested from mice and immediately placed into 10% FBS-containing Dulbecco's modified Eagle's medium. A part of the aorta was treated with a cotton stick to wipe off the endothelium and used as endothelium-removed aortas (EC−). The endothelium-intact aortas (EC+) and endothelium-removed aortas (EC−) were homogenized and analyzed by Western blotting with antibodies specific to AMPK, eNOS, VCAM-1, or their phosphate forms (where indicated). The ex vivo monocyte adhesion assay was performed as described previously (24). In brief, the aortas were opened up longitudinally and pinned onto sterile agar. All aortas were incubated for 15 min with 1 × 106 WEHI-241.1 mouse monocytes that were fluorescently labeled with calcein-AM (Molecular Probes). After incubation, unbound monocytes were rinsed away, and the number of monocytes firmly bound to aorta was counted in four fields using fluorescent microscopy. Data are represented as the mean ± S.E. of four areas of aorta.
Data Presentation
All experiments were performed in duplicate or triplicate. The results of multiple observations are presented as the mean ± S.E. or as a representative result of more than two different separate experiments, unless otherwise stated. Statistical significance was assessed by analysis of variance or the Student's t test; values were considered significant at p < 0.05.
RESULTS
HDL Stimulates AMPK Phosphorylation through the S1P Receptor/Gi Protein and SR-BI/PDZK1 Systems
Consistently with previous studies, native HDL (8) and S1P (9) stimulated AMPK phosphorylation (Fig. 1A), reflecting enzyme activation. We also tested the effects of lipid-free apoA mixture, which was prepared by delipidation of HDL, rHDL, which was prepared by reconstitution of apoA mixture with phosphatidylcholine, and cholesterol-loaded rHDL, which was prepared by reconstitution of the apoA mixture with phosphatidylcholine and cholesterol. Among them, only rHDL was effective in stimulating AMPK phosphorylation to an extent similar to that in HDL and S1P (Fig. 1A). Henceforth, we examined the effects of S1P, rHDL, and native HDL in comparison with PBS (Fig. 1A, none). Simultaneous transfection of the respective siRNAs specific to S1P1 and S1P3 receptors decreased mRNA expression of these receptors to less than 10% (supplemental Fig. S1, A and B). Moreover, Western blotting with S1P1 receptor-specific antibody showed that S1P1 receptor protein expression was markedly decreased by the siRNA treatment (supplemental Fig. S1C). Unfortunately, however, we have not yet succeeded in detecting the S1P3 receptor-specific protein band by Western blotting. Under the conditions of down-regulation of S1P receptors, at least at their mRNA expression levels, S1P-induced AMPK activation was almost completely lost (Fig. 1B), whereas rHDL- and HDL-induced enzyme activation was hardly and partially (about 50%) inhibited, respectively (Fig. 1B). A similar AMPK activity profile was observed by PTX treatment of the cells (Fig. 1C), suggesting that S1P receptors and Gi proteins are at least partly involved in HDL-induced AMPK activation. On the other hand, siRNA against SR-BI (siSR-BI) or siRNA against PDZK1 (siPDZK1), an adapter protein for SR-BI, which specifically inhibited expression of the respective protein (supplemental Fig. S1D), also partly inhibited HDL-induced AMPK activation (Fig. 1D). As expected, enzyme activation induced by rHDL, but not by S1P, was almost completely inhibited under the conditions. The treatment of either siSR-BI or siPDZK1, together with PTX, inhibited all stimulus-induced AMPK phosphorylation (Fig. 1E). These results suggest that HDL induces AMPK activation through both S1P receptor/Gi protein and SR-BI/PDZK1 systems via lipoprotein-associated S1P and apoA, respectively.
Role of CaMKK and LKB1 in HDL-induced Activation of AMPK, Akt, and eNOS
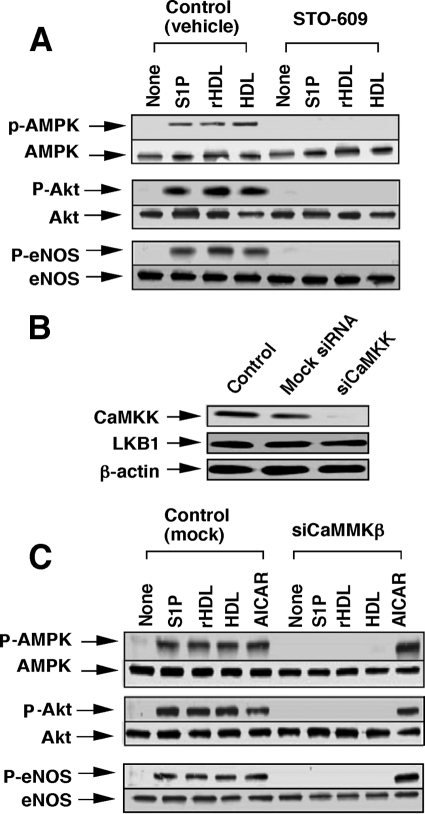
We investigated whether upstream kinases of AMPK, such as CaMKK and LKB1, are involved in AMPK activation by the S1P receptor and SR-BI stimulation. Consistently with a previous study (9), S1P-induced phosphorylation and hence the activation of AMPK, Akt, and eNOS were all almost completely inhibited by STO-609, a specific inhibitor of CaMKK (Fig. 2A). Similarly to the S1P-induced actions, HDL- and rHDL-induced activation of AMPK, Akt, and eNOS were also suppressed by the CaMKK inhibitor (Fig. 2A). We further examined the role of CaMKK by using siRNA strategy. As shown in Fig. 2B, siRNAs for CaMKKβ specifically attenuated the expression of CaMKK without effect on the expression of LKB1, another regulator of AMPK (5–7). Consistently with the results of Fig. 2A, CaMKKβ-siRNA inhibited AMPK, Akt, and eNOS activation induced by S1P, rHDL, and HDL (Fig. 2C). In this experiment, we also examined the effect of AICAR, an AMPK activator (30, 31). Similarly to HDL and S1P, AICAR activated phosphorylation of AMPK, Akt, and eNOS; however, the ribonucleoside-induced actions were hardly affected by the knockdown of CaMKKβ (Fig. 2C).
FIGURE 2.
CaMKK is involved in both S1P receptor- and SR-BI-mediated phosphorylation of AMPK, Akt, and eNOS. A, HUVECs were treated with vehicle or 10 μm STO-609 for 20 min. The cells were then incubated for 10 min with S1P, rHDL, native HDL, or PBS (None) at the concentrations shown in Fig. 1 to measure phosphorylated (p) form and total of AMPK, Akt, and eNOS by Western blotting. B, cells were treated with PBS (Control), nonsilencing siRNA (Mock), or siRNA for CaMKKβ (siCaMKKβ) for 48 h, as described under “Experimental Procedures,” and then CaMKKβ and LKB1 protein expression was measured. C, cells were similarly treated with nonsilencing RNA (mock) or siRNA for CaMKKβ (siCaMKKβ), and phosphorylation activities of the enzymes were measured as in A except for including AICAR (1 mm). A representative of three separate experiments is shown in A–C.
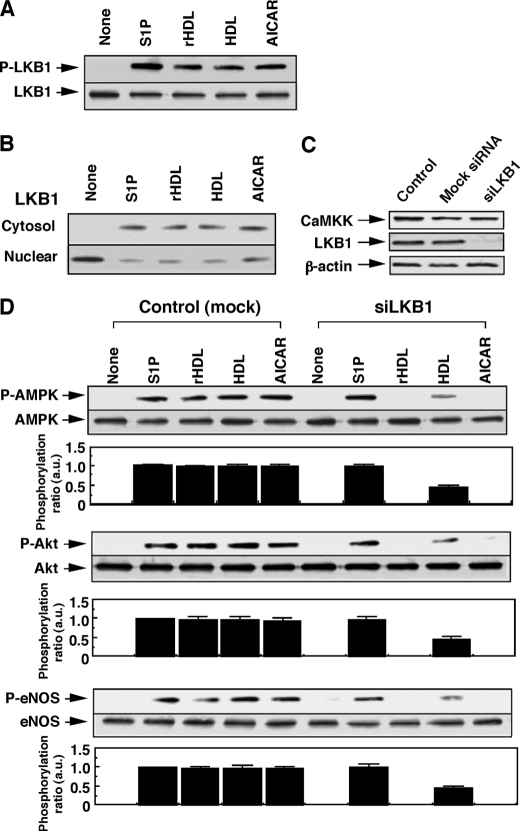
We examined the role of LKB1 in Fig. 3. All the stimuli employed were effective for stimulating LKB1 phosphorylation (Fig. 3A). Consistent with the previous study (32), phosphorylation of LKB1 at Ser-428 was accompanied by nuclear export of LKB1 into cytosol (Fig. 3B). LKB1-siRNA specifically attenuated the expression of LKB1 protein without appreciable effect on the expression of CaMKKβ (Fig. 3C). AICAR has been shown to be sensitive to LKB1 for AMPK activation (31). Consistent with this report, in the cells treated with LKB1-siRNA, phosphorylation activities on AMPK, Akt, and eNOS by AICAR were almost completely lost. In contrast to the results with knockdown and inhibition of CaMKK, however, S1P-induced activation of these enzymes was hardly affected by LKB1-siRNA, whereas these activities by rHDL and HDL were almost completely and partly inhibited, respectively. These results suggest that LKB1 is involved in the SR-BI-mediated, but not the S1P receptor-mediated, activation of AMPK, Akt, and eNOS.
FIGURE 3.
LKB1 is involved in SR-BI-mediated but not in S1P receptor-mediated phosphorylation of AMPK, Akt, and eNOS. A, HUVECs were incubated for 10 min with S1P (1 μm), rHDL (500 μg of protein/ml), native HDL (500 μg of protein/ml), AICAR (1 mm), or PBS (None) to measure phosphorylation activity at Ser-428 of LKB1. B, distribution of LKB1 in nuclear and cytosol fractions was analyzed as described under “Experimental Procedures.” A representative of three separate experiments is shown in A and B. C, cells were treated with PBS (Control), nonsilencing siRNA (Mock), or siRNA for LKB1 (siLKB1) for 48 h, as described under “Experimental Procedures.” LKB1 and CaMKKβ protein expression was measured. A representative of three separate experiments is shown. D, cells were treated with nonsilencing RNA (mock) or siRNA for LKB1 (siLKB1), and then the phosphorylation activities of the enzymes were measured as described in Fig. 2A. Upper column shows a typical Western blotting of the phosphorylated (p) form and total of the indicated enzymes of three separate experiments, and the lower column shows the results of densitometer analysis from pooled data, in which the ratio (a.u., arbitrary unit) of phosphorylated form and total enzyme was determined. The results are expressed as fold of the value obtained by S1P (1 μm) in each control experiment. *, effect of siLKB1 on the responses to each agents compared with those in control cells was significant.
AMPK May Be an Upstream Regulator for PI3K/Akt, Migration, and Inhibition of Adhesion Molecule Expression
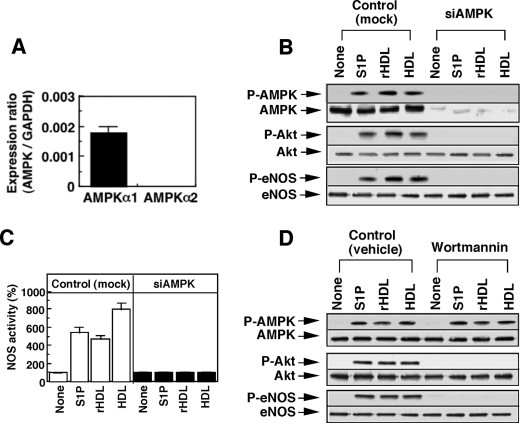
The results described above raised the possibility that CaMKK and/or LKB1 is involved in AMPK, Akt, and eNOS activation. However, the relationship between AMPK and PI3K/Akt remains controversial (9–12, 33–35). In HUVECs, AMPKα1, but not AMPKα2, is expressed (Fig. 4A). To knock down the AMPKα1, we employed AMPKα1-siRNA, which inhibited AMPKα protein expression by more than 80% (Fig. 4B). Under the conditions, the phosphorylation of Akt and eNOS (Fig. 4B) and the activation of NOS (Fig. 4C) in response to any stimulant were almost completely inhibited. When PI3K was inhibited by wortmannin, however, AMPK activity was hardly affected, whereas activation of Akt and eNOS was almost completely inhibited (Fig. 4D). These results suggest that AMPK is an upstream regulator of PI3K/Akt.
FIGURE 4.
AMPK is an upstream regulator for PI3K/Akt and eNOS. A, HUVECs express AMPKα1 but not AMPKα2. Expression of mRNA of either AMPKα1 or AMPKα2 was measured by a quantitative reverse transcription-PCR using real time TaqMan technology. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. B and C, cells were treated with nonsilencing siRNA (Control or mock) or siRNA for AMPK (siAMPK) and then incubated for 10 min with S1P (1 μm), rHDL (500 μg of protein/ml), native HDL (500 μg of protein/ml), or PBS (None) to analyze phosphorylation (p) of AMPK, Akt, and eNOS by Western blotting (B) and to measure NOS activity (C). Results are expressed as percentage of the basal activity (65 ± 7 pmol/mg protein/min), which was unchanged by siAMPK treatment, in (C). D, cells were treated with or without wortmannin for 20 min and then incubated for 10 min with the indicated agents at the concentrations shown in B to analyze phosphorylation of AMPK, Akt, and eNOS by Western blotting. A representative (B and D) or mean ± S.E. (A and C) of three separate experiments is shown.
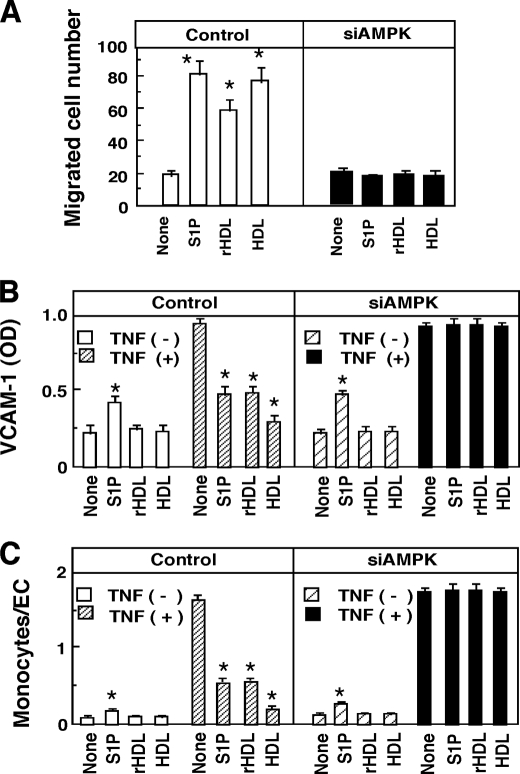
Migration of ECs, which may be important for repairing injured endothelium (36), was almost completely inhibited by AMPK-siRNA (Fig. 5A). As for adhesion activity, S1P and HDL displayed different response patterns; S1P, but not rHDL or HDL, slightly stimulated VCAM-1 expression (Fig. 5B) and monocyte adhesion (Fig. 5C) in the absence of TNF-α. The stimulatory actions of S1P on the adhesion activities, which are mediated mainly by S1P3 receptors/G12/13 proteins (19), were not affected by AMPK-siRNA (Fig. 5, B and C). In the presence of TNF-α, however, all stimuli inhibited the cytokine-induced actions. The inhibitory activities were completely blocked by AMPK-siRNA treatment (Fig. 5, B and C). Thus, AMPK may play important roles in HDL- and S1P-induced PI3K/Akt activation, migration, and the inhibition of adhesion molecule expression.
FIGURE 5.
AMPK plays a key role in migration and inhibition of VCAM-1 expression and monocyte adhesion in HUVECs. HUVECs were treated with nonsilencing siRNA (Control) or siRNA for AMPK (siAMPK). A, cells were incubated for 4 h with S1P (1 μm), rHDL (500 μg protein/ml), native HDL (500 μg protein/ml), or PBS (None) to measure migration activity. The cell number migrated to the lower surface of the filter was counted. B, cells were incubated for 8 h with the indicated agents in the presence or absence of 60 pm TNF-α to measure VCAM-1 expression. C, cells were similarly treated with agents as in B and then incubated for 15 min to measure THP-1 monocyte adhesion activity. Results are means ± S.E. of three separate experiments. *, effect of test agents was significant.
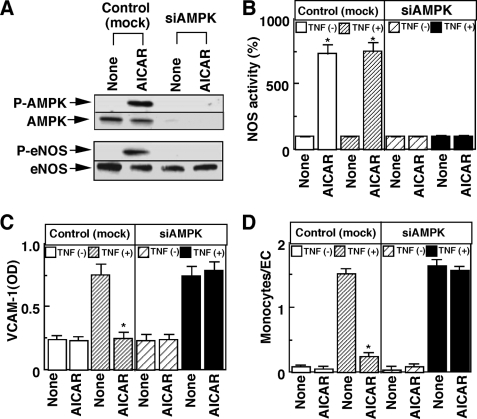
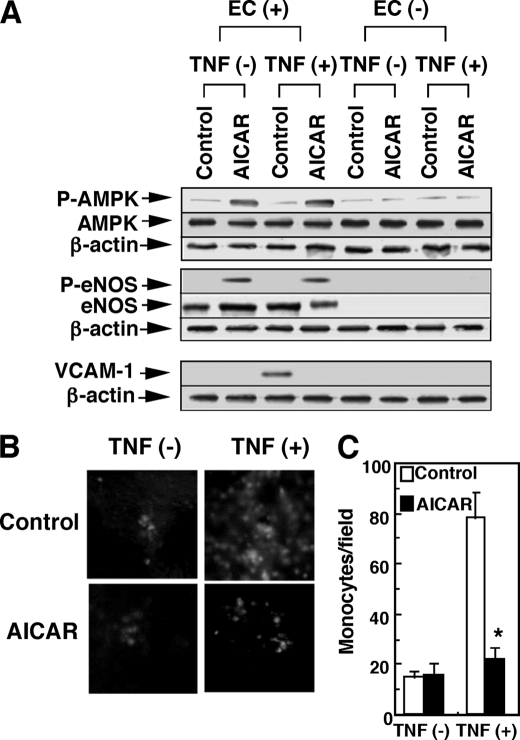
We finally confirmed the role of AMPK in the endothelium by using an AMPK-specific activator, AICAR, in vitro and in vivo. Consistently with results of Fig. 2C, AICAR stimulated the phosphorylation of AMPK and eNOS (Fig. 6A), in association with NOS activation (Fig. 6B). AICAR also inhibited TNF-α-induced VCAM-1 expression (Fig. 6C), which was accompanied by the inhibition of cytokine-induced monocyte adhesion (Fig. 6D). All the AICAR-induced actions were almost completely abolished by the AMPK-siRNA treatment (Fig. 6, A–D). The in vivo treatment of mice with AICAR for 3 days stimulated AMPK phosphorylation in the aorta with endothelium but only slightly, if any, in the aorta without endothelium regardless of treatment with TNF-α (Fig. 7A), suggesting that AMPK in ECs is selectively phosphorylated by AICAR treatment in our experimental conditions. As expected, eNOS was not detected in aortas without endothelium. TNF-α treatment for 2 h in vivo stimulated VCAM-1 expression (Fig. 7A), which was associated with the stimulation of monocyte adhesion on the endothelium of the aorta ex vivo (Fig. 7, B and C). The AICAR pretreatment almost completely inhibited the TNF-α-induced VCAM-1 expression (Fig. 7A) and ex vivo monocyte adhesion in endothelium of the aorta (Fig. 7, B and C). These results strongly support the role of AMPK in eNOS activation and the resulting inhibition of VCAM-1 expression and monocyte adhesion.
FIGURE 6.
AICAR induces activation of eNOS and inhibition of VCAM-1 expression and monocyte adhesion in vitro. HUVECs were treated with nonsilencing siRNA (Control or mock) or siRNA for AMPK (siAMPK) to measure phosphorylation (P) of AMPK and eNOS (A), NOS activity (B), VCAM-1 expression (C), and THP-1 monocyte adhesion (D). The experimental conditions were the same as those for Fig. 5 except that 1 mm AICAR was used as a test agent. A representative (A) or mean ± S.E. (B–D) of three separate experiments is shown. *, effect of AICAR was significant.
FIGURE 7.
AICAR induces activation of eNOS and inhibition of VCAM-1 expression and monocyte adhesion in vivo and ex vivo. Mice were treated with AICAR (500 mg/kg) or PBS (Control) as a vehicle for 3 days and then injected with TNF-α (20 μg/kg) or PBS as a vehicle. Two h later, aortas were harvested from mice. A, AMPK, eNOS, VCAM-1, β-actin, and their phosphorylated (P) form (where indicated) were detected by Western blotting in the endothelium-intact aortas (EC+) or endothelium-removed aortas (EC−). The results shown are representative of four separate experiments. B and C, aortas were harvested from mice treated with or without AICAR and TNF-α, and then ex vivo monocyte adhesion assay was performed using longitudinally opened aortas. Aortas were incubated for 15 min with WEHI-241.1 mouse monocytes that were fluorescently labeled with calcein-AM. Representative images of fluorescently labeled monocytes (shown as white dots) adhered to aortas injected with or without TNF-α and/or AICAR are shown in B. The number of monocytes firmly bound to aorta was counted in four fields using fluorescent microscopy in C. Results are expressed as the number of monocytes attached to endothelium per field. Data are representative (B) or means ± S.E. of four aortas (C). *, effect of AICAR was significant.
HDL-induced ERK Activation and Proliferation Are Independent of AMPK but Dependent on Ras
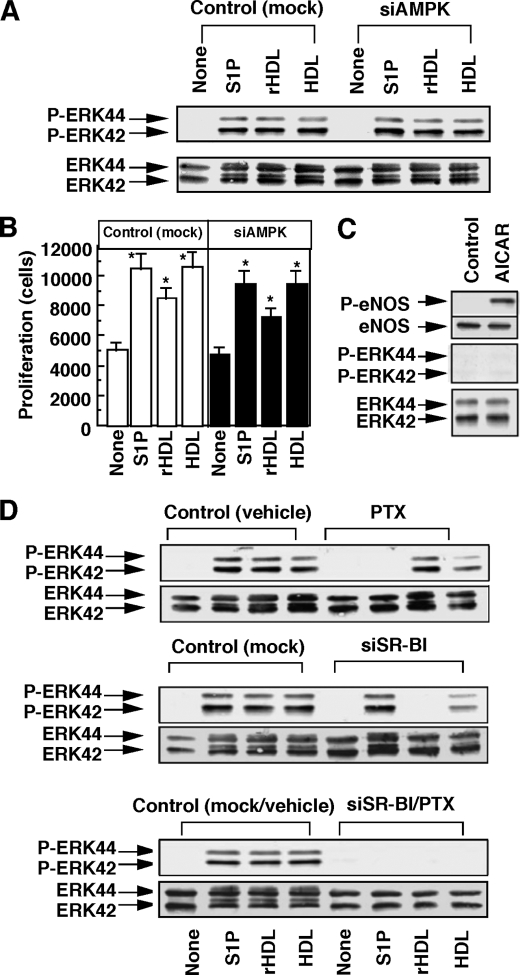
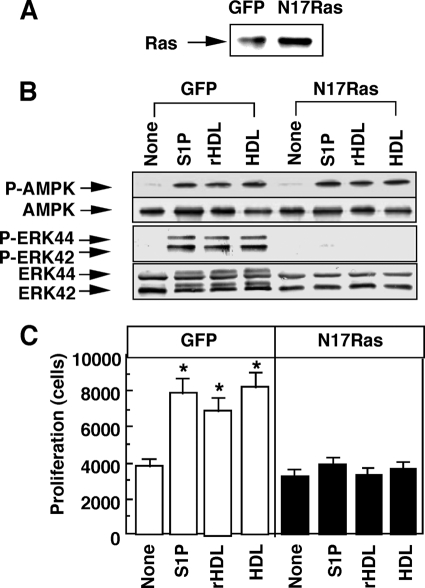
Repairing injured endothelium requires proliferation in addition to migration of endothelial cells. Interestingly, ERK activation (Fig. 8A) and proliferation (Fig. 8B) were hardly affected by knockdown of AMPK regardless of any stimulant employed. Moreover, an AMPK activator AICAR was ineffective in stimulating ERK under the condition where eNOS was activated (Fig. 8C). These results suggest that AMPK is not a critical player of ERK activation and proliferation. We characterized upstream signaling pathways leading to ERK activation. Similarly to the results of AMPK activation (Fig. 1), S1P- and rHDL-induced ERK activation was almost completely inhibited by PTX and SR-BI siRNA, respectively (Fig. 8D). On the other hand, HDL-induced ERK activation was partly inhibited by either PTX or SR-BI-siRNA, and completely inhibited by the combination of PTX and SR-BI-siRNA (Fig. 8D). These results suggest that both SR-BI/PZDK1 and S1P receptor/Gi protein systems equally contribute to the regulation of ERK. We speculated that Ras could be an upstream activator of ERK (37). To block the function of Ras, we employed a dominant-negative Ras, N17Ras. In the cells overexpressing dominant-negative Ras (Fig. 9A), ERK activation (Fig. 9B) and proliferation (Fig. 9C) induced by S1P, rHDL, and HDL were almost completely inhibited. Dominant-negative Ras, however, failed to inhibit AMPK activation (Fig. 9B). These results suggest that Ras, but not AMPK, plays a critical role in SR-BI- and S1P receptor-mediated ERK activation and proliferation.
FIGURE 8.
S1P receptor- and SR-BI-mediated ERK activation and proliferation are independent of AMPK. A and B, HUVECs were treated with nonsilencing siRNA (Control or mock) or siRNA for AMPK (siAMPK). The cells were incubated for 10 min to measure phosphorylation (P) of ERK in A and for 48 h to measure proliferation activity in B as described under “Experimental Procedures.” A representative (A) or mean ± S.E. (B) of three separate experiments is shown. C, HUVECs were treated with AICAR (1 mm) and then incubated for 10 min to measure phosphorylation of eNOS and ERK. D, HUVECs were treated with PTX and/or siRNA for SR-BI (siSR-BI). As control, PBS and/or nonsilencing siRNA were used. The cells were then incubated with the indicated agents to measure ERK phosphorylation activity. The results shown are representative of three separate experiments. *, effect of the test agents was significant.
FIGURE 9.
HDL-induced ERK activation and proliferation are dependent on Ras. HUVECs were infected with adenovirus encoding green fluorescent protein (GFP) or N17Ras for 48 h. A, forced expression of dominant-negative Ras was confirmed as an increase in the density in the band (∼20 kDa) corresponding to the native Ras or N17Ras. B, phosphorylation of AMPK and ERK was examined. C, proliferation activity was measured. Other experimental conditions are the same as those shown in Fig. 8. Results are expressed as a representative (A and B) or mean ± S.E. (C) of three separate experiments. *, effect of the test agents was significant.
DISCUSSION
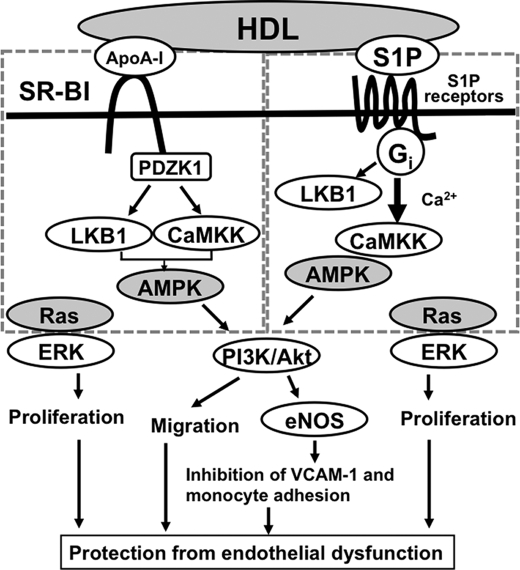
In this study, we obtained the following important new findings with respect to the mechanism and role of HDL-induced activation of AMPK in ECs. First, HDL stimulated AMPK activation through both S1P receptors/Gi proteins and SR-BI/PDZK1. Second, CaMKK plays a role in the activation of AMPK by both receptor systems, but LKB1 may be involved in SR-BI signaling but not in S1P receptor signaling. Third, the HDL-induced activation of AMPK resulted in eNOS activation, through PI3K/Akt, and the subsequent inhibition of expression of the adhesion molecule, VCAM-1, thereby inhibiting monocyte adhesion to ECs. The role of AMPK in adhesion molecule expression was confirmed by in vivo and ex vivo experiments of mouse aorta with AICAR, an AMPK activator. Finally, HDL-induced antiatherogenic actions seem to be regulated by two independent signaling molecules, i.e. AMPK and Ras. Postulated scheme for signaling pathways for HDL-induced protection of endothelial dysfunction is shown in Fig. 10.
FIGURE 10.
Postulated mechanisms of HDL-induced antiatherogenic protection from endothelial dysfunction. HDL activates AMPK, through the apoA-I/SR-BI/PDZK1 and the S1P/S1P receptor/Gi-protein systems, thereby stimulating PI3K/Akt and subsequent eNOS activation, cell migration, and inhibition of adhesion molecule expression. Both systems seem to be regulated by CaMKK and, for SR-BI system, additionally by LKB1. HDL also stimulates ERK/proliferation through both SR-BI and S1P receptors in a manner dependent on Ras but not on AMPK. See text for more detail.
Although previous studies have shown that HDL (8) and S1P (9) activate AMPK and eNOS in ECs, the action mechanism of HDL is controversial. Yuhanna et al. (38) first reported that HDL activates eNOS through SR-BI; however, they failed to detect eNOS activation by lipid-free apoA-I in ECs. Their group later reported that reconstituted apoA-I with phosphatidylcholine but without or with low concentrations of cholesterol can stimulate eNOS activation (18). On the other hand, Drew et al. (8) reported that lipid-free apoA-I stimulated eNOS by their interaction, in association with AMPK activation. The present results (Fig. 1A) and the results of the previous study (19) are consistent with those from Assanasen et al. (18), who suggested that cholesterol movement through SR-BI is critical for eNOS activation by HDL. Thus, there are many reports as to HDL-induced activation of eNOS; however, the participation of HDL receptors, i.e. SR-BI and S1P receptors, in HDL-induced AMPK activation has not yet been investigated. This study showed that the SR-BI/PZDK1 system and S1P receptor/Gi protein system contribute equally to the HDL-induced activation of AMPK in ECs. Whether internalization of apoA-I is essential, as suggested by the previous study (8), remains unknown. It should be noted, however, that the internalization of apoA-I was examined by lipid-free apoA-I but not by native HDL (8).
The role of CaMKK in calcium-mobilizing G protein-coupled receptors, including S1P receptors (9), in AMPK activation has been reported (39). Indeed, S1P has been shown to remarkably elevate the intracellular Ca2+ concentration in ECs (4). In contrast, we failed to detect a significant increase in the intracellular Ca2+ concentration by rHDL, an SR-BI ligand (data not shown). The participation of CaMKK in the SR-BI-mediated AMPK signaling, however, was suggested by the finding that knockdown of CaMKKβ and inhibition of the enzyme by STO-609 remarkably attenuated either S1P- or rHDL-induced activation of AMPK, Akt, and eNOS. It should be noted, however, that CaMKKβ is known to be substantially activated at the resting intracellular Ca2+ concentration, although the enzyme activity is further stimulated by the increase in its concentration (40). The components of the SR-BI-mediated eNOS regulatory system seem to be localized in caveolae, the cholesterol-rich microdomain of the plasma membrane (36), which are also known to be a critical platform for signaling molecules involved in a variety of signal transduction systems, including intracellular Ca2+ homeostasis (41). Thus, it is not surprising that calcium/calmodulin-sensitive eNOS and CaMKK are activated without global increases in intracellular Ca2+ concentration in response to SR-BI stimulation, as is the case for the estrogen activation of eNOS (42).
In addition, LKB1 also appears to be involved in the AMPK activation through SR-BI. Similarly to CaMKK, LKB1 is also reported to be constitutively active (43). Fogarty and Hardie (44) have recently shown that even though LKB1 is phosphorylated at Ser-431 (Ser-428 in human), its phosphorylation does not change the enzyme activity. Xie et al. (32) have also shown that phosphorylation of Ser-428/431 resulted in only a marginal increase in the enzyme activity. Interestingly, however, they observed that Ser-428/431 phosphorylation by metformin is necessary for LKB1 nuclear export to cytosol, where LKB1 interacts with AMPK in ECs (32). Thus, Ser-428/431 phosphorylation is essential for the LKB1 nuclear export and hence LKB1 activation of AMPK at least in ECs. The export of LKB1 into cytosol has also been shown to be associated with adiponectin activation of AMPK in muscle cells (45) and breast cancer cells (46). Consistent with these reports, HDL, S1P, and rHDL stimulated LKB1 nuclear export in association with phosphorylation of Ser-428 in HUVECs in this study. Thus, stimulation of either S1P receptors or SR-BI causes the translocation of LKB1 and hence potentially activates AMPK.
Nevertheless, LKB1 was unable to activate AMPK when CaMKK was inhibited by a specific inhibitor or down-regulated by CaMKK-siRNA. The peculiar observation may be deeply related to the regulatory mechanism of AMPK activity. It is well known that AMPK activity is regulated by the balance of phosphorylation by kinases, such as LKB1 and CaMKK, and dephosphorylation by protein phosphatase, possibly PP2Cα (7, 43). Under the low CaMKK activity status, phosphorylation activity by LKB1 may be too low to overcome the dephosphorylation activity when either S1P receptors or SR-BI was stimulated. Similarly, under the low LKB1 activity status, CaMKK activity is too low to overcome the phosphatase activity in the case of SR-BI stimulation. Thus, SR-BI-mediated AMPK activation requires simultaneous activation of both CaMKK and LKB1. In the case of S1P receptor stimulation, however, strong activation of CaMKK by high Ca2+ supply may cause the AMPK activation without the aid of LKB1. In relation to this, it is interesting that AICAR can stimulate AMPK in association with LKB1 phosphorylation and nuclear export even under the low CaMKK activity status (Fig. 2C and Fig. 3, A and B). AMP binds to the γ-subunit of AMPK and thereby inhibits dephosphorylation by phosphatase. Thus, although LKB1 is not the AMP-binding site, the phosphorylation enzyme is necessary for the activation of AMPK by AMP. In the case of AICAR, the ribonucleoside is transported into cells by the adenosine transporter and metabolized by adenosine kinase into ZMP, an AMP analogue. ZMP then functions like endogenous AMP. Thus, similar to endogenous AMP, ZMP would prevent dephosphorylation of AMPK by phosphatase, such as PP2Cα (7, 30). Under the condition of inhibiting dephosphorylation of AMPK, LKB1 may be able to phosphorylate and activate AMPK without the aid of other phosphorylation enzymes. It remains unknown, however, how AICAR stimulates LKB1 phosphorylation and nuclear export.
This study showed that wortmannin, a PI3K inhibitor, inhibited HDL- and S1P-induced phosphorylation of Akt and eNOS without any significant change in AMPK phosphorylation. Knockdown of AMPK almost completely inhibited the phosphorylation of Akt and eNOS, suggesting that AMPK is an upstream signaling molecule of PI3K/Akt, which regulates the eNOS activity. The finding that an AMPK activator, AICAR, stimulated Akt and eNOS further supports this conclusion. The signaling pathway of AMPK/PI3K/Akt/eNOS is consistent with those suggested for adiponectin-induced eNOS activation in HUVECs (12) and S1P- and vascular endothelial growth factor-induced eNOS activation in bovine aortic ECs (9). However, the eNOS activation mechanism in ECs is complex. Thors et al. (10) showed that thrombin and histamine stimulate eNOS via the AMPK-mediated pathway independent of PI3K/Akt in HUVECs. On the other hand, it was reported that AMPK is not involved in eNOS activation by insulin (33) and vascular endothelial growth factor (34). Further complicating the issue, PI3K works as an upstream regulator of AMPK as indicated by the finding that PI3K inhibitors inactivated AMPK (11). Thus, the signaling pathways of AMPK, PI3K/Akt, and eNOS seem to be differentially regulated depending on the differences in sources (or sites) or species, even in vascular ECs. The differences in time and stimuli employed may also partly explain the different regulatory mechanism of eNOS activation (35). Further experiments are required to determine the relationship of the regulation of these important signaling enzyme activities.
ATP-consuming proliferation is expected to be inhibited by AMPK activation (5–7). On the other hand, recent studies have shown that AMPK (34) and LKB1, an upstream regulator of AMPK (47), are essential for angiogenesis, which must be associated with migration and proliferation of ECs. Moreover, AICAR, an AMPK stimulator, has been shown to activate ERK in osteoblasts (48, 49). Thus, the activation of AMPK potentially acts on ERK and proliferation in an inhibitory or stimulatory manner. Our results showed that HDL-induced ERK activation and proliferation were independent of AMPK, whereas migration response to HDL was depending on AMPK, in ECs. Instead, HDL utilizes the Ras system, which is an independent signaling molecule from AMPK, to induce proliferation. This signaling cascade resembles the insulin signaling pathways; insulin stimulates the PI3K/Akt pathway, which usually regulates a variety of differentiated functions of the cells, and the Ras/ERK pathway, which regulates proliferation (50). In conclusion, HDL activates AMPK through the apoA-I/SR-BI/PDZK1 and the S1P/S1P receptor/Gi protein systems, thereby stimulating PI3K/Akt and the subsequent eNOS activation, cell migration, and the inhibition of adhesion molecule expression. Both systems seem to be regulated by CaMKK and, for the SR-BI system, additionally by LKB1. HDL also stimulates ERK and proliferation, which are mediated by Ras but not by AMPK. Thus, HDL exerts antiatherogenic actions through dual systems involving AMPK and Ras.
Supplementary Material
This work was supported by Grants-in-aid for Scientific Research 20591077 (to T. K.), 21390016, 20054003, and 20015008 (to F. O.), 21591157 and 21026004 (to H. T.), 21591158 (to K. S.), and 20790636 (to C. M.) from the Japan Society for the Promotion of Science and grants from Global Center of Excellence Program (to K. S. and C. M.) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, ONO Medical Research Foundation (to F. O.), and Takeda Science Foundation (to T. K. and C. M.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
- HDL
- high density lipoprotein
- AMPK
- AMP-activated protein kinase
- NO
- nitric oxide
- NOS
- NO synthase
- eNOS
- endothelial NO synthase
- rHDL
- reconstituted HDL
- apoA
- apolipoprotein A
- S1P
- sphingosine 1-phosphate
- EC
- endothelial cell
- HUVEC
- human umbilical vein EC
- CaMKK
- calcium/calmodulin-dependent protein kinase kinase
- ERK
- extracellular signal-regulated kinase
- SR-BI
- scavenger receptor class B type I
- VCAM-1
- vascular cell adhesion molecule
- PI3K
- phosphatidylinositol 3-kinase
- PBS
- phosphate-buffered saline
- PTX
- pertussis toxin
- PDZ
- PSD-95/Dlg/ZO-1
- BSA
- bovine serum albumin
- AICAR
- 5-aminoimidazole-4-carboxamide ribonucleoside
- ZMP
- 5-aminoimidazole-4-carboxamide 1-β-d-ribofuranosyl monophosphate
- siRNA
- small interfering RNA
- MES
- 4-morpholineethanesulfonic acid
- TNF-α
- tumor necrosis factor-α
- FBS
- fetal bovine serum.
REFERENCES
- 1.Assmann G., Gotto A. M., Jr. (2004) Circulation 109, III8–III13 [DOI] [PubMed] [Google Scholar]
- 2.Rader D. J. (2006) J. Clin. Invest. 116, 3090–3100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nofer J. R., Walter M., Assmann G. (2005) Expert Rev. Cardiovasc. Ther. 3, 1071–1086 [DOI] [PubMed] [Google Scholar]
- 4.Okajima F., Sato K., Kimura T. (2009) Endocr. J. 56, 317–334 [DOI] [PubMed] [Google Scholar]
- 5.Witters L. A., Kemp B. E., Means A. R. (2006) Trends Biochem. Sci. 31, 13–16 [DOI] [PubMed] [Google Scholar]
- 6.Towler M. C., Hardie D. G. (2007) Circ. Res. 100, 328–341 [DOI] [PubMed] [Google Scholar]
- 7.Witczak C. A., Sharoff C. G., Goodyear L. J. (2008) Cell. Mol. Life Sci. 65, 3737–3755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drew B. G., Fidge N. H., Gallon-Beaumier G., Kemp B. E., Kingwell B. A. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 6999–7004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levine Y. C., Li G. K., Michel T. (2007) J. Biol. Chem. 282, 20351–20364 [DOI] [PubMed] [Google Scholar]
- 10.Thors B., Halldórsson H., Thorgeirsson G. (2004) FEBS Lett. 573, 175–180 [DOI] [PubMed] [Google Scholar]
- 11.Youn J. Y., Wang T., Cai H. (2009) Circ. Res. 104, 50–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ouchi N., Kobayashi H., Kihara S., Kumada M., Sato K., Inoue T., Funahashi T., Walsh K. (2004) J. Biol. Chem. 279, 1304–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hattori Y., Suzuki K., Hattori S., Kasai K. (2006) Hypertension 47, 1183–1188 [DOI] [PubMed] [Google Scholar]
- 14.Prasad R., Giri S., Nath N., Singh I., Singh A. K. (2006) J. Neurosci. Res. 84, 614–625 [DOI] [PubMed] [Google Scholar]
- 15.Gaskin F. S., Kamada K., Yusof M., Korthuis R. J. (2007) Am. J. Physiol. Heart Circ. Physiol. 292, H326–H332 [DOI] [PubMed] [Google Scholar]
- 16.Ewart M. A., Kohlhaas C. F., Salt I. P. (2008) Arterioscler. Thromb. Vasc. Biol. 28, 2255–2257 [DOI] [PubMed] [Google Scholar]
- 17.Nofer J. R., van der Giet M., Tölle M., Wolinska I., von Wnuck Lipinski K., Baba H. A., Tietge U. J., Gödecke A., Ishii I., Kleuser B., Schäfers M., Fobker M., Zidek W., Assmann G., Chun J., Levkau B. (2004) J. Clin. Invest. 113, 569–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Assanasen C., Mineo C., Seetharam D., Yuhanna I. S., Marcel Y. L., Connelly M. A., Williams D. L., de la Llera-Moya M., Shaul P. W., Silver D. L. (2005) J. Clin. Invest. 115, 969–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimura T., Tomura H., Mogi C., Kuwabara A., Damirin A., Ishizuka T., Sekiguchi A., Ishiwara M., Im D. S., Sato K., Murakami M., Okajima F. (2006) J. Biol. Chem. 281, 37457–37467 [DOI] [PubMed] [Google Scholar]
- 20.Zhu W., Saddar S., Seetharam D., Chambliss K. L., Longoria C., Silver D. L., Yuhanna I. S., Shaul P. W., Mineo C. (2008) Circ. Res. 102, 480–487 [DOI] [PubMed] [Google Scholar]
- 21.Kimura T., Sato K., Kuwabara A., Tomura H., Ishiwara M., Kobayashi I., Ui M., Okajima F. (2001) J. Biol. Chem. 276, 31780–31785 [DOI] [PubMed] [Google Scholar]
- 22.Murata N., Sato K., Kon J., Tomura H., Yanagita M., Kuwabara A., Ui M., Okajima F. (2000) Biochem. J. 352, 809–815 [PMC free article] [PubMed] [Google Scholar]
- 23.Kimura T., Tomura H., Mogi C., Kuwabara A., Ishiwara M., Shibasawa K., Sato K., Ohwada S., Im D. S., Kurose H., Ishizuka T., Murakami M., Okajima F. (2006) Cell. Signal. 18, 841–850 [DOI] [PubMed] [Google Scholar]
- 24.Kimura T., Mogi C., Tomura H., Kuwabara A., Im D. S., Sato K., Kurose H., Murakami M., Okajima F. (2008) J. Immunol. 181, 7332–7340 [DOI] [PubMed] [Google Scholar]
- 25.Kimura T., Sato K., Malchinkhuu E., Tomura H., Tamama K., Kuwabara A., Murakami M., Okajima F. (2003) Arterioscler. Thromb. Vasc. Biol. 23, 1283–1288 [DOI] [PubMed] [Google Scholar]
- 26.Arai K., Maruyama Y., Nishida M., Tanabe S., Takagahara S., Kozasa T., Mori Y., Nagao T., Kurose H. (2003) Mol. Pharmacol. 63, 478–488 [DOI] [PubMed] [Google Scholar]
- 27.Pace M. C., Chambliss K. L., German Z., Yuhanna I. S., Mendelsohn M. E., Shaul P. W. (1999) Am. J. Physiol. 277, L106–L112 [DOI] [PubMed] [Google Scholar]
- 28.Kimura T., Watanabe T., Sato K., Kon J., Tomura H., Tamama K., Kuwabara A., Kanda T., Kobayashi I., Ohta H., Ui M., Okajima F. (2000) Biochem. J. 348, 71–76 [PMC free article] [PubMed] [Google Scholar]
- 29.Rikitake Y., Kawashima S., Yamashita T., Ueyama T., Ishido S., Hotta H., Hirata K., Yokoyama M. (2000) Arterioscler. Thromb. Vasc. Biol. 20, 1006–1012 [DOI] [PubMed] [Google Scholar]
- 30.Corton J. M., Gillespie J. G., Hawley S. A., Hardie D. G. (1995) Eur. J. Biochem. 229, 558–565 [DOI] [PubMed] [Google Scholar]
- 31.Stahmann N., Woods A., Carling D., Heller R. (2006) Mol. Cell. Biol. 26, 5933–5945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie Z., Dong Y., Scholz R., Neumann D., Zou M. H. (2008) Circulation 117, 952–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morrow V. A., Foufelle F., Connell J. M., Petrie J. R., Gould G. W., Salt I. P. (2003) J. Biol. Chem. 278, 31629–31639 [DOI] [PubMed] [Google Scholar]
- 34.Nagata D., Mogi M., Walsh K. (2003) J. Biol. Chem. 278, 31000–31006 [DOI] [PubMed] [Google Scholar]
- 35.Hu Z., Chen J., Wei Q., Xia Y. (2008) J. Biol. Chem. 283, 25256–25263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mineo C., Shaul P. W. (2006) Cardiovasc. Res. 70, 31–41 [DOI] [PubMed] [Google Scholar]
- 37.Miura S., Fujino M., Matsuo Y., Kawamura A., Tanigawa H., Nishikawa H., Saku K. (2003) Arterioscler. Thromb. Vasc. Biol. 23, 802–808 [DOI] [PubMed] [Google Scholar]
- 38.Yuhanna I. S., Zhu Y., Cox B. E., Hahner L. D., Osborne-Lawrence S., Lu P., Marcel Y. L., Anderson R. G., Mendelsohn M. E., Hobbs H. H., Shaul P. W. (2001) Nat. Med. 7, 853–857 [DOI] [PubMed] [Google Scholar]
- 39.Hutchinson D. S., Summers R. J., Bengtsson T. (2008) Pharmacol. Ther. 119, 291–310 [DOI] [PubMed] [Google Scholar]
- 40.Anderson K. A., Means R. L., Huang Q. H., Kemp B. E., Goldstein E. G., Selbert M. A., Edelman A. M., Fremeau R. T., Means A. R. (1998) J. Biol. Chem. 273, 31880–31889 [DOI] [PubMed] [Google Scholar]
- 41.Isshiki M., Anderson R. G. (2003) Traffic 4, 717–723 [DOI] [PubMed] [Google Scholar]
- 42.Caulin-Glaser T., García-Cardeña G., Sarrel P., Sessa W. C., Bender J. R. (1997) Circ. Res. 81, 885–892 [DOI] [PubMed] [Google Scholar]
- 43.Sanders M. J., Grondin P. O., Hegarty B. D., Snowden M. A., Carling D. (2007) Biochem. J. 403, 139–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fogarty S., Hardie D. G. (2009) J. Biol. Chem. 284, 77–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou L., Deepa S. S., Etzler J. C., Ryu J., Mao X., Fang Q., Liu D. D., Torres J. M., Jia W., Lechleiter J. D., Liu F., Dong L. Q. (2009) J. Biol. Chem. 284, 22426–22435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taliaferro-Smith L., Nagalingam A., Zhong D., Zhou W., Saxena N. K., Sharma D. (2009) Oncogene 28, 2621–2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Londesborough A., Vaahtomeri K., Tiainen M., Katajisto P., Ekman N., Vallenius T., Mäkelä T. P. (2008) Development 135, 2331–2338 [DOI] [PubMed] [Google Scholar]
- 48.Kim J. E., Ahn M. W., Baek S. H., Lee I. K., Kim Y. W., Kim J. Y., Dan J. M., Park S. Y. (2008) Bone 43, 394–404 [DOI] [PubMed] [Google Scholar]
- 49.Kanazawa I., Yamaguchi T., Yano S., Yamauchi M., Sugimoto T. (2009) Am. J. Physiol. Endocrinol. Metab. 296, E139–E146 [DOI] [PubMed] [Google Scholar]
- 50.Saltiel A. R., Pessin J. E. (2002) Trends Cell Biol. 12, 65–71 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.