Abstract
Specific oxidized phospholipids (oxPCCD36) accumulate in vivo at sites of oxidative stress and serve as high affinity ligands for scavenger receptors class B (CD36 and SR-BI). Recognition of oxPCCD36 by scavenger receptors plays a role in several pathophysiological processes. The structural basis for the recognition of oxPCCD36 by CD36 and SR-BI is poorly understood. A characteristic feature of oxPCCD36 is an sn-2 acyl group that incorporates a terminal γ-hydroxy (or oxo)-α,β-unsaturated carbonyl. In the present study, a series of model oxidized phospholipids were designed, synthesized, and tested for their ability to serve as ligands for CD36 and SR-BI. We demonstrated that intact the sn-1 hydrophobic chain, the sn-3 hydrophilic phosphocholine or phosphatidic acid group, and the polar sn-2 tail are absolutely essential for high affinity binding. We further found that a terminal negatively charged carboxylate at the sn-2 position suffices to generate high binding affinity to class B scavenger receptors. In addition, factors such as polarity, rigidity, optimal chain length of sn-2, and sn-3 positions and negative charge at the sn-3 position of phospholipids further modulate the binding affinity. We conclude that all three positions of oxidized phospholipids are essential for the effective recognition by scavenger receptors class B. Furthermore, the structure of residues in these positions controls the affinity of the binding. The present studies suggest that, in addition to oxPCCD36, other oxidized phospholipids observed in vivo may represent novel ligands for scavenger receptors class B.
Keywords: Atherosclerosis, Lipoprotein receptor, Macrophage, Oxidative Stress, Phospholipid, CD36, SR-BI, Cardiovascular Pathology, Oxidized Low Density Lipoproteins, Oxidized Phospholipids
Introduction
Specific oxidized phospholipids (oxPCCD36)3 accumulate at sites of oxidative stress in vivo such as within atherosclerotic lesions and plasma in dyslipidemia (1, 2). They serve as high affinity ligands for scavenger receptors class B: CD36 and SR-BI (3, 4). Recognition of oxPCCD36 on the surface of cell membranes and lipoprotein particles by scavenger receptors class B plays an important role in several pathophysiological processes, including atherosclerosis and thrombosis. oxPCCD36 phospholipids mediate uptake of oxidized low density lipoprotein (oxLDL) by macrophages via CD36 and promote a pro-thrombotic state via platelet scavenger receptor CD36 (1, 2). oxPCCD36 phospholipids also prevent binding of high density lipoprotein by SR-BI because of the close proximity of the binding sites for these two ligands on SR-BI. Furthermore, oxPCCD36 interfere with SR-BI-mediated selective uptake of cholesteryl esters in hepatocytes (4). These data demonstrate that oxidative stress and accumulation of specific oxidized phospholipids may have a detrimental effect due to specific interaction with scavenger receptors class B. However, the exact molecular mechanism of the recognition of oxPCCD36 by scavenger receptors class B is poorly understood.
Initial studies have demonstrated that the sn-2 acyl group of oxPCCD36 that incorporates a terminal γ-hydroxy (or oxo)-α,β-unsaturated carbonyl is essential for high affinity binding to CD36 (3). Recent studies have shown that the truncated oxidized sn-2 fatty acid moiety protrudes into the aqueous phase rendering it accessible for recognition (5). It has been shown that electrophilic reactivity is not a prerequisite for high affinity CD36 binding, because the relatively unreactive oxPCCD36 with γ-hydroxy-α,β-unsaturated enoic acid groups at the sn-2 position are excellent ligands (3). We have recently demonstrated that two lysine groups in CD36 (Lys-164/166) are indispensable for the binding of oxPCCD36 to CD36 (6). These studies suggested an electrostatic interaction mechanism of the binding, where the negative charges in the oxidized phospholipids form salt bridges with the positive lysine groups in the binding domain of CD36. Further elucidation of the structural basis for the recognition of oxidized phospholipids by scavenger receptors class B is required to better understand the contribution of oxidative stress and specifically oxidized phospholipids to cardiovascular pathology.
In the present study, a series of phospholipids having various functional groups at sn-1, -2, and -3 positions was designed, synthesized, and used to elucidate the structural basis for the recognition of oxPCCD36 by scavenger receptors CD36 and SR-BI. We demonstrated that a negative carboxylate at the terminal of sn-2 position of glycerophospholipid is sufficient to generate high binding affinity to class B scavenger receptors. However, we demonstrated that structural modifications of either of three parts of oxidized phospholipids at sn-1, sn-2, and sn-3 positions can significantly modulate binding.
EXPERIMENTAL PROCEDURES
Materials
Tissue culture media and additives were purchased from Invitrogen. Na[125I] was supplied by ICN Pharmaceutical, Inc. (Costa Mesa, CA). [3H]DPPC were from American Radiolabel Chemicals, Inc. (St. Louis, MO). 1-Hexadecanoyl-2-eicosatetra-5′,8′,11′,14′-enoyl-sn-glycero-3-phosphocholine (PAPC) and 1,2-dihexadecanoyl-sn-glycero-3-phosphocholine (DPPC) were purchased from Avanti Polar Lipids (Alabaster, AL). The 9-keto-10-dodecendioic acid and 5-keto-6-octendioic acid esters of 2-lysoPC (KDdiA-PC and KOdiA-PC, respectively) were synthesized as described earlier (7). All other reagents were obtained from Sigma unless otherwise specified. Synthesis of phospholipids was performed as described in the supplemental Methods. The purity of the synthetic phospholipids was routinely assessed by high-performance liquid chromatography with on-line electrospray ionization tandem mass spectrometry and was found to be higher than 98%.
Methods
General Procedures
All buffers were passed over a column of Chelex-100 resin (Bio-Rad) and supplemented with diethylenetriamine pentaacetic acid to remove any potential transition metal ions, which might catalyze LDL and phospholipid oxidation during incubation. Protein content was determined by the Markwell-modified Lowry protein assay with bovine serum albumin as a standard (8). LDL was isolated from fresh plasma by sequential ultracentrifugation (9), and iodination with Na [125I] was performed as previously described (10).
Lipoprotein Modification
LDL was modified by MPO-H2O2-NO2− system by incubating LDL (0.2 mg of protein/ml) at 37 °C in 50 mm sodium phosphate, pH 7.0, 100 μm diethylenetriamine pentaacetic acid, 30 nm MPO, 100 μg/ml glucose, 20 ng/ml glucose oxidase, and 0.5 mm NaNO2 for 8 h (3). Oxidation reactions were terminated by addition of 40 μm butylated hydroxytoluene and 300 nm catalase to the reaction mixture.
Preparation of Phospholipid Vesicles
Stock solutions (0.6 mm) of small unilamellar vesicles comprised of PAPC, PLPC, or POPC with indicated molar ratio of specific synthetic oxidized phospholipids were prepared in argon-sparged sodium phosphate buffer by multiple extrusions through a 0.1-μm polycarbonate filter using an Avanti Mini-Extruder Set (Avanti Polar Lipids, Inc., Alabaster, AL) at 37 °C (3). For direct binding experiments, [3H]DPPC (25 μCi/mg of phospholipids) was added as a tracer (3). Vesicle size analysis was performed using a light-scattering particle analyzer equipped with a helium-neon laser (15 milliwatts), a goniometer (BI-240), and BI-9000 digital correlator (Brookhaven Instruments, Holtsville, NY). Measurements were conducted at 25 °C, and data were collected at a scattering angle of 90°. The effective hydrodynamic diameters of vesicles made of various synthetic phospholipids were very similar. The average diameter for the seven tested phospholipids was 101.6 ± 1.2 nm, and the average size distribution was 0.15 ± 0.012.
Cells
CD36- or SR-BI-expressing HEK-293 cells were generated as described before (4, 11). Experiments were performed on confluent cell monolayer in Dulbecco's modified Eagle's medium/F-12 medium containing fetal bovine serum (10%), butylated hydroxytoluene (20 μm), diethylenetriamine pentaacetic acid (100 μm), and catalase (300 nm).
Lipoprotein Binding and Competition Experiments
Binding of [125I]LDL oxidized by the MPO-H2O2-NO2− system to CD36-overexpressing or SR-BI-overexpressing HEK-293 cells or control vector cells was determined following 3-h incubations at 4 °C. In competition experiments, [125I]LDL oxidized by the MPO-H2O2-NO2− system (5 μg/ml) was incubated with cells in the presence of indicated concentrations of unlabeled oxidized phospholipids (3).
GST Fusion Proteins
All the GST fusion proteins were made in RosettaTM(DE3)pLacI strain of Escherichia coli (EMD Biosciences-Novagen, San Diego, CA) and purified using glutathione-Sepharose 4B beads (Amersham Biosciences), as described before (6). The size, amount, and purity of the fusion proteins were examined by SDS-PAGE. The molecular weight was found to be close to the predicted value, and purity was typically >95%.
Direct Binding Assays
Binding of phospholipid vesicles to GST fusion proteins was assessed by incubating [3H]DPPC-labeled phospholipids vesicles (10 μm) with the glutathione-Sepharose-bound proteins (2.5 μg of protein/tube) in phosphate-buffered saline for 3 h at 4 °C with gentle rocking. Unbound phospholipid vesicles were removed by repeated washing of the beads with phosphate-buffered saline using low speed centrifugation, and then the bound radioactivity was quantified.
Cholesteryl Ester Synthesis Assay
Thioglycollate-elicited mouse peritoneal macrophages were isolated and plated in 12-well cell culture clusters in RPMI 1640/10% fetal bovine serum (10). Confluent mouse peritoneal macrophage were incubated with synthetic phospholipids (30 μm), [14C]oleate (1.5 μCi/ml), NO2-LDL (25 μg/ml) at 37 °C. 24 h later, the supernatant was removed, cells were washed with phosphate-buffered saline, cholesterol and cholesteryl esters were extracted and separated by TLC, and incorporation of [14C]oleate in cholesteryl esters was quantified by liquid scintillation counting (12).
Statistical Analysis
The inhibition of the 125I-NO2-LDL binding to cells by synthetic phospholipids was studied at seven different phospholipids concentrations. Results were expressed as the percentage of control binding and calculated as 100 × (r/c), where c is the radioactivity count in control samples incubated without synthetic phospholipids competitor and r is the radioactivity count in samples incubated with phospholipids competitor. The percentage of control binding versus log [synthetic phospholipids] data were plotted using Prism software (GraphPad Inc., San Diego, CA), and the IC50 values were calculated using nonlinear regression curve fitting with one-site competition. Values are expressed as means ± S.E. The statistical significance was evaluated using a two-tailed unpaired Student's test. Results were considered statistically significant with p values <0.05.
RESULTS
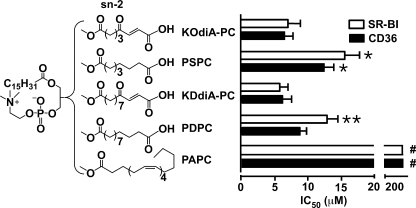
A Terminal Carboxylate Group in the sn-2 Position of Phospholipids Engenders High Binding Activity to Scavenger Receptor Class B
Our recent observations indicated that the negative charge on the phospholipid is crucial for the binding activity to CD36 (6). To see if the terminal carboxylate group alone is sufficient to generate high binding affinity to class B scavenger receptors, we designed and synthesized 1-palmitoyl-2-suberoyl-sn-glycero-3-phospho-choline (PSPC) and 1-palmitoyl-2-dodecanedioyl-sn-glycero-3-phosphocholine (PDPC) (Fig. 1). These phospholipids lack the γ-oxo-α,β-double bond present in the original oxPCCD36 lipids. PSPC and PDPC were incorporated into inert PAPC vesicles (models of oxLDL phospholipid shell), and their activity was compared with their oxPCCD36 analogs KOdiA-PC and KDdiA-PC (1, 3). Vesicles made of PAPC served as a negative control. 125I-LDL oxidized by the MPO-H2O2-NO2− system (125I-NO2-LDL) binds specifically to scavenger receptors CD36 and SR-BI via oxPCCD36, therefore we assessed the binding activity of synthetic phospholipids by their ability to block the binding of 125I-NO2-LDL to cells overexpressing CD36 or SR-BI. We found that PSPC and PDPC have IC50 values comparable to their oxPCCD36 analogs for both CD36 and SR-BI (Fig. 1), whereas vesicles made of native unoxidized phospholipids had no detectable binding activity (Fig. 1, data for PAPC are shown). Thus, the negative carboxylate group in the sn-2 position of oxPC suffices to generate high binding affinity to class B scavenger receptors. It should be noted, though, that the presence of γ-oxo-α,β-unsaturation in addition to the carboxylate group as in oxPCCD36 was usually associated with notable increases in the binding activity of the lipids (Fig. 1).
FIGURE 1.
A negative carboxylate group at the terminal of the sn-2 position of phospholipid confers significant binding activity to CD36 and SR-BI. The synthetic phospholipids were analyzed for their ability to compete for the binding of 125I-NO2-LDL (5 μg/ml) to CD36 and SR-BI transfected 293 cells as described under experimental procedures. Binding abilities of the synthetic phospholipids to both receptors were determined by assessing the concentrations of synthetic phospholipids (presented as an equimolar mixture of synthetic phospholipids and PAPC) required to block 50% of 125I-NO2-LDL binding (IC50). Results represent the mean ± S.E. of three independent experiments. #, p < 0.0001 for comparison versus KOdiA-PC, PSPC, KDdiA-PC, and PDPC; *, p < 0.05 for comparison versus KOdiA-PC and **, p < 0.05 for comparison versus KDdiA-PC.
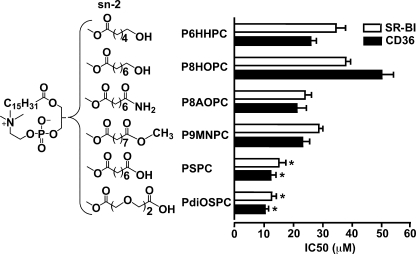
Neutral Polar Functional Groups in the Distal End of the sn-2 Position of Phospholipids Generate Weak Binding Activity
To see the effects of neutral polar functional groups in the distal end of the sn-2 position on receptor-binding activity, we designed and synthesized a series of phospholipids that are similar to PSPC but possess different functional groups at the sn-2 position (Fig. 2). 1-Palmitoyl-2-(6′-hydroxyhexanoyl)-sn-glycero-3-phosphocholine (P6HHPC), 1-palmitoyl-2-(8′-hydroxyoctanoyl)-sn-glycero-3-phosphocholine (P8HOPC), 1-palmitoyl-2-(8′-amino-8′-oxo-octanoyl)-sn-glycero-3- phosphocholine (P8AOPC), and 1-palmitoyl-2-(9′-methoxyl-9′-oxo-nonanoyl)-sn-glycero-3-phosphocholine (P9MNPC) have hydroxyl, amide, and methyl ester neutral polar groups at the terminus of sn-2 position (Fig. 2). Although the negatively charged carboxylate group can form hydrogen bonds and salt bridges with the amino group of lysines in the binding domain of the receptor (6), these neutral groups can only form weak hydrogen bonds with the amino group of lysines. In addition, we synthesized 1-palmitoyl-2-(3′,6′-dioxo-suberoyl)-sn-glycero-3-phosphocholine (PdiOSPC) (Fig. 2) with two oxygen atoms incorporated into the carbon chain at the sn-2 position which, theoretically, could make the chain more polar, serve as additional hydrogen bond acceptors, and thus increase the binding. The IC50 values (Fig. 2) showed that the phospholipids with terminal neutral polar groups have noticeable binding activity to both receptors; however, the activity was much weaker compared with phospholipids with negative carboxylate at the distal end of the sn-2 position (PSPC and PdiOSPC). The activity of PdiOSPC was similar to that of PSPC, suggesting that, in the presence of a negative carboxylate, additional oxygen atoms in the sn-2 chain do not play a significant role in binding activity (Fig. 2).
FIGURE 2.
Phospholipids with neutral polar functional groups in the distal end of sn-2 position of phospholipids have weak binding activity. The synthetic phospholipids were analyzed for their ability to compete for the binding of 125I-NO2-LDL (5 μg/ml) to CD36 and SR-BI transfected 293 cells as in Fig. 1. Results represent the mean ± S.E. of three independent experiments. *, p < 0.05 for comparison versus P6HHPC, P8HOPC, P8AOPC, and P9MNPC.
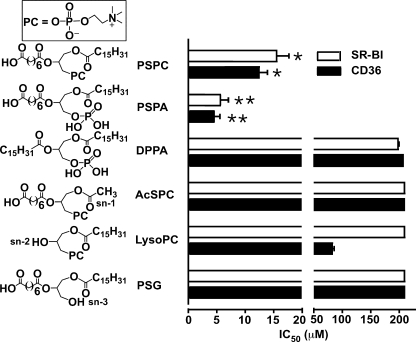
Cooperation of Functional Groups at sn-1, -2, and -3 Positions of Oxidized Phospholipids Is Required for the High Affinity Recognition by the CD36 and SR-BI
Previous experiments demonstrated that a negative group at the sn-2 position of the phospholipid is required for high binding affinity to CD36 and SR-BI. To test whether additional negative charge at the sn-3 position could further increase binding activity, we designed PSPA (Fig. 3). PSPA has a negatively charged phosphate group at the sn-3 position instead of a neutral phosphocholine group as in PSPC. PSPA was found to have a higher activity than PSPC (Fig. 3), further demonstrating the critical importance of a negative charge for high affinity binding to scavenger receptors class B. To test whether a negative group at sn-3 alone is sufficient for the high affinity binding, we designed 1,2-dipalmitoyl-sn-glycero-3-phosphatidic acid sodium salt (DPPA) possessing a negative phosphate group at the sn-3 position and two long hydrophobic chains at sn-1 and sn-2 positions (Fig. 3). DPPA was found to have very poor binding affinity (Fig. 3), demonstrating that a negative group alone at sn-3 is not sufficient to induce the high affinity binding. These data suggest that the cooperation of all three parts of the oxidized phospholipids may be important for the binding to CD36 and SR-BI. To test this hypothesis, we designed three phospholipids similar to PSPC in which one functional group either at the sn-1, sn-2, or sn-3 positions was significantly shortened or removed, as shown in Fig. 3. 1-Acetyl-2-suberoyl-sn-3-phosphocholine (AcSPC) at the sn-1 position has an acetyl group instead of long hydrophobic chain. l-α-Lysophosphatidylcholine (LysoPC) and 1-palmitoyl-2-suberoyl-sn-glycerol (PSG) contain only a hydroxyl group at sn-2 or sn-3 position, respectively. All three phospholipids (AcSPC, LysoPC, and PSG) exhibit a near complete lack of binding activity to both CD36 and SR-BI (Fig. 3). These results strongly suggest that an optimal structure of the sn-1, sn-2, and sn-3 positions of the oxidized phospholipids is important for the recognition by CD36 and SR-BI.
FIGURE 3.
All three parts of oxidized phospholipids are indispensable for the recognition by the CD36 and SR-BI. The synthetic phospholipids were analyzed for their ability to compete for the binding of 125I-NO2-LDL (5 μg/ml) to CD36 and SR-BI transfected 293 cells as in Fig. 1. Results represent the mean ± S.E. of three independent experiments. *, p < 0.001 for comparison versus DPPA, AcSPC, LysoPC, and PSG; **, p < 0.05 for comparison versus PSPC.
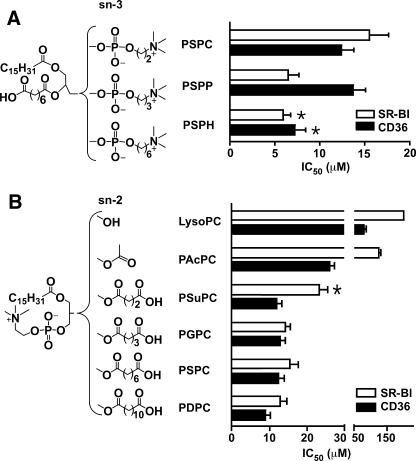
The Effect of Chain Length at sn-2 and sn-3 Positions of oxPC on the Receptor Binding Activity
To assess whether the chain length in sn-2 and sn-3 positions is important for optimal binding, we designed, synthesized, and tested a series of phospholipids with varying chain lengths at these positions, as shown in Fig. 4(A and B). The IC50 data suggest that the chain length at the sn-3 position moderately affects the binding activity. Elongation of the carbon chain from 2 to 6 resulted in a 20–40% reduction in IC50 for the phospholipids (Fig. 4A) on both CD36 and SR-BI. A comparison between 1-palmitoyl-2-succinyl-sn-glycero-3-phosphocholine (PSuPC), 1-palmitoyl-2-glutaroyl-sn-glycero-3-phospho-choline (PGPC), PSPC, and PDPC (Fig. 4B) shows that a chain length of four carbons at the sn-2 position of the phospholipids is sufficient for high affinity binding, with only moderate changes following further elongation. Compared with CD36, SR-BI is more sensitive to the chain length at the sn-2 position. For SR-BI, reduction of the chain length to four carbons almost doubles the IC50. In addition, SR-BI shows significantly less binding activity than CD36 to PAcPC that has a very short sn-2 chain length. For CD36, phospholipids with carbon chain lengths from 4 (e.g. PSuPC) to 12 (e.g. PDPC) have similar binding affinity.
FIGURE 4.
Chain length at sn-2 and -3 positions modulates the binding affinity of oxidized phospholipids. A, longer chain length at sn-3 position provides better binding activity. Results represent the mean ± S.E. of three independent experiments. *, p < 0.05 for comparison versus PSPC. B, SR-BI is more sensitive to the chain length at sn-2 position than CD36. The synthetic phospholipids were analyzed for their ability to compete for the binding of 125I-NO2-LDL (5 μg/ml) to CD36- and SR-BI-transfected 293 cells as in Fig. 1. Results represent the mean ± S.E. of three independent experiments. *, p < 0.05 for comparison versus PGPC, PSPC, and PDPC.
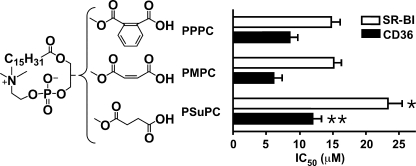
The Role of γ-Oxo-α,β-unsaturation in oxPCCD36 Activity
To test whether it is the electrophilic reactivity of the γ-oxo-α,β-double bond or other properties that contribute to increased activity of oxPCCD36 such as KOdiA-PC and KDdiA-PC compared with PSPC and PDPC, we designed 1- palmitoyl-2-maleoyl-sn-glycero-3-phosphocholine (PMPC) and 1-palmitoyl-2-phthalyl-sn-glycero-3-phosphocholine (PPPC) and compared their activity to the activity of PSuPC. PMPC and PPPC contain the structural moieties at the sn-2 position, which are similar to γ-oxo-α,β-unsaturation of oxPCCD36, but are not electrophilically reactive toward the lysine amino groups. The PSuPC used for comparison has an identical chain length, but lacks the sn-2 α,β-double bond (Fig. 5). The competition assay showed that PPPC and PMPC have higher binding affinity to both receptors compared with PSuPC. This result suggests that electrophilic reactivity is not critical for the binding activity of phospholipids with carboxylate group in the sn-2 position and that other properties are responsible for the binding.
FIGURE 5.
The rigidity of γ-oxo-α,β-unsaturated carbonyl in oxPCCD36 increases the binding activities to both receptors. The synthetic phospholipids were analyzed for their ability to compete for the binding of 125I-NO2-LDL (5 μg/ml) to CD36- and SR-BI-transfected 293 cells as in Fig. 1. Results represent the mean ± S.E. of three independent experiments. *, p < 0.05 for comparison versus PPPC and PMPC; **, p < 0.05 for comparison versus PMPC.
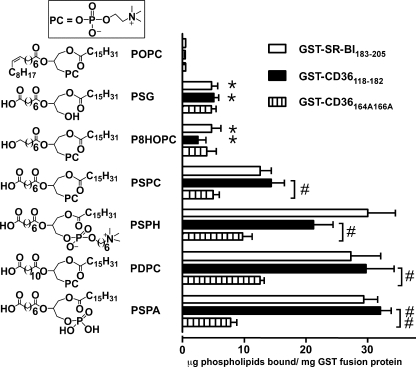
Model Oxidized Phospholipids Species Directly Bind to Scavenger Receptors Class B
So far we have employed competition assays to determine the activity of the synthesized lipids. To directly demonstrate the binding activity of the model synthetic oxPCs to scavenger receptors class B, we performed a parallel series of studies using direct binding assays. Each of a selected number of model synthetic oxPC species covering a wide range of IC50 was incorporated into small unilamellar vesicles composed of unoxidized parent lipid as a carrier and a tracer level of [3H]DPPC. Vesicles were then tested for their capacity to specifically bind to CD36118–182 or SR-BI183–205 GST fusion proteins that are shown to contain the binding domain for oxidized lipoproteins and oxidized phospholipids (4, 6). Although vesicles composed of PAPC, PLPC, or POPC alone failed to demonstrate specific binding (data for POPC are shown), vesicles containing PSPC, PDPC, PSPH, or PSPA bound to GST-CD36118–182 or GST-SR-BI183–205 at significantly greater levels than to GST (Fig. 6). At the same time, PSG or P8HOPC, which had weak competing activity, showed weak but detectable specific direct binding. In general, the ranking order of direct binding activity among the synthetic oxidized PC species was consistent with their inhibitory capacity noted in competition assays. These experiments demonstrate that model oxidized phospholipids directly interact with scavenger receptors. They also strongly suggest that the inhibitory effect of model synthetic oxPC species on the binding of NO2-LDL is not due to indirect interaction with NO2-LDL.
FIGURE 6.
Model synthetic phospholipids bind directly to CD36 and SR-BI peptides containing binding site for oxidized LDL. Binding of POPC vesicles containing 20 mol% of synthetic phospholipids and tracer amount of [3H]DPPC to the indicated glutathione-Sepharose-bound GST fusion proteins (GST-CD36118–182, GST-CD36164A166A, or GST-SR-BI183–205) was assessed as described under “Experimental Procedures.” Results represent the mean ± S.E. of three independent experiments. *, p < 0.01 for comparison versus PSPC, PSPH, PDPC, and PSPA; #, p < 0.05 and ##, p < 0.01.
To show the role of the interaction between the negative carboxylate group of the phospholipids and the positive lysine amino group in the scavenger receptor binding domain, two lysine groups (Lys-164 and Lys-166) in GST-CD36118–182 were replaced by alanine groups (Ala-164 and Ala-166), and the mutated GST-CD36164A166A was used in a direct binding assay. The test result demonstrated that replacing the two positive lysine groups with two neutral alanine groups led to the remarkable decrease in the direct binding capacity of model phospholipids with high binding activity and a negative carboxylate group at the sn-2 position (Fig. 6).
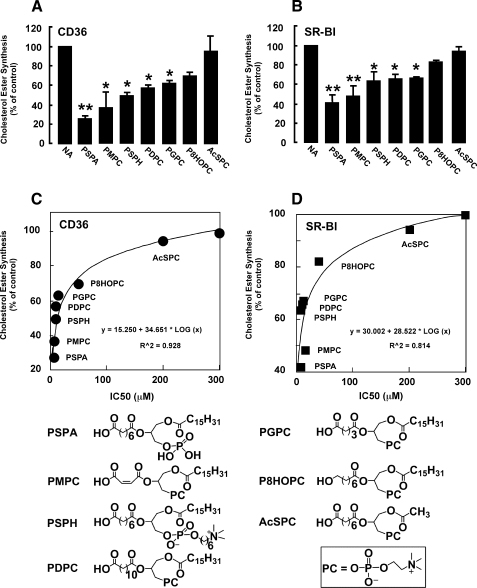
Model Oxidized Phospholipids Interfere with Macrophage Foam Cell Formation Induced by Oxidized LDL
Our data suggest that synthetic oxidized phospholipids may be able to interfere with foam cell formation by inhibiting the binding and subsequent uptake of oxidized lipoproteins mediated by scavenger receptors class B. Thus, we examined the effects of a select number of model synthetic oxPC species, covering a wide range of IC50 values (AcSPC, P8HOPC, PSPA, PGPC, PDPC, PSPH, and PMPC) on macrophage foam cell formation. Murine peritoneal macrophages were incubated with indicated oxidized phospholipids, [14C]oleate and LDL modified by the MPO-H2O2-NO2− system (NO2-LDL), which is a specific high affinity ligand for class B scavenger receptors, but not for class A scavenger receptors (9). Macrophage cholesteryl ester formation was monitored by measuring the [14C]oleate incorporation into cholesteryl ester fraction of macrophage lipids. NO2-LDL induced a significant [14C]oleate incorporation into cellular cholesteryl ester pools in macrophages, as anticipated (Fig. 7). In contrast, cells incubated with NO2-LDL in the presence of phospholipids with high binding activity (PSPA, PSPH, and PMPC) had significantly reduced [14C]oleate incorporation into cellular cholesteryl ester pools (Fig. 7A). In agreement with binding studies, AcSPC had no effect and P8HOPC had a modest effect on cholesteryl ester accumulation in macrophages exposed to NO2-LDL (Fig. 7). Similar results were obtained in CD36-deficient macrophages where uptake of NO2-LDL was mediated by SR-BI (Fig. 7B). IC50 values of model oxidized phospholipids correlated strongly with the capacity to inhibit cholesteryl ester accumulation (Fig. 7, C and D).
FIGURE 7.
Model synthetic phospholipids interfere with foam cell formation. Murine thioglycollate-elicited peritoneal macrophages were isolated from mice of indicated phenotype, cultured, and incubated with NO2-LDL (25 μg/ml) and [14C]oleate (1.5 μCi/ml) in the presence or absence of indicated synthetic phospholipids (30 μm). A and B, [14C]cholesteryl ester synthesis assay was carried out as described under “Experimental Procedures.” C and D, IC50 of synthetic phospholipids correlate with the capacity to inhibit cholesteryl ester accumulation in macrophages. *, p < 0.05; **, p < 0.01 versus NA (no addition).
DISCUSSION
Recognition of oxPCCD36 by scavenger receptors class B plays a role in several pathophysiological processes associated with oxidative stress (2, 4). We recently showed that the mechanism of interaction with CD36 is mostly electrostatic and involves two conserved, positively charged amino acids in the binding site of CD36 (6). Using specifically designed synthetic phospholipids, the present studies systematically investigated the structural basis for the recognition of oxidized phospholipids by two class B scavenger receptors: CD36 and SR-BI. We now demonstrate that the electrostatic interaction between the negative charges at the sn-2 position of phospholipids, and the positive charges in the scavenger receptor binding domain, is the most critical element of the recognition. We also showed that the negative charge at the sn-2 position requires the cooperation of both the sn-1 and sn-3 positions to promote the recognition. Finally, we found that a conjugated rigid structure at the sn-2 position, the presence of an additional negative charge at the sn-3 position, and chain length of both the sn-2 and sn-3 positions all modulate significantly the binding affinity.
Recent studies on the conformation and orientation of oxidized phospholipids in cell membranes and oxidized lipoproteins demonstrated that the truncated polar sn-2 residue protrudes into the aqueous phase, making it accessible for the receptors (5, 13). This exposed sn-2 fatty acid moiety is very likely a prerequisite for detection of oxidized phospholipids by scavenger receptors. However, the present study clearly demonstrates that all three parts of the oxidized phospholipids play a critical role in the high affinity binding to class B scavenger receptors. Deletion of functional groups at any position, as in AcSPC, LysoPC, and PSG, leads to the loss of binding affinity to scavenger receptors class B. It is possible that all three positions of the oxidized phospholipids are required to maintain the proper conformation and orientation of the oxidized phospholipid molecule in the shell of oxLDL or oxidized phospholipids in the membrane. Our findings are similar to the report of the Berliner group, who reported that all parts of the oxidized phospholipid molecules are required for activation of endothelial cells (14).
The negative carboxylate group at the sn-2 position of phospholipids can form both salt bridges and hydrogen bonds with the positively charged amino groups of lysines in the receptor binding domain, which can explain the higher binding affinity generated by the carboxylate group. Indeed, the role of positively charged lysines was demonstrated in the direct binding assay with mutated GST-CD36164A166A, where lysines were substituted with neutral alanines. GST-CD36164A166A showed only residual binding of oxidized phospholipids with a negative carboxylate group at sn-2 position.
The contribution of γ-hydroxy (or oxo)-α,β-unsaturation at sn-2 position to binding activity of oxPCCD36 was not established before. In this study, we observed that the presence of γ-oxo-α,β-unsaturation is associated with noticeable increase in the binding activity. The electrophilic reactivity of some of oxPCCD36 was previously ruled out as playing a role in high affinity CD36 binding, because the relatively unreactive oxPCCD36 with γ-hydroxy-α,β-unsaturated enoic acid groups at sn-2 position are excellent ligands (3). The common carboxylic acid missing the γ-oxo-α,β-unsaturated double bond is less acidic with a much higher pKa (∼5). However, this probably does not play a significant role, because at physiological pH of 7.4, >99% of carboxylic acids with pKa of ∼5 would dissociate and exist in the form of the negative carboxylate group. Another difference between the γ-oxo-α,β-unsaturated carboxylate group and the common carboxylate group is that the former has more rigidity, because of its conjugated structure. Our data in Fig. 5 support the conclusion that rigidity could be the mechanism contributing to the higher binding affinity generated by the γ-oxo-α,β-unsaturated carboxylate group. Furthermore, the noticeable reduction in the binding activity of γ-hydroxy-containing oxPCCD36 compared with γ-oxo-containing oxPCCD36 (3) can now be explained by the lesser rigidity of the former. Our data in Fig. 2 also support the conclusion that additional polarity of sn-2 position as such does not play a significant role in the binding activity.
In the present studies, CD36 and SR-BI were systematically compared in their recognition of phospholipids containing different functional groups. Generally speaking, CD36 and SR-BI show comparable binding affinity to various synthetic phospholipids, show limited binding affinity to neutral phospholipids, and exhibit much higher binding affinity to negatively charged oxidized phospholipids (Fig. 2). SR-BI has a slightly lower affinity for the oxidized phospholipids. This difference is especially clear for phospholipids of relatively low binding activity. Compared with CD36, SR-BI seems more sensitive to the chain length at the sn-2 position (Fig. 4B). Although CD36 still recognizes oxPC with a 1–4 carbon chain, SR-BI rapidly loses its recognition capacity when the sn-2 chain is shortened. This phenomenon suggests that SR-BI may be more selective in the recognition of ligands containing oxidized phospholipids compared with CD36. The binding domain of SR-BI for oxidized phospholipids has not yet been identified, thus it is not clear what may explain the binding differences of the two receptors.
Many of the lipids tested in the current study are model lipids, and their presence in vivo is either unknown or unlikely. Nevertheless, the information inferred by this work can be applied to lipids that are observed in vivo. Two biologically active oxidized phospholipids, glutaroyl (PGPC) and oxovaleroyl phospholipid, were previously described by Berliner and coauthors (15). Oxovaleroyl has a group in the sn-2 position that has a polarity similar to P6HHPC and P8HOPC; correspondingly, in its free nonprotein-bound form it has a weak affinity to CD36 as we showed earlier (1, 3). In contrast, PGPC possesses a carboxylate group at the sn-2 position, which, according to our results, suffices for recognition by class B scavenger receptors. Indeed, PGPC was found to be a good ligand for CD36 and SR-BI (Fig. 4B).
Our present study adds new information useful for the development of phospholipid or small molecule analogs capable of inhibiting the uptake of oxLDL and foam cell formation mediated by scavenger receptors and, potentially, suppressing atherogenesis in vivo. According to our study, a phosphatidic acid derivative incorporating a long hydrophobic chain at the sn-1 position and a relatively rigid (a conjugated double bond can be used to strengthen the rigidity) 6–12 carbon chain with a terminal carboxylate group at the sn-2 position would be a strong inhibitor. Because oxidized phospholipids are hydrolyzed by phospholipase 2, having an sn-2 ether bond would significantly increase the in vivo stability of such a phospholipid. However, several additional considerations should be taken into account. We have shown previously that oxPCCD36 activates platelets via the scavenger receptor CD36. We have also found that oxPCCD36 may potentially inhibit reverse cholesterol transport due to interference with high density lipoprotein binding to SR-BI. Thus, the effects of such inhibitors on atherosclerosis in vivo need further systematic studies.
In summary, the present systematic studies significantly extend the understanding about the structural factors governing the recognition of oxidized phospholipids by CD36 and SR-BI receptors. Our findings clearly demonstrate that, in addition to oxPCCD36, other oxidized phospholipids observed in vivo at sites of oxidative stress may be novel ligands for scavenger receptors class B.
Supplementary Material
Acknowledgments
We thank V. Verbovetskaya for technical assistance; Dr. Robert Salomon, Jim Laird, and Jamie Ott for their help in preparation of the manuscript; and Dr. Alexander M. Jamieson and Zheng Li for their help in the size analysis of phospholipid vesicles.
This work was supported, in whole or in part, by National Institutes of Health Grants HL053315, HL077213 and 2P01HL073311-06 (to E. A. P.) and HL053315 (to L. M. S.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Methods, Schemes S1–S5, and additional references.
- oxPCCD36
- specific oxidized phospholipids
- MPO
- myeloperoxidase
- KOdiA-PC
- 5-keto-6-octendioic acid ester of 2-lyso-PC
- KDdiA-PC
- 9-keto-10-dodecendioic acid ester of 2-lyso-PC
- P6HHPC
- 1-palmitoyl-2-(6′-hydroxyhexanoyl)-sn-glycero-3-phosphocholine
- P8HOPC
- 1-palmitoyl-2-(8′-hydroxyoctanoyl)-sn-glycero-3-phosphocholine
- P8AOPC
- 1-palmitoyl-2-(8′-amino-8′-oxo-octanoyl)-sn-glycero-3-phosphocholine
- P9MNPC
- 1-palmitoyl-2-(9′-methoxyl-9′-oxo-nonanoyl)-sn-glycero-3-phosphocholine
- LDL
- low density lipoprotein
- oxLDL
- oxidized LDL
- DPPC
- 1,2-dihexadecanoyl-sn-glycero-3-phosphocholine
- PAPC
- 1-hexadecanoyl-2-eicosatetra-5′,8′,11′,14′-enoyl-sn-glycero-3-phosphocholine
- GST
- glutathione S-transferase
- PSPC
- 1-palmitoyl-2-suberoyl-sn-glycero-3-phospho-choline
- PDPC
- 1-palmitoyl-2-dodecanedioyl-sn-glycero-3-phosphocholine
- DPPA
- 1,2-dipalmitoyl-sn-glycero-3-phosphatidic acid sodium salt
- P9MNPC
- 1-palmitoyl-2-(9′-methoxyl-9′-oxo-nonanoyl)-sn-glycero-3-phosphocholine
- PDiOSPC
- 1-palmitoyl-2-(3′,6′-dioxo-suberoyl)-sn-glycero-3-phosphocholine
- AcSPC
- 1-acetyl-2- suberoyl-sn-3-phosphocholine
- LysoPC
- l-α-lysophosphatidylcholine
- PSG
- 1-palmitoyl-2-suberoyl-sn-glycerol
- oxPC
- oxidized PC
- PSuPC
- 1-palmitoyl-2-succinyl-sn-glycero-3-phosphocholine
- PGPC
- 1-palmitoyl-2-glutaroyl-sn-glycero-3-phosphocholine
- PMPC
- 1-palmitoyl-2-maleoyl-sn-glycero-3-phosphocholine
- PPPC
- 1-palmitoyl-2-phthalyl-sn-glycero-3-phosphocholine
- PSPA
- 1-palmitoyl-2-suberoyl-sn-glycerophosphatidic acid
- PSPH
- 1-palmitoyl-2-suberoyl-sn-glycero-3-phosphatidyl-(N,N,N-trimethylamino)-hexanol
- PLPC
- 1-hexadecanoyl-2-octadecadi-9′,12′-enoyl-sn-glycero-3-phosphocholine
- POPC
- 1-hexadecanoyl-2-octadecanoyl-2- octadec-9′-enoyl-sn-glycero-3-phosphocholine.
REFERENCES
- 1.Podrez E. A., Poliakov E., Shen Z., Zhang R., Deng Y., Sun M., Finton P. J., Shan L., Febbraio M., Hajjar D. P., Silverstein R. L., Hoff H. F., Salomon R. G., Hazen S. L. (2002) J. Biol. Chem. 277, 38517–38523 [DOI] [PubMed] [Google Scholar]
- 2.Podrez E. A., Byzova T. V., Febbraio M., Salomon R. G., Ma Y., Valiyaveettil M., Poliakov E., Sun M., Finton P. J., Curtis B. R., Chen J., Zhang R., Silverstein R. L., Hazen S. L. (2007) Nat. Med. 13, 1086–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Podrez E. A., Poliakov E., Shen Z., Zhang R., Deng Y., Sun M., Finton P. J., Shan L., Gugiu B., Fox P. L., Hoff H. F., Salomon R. G., Hazen S. L. (2002) J. Biol. Chem. 277, 38503–38516 [DOI] [PubMed] [Google Scholar]
- 4.Ashraf M. Z., Kar N. S., Chen X., Choi J., Salomon R. G., Febbraio M., Podrez E. A. (2008) J. Biol. Chem. 283, 10408–10414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greenberg M. E., Li X. M., Gugiu B. G., Gu X., Qin J., Salomon R. G., Hazen S. L. (2008) J. Biol. Chem. 283, 2385–2396 [DOI] [PubMed] [Google Scholar]
- 6.Kar N. S., Ashraf M. Z., Valiyaveettil M., Podrez E. A. (2008) J. Biol. Chem. 283, 8765–8771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun M., Deng Y., Batyreva E., Sha W., Salomon R. G. (2002) J. Org. Chem. 67, 3575–3584 [DOI] [PubMed] [Google Scholar]
- 8.Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. (1978) Anal. Biochem. 87, 206–210 [DOI] [PubMed] [Google Scholar]
- 9.Podrez E. A., Schmitt D., Hoff H. F., Hazen S. L. (1999) J. Clin. Invest. 103, 1547–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoppe G., O'Neil J., Hoff H. F. (1994) J. Clin. Invest. 94, 1506–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Podrez E. A., Febbraio M., Sheibani N., Schmitt D., Silverstein R. L., Hajjar D. P., Cohen P. A., Frazier W. A., Hoff H. F., Hazen S. L. (2000) J. Clin. Invest. 105, 1095–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldstein J. L., Dana S. E., Brown M. S. (1974) Proc. Nat. Acad. Sci. U.S.A. 71, 4288–4292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li X. M., Salomon R. G., Qin J., Hazen S. L. (2007) Biochemistry 46, 5009–5017 [DOI] [PubMed] [Google Scholar]
- 14.Subbanagounder G., Leitinger N., Schwenke D. C., Wong J. W., Lee H., Rizza C., Watson A. D., Faull K. F., Fogelman A. M., Berliner J. A. (2000) Arterioscler. Thromb. Vasc. Biol. 20, 2248–2254 [DOI] [PubMed] [Google Scholar]
- 15.Watson A. D., Leitinger N., Navab M., Faull K. F., Hörkkö S., Witztum J. L., Palinski W., Schwenke D., Salomon R. G., Sha W., Subbanagounder G., Fogelman A. M., Berliner J. A. (1997) J. Biol. Chem. 272, 13597–13607 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.