Abstract
The central enzyme of the visual transduction cascade, cGMP phosphodiesterase (PDE6), is regulated by its γ-subunit (Pγ), whose inhibitory constraint is released upon binding of activated transducin. It is generally believed that the last four or five C-terminal amino acid residues of Pγ are responsible for blocking catalysis. In this paper, we showed that the last 10 C-terminal residues (Pγ78–87) are the minimum required to completely block catalysis. The kinetic mechanism of inhibition by the Pγ C terminus depends on which substrate is undergoing catalysis. We also discovered a second mechanism of Pγ inhibition that does not require this C-terminal region and that is capable of inhibiting up to 80% of the maximal cGMP hydrolytic rate. Furthermore, amino acids 63–70 and/or the intact α2 helix of Pγ stabilize binding of C-terminal Pγ peptides by 100-fold. When PDE6 catalytic subunits were reconstituted with portions of the Pγ molecule and tested for activation by transducin, we found that the C-terminal region (Pγ63–87) by itself could not be displaced but that transducin could relieve inhibition of certain Pγ truncation mutants. Our results are consistent with two distinct mechanisms of Pγ inhibition of PDE6. One involves direct interaction of the C-terminal residues with the catalytic site. A second regulatory mechanism may involve binding of other regions of Pγ to the catalytic domain, thereby allosterically reducing the catalytic rate. Transducin activation of PDE6 appears to require interaction with both the C terminus and other regions of Pγ to effectively relieve its inhibitory constraint.
Keywords: Allosteric Regulation, Cyclic GMP (cGMP), Enzyme Inhibitors, G Proteins, Heterotrimeric G Proteins, Phosphodiesterases, Photoreceptors, Protein Conformation, PDE6
Introduction
The photoreceptor cyclic nucleotide phosphodiesterase (PDE6)2 is the central enzyme in the vertebrate visual signaling pathway in rods and cones. Phototransduction is initiated when light induces the isomerization of the 11-cis-retinal chromophore of rhodopsin, which leads to activation of the photoreceptor-specific G-protein, transducin. Transducin binds GTP and releases its activated α-subunit (Tα*-GTP) to activate membrane-associated rod PDE6 by relieving the inhibition of the γ-subunit at the active sites. The activation of PDE6 results in rapid lowering of cGMP levels, closure of cGMP gated ion channels, and hyperpolarization of the cell membrane (1–5).
The PDE6 holoenzyme consists of a catalytic dimer of α- and β-subunits (Pαβ) and two inhibitory γ-subunits (Pγ) that are tightly bound to Pαβ. Considering its small size, the Pγ subunit of rod and cone PDE6 serves a remarkable number of functions (reviewed in Refs. (6 and 7): 1) a primary function of the Pγ subunit is to inhibit catalysis of cGMP by binding to the catalytic domain of PDE6; 2) the Pγ subunit also enhances the binding affinity of cGMP to the regulatory GAF (cGMP-regulated PDEs, certain adenylate cyclases, and the transcription factor Fh1A of bacteria) domain; 3) activated transducin binds to the Pγ subunit, leading to deinhibition of PDE6 at its active site; and 4) during deactivation, the γ-subunit participates in a protein complex with the RGS9 and other proteins to accelerate the GTPase activity of activated transducin.
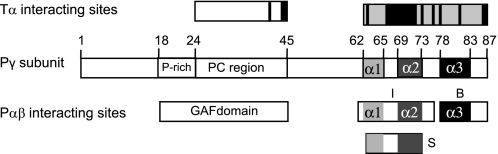
The 10-kDa Pγ inhibitory subunit has two major functional domains. The proline-rich and polycationic region (amino acids 18–45; see Fig. 1A) interacts with the GAF domains of the PDE6 catalytic dimer (8–11) and stabilizes noncatalytic cGMP binding to the GAF domains (12). The C-terminal region (broadly defined as amino acids 62–87) interacts with the catalytic domain and blocks the catalytic activity (8, 9, 13–17). Structural studies of Pγ in solution indicate that this protein is overall an intrinsically disordered protein (18) but contains α-helical secondary structure (α1, α2, and α3; Fig. 1A) in its C-terminal region (19); the α-helical content within the C-terminal region of Pγ is also observed when this region of Pγ forms a complex with either transducin (20) or a chimeric PDE5/6 catalytic domain (21).
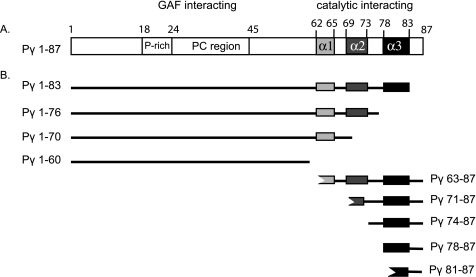
FIGURE 1.
Domain organization of inhibitory Pγ subunit. A, the 10-kDa Pγ inhibitory subunit has two major functional domains (6, 7). The proline-rich and polycationic region (amino acids 18–45, open box) serves as the primary interaction site with the GAF domains of the PDE6 catalytic dimer. The C-terminal domain of Pγ (amino acids ∼62–87) is shown as consisting of three α-helical sequences (α1, light gray; α2, dark gray; α3, black) based on structural studies of its complex with transducin α-subunit (20) or free in solution (19); the functional interaction of these putative structural elements with PDE6 catalytic dimer is a focus of the experiments in this paper. B, some of the Pγ truncation mutants and synthetic peptides used in this study are depicted.
Despite a wealth of biochemical and structural data, there is currently no consensus in the literature on the exact structural element(s) responsible for inhibition of cyclic nucleotide hydrolysis at the PDE6 active site. For example, C-terminally truncated Pγ mutants lacking the last 5–10 amino acids have been reported to result in no inhibition (9, 22, 23) up to 50% inhibition (15) of catalytic activity. There are also reports that inhibition of PDE6 catalysis can occur without an absolute requirement of the extreme C-terminal amino acids (8, 15–17).
The kinetic mechanism of Pγ inhibition of PDE6 catalysis is generally believed to occur by a simple competition of Pγ and substrate for access to the active site. Although this model is supported by kinetic studies showing competition between cAMP hydrolysis and Pγ binding at the active site (12), similar experiments using cGMP as the substrate are not consistent with a simple competitive inhibition mechanism (24). Pharmacological studies with active site inhibitors also reveal complex kinetic behavior of Pγ, PDE inhibitors, and substrate at the PDE6 active site (25–27), suggesting that a more complex mechanism of inhibition by Pγ may be occurring.
The same two functional domains of Pγ that interact with PDE6 have also been shown to bind to activated transducin α-subunit. The polycationic, GAF domain-interacting region of Pγ (amino acids 24–45) interacts with transducin, although the functional significance of this interaction for the mechanism of transducin activation is not clear (28–31). The C-terminal region of Pγ is bound by activated transducin during PDE6 activation (8, 9, 20, 32, 33). Furthermore, during the deactivation process, this same C-terminal region of Pγ forms a complex with the RGS9-1 complex to help accelerate the GTPase activity of transducin (20) and speed the deactivation process.
In this paper, we documented two distinct mechanisms of Pγ inhibition: one using the last 10 C-terminal residues of Pγ to “block” the active site and a second one in which amino acid residues between positions 61 and 76 of Pγ can “induce” an inhibitory conformational change in the catalytic domain. Furthermore, we defined a similar region of Pγ (beginning approximately at amino acid 61 and extending to the end of the α2 helical region) that appears able to “sense” conformational changes in the catalytic domain. The ability of transducin to relieve inhibition of some—but not all—of our Pγ constructs suggests that transducin requires multiple sites of interaction with Pγ, including interactions distinct from its role in displacing the Pγ C terminus from the enzyme active site.
EXPERIMENTAL PROCEDURES
Materials
Bovine retinas were purchased from W. L. Lawson, Inc. Synthetic peptides Pγ63–87, Pγ71–87 Pγ74–87, Pγ78–87, and Pγ81–87 were purchased from New England Peptide and repurified to >95% purity by reverse phase high pressure liquid chromatography. Ultima Gold scintillation fluid was from PerkinElmer Life Sciences. The filtration membranes were from Millipore, the bicinchoninic acid protein assay reagents were from Pierce, and all of other chemicals were from Sigma-Aldrich. The primers for the construction of C-terminally truncated Pγ mutants were from Invitrogen. The plasmid mini and midi preparation kits were from Qiagen.
Construction of Pγ Mutants
The Pγ truncation mutants lacking various lengths of the C-terminal amino acids were generated using PCR; Pγ1–83 and Pγ1–86 were constructed as previously described (15). For the mutants Pγ1–60, Pγ1–70, and Pγ1–80, the constructs were chemically synthesized (Blue Heron Biotechnology). For PCR constructs, the primers were designed based on the sequence of rod bovine Pγ (primer sequences available on request), and the PCR products were inserted into the NdeI and BamHI sites of the pET11a vector (Novagen). The sequences of all Pγ mutants were confirmed by DNA sequencing using Applied Biosystems BigDye terminator cycle sequencing kits in the Hubbard Center for Genome Studies at the University of New Hampshire.
Purification of Pγ Truncation Mutants
Recombinant Pγ truncation mutants were expressed in Escherichia coli BL21/DE3. The bacterial extract was partially purified by cation exchange chromatography using SP Sepharose, followed by C4 reverse phase high pressure liquid chromatography (34). The purity (>95%) and size of these proteins were evaluated by SDS-polyacrylamide gel electrophoresis. Most mutants were also examined by analytical ultracentrifugation using sedimentation velocity analysis to confirm the absence of aggregates or self-association of the constructs. Protein concentrations were determined by the bicinchoninic acid protein assay (35) using bovine γ-globulin as a standard.
PDE6 and Pαβ Purification and Functional Assays
Bovine rod PDE6 was purified from bovine retinas as described (36). Pαβ catalytic dimers lacking Pγ were prepared by limited trypsin proteolysis and repurified by Mono Q anion exchange chromatography to remove proteolytic fragments of Pγ (36). The PDE6 concentration was estimated based on the rate of cGMP hydrolysis of trypsin-activated PDE6 and a knowledge of the kcat of the enzyme (5600 mol cGMP hydrolyzed per Pαβ per s) (37); this estimate was validated by determining stoichiometric binding of [3H]cGMP to PDE6 with a filter binding assay (38).
To prepare reconstituted PDE6 with cGMP bound to the regulatory GAF domains, purified Pαβ was incubated with 10 mm EDTA for 2 h at room temperature, followed by the addition of 1 μm cGMP in the presence of truncation mutants (Pγ1–80); the inclusion of Pγ constructs containing the proline-rich and polycationic region (see Fig. 1A) with Pαβ served to sufficiently stabilize cGMP binding to the noncatalytic binding sites so that these sites remained liganded for the duration of the activity assay. Occupancy of the GAP domains with bound cGMP was verified experimentally as described previously (39). After adding 10 mm MgCl2 to restore the ability of PDE6 to undergo catalysis, cyclic nucleotide hydrolytic rates were measured in 20 mm Tris, 10 mm MgCl2, 0.5 mg/ml bovine serum albumin, and the indicated concentration of cyclic nucleotides using either a phosphate release microplate assay or a radiotracer assay (40).
Purification of Activated Transducin α-Subunit (Tα*) and Transducin Activation of Reconstituted Pαβ and Pγ Mutants
Transducin α-subunits were extracted from the PDE6-depleted bovine ROS membranes by the addition of 50 μm GTPγS. The extracted Tα*-GTPγS was purified on a Blue Sepharose column as described (41, 42), followed by gel filtration chromatography to completely remove PDE6. The concentration of Tα*-GTPγS was determined by a colorimetric protein assay. Purified T*α-GTPγS was stored at 4 °C and used for a few weeks. For the transducin activation measurement, purified Pαβ was preincubated with Pγ mutants or peptides at the indicated concentrations to maximally inhibit PDE activity. For the case of Pγ63–87, the concentration (10 μm) was chosen to suppress ∼70% of PDE activity, enabling us to detect the ability of transducin to stimulate PDE activity. 9 μm activated transducin (supplemented with 50 μm GTPγS) was added to above mixture and incubated for 5 min. The PDE activity was measured using radiotracer assay using 0.1 mm [3H]cAMP as substrate.
Data Analysis
Except where noted, the experiments were repeated at least three times, and the average results were reported as the means ± S.D. The curve fitting was performed with the computer program SigmaPlot (SPSS, Inc., Chicago, IL). The affinity of peptides (IC50) was determined by fitting experimental data to a three- or four-parameter logistic dose response function (43).
RESULTS
The Last 10 Amino Acids of Pγ Are Sufficient to Fully Inhibit Catalysis
Although it is known that the C-terminal region of Pγ interacts with the catalytic pocket and blocks catalysis (see the Introduction), it is surprising that the structural requirements for complete suppression of PDE6 catalytic activity have never been determined. To better understand the structure/function relationship of Pγ to block catalysis of the PDE6 active sites, we synthesized a series of synthetic peptides differing in the length of the C-terminal amino acid sequence (Fig. 1B) and examined their ability to inhibit PDE6 catalytic activity.
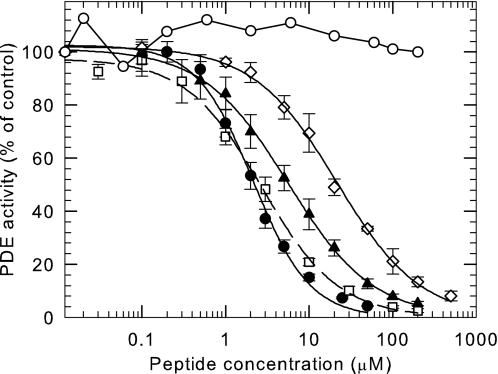
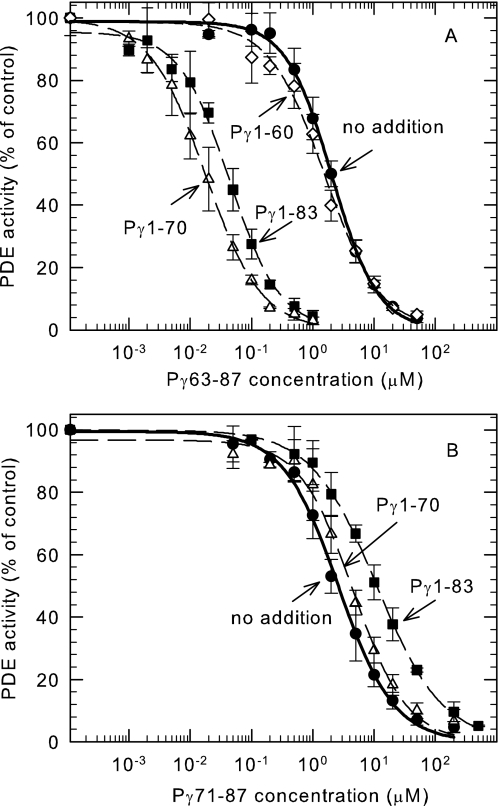
Although the last few C-terminal residues of Pγ have been reported to physically interact with the catalytic domain to block catalysis (9, 13–17), the shortest peptide we tested (Pγ81–87) showed no ability to inhibit the catalytic activity of PDE6 under our experimental conditions (IC50 > 2 mm; Fig. 2). However, a synthetic peptide containing three additional amino acids to include the α3 helix (Pγ78–87) was sufficient to completely suppress the catalytic activity of PDE6 (IC50 = 21.4 ± 1.2 μm). This result demonstrates the central importance of the α3 helical region of Pγ to stabilize binding at least 100-fold so that this segment of the Pγ structure can bind to and inhibit catalysis at the active site of PDE6.
FIGURE 2.
The last 10 amino acids of Pγ are sufficient to fully inhibit PDE6 catalytic activity. Purified Pαβ (0.2 nm) was preincubated for 20 min with the indicated C-terminal Pγ synthetic peptides, followed by addition of 2 mm cGMP substrate. ●, Pγ63–87; □, Pγ71–87; ▴, Pγ74–87; ♢, Pγ78–87; ○, Pγ81–87. Catalytic activity was measured by the phosphate release assay. The data are the means ± S.D. of three experiments. The lines represent the fit to a three-parameter logistic dose-response equation with IC50 values of 2.2 ± 0.1 μm (●, Pγ63–87), 2.8 ± 0.2 μm (□, Pγ71–87), 5.6 ± 0.2 μm (▴, Pγ74–87), and 21.4 ± 1.2 μm (♢, Pγ78–87).
Compared with the dramatic stabilizing interactions conferred by the α3 helical region of Pγ, we observed only modest additional stabilizing interactions when longer Pγ synthetic peptides were tested. As shown in Fig. 2, a 4-fold increase in inhibitory potency was observed for Pγ74–87 compared with Pγ78–87. Inclusion of part (Pγ71–87) or all (Pγ63–87) of the α2 helical region of Pγ had an even smaller effect on the IC50 of inhibition of these Pγ peptides (Fig. 2). We conclude that the region between amino acids 63 and 77 of the Pγ sequence contain minor stabilizing interactions with the PDE6 catalytic domain, compared with the important stabilizing influence of the α3 helical region that comprises amino acids 78–83 of Pγ.
The C-terminal Region of Pγ Is a Classical Noncompetitive Inhibitor of cGMP Hydrolysis, but a Competitive Inhibitor When cAMP Is the Substrate
While investigating the dose-response relationship of the C-terminal synthetic peptides of Pγ shown in Fig. 2, we were surprised to find that the IC50 for inhibition was independent of the cGMP substrate concentration used for the activity measurements (data not shown). This is inconsistent with the commonly held model that the C terminus of Pγ is a competitive inhibitor of the active site of PDE6 (see the Introduction).
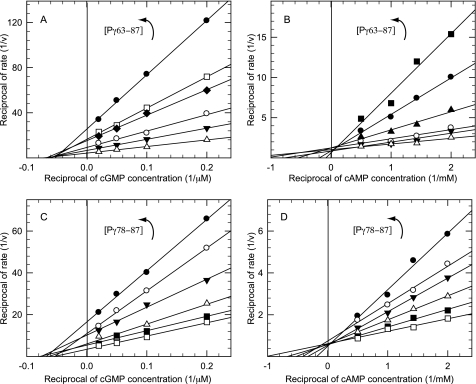
To examine this further, we assayed the inhibitory effects of our C-terminal synthetic peptides over a range of cGMP concentrations to determine the kinetic mechanism of inhibition. Surprisingly, a double-reciprocal plot of these data visually demonstrates that Pγ63–87 was a simple noncompetitive inhibitor of catalysis (KI = 1.8 ± 0.7 μm) when cGMP was the substrate (Fig. 3A). When cAMP was used as the substrate, the mechanism of inhibition by Pγ63–87 changed to that of a simple competitive inhibitor (Fig. 3B; KI = 2.4 ± 0.4 μm).
FIGURE 3.
C-terminal peptides of Pγ are competitive for cGMP hydrolysis and noncompetitive for cAMP hydrolysis. Purified Pαβ was incubated with increasing amounts of Pγ63–87 for 10 min at room temperature. ▵, 0 μm; ▾, 1 μm; ○, 2 μm; ♦, 4 μm; ▴, 5 μm; □, 6 μm; ●, 10 μm; ■, 20 μm. The initial rate was determined by adding the indicated amount of [3H]cGMP (A) or [3H]cAMP (B). In separate experiments, purified Pαβ was incubated with increasing amounts of Pγ78–87 for 10 min at room temperature. □, 0 μm; ■, 10 μm; ▵, 20 μm; ▾, 40 μm; ○, 60 μm; ●, 100 μm. The initial rate was determined by adding the indicated amount of [3H]cGMP (C) or [3H]cAMP (D). The data were plotted as a double reciprocal plot (1/v versus 1/[S]) and are representative of at least two experiments.
To evaluate whether the inhibition mechanism was dependent on the length of the Pγ synthetic peptide, we repeated this analysis with the shorter Pγ78–87 peptide. We observed the same double-reciprocal pattern indicative of noncompetitive inhibition when cGMP was the substrate (Fig. 3C, KI = 28.1 ± 3.2 μm) and competitive inhibition when cAMP was the substrate (Fig. 3D; KI = 48.5 ± 2.1 μm). Although shortening the Pγ C-terminal peptide by 15 residues reduced the inhibitory potency ∼10-fold in both cases, the mechanism of inhibition of the Pγ peptide (noncompetitive with cGMP, competitive with cAMP) was unchanged by reducing its size.
Because cGMP is a ligand for the noncatalytic cGMP-binding site within the regulatory GAF domains, but cAMP is not (44), we attempted to test the hypothesis that occupancy of the GAF domain by cGMP allosterically alters the catalytic domain and how it interacts with Pγ. Unfortunately, we were unable to find experimental conditions that retained bound cGMP in the GAF domain-binding site in the absence of the GAF-interacting region of Pγ for the duration of time needed to evaluate the kinetic mechanism of inhibition by Pγ.
Inhibition of Catalysis Can Occur by a Distinct Mechanism Induced by the First 76 Amino Acids of Pγ
Although the above results demonstrate that the last 10 amino acids are sufficient to suppress 100% of the catalytic activity of PDE6, previous work suggested that other regions of Pγ could modulate catalytic activity (see the Introduction). Recently, we showed that Pγ1–60 had no inhibitory activity by itself but was able to induce an allosteric change that was communicated between the regulatory GAF domains and the catalytic domains (39). To examine evidence for an alternative mechanism of Pγ inhibition, we used a set of longer truncation mutants (Fig. 1B) to identify structural elements that induce a reduction in catalytic activity of the PDE6 catalytic dimer.
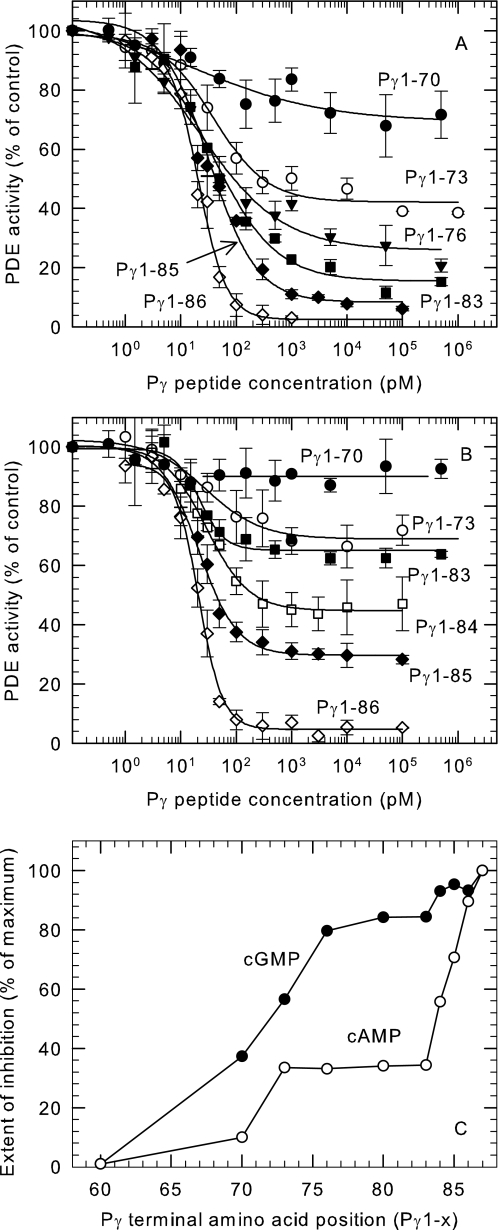
Surprisingly, the truncation mutant Pγ1–76 that lacks the last 11 amino acids (including the entire α3 helix) showed 80% inhibition of cGMP hydrolysis (Fig. 4A). The extent to which truncated Pγ was able to inhibit cGMP hydrolysis depended on the length of the protein. Whereas Pγ1–60 had no detectible inhibitory activity (data not shown), Pγ1–70 was capable of partially inhibiting cGMP hydrolysis (37%), and adding an additional three amino acids (Pγ1–73) led to inhibition of 56% of the total catalytic activity (Fig. 4A). We conclude that Pγ truncation mutants containing the region between amino acids 61–76 (“inducer” region; see “Discussion”) are able to inhibit cGMP hydrolysis by a different mechanism than that used by the last 10 C-terminal residues of Pγ.
FIGURE 4.
Inhibition studies of series of truncation mutants revealed a second inhibition mechanism of Pγ. Purified Pαβ (10 pm) was incubated with the Pγ truncation mutants for 10 min at room temperature. The PDE activity was measured by the addition of 1 μm [3H]cGMP (A) or 0.1 mm [3H]cAMP (B) using the radiotracer assay. PDE activity is normalized to the percentage of Pαβ activity in the absence of Pγ truncation mutants. The data are the means ± S.D. of three experiments. The lines represent the fit to a four-parameter logistic dose-response equation, with the values for the IC50 and the maximum extent of inhibition summarized in supplemental Table S1. C, summary of the maximum inhibition of the truncation mutants when cGMP (●) or cAMP (○) was used as substrate. The x axis represents the position of the last amino acid at the C-terminal end of the Pγ mutants.
Because this result was entirely unexpected, we were concerned that contaminating fragments of Pγ (resulting from trypsin proteolysis that was used to prepare Pαβ; see “Experimental Procedures”) might account for this behavior. To rule out this possibility, we added the C-terminal peptide (Pγ63–87) in an amount stoichiometric with the Pαβ concentration used in these experiments (10 pm). We observed no inhibition of catalysis by this amount of Pγ63–87 in the absence (Fig. 2, filled circles) or presence of the Pγ truncation mutants (data not shown).
The effectiveness of this so-called inducer region of Pγ (i.e. amino acids 61–76) to inhibit catalysis was found to depend on the substrate being tested. Fig. 4B shows that most Pγ truncation mutants were less effective in inhibiting cAMP hydrolysis than cGMP hydrolysis. Furthermore, the extent of maximal inhibition of cAMP hydrolysis was similar for the Pγ truncation mutants varying in length from Pγ1–73 to Pγ1–83 (Fig. 4C).
To test whether the greater effectiveness of Pγ truncation mutants to inhibit cGMP hydrolysis resulted from allosteric regulation of the catalytic domains by cGMP binding to the regulatory GAF domains, we prepared PDE6 catalytic dimers reconstituted with the Pγ1–80 truncation mutant to which cGMP was either present or absent at the regulatory GAF domains (see “Experimental Procedures”) and then measured the rate of cAMP hydrolysis. We found no difference in cAMP hydrolytic rates as a function of cGMP occupancy of the GAF domains (data not shown), indicating that the occupation of the GAF domain by cGMP was not responsible for this effect.
We also noted that the effectiveness of Pγ truncation mutants to inhibit catalysis was dependent on the cGMP substrate concentration. When several different Pγ truncation mutants were tested with millimolar levels of cGMP as substrate (Table 3 of Ref. 15; see also supplemental Table S1), lower maximal extents of inhibition were seen compared with the results in Fig. 4A using 1 μm cGMP as substrate. Although the mechanism by which high cGMP concentrations reduces the maximum inhibitory effect of these Pγ truncation mutants is not understood, we speculate that high cGMP concentrations may induce a conformational change in the PDE6 catalytic dimer, similar to that recently reported for the related PDE5 enzyme (45), that alters the effectiveness of Pγ truncation mutants to inhibit catalysis.
A Short Segment within the C-terminal Region of Pγ Can Detect Conformational Changes Induced by the Binding of Pγ Truncation Mutants
The previous sections defined two different regions of Pγ that are capable of inhibiting catalysis. The C-terminal 10 amino acids (“blocking” region) are, by themselves, sufficient to fully inhibit catalysis, whereas the interval between amino acids 61 and 76 (inducer region) can inhibit most of the PDE6 activity in the absence of the blocking region. To examine whether these two different inhibitory activities interact with each other in a cooperative manner, we tested the hypothesis that the N-terminal portion of Pγ would enhance the binding affinity of C-terminal peptides.
When Pγ1–60 was added to Pαβ in the presence of Pγ63–87, the apparent binding affinity of the C-terminal peptide was only slightly increased (∼2-fold; Fig. 5A). However, when an additional 10 amino acids were present (Pγ1–70), the affinity of Pγ63–87 was dramatically increased ∼100-fold (Fig. 5A). Lengthening the N-terminal portion of Pγ another 3 (Pγ1–73; data not shown), 6 (Pγ1–76; data not shown), or 13 amino acids (Pγ1–83; Fig. 5A) showed a similar ability to enhance the inhibitory potency of the C-terminal Pγ63–87 peptide.
FIGURE 5.
The truncation mutants of Pγ greatly enhanced the affinity of Pγ63–87 but not Pγ71–87. Purified Pαβ (10 pm) was preincubated with the indicated concentration of Pγ63–87 (A) or Pγ71–87 (B) in the presence of Pγ1–60 (♢), Pγ1–70 (▵), or Pγ1–83 (■) or 10 mm Tris (●) for 10 min at room temperature. The PDE activity was measured by the addition of 0.1 mm [3H]cAMP using the radiotracer assay and normalized to the percentage of the activity in the absence of C-terminal peptide (Pγ1–60, no inhibition; Pγ1–70, 10% inhibition; Pγ1–83, 35% inhibition). The data are the means ± S.D. of three experiments. The lines represent the fit to a three-parameter logistic dose-response equation. The following IC50 values were determined for A: 2.1 ± 0.1 μm (no addition), 1.6 ± 0.1 μm (Pγ1–60), 0.018 ± 0.001 μm (Pγ1–70), and 0.045 ± 0.003 μm (Pγ1–83). For B, the IC50 values were: Pγ71–87: 2.6 ± 0.2 μm (no addition), 4.6 ± 0.4 μm (Pγ1–70), and 11.0 ± 0.4 μm (Pγ1–83).
Having determined that the inhibitory activity of Pγ63–87 was potentiated by the presence of Pγ1–70, we next asked whether shorter C-terminal peptides of Pγ would also be sensitive to the presence of N-terminal truncation mutants. As seen in Fig. 5B, shortening the C-terminal fragment by eight amino acids (Pγ71–87) abolished the ability of Pγ1–70 or Pγ1–83 to enhance the binding of the C-terminal peptide; in the latter case Pγ1–83 actually reduced by ∼4-fold the effectiveness of Pγ71–87 to inhibit catalysis. The ability of Pγ63–87, but not Pγ71–87, to be stabilized by the Pγ1–70 N-terminal fragment defines a region of 7–10 amino acid residues starting at position 63 that is capable of detecting the presence of the N-terminal fragment and/or a conformational change of the catalytic domain caused by this fragment (“sensor” region; see “Discussion”).
The fact that the potentiation effect of the N-terminal truncation mutant was only observed when combined with a C-terminal peptide with which it shared 8 amino acids (residues 63–70) raised a concern about a potential artificial interaction between Pγ1–70 and Pγ63–87 being responsible for this effect. To directly address this, sedimentation equilibrium measurements were performed with the two individual Pγ proteins and with an equimolar mixture of them. Even at the highest concentration tested (50 μm), no evidence for self-association or interaction of the two Pγ fragments could be detected (data not shown). Furthermore, we have conducted an additional experiment in which a 100-fold enhancement of the inhibitory potency of Pγ67–87 could be observed with the N-terminal mutant Pγ1–68 (data not shown); in this instance, the overlap was reduced to 2 amino acids. Together, these two observations greatly reduce the likelihood that the observed potentiation of the inhibitory effectiveness of the C-terminal region of Pγ by these N-terminal Pγ truncation mutants can be attributed to an artificial interaction of the overlapping sequence of the two Pγ constructs.
Transducin Activation of PDE6 Holoenzyme Requires Additional Sites of Interaction besides the C-terminal Blocking Region of Pγ
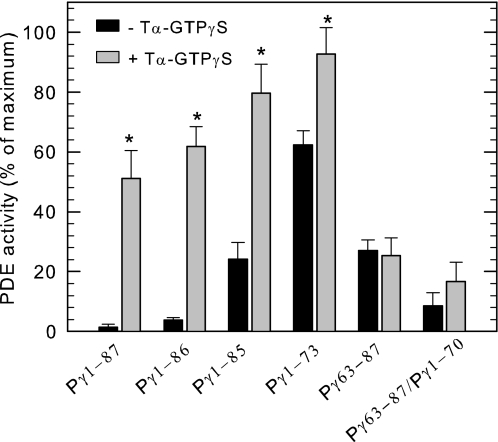
It is known that transducin α-subunit has multiple sites of interaction with PDE6 holoenzyme, and the prevailing view is that Tα* binds to the C-terminal region of Pγ to displace these blocking residues and thereby relieve its inhibitory action. Having documented above two different mechanisms of regulation of PDE6 activity by Pγ, we questioned whether Tα* interacts with one or both regions responsible for these different mechanisms of inhibition. To study this, we mixed Pαβ with various fragments of Pγ and tested the ability of the reconstituted PDE6 to become activated upon exposure to saturating amounts of Tα*-GTPγS. Because Pαβ binds with high affinity to the N-terminal truncation mutants of Pγ that we tested for this experiment (Fig. 4 and supplemental Table S1), we were able to evaluate the ability of activated transducin to displace the Pγ mutants bound to the Pαβ dimer and thereby relieve inhibition of catalysis. Mutants missing the last one or two C-terminal amino acids were also able to be activated and in fact showed a greater extent of activation than wild-type Pγ bound to Pαβ (Fig. 6). In contrast, even high concentrations of Tα*-GTPγS were incapable of activating Pαβ that had been reconstituted with the C-terminal peptide Pγ63–87 (Fig. 6). The inclusion of Pγ1–70 (to enhance 100-fold the C-terminal peptide binding affinity; see Fig. 5A) with Pγ63–87 was also ineffective in restoring the ability of Tα*-GTPγS to activate the reconstituted PDE6 enzyme (Fig. 6). This result indicates that the multiple sites of interaction observed in the crystal structure complex of Tα*-GTPγS with the C-terminal half of Pγ (20) were not sufficient to effectively displace the C-terminal blocking region from the PDE6 catalytic pocket under our experimental conditions.
FIGURE 6.
Activated transducin reverses the inhibition of truncation mutants, but not C-terminal peptide Pγ63–87. Purified Pαβ (1 nm) was incubated with 10 nm Pγ1–87, 30 nm Pγ1–86, 30 nm Pγ1–85, 30 nm Pγ1–73, 10 μm Pγ63–87, or a mixture of 10 μm Pγ63–87 and 0.25 μm Pγ1–70 for 5 min at room temperature. The activated transducin α-subunit (9 μm) was added to the above mixture, followed by the addition of 0.1 mm [3H]cAMP for PDE activity measurements. The PDE activity was expressed as the percentage of Pαβ activity in the absence of Pγ peptides. The data are the means ± S.D. of three experiments, except Pγ63–87 and Pγ1–70 where n = 2. The asterisks indicate that the PDE activity in the presence of transducin was statistically significant (p < 0.01) compared with the control, in which no transducin was added.
When we tested the ability of Tα*-GTPγS to relieve the partial inhibition imparted by Pγ1–73 reconstituted with Pαβ, we observed that activated transducin could activate PDE6 to nearly the same extent as the Pαβ catalytic dimer (Fig. 6). This indicates that in the absence of the C-terminal blocking region of Pγ, Tα*-GTPγS can interact with sufficient affinity to reverse the inhibition that is induced by the region of Pγ ranging from amino acids 61 to 76. It is reasonable to conclude from Fig. 6 that activated transducin can bind to full-length Pγ at multiple sites and that the N-terminal interacting sites confer binding stability that permits relief of inhibition by both reversing conformational changes induced by amino acids 61–76 as well as displacing the C-terminal blocking peptide from the catalytic pocket.
DISCUSSION
Three major conclusions emerge from this paper: 1) the minimum structural requirement for complete inhibition of catalysis is the last 10 amino acids (including the α3 helical segment) of the Pγ subunit; 2) a novel mechanism for inhibiting catalysis results from an apparent conformational change in the catalytic domain that can be induced by a region of Pγ consisting of amino acids 61–76 (including the α1 and α2 helical regions); and 3) activated transducin relieves both types of Pγ inhibition, but to displace the extreme C-terminal blocking residues the N-terminal domain of Pγ must be linked to the C-terminal region.
Two Distinct Inhibition Mechanisms Utilize Different Regions of the Pγ Molecule
Previous work established that the last several amino acids of the Pγ sequence are critical for inhibiting catalysis of PDE6 (9, 14–17), and the preponderance of evidence has suggested a classical competitive inhibition mechanism between the Pγ C terminus and substrate (12, 17, 26). Our results demonstrate that in addition to the last several amino acids of Pγ, an intact α3 helical region is also required, because Pγ81–87 was completely ineffective in inhibiting catalysis, whereas Pγ78–87 could completely suppress catalytic activity (Fig. 2). Our functional definition of the blocking region of Pγ in this study (Fig. 7, region B) is consistent with structural studies revealing that Pγ residues 78–87 assume a nearly identical conformation when bound to the α-subunit of transducin (20) or when complexed with a PDE5/6 chimeric catalytic domain (21).
FIGURE 7.
Interacting sites of Pγ subunit with PDE6 catalytic subunits and transducin α-subunit. Above the schematic of the Pγ subunit domain organization, two interacting regions of transducin α-subunit with Pγ are depicted, one between the polycationic region of Pγ with the α3-β5 region of Tα* (32) and a second between the C-terminal region of Pγ and the switch II/α3 region of Tα* (20). The black bars represent the known interacting amino acid residues of Pγ with transducin α-subunit based on structural and cross-linking studies (14, 20, 33). Below the Pγ domain organization are shown the two major regions of interaction with the PDE6 catalytic dimer: the GAF-interacting region and the catalytic interacting region. Based on the work in this paper, we define the following functional attributes within the catalytic domain interacting region: the inducer region, amino acids 61–76 (I), serves to induce an inhibited state of the enzyme that likely involves conformational changes in the catalytic domain; the sensor region, amino acids 63–70 (S) and/or an intact α2 helical segment (amino acids 69–73), is responsible for sensing conformational changes within the catalytic domain that result in increased affinity of the C-terminal peptide for the catalytic domain; and the blocking region (B), amino acids 78–87, directly interacts with the catalytic domains of PDE6 to suppress cyclic nucleotide hydrolysis.
However, close examination of the kinetic mechanism by which this blocking region of Pγ inhibits catalysis does not support a simple direct competitive inhibition between Pγ and substrate except when cAMP is the substrate (Fig. 3). When cGMP is the substrate, a pattern consistent with noncompetitive inhibition is found for both the minimal blocking region (Pγ78–87) as well as for a larger C-terminal peptide containing additional sites of interaction with the catalytic domains. We suggest that cGMP binding either to the noncatalytic binding sites in the regulatory GAF domain (39) or to the catalytic domain induce a conformational change that alters the interaction of Pγ with the PDE6 catalytic domain. The idea that cGMP binding to the catalytic domains of PDE6 might be responsible for some of the novel observations in this study is consistent with a recent study of the closely related PDE5 enzyme in which large conformational changes are observed upon cGMP binding to the catalytic domain (45). Future experiments will be needed to discriminate the locus of this proposed effect of cGMP binding, as well as its physiological relevance for PDE6 regulation during phototransduction.
In addition to the ability of the blocking region of Pγ to inhibit PDE6 activity, we have defined a second, allosteric mechanism of inhibition and have localized the region of Pγ capable of inducing partial inhibition of catalysis to Pγ amino acids 61–76 (Fig. 7, segment I). The comparison between Pγ1–60 and Pγ1–70 (Fig. 4) clearly demonstrated that the additional 10 amino acids in the longer truncation mutant induced a partial inhibition of catalytic activity in the absence of the blocking C-terminal segment. This region encompasses the α1 helical region previously defined in structural studies (19, 20). Furthermore, the ability of the region comprising amino acids 71–76 to further increase the inhibition of cGMP hydrolysis (Fig. 4C) suggests additional inhibitory interactions of the α2 helical region of Pγ with the PDE6 catalytic dimer.
Our identification of a novel allosteric inhibitory mechanism that occurs upon binding of the so-called inducer region of Pγ (Fig. 7, segment I) to the catalytic domain of PDE6 may be consistent with two recent structural determinations of related PDEs. Of immediate relevance is the structural determination of a PDE5/6 chimeric catalytic domain complexed with Pγ70–87 (21) that shows that Pγ residues 71 and 73 interacting with the PDE5/6 H-loop and its adjacent α12 helix within the catalytic domain. Intriguingly, this H-loop (found in all PDE family members whose crystal structures have been determined) is believed to be a major structural element responsible for allosteric regulation of the cGMP-stimulated PDE2 enzyme (46). Although the relevance of the PDE5/6 chimera and PDE2 structures to the structure and regulation of photoreceptor PDE6 remains to be determined, our biochemical study supports the idea that Pγ can interact with the H-loop to modulate catalytic activity distinct from the blocking action of the extreme C terminus of Pγ.
Distinct Segments of the Pγ Sequence Induce and Detect Conformational Changes in the Catalytic Domain of PDE6
The 100-fold increase in binding affinity of Pγ63–87 when Pγ1–70 is bound to PDE6 catalytic subunits (Fig. 5A) reveals a much larger allosteric effect of Pγ on the conformation of the catalytic domain than the 2-fold allosteric effect previously reported (39) for the shorter Pγ1–60 (Fig. 5A). This requirement for amino acids 61–70 suggests that an intact α1 helix on the N-terminal Pγ fragment is essential for causing this large enhancement of binding affinity of the C-terminal Pγ fragment.
The observation that the shorter C-terminal Pγ fragment, Pγ71–87, fails to show this 100-fold enhancement of binding affinity (Fig. 5B) that occurs with the Pγ63–87 peptide suggests that amino acids 63–70 contribute to sensing the conformational change in the catalytic domain that is induced by Pγ1–70. Alternatively, the fact that Pγ63–87 contains an intact α2 helical segment (amino acids 69–73), whereas Pγ71–87 does not, suggests the potential importance of the α2 helical structure in sensing the conformational changes induced by binding of the α1 helical region of Pγ to the catalytic domain of Pαβ. Although our current results do not pinpoint which amino acids within this region are responsible for this effect, the conformational changes inferred from these experiments reflect a previously unappreciated aspect of PDE6 regulation that warrants further study.
Transducin Interaction with the N-terminal Half of Pγ Is Required for Efficient Relief of Inhibition of the PDE6 Holoenzyme
The C-terminal region of Pγ has been documented to interact with transducin (8, 9, 20, 32, 33); however, our functional assay demonstrated that these interaction sites alone were not sufficient to relieve the inhibition of the reconstituted enzyme (Fig. 6). In addition, activated transducin failed to relieve the inhibition of Pγ63–87 in the presence of the truncation mutant (Pγ1–70), demonstrating the requirement of a physical linkage of the N- and C-terminal regions of Pγ for effective transducin activation of PDE6. Furthermore, the ability of transducin to reverse the inhibition of the Pγ truncation mutants lacking the C-terminal blocking region (Fig. 6) demonstrates an effective interaction of transducin with the N-terminal fragment of Pγ to displace Pγ from the catalytic subunit and allow catalysis to occur. This functional assay of Pγ-transducin interaction agrees well with previous studies that localize an important site of interaction of transducin with the polycationic region of Pγ (see the Introduction).
Interestingly, the polycationic region (and the adjacent proline-rich region) of Pγ (Fig. 1) also interact with the GAF domains of PDE6 with high affinity and enhance the binding affinity of cGMP to the GAF domain (12). Our results suggest that the activation of PDE6 by transducin is a competition between transducin and PDE6 for binding of Pγ in two distinct regions, namely its C-terminal blocking region and the central region of Pγ. Moreover, the fact that the binding affinity of the Pγ central region depends on cGMP occupancy of the PDE6 GAF domains opens the possibility that the effectiveness of transducin to compete with PDE6 to activate catalysis may be regulated by cGMP levels in the photoreceptor outer segment. However, it is important to recognize that our functional assays of transducin activation of PDE6 catalytic dimers associated with Pγ fragments are conducted in solution—not on the rod outer segment disk membrane—with purified, reconstituted components, and caution is required in relating these results to the situation in living photoreceptors.
Conclusion
This paper demonstrates the functional importance of the three α-helical domains in the C-terminal region of Pγ to bind to the catalytic domains of PDE6 to directly block catalysis at the active site and to induce and sense conformational changes in the catalytic domain that alter its interactions with Pγ. We further show the functional relevance of these Pγ structural elements to the mechanism of transducin activation of PDE6.
Mutations in Pγ have been shown to lead to defects in phototransduction as well as retinal degeneration in animal models (reviewed in Refs. 7 and 47) but has not been seen in humans thus far. Importantly, some of these mutations are located in the C-terminal region (48–51) that we have investigated in this study. Furthermore, a single amino acid substitution in the transducin α-subunit (i.e. G38D) disrupts the ability of transducin to bind Pγ (52, 53) and is known to cause the Nougaret form of dominant stationary night blindness (54). The results in this study may provide a molecular basis for understanding how alterations in different regions of the Pγ molecule may adversely affect the transducin-mediated regulation of PDE6, as well as the ability of PDE6 to regulate cGMP homeostasis in the dark-adapted state and/or during visual excitation, recovery, and adaptation of photoreceptors.
Supplementary Material
Acknowledgments
We thank Karyn B. Cahill for kindly providing help on the construction of the truncation mutants of Pγ and Suzanne L. Matte for providing purified transducin and performing analytical ultracentrifugation experiments with Pγ mutants.
This work was supported, in whole or in part, by National Institutes of Health Grant EY-05798.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table S1.
- PDE6
- photoreceptor cyclic nucleotide phosphodiesterase
- Pαβ
- catalytic dimer of PDE6 α- and β-subunits
- Pγ
- inhibitory γ subunit of PDE6
- Tα*
- activated transducin α-subunit
- GTPγS
- guanosine 5′-O-(thiotriphosphate).
REFERENCES
- 1.Pugh E. N., Lamb T. D. (2000) in Molecular Mechanisms in Visual Transduction (Stavenga D. G., DeGrip W. J., Pugh E. N. eds) pp. 183–255, Elsevier Science Publishing Co., Inc., New York [Google Scholar]
- 2.Arshavsky V. Y., Lamb T. D., Pugh E. N., Jr. (2002) Annu. Rev. Physiol. 64, 153–187 [DOI] [PubMed] [Google Scholar]
- 3.Fu Y., Yau K. W. (2007) Pflugers Arch. 454, 805–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wensel T. G. (2008) Vision Res. 48, 2052–2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cote R. H. (2008) in Visual Transduction and Non-Visual Light Perception (Tombran-Tink J., Barnstable C. J. eds) pp. 141–169, Humana Press, Totawa, NJ [Google Scholar]
- 6.Cote R. H. (2006) in Cyclic Nucleotide Phosphodiesterases in Health and Disease (Beavo J. A., Francis S. H., Houslay M. D. eds) pp. 165–193, CRC Press, Boca Raton, FL [Google Scholar]
- 7.Guo L. W., Ruoho A. E. (2008) Curr. Protein Pept. Sci. 9, 611–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Artemyev N. O., Hamm H. E. (1992) Biochem. J. 283, 273–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takemoto D. J., Hurt D., Oppert B., Cunnick J. (1992) Biochem. J. 281, 637–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lipkin V. M., Bondarenko V. A., Zagranichny V. E., Dobrynina L. N., Muradov K. G., Natochin M. Y. (1993) Biochim. Biophys. Acta 1176, 250–256 [DOI] [PubMed] [Google Scholar]
- 11.Natochin M., Artemyev N. O. (1996) J. Biol. Chem. 271, 19964–19969 [DOI] [PubMed] [Google Scholar]
- 12.Mou H., Cote R. H. (2001) J. Biol. Chem. 276, 27527–27534 [DOI] [PubMed] [Google Scholar]
- 13.Lipkin V. M., Dumler I. L., Muradov K. G., Artemyev N. O., Etingof R. N. (1988) FEBS Lett. 234, 287–290 [DOI] [PubMed] [Google Scholar]
- 14.Brown R. L. (1992) Biochemistry 31, 5918–5925 [DOI] [PubMed] [Google Scholar]
- 15.Skiba N. P., Artemyev N. O., Hamm H. E. (1995) J. Biol. Chem. 270, 13210–13215 [DOI] [PubMed] [Google Scholar]
- 16.Artemyev N. O., Natochin M., Busman M., Schey K. L., Hamm H. E. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 5407–5412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Granovsky A. E., Natochin M., Artemyev N. O. (1997) J. Biol. Chem. 272, 11686–11689 [DOI] [PubMed] [Google Scholar]
- 18.Uversky V. N., Permyakov S. E., Zagranichny V. E., Rodionov I. L., Fink A. L., Cherskaya A. M., Wasserman L. A., Permyakov E. A. (2002) J. Proteome Res. 1, 149–159 [DOI] [PubMed] [Google Scholar]
- 19.Song J., Guo L. W., Muradov H., Artemyev N. O., Ruoho A. E., Markley J. L. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 1505–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slep K. C., Kercher M. A., He W., Cowan C. W., Wensel T. G., Sigler P. B. (2001) Nature 409, 1071–1077 [DOI] [PubMed] [Google Scholar]
- 21.Barren B., Gakhar L., Muradov H., Boyd K. K., Ramaswamy S., Artemyev N. O. (2009) EMBO J. 28, 3613–3622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown N. G., Fowles C., Sharma R., Akhtar M. (1992) Eur. J. Biochem. 208, 659–667 [DOI] [PubMed] [Google Scholar]
- 23.Yamazaki M., Li N., Bondarenko V. A., Yamazaki R. K., Baehr W., Yamazaki A. (2002) J. Biol. Chem. 277, 40675–40686 [DOI] [PubMed] [Google Scholar]
- 24.Hurley J. B., Stryer L. (1982) J. Biol. Chem. 257, 11094–11099 [PubMed] [Google Scholar]
- 25.Gillespie P. G., Beavo J. A. (1989) Mol. Pharmacol. 36, 773–781 [PubMed] [Google Scholar]
- 26.D'Amours M. R., Granovsky A. E., Artemyev N. O., Cote R. H. (1999) Mol. Pharmacol. 55, 508–514 [PubMed] [Google Scholar]
- 27.Zhang X., Feng Q., Cote R. H. (2005) Invest. Ophthalmol. Vis. Sci. 46, 3060–3066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morrison D. F., Cunnick J. M., Oppert B., Takemoto D. J. (1989) J. Biol. Chem. 264, 11671–11681 [PubMed] [Google Scholar]
- 29.Artemyev N. O., Rarick H. M., Mills J. S., Skiba N. P., Hamm H. E. (1992) J. Biol. Chem. 267, 25067–25072 [PubMed] [Google Scholar]
- 30.Artemyev N. O., Mills J. S., Thornburg K. R., Knapp D. R., Schey K. L., Hamm H. E. (1993) J. Biol. Chem. 268, 23611–23615 [PubMed] [Google Scholar]
- 31.Artemyev N. O. (1997) Biochemistry 36, 4188–4193 [DOI] [PubMed] [Google Scholar]
- 32.Skiba N. P., Bae H., Hamm H. E. (1996) J. Biol. Chem. 271, 413–424 [DOI] [PubMed] [Google Scholar]
- 33.Grant J. E., Guo L. W., Vestling M. M., Martemyanov K. A., Arshavsky V. Y., Ruoho A. E. (2006) J. Biol. Chem. 281, 6194–6202 [DOI] [PubMed] [Google Scholar]
- 34.Artemyev N. O., Arshavsky V. Y., Cote R. H. (1998) Methods 14, 93–104 [DOI] [PubMed] [Google Scholar]
- 35.Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. (1985) Anal. Biochem. 150, 76–85 [DOI] [PubMed] [Google Scholar]
- 36.Pentia D. C., Hosier S., Collupy R. A., Valeriani B. A., Cote R. H. (2005) Methods Mol. Biol. 307, 125–140 [DOI] [PubMed] [Google Scholar]
- 37.Mou H., Grazio H. J., 3rd, Cook T. A., Beavo J. A., Cote R. H. (1999) J. Biol. Chem. 274, 18813–18820 [DOI] [PubMed] [Google Scholar]
- 38.Cote R. H. (2005) Methods Mol. Biol. 307, 141–154 [DOI] [PubMed] [Google Scholar]
- 39.Zhang X. J., Cahill K. B., Elfenbein A., Arshavsky V. Y., Cote R. H. (2008) J. Biol. Chem. 283, 29699–29705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cote R. H. (2000) Methods Enzymol. 315, 646–672 [DOI] [PubMed] [Google Scholar]
- 41.Kleuss C., Pallast M., Brendel S., Rosenthal W., Schultz G. (1987) J. Chromatogr. 407, 281–289 [DOI] [PubMed] [Google Scholar]
- 42.Wensel T. G., He F., Malinski J. A. (2005) Methods Mol. Biol. 307, 289–313 [DOI] [PubMed] [Google Scholar]
- 43.Jungbauer A., Graumann K. (2001) J. Clin. Ligand Assay 24, 270–274 [Google Scholar]
- 44.Hebert M. C., Schwede F., Jastorff B., Cote R. H. (1998) J. Biol. Chem. 273, 5557–5565 [DOI] [PubMed] [Google Scholar]
- 45.Corbin J. D., Zoraghi R., Francis S. H. (2009) Cell. Signal. 21, 1768–1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pandit J., Forman M. D., Fennell K. F., Dillman K. S., Menniti F. S. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 18225–18230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Farber D. B., Tsang S. H. (2003) Front. Biosci. 8, s666–s675 [DOI] [PubMed] [Google Scholar]
- 48.Tsang S. H., Burns M. E., Calvert P. D., Gouras P., Baylor D. A., Goff S. P., Arshavsky V. Y. (1998) Science 282, 117–121 [DOI] [PubMed] [Google Scholar]
- 49.Salchow D. J., Gouras P., Doi K., Goff S. P., Schwinger E., Tsang S. H. (1999) Invest. Ophthalmol. Vis. Sci. 40, 3262–3267 [PMC free article] [PubMed] [Google Scholar]
- 50.Tsang S. H., Yamashita C. K., Doi K., Salchow D. J., Bouvier N., Mendelsohn M., Gouras P., Farber D. B., Goff S. P. (2001) Biochem. J. 353, 467–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsang S. H., Yamashita C. K., Lee W. H., Lin C. S., Goff S. P., Gouras P., Farber D. B. (2002) Vision Res. 42, 439–445 [DOI] [PubMed] [Google Scholar]
- 52.Muradov K. G., Artemyev N. O. (2000) J. Biol. Chem. 275, 6969–6974 [DOI] [PubMed] [Google Scholar]
- 53.Moussaif M., Rubin W. W., Kerov V., Reh R., Chen D., Lem J., Chen C. K., Hurley J. B., Burns M. E., Artemyev N. O. (2006) J. Neurosci. 26, 6863–6872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dryja T. P., Hahn L. B., Reboul T., Arnaud B. (1996) Nat. Genet. 13, 358–360 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.