Abstract
The Golgi-specific zinc finger protein GODZ (palmitoyl acyltransferase/DHHC-3) mediates the palmitoylation and post-translational modification of many protein substrates that regulate membrane-protein interactions. Here, we show that GODZ also mediates Ca2+ transport in expressing Xenopus laevis oocytes. Two-electrode voltage-clamp, fluorescence, and 45Ca2+ isotopic uptake determinations demonstrated voltage- and concentration-dependent, saturable, and substrate-inhibitable Ca2+ transport in oocytes expressing GODZ cRNA but not in oocytes injected with water alone. Moreover, we show that GODZ-mediated Ca2+ transport is regulated by palmitoylation, as the palmitoyl acyltransferase inhibitor 2-bromopalmitate or alteration of the acyltransferase DHHC motif (GODZ-DHHS) diminished GODZ-mediated Ca2+ transport by ∼80%. The GODZ mutation V61R abolished Ca2+ transport but did not affect palmitoyl acyltransferase activity. Coexpression of GODZ-V61R with GODZ-DHHS restored GODZ-DHHS-mediated Ca2+ uptake to values observed with wild-type GODZ, excluding an endogenous effect of palmitoylation. Coexpression of an independent palmitoyl acyltransferase (HIP14) with the GODZ-DHHS mutant also rescued Ca2+ transport. HIP14 did not mediate Ca2+ transport when expressed alone. Immunocytochemistry studies showed that GODZ and HIP14 co-localized to the Golgi and the same post-Golgi vesicles, suggesting that heteropalmitoylation might play a physiological role in addition to a biochemical function. We conclude that GODZ encodes a Ca2+ transport protein in addition to its ability to palmitoylate protein substrates.
Keywords: Calcium Channels, Golgi, Membrane, Protein Palmitoylation, Subcellular Organelles, 45Ca2+ Isotopic Uptake, GODZ Protein, Golgi-specific Zinc Finger Protein, Palmitoylation, Xenopus oocyte expression
Introduction
Many proteins undergo post-translational palmitoylation, a process that is important in regulation of membrane-protein interactions and intracellular protein stability (1, 2). Protein palmitoylation is determined by the balance of palmitoyl acyltransferase and palmitoyl thioesterase activities. Palmitoyl acyltransferases are characterized by the presence of a cysteine-rich domain with a signature Asp-His-His-Cys (DHHC) motif (1). Using yeast two-hybrid screening with the SOS recruitment system, Uemura et al. (3) identified a Golgi-specific protein with a DHHC zinc finger domain and four predicted transmembrane regions that they designated as GODZ. With a similar approach using the γ2-subunit of GABAA3 (a determinant of subcellular GABAA receptor trafficking) as bait, Keller et al. (4) isolated GODZ (palmitoyl acyltransferase/DHHC-3). As reviewed by Keller et al. (4), GODZ has three closely related paralogs, and all four are members of the cysteine-rich domain DHHC superfamily of proteins. GODZ protein is predominately localized to the Golgi complex but also traffics to the subplasma membrane region (2, 3). GODZ has been shown to palmitoylate a variety of proteins, including transmembrane proteins (3–11). Furthermore, GODZ undergoes autopalmitoylation, which may be a general feature of this class of enzymes (4).
Using microarray analysis, we have recently shown that HIP14 (huntingtin-interacting protein 14) is differentially expressed in response to changes in extracellular magnesium (12). Moreover, we used heterologous expression studies with voltage-clamp determinations and fluorescence measurements to demonstrate that HIP14 protein mediated Mg2+ transport. Like GODZ, HIP14 possesses an intracellular DHHC motif that palmitoylates a number of different proteins, including itself, by a process of autoacylation (13–15). The transport activity of HIP14 is modulated by the palmitoylation state of the protein either via autoacylation or GODZ-mediated heteroacylation (12). In the performance of these studies, we noticed that GODZ diminished the resting transmembrane voltage in expressing oocytes, as would be expected if it encoded an ionic transport protein. This observation led us to test the notion that GODZ might mediate electrolyte transport. GODZ was expressed in Xenopus oocytes, and two-electrode voltage-clamp, fluorescence, and 45Ca2+ uptake assays were performed to determine what ions may be transported. We confirmed that GODZ is a transport protein that mediates Ca2+ flux in expressing oocytes. Unlike HIP14, GODZ did not transport Mg2+, and the expression of GODZ protein was not differentially regulated in response to changes in magnesium. However, like HIP14, GODZ-mediated cationic transport activity was modulated by palmitoylation of the transport protein. We conclude that GODZ mediates membrane Ca2+ uptake, so in addition to its enzymatic functions, it possesses Ca2+ transport activity.
MATERIALS AND METHODS
Construction of Expression Vectors Encoding GODZ
Human and mouse GODZ-FLAG cDNA fusion constructs were obtained from Dr. Alaa El-Husseini (14). Mouse GODZ-DHHS-FLAG (GODZ-C157S-FLAG mutant) was from Dr. Bernhard Lüscher (7). The mouse mutant construct GODZ-V61R-FLAG, in which Val61 was replaced with Arg, was produced from wild-type GODZ-FLAG using the QuikChange II XL site-directed mutagenesis kit (Stratagene). Human HIP14-GFP, corresponding to DHHC-17, and human HIP14ΔDHHC-GFP constructs were gifts from Dr. Alaa El-Husseini (14). The human truncation mutant HIP14ΔDHHC-GFP had only the DHHC motif removed as designed and described by El-Husseini (14).
Sequence Analysis
The GODZ cDNA sequences were determined by standard methods. Protein motifs were identified using BLASTP and the Swiss-Prot Database. Membrane topology was predicted by the SOSUI program based on Kyte-Doolittle hydrophobicity analysis.
Expression of Mouse GODZ cRNA in Xenopus Oocytes and Characterization of GODZ-mediated Ca2+ Transport
We used heterologous expression of GODZ in Xenopus laevis oocytes to show that GODZ mediates Ca2+ transport. Xenopus oocytes have the advantage that they express intracellular mammalian proteins on the surface membrane, making them available for study. Both GODZ and HIP14 are intracellular proteins. Moreover, the expression of these proteins is efficient with high fidelity, so fluxes are readily measured. Thus, oocytes are ideal cells to study intracellular transporters.
For Xenopus oocyte expression, cRNA was synthesized from mouse cDNA constructs, linearized, and then transcribed with T7 polymerase in the presence of m7GpppG cap using the mMESSAGE MACHINETM T7 kit transcription system for wild-type GODZ and T3 polymerase with the T3 kit for mutant GODZ-DHHS (Ambion). Preparation of oocytes and injection with cRNA were as described previously (16). Oocytes were studied 3–5 days following either water or cRNA injection.
In the majority of experiments, we determined Ca2+ uptake with the use of radioisotopic 45Ca2+. The method of 45Ca2+ uptake was performed as described by Peng et al. (17). Defolliculated oocytes were injected with 50 nl of either water or cRNA. 45Ca2+ uptake was assayed 3–5 days after injection of cRNA. The standard uptake solution contained 100 mm NaCl, 1.0 mm MgCl2, 0.5 mm CaCl2, and 10 mm Hepes, pH 7.5. Uptake was performed at 21 °C for up to 240 min, and oocytes were washed with 6× ice-cold uptake solution containing 20 mm MgCl2. The results obtained with single oocytes are presented as means ± S.E. from at least three experiments using 5–10 individual oocytes/data point. Statistical significance was taken as p < 0.05 (determined with Student's t test).
In some experiments, we used two-electrode voltage-clamp assays to determine the expressed currents. Two-electrode voltage-clamp assays were as described previously and performed at 21 °C (16). In these experiments, we confirmed that the measured currents were due to Ca2+ flux with the use of epifluorescence microscopy and the Ca2+-responsive fura-2 fluorescence dye (16). Oocytes were injected with 50 μm fura-2 acid (Molecular Probes) 20 min prior to experimentation. The chamber (0.5 ml) was mounted on an inverted Nikon Diaphot TMD microscope with a fluor 10× objective, and a current-voltage (I-V) plot was determined. Subsequently, the oocytes were clamped at −70 mV for fluorescence measurements for the indicated times. Fluorescence was continuously recorded using a dual excitation wavelength spectrofluorometer (DeltaScan, Photon Technology) with excitation for fura-2 at 340 and 385 nm (chopper speed set at 100 Hz) and emission at 505 nm. The results are presented as the 340/385 nm ratio, which reflects the intracellular Ca2+ concentration.
Palmitoylation Assay
Palmitoylation assays were performed by previously reported techniques (18). Briefly, COS-7 cells were transiently transfected with GODZ-FLAG or GODZ-V61R-FLAG with the aid of Lipofectamine 2000 (Invitrogen). After 24–48 h post-transfection, cells were harvested for FLAG immunoprecipitation and biotin-BMCC palmitoylation assays. COS-7 cells were lysed in ice-cold buffer containing 50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1% Triton, 50 mm N-ethylmaleimide, one tablet of protease inhibitor (Roche Applied Science), and 0.25 mg/ml phenylmethylsulfonyl fluoride. Cell lysates were rotated at 4 °C for 30 min before the insoluble material was removed by centrifugation at 14,000 rpm for 15 min. Lysates were incubated with EZviewTM Red anti-FLAG M2 affinity gel (Sigma) for 60 min at 4 °C with rocking. Following immunoprecipitation, beads were incubated three times with wash buffer (50 mm Tris, pH 7.4, 5 mm EDTA, and 150 mm NaCl containing 1% Triton) to completely remove N-ethylmaleimide. Subsequently, half of the immunoprecipitations were treated with 1 m hydroxylamine in lysis buffer for 1 h at room temperature. The other half of the immunoprecipitations were added to lysis buffer without hydroxylamine. Subsequently, the beads were incubated with 0.5 mm biotin-BMCC, pH 6.2, at 4 °C for 1 h to label reactive cysteine residues. Following SDS-PAGE and transfer to nitrocellulose membranes, the blots were reacted with horseradish peroxidase-conjugated streptavidin to detect biotin-BMCC-labeled proteins (palmitoylated protein) and anti-FLAG antibody to detect total immunoprecipitated proteins.
Immunofluorescence Confocal Microscopy
Oocytes were mounted in OCT cryostat medium and flash-frozen in isopentane cooled in liquid nitrogen. Eighteen-μm-thick sections were cut into frozen oocytes and mounted directly onto Superfrost Plus slides (Fisher). Sections were fixed in −20 °C methanol and processed for immunohistochemistry using either rabbit anti-FLAG or anti-GFP primary antibodies and anti-rabbit Alexa 568-conjugated secondary antibody.
Mammalian cell immunolocalizations were performed in COS-7 cells cultured in minimal essential medium supplemented with 10% fetal bovine serum, 110 mg/liter sodium pyruvate, 5 mm l-glutamine, 50 units/ml penicillin, and 50 μg/ml streptomycin in an humidified environment of 5% CO2 and 95% air at 37 °C. The COS-7 cells were transiently transfected with pCI-GODZ-FLAG, pCI-GODZ-DHHS-FLAG mutant, pCI-HIP14-GFP, or pCI-HIP14-DHHC-GFP mutant using Lipofectamine 2000. Coverslips of cultured COS-7 cells were fixed at room temperature for 10 min in 2% paraformaldehyde. Cells were washed three times with phosphate-buffered saline containing 0.3% Triton X-100 before each antibody incubation. Alexa 488- and Alexa 568-conjugated secondary antibodies were obtained from Molecular Probes. Alexa 350-conjugated phalloidin (Molecular Probes) was used to stain for actin in the indicated experiments to aid in delimiting the peripheral membrane ruffles. All antibody reactions were performed in blocking solution composed of 2% normal goat serum in phosphate-buffered saline containing 0.3% Triton X-100 for 1.5 h at room temperature. Following staining, coverslips were then mounted on slides with Fluoromount-G glycerol-based mounting medium (Southern Biotechnology).
Cell images were taken using a 63× water lens affixed to a Zeiss LSM 510 Meta microscope and AxioVision (epifluorescent) software. Cells were selected from 10–12 fields of view and used for assessment of co-localization of antibody staining. Oocyte images were performed using 20× dry lens affixed to a Zeiss LSM 510 Meta microscope and LSM Meta (confocal) software.
RESULTS
Characterization of the Predicted GODZ Protein
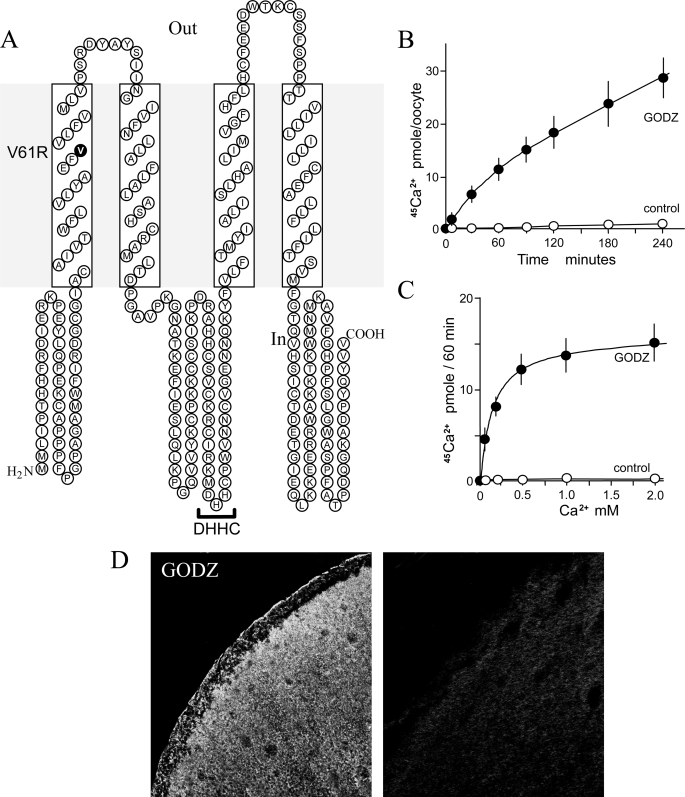
The full-length GODZ cDNA encodes a protein of 229 amino acids carrying a DHHC zinc finger domain (3). Mouse GODZ shares 97% similarity with human GODZ (palmitoyl acyltransferase/DHHC-3). Hydrophobicity plots using the SOSUI program predicted a secondary amino acid structure with four predicted transmembrane domains (TMDs) conforming to a prototypical membrane receptor/transporter (Fig. 1A). The DHHC consensus sequence is located within the cytoplasmic region between TMD2 and TMD3. The GODZ transcript has a wide tissue distribution, being relatively rich in brain tissue (3).
FIGURE 1.
GODZ-mediates Ca2+ transport. A, predicted secondary structure of mouse GODZ. GODZ resembles a typical membrane transporter with four TMDs and possesses a palmitoyl acyltransferase motif (indicated by DHHC) between TMD2 and TMD3. The transmembrane mutation site of the GODZ-V61R construct is indicated in boldface. B, 45Ca2+ uptake in oocytes expressing GODZ cRNA as a function of time. Oocytes were incubated with 0.5 mm 45CaCl2 for the indicated times. Values are means ± S.E. for 5–16 individual oocytes from three different preparations. C, summary of concentration-dependent Ca2+-evoked currents in GODZ-expressing oocytes. Oocytes were incubated with the indicated concentrations of Ca2+ for 1.0 h. The Michaelis constant determined by nonlinear regression analysis was 0.17 ± 0.01 mm. Values are means ± S.E. for 5–18 individual oocytes from three different preparations. D, surface expression of GODZ-FLAG protein in X. laevis oocytes as determined by immunofluorescence. Left panel, GODZ-FLAG-injected oocyte treated with anti-FLAG antibody showing intense surface staining; right panel, control water-injected oocyte tested with anti-FLAG antibody.
GODZ Mediates Ca2+ Transport in Expressing Xenopus Oocytes
To determine whether GODZ encodes a functional Ca2+ transporter, we prepared cRNA, injected it into Xenopus oocytes, and measured Ca2+ uptakes using 45Ca2+ measurements. 45Ca2+ uptake was linear for up to 1.0 h in oocytes expressing GODZ cRNA (Fig. 1B). There was no detectable 45Ca2+ uptake in control water-injected oocytes (Fig. 1B). Among the inherent properties of all transporters is the property of substrate saturation (17). The 45Ca2+ uptake was saturable, demonstrating a Michaelis constant (Km) of 0.17 ± 0.01 mm (Fig. 1C). Immunofluorescence using a GODZ-FLAG construct and anti-FLAG antibody showed predominantly surface localization of the GODZ fusion protein in GODZ-FLAG-expressing oocytes, whereas there were no staining in control water-injected oocytes (Fig. 1D).
We next performed a number of experiments to characterize GODZ-mediated Ca2+ transport. Using voltage-clamp techniques, we first showed that the Ca2+-induced currents measured were dependent on the external Ca2+ concentration (supplemental Fig. S1A). In addition to Ca2+, GODZ mediated Sr2+ and Ba2+ but not Mg2+ ions (supplemental Fig. S1B). The other palmitoyl acyltransferases that we have previously studied, HIP14 and HIP14L, transported Mg2+ in addition to a number of divalent cations, but they did not mediate Ca2+ transport (12). Thus, we thought it prudent to further test the ability of GODZ to mediate Mg2+ flux (supplemental Fig. S1B). Expressed GODZ did not mediate Mg2+ transport as determined by voltage-clamp assay (supplemental Fig. S1C, upper panel) or fluorescence analysis using the Mg2+-selective dye mag-fura-2 (supplemental Fig. S1C, lower panel). A general property of transporters is the ability to be inhibited by related substrates. We tested if a number of cations would inhibit GODZ-mediated 45Ca2+ influx. Mn2+, La3+, Ba2+, and Sr2+ significantly inhibited GODZ-mediated 45Ca2+ influx, but Mg2+ did not (supplemental Fig. S1D). Sodium-coupled Ca2+ transport has been demonstrated in many cells. Removal of external Na+ did not significantly affect GODZ-mediated Ca2+ currents as determined by two-electrode voltage-clamp assay (supplemental Fig. S1D). Ca2+/H+ exchange has also been demonstrated in some cells. 45Ca2+ uptake did not appear to be coupled to H+, as acid pH inhibited influx rather than stimulated uptake (supplemental Fig. S1E). Finally, GODZ-mediated Ca2+ transport was not inhibited by the calcium channel blocker nifedipine (supplemental Fig. S1F). Together, these data indicate that GODZ mediates voltage-dependent Ca2+ transport that is not coupled to external Na+ or H+ but is influenced by the external pH. Furthermore, GODZ mediates the transport of some other divalent cations but not Mg2+ ions. As expected with a membrane transporter, GODZ-mediated Ca2+ transport is inhibited by some divalent cations.
Palmitoyl Acyltransferase Modulates GODZ-mediated Ca2+ Transport
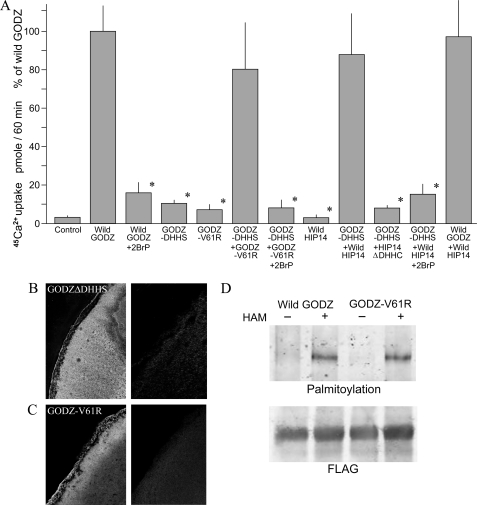
It was of interest that GODZ demonstrates protein acyltransferase activity, and metabolic labeling studies showed that GODZ itself was palmitoylated through its innate DHHC domain (3, 6). GODZ contains 11 cysteine residues in the predicted cytoplasmic loop region that are highly conserved and might function as palmitoylation sites. As palmitoylation has been shown to influence the function of a number of diverse transporters, we tested the notion that palmitoylation might influence GODZ-mediated Ca2+ transport (4, 5, 18–24). To determine whether the protein acyltransferase modulates transport function, we performed two types of experiments. First, we used the specific antagonist 2-bromopalmitate to block palmitoylation and determined the effect on GODZ-mediated Ca2+ transport (6). Treatment of GODZ-expressing oocytes with 75 μm 2-bromopalmitate for 3 h diminished Ca2+ transport by ∼80% as determined by 45Ca2+ uptake measurements (Fig. 2A). Second, we obtained an analogous Asp-His-His-Ser (DHHS) mutant form of GODZ (GODZ-DHHS-FLAG) that lacked palmitoylation activity (6). Ca2+ transport was decreased by 80% in mutant GODZ-DHHS-expressing cells relative to wild-type GODZ-expressing oocytes, again using 45Ca2+ uptake determinations (Fig. 2A). The residual Ca2+ transport observed with the DHHC deletion was similar to that observed with maximal 2-bromopalmitate inhibition. Furthermore, the residual Ca2+ transport observed with GODZ-DHHS was not significantly inhibited by 2-bromopalmitate, indicating that both approaches, inhibition and deletion, have the same effect (data not shown). The GODZ-DHHS-FLAG construct localized to the surface membrane of the oocyte (Fig. 2B). Taken together, these data suggest that palmitoylation activates GODZ-mediated Ca2+ transport.
FIGURE 2.
Palmitoyl acyltransferase modulates GODZ-mediated Ca2+ transport. Where indicated, oocytes were injected with wild-type GODZ, GODZ-DHHS, mutant GODZ-V61R, wild-type HIP14, or HIP14ΔDHHC cRNA. Also where indicated, expressing oocytes were treated with and without 75 μm 2-bromopalmitate (2BrP) for 3 h prior to experimentation. A, summary of 45Ca2+ uptake rates. The methods used were those described in the legend to Fig. 1B. The results were normalized to wild-type GODZ and are represented as means ± S.E. for n > 12 oocytes. *, p < 0.01, from determinations with wild-type GODZ-expressing oocytes. B, left panel, surface expression of GODZ-DHHS mutant protein in cRNA-injected X. laevis oocytes as determined by immunofluorescence; right panel, absence of GODZ-DHHS expression in water-injected oocytes. C, left panel, surface expression of GODZ-V61R protein in oocytes as determined by immunofluorescence; right panel, absence of GODZ-V61R expression in water-injected oocytes. D, mutant GODZ-V61R retains the palmitoyl acyltransferase function as indicated by biotin-BMCC bands in the presence of hydroxylamine (HAM). Gels are representative of three independent experiments.
It could be argued that heterologous expression of palmitoyl acyltransferase might activate endogenous oocyte Ca2+ transport. To test this notion, we synthesized a GODZ mutant with a V61R amino acid replacement within a predicted transmembrane region highly conserved among the known species (Fig. 1A). The rational is that this region would perform a significant if not essential function in membrane transport. Substitution of a polar amino acid for a neutral one would be expected to have functional consequences. GODZ-V61R did not mediate Ca2+ transport (Fig. 2A) even though it was expressed on the oocyte surface membrane (Fig. 2C) but retained palmitoyl acyltransferase activity (Fig. 2D). This indicates that the Val61 site in wild-type GODZ is essential for the ability of GODZ to mediate Ca2+ transport. There is no similarity in the transmembrane regions of GODZ compared with the Mg2+ transporters HIP14 and HIP14L, which do not mediate Ca2+ transport. Accordingly, the Ca2+ uptake observed in GODZ-expressing oocytes is the result of the ability of GODZ to mediate Ca2+ transport rather than to activate endogenous transport proteins. Moreover, GODZ-V61R with its palmitoyl acyltransferase activity was capable of restoring GODZ-DHHS-mediated Ca2+ transport (Fig. 2A). These observations support the idea that GODZ-mediated Ca2+ transport is influenced by palmitoylation.
In support of this conclusion, overexpression of wild-type HIP14, an independent acyltransferase that does not transport Ca2+, with mutant GODZ-DHHS stimulated 45Ca2+ uptake to levels not unlike those observed for wild-type GODZ alone (Fig. 2A). It should be noted that HIP14 was overexpressed in these oocytes. HIP14 (DHHC-17) and GODZ (DHHC-3) are distinctive acyltransferases with distinctive substrates (3, 4, 13). However, they may palmitoylate multiple substrates if overexpressed within cells (25, 26). Moreover, overexpression may bypass acyltransferase regulators, which otherwise ensure palmitoylation by a selected DHHC protein (26). As HIP14 alone did not stimulate Ca2+ transport in oocytes, it is apparent that palmitoylation was not a general endogenous effect but required the heterologous expression of HIP14 protein (12). Truncated HIP14 lacking the DHHC motif (HIP14ΔDHHC) did not rescue GODZ-DHHS-mediated Ca2+ transport. This further supports the notion that native oocytes do not possess endogenous protein acyltransferase that is capable of palmitoylation of the GODZ protein. Additionally, 2-bromopalmitate inhibited the stimulation of transport by HIP14 in GODZ-DHHS-expressing oocytes again to transport rates similar to those observed with wild-type GODZ plus 2-bromopalmitate. These studies strongly argue for a role of the GODZ palmitoyl acyltransferase in modulating GODZ-mediated Ca2+ transport function through autoacylation. Finally, coexpression of wild-type HIP14 with wild-type GODZ in the oocytes did not stimulate Ca2+ transport rates above those observed for GODZ alone (Fig. 2A). This suggests that heterologously expressed GODZ is normally fully activated (autoacylated), so regulation of transport function involves deacylation.
Immunolocalization of GODZ and HIP14 Proteins
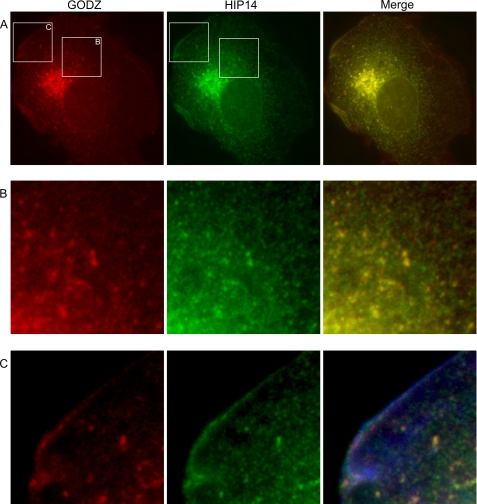
GODZ protein is thought to be localized to the Golgi complex and post-Golgi vesicles involved with protein sorting and recycling to the Golgi (3, 4, 7). We and others (12–14) have also shown that HIP14 localizes to Golgi and post-Golgi subplasma membrane vesicles. This led us to determine whether GODZ and HIP14 proteins are localized to similar regions within the cell. COS-7 cells were transfected with GODZ-FLAG and HIP14-GFP constructs, and the fusion proteins were localized by immunofluorescence. The predominate amounts of GODZ-FLAG and HIP14-GFP proteins were localized to the Golgi complex (Fig. 3A). However, both proteins were also present in post-Golgi vesicles, as shown in the inset at higher magnification in Fig. 3B. The merged images show that GODZ-FLAG and HIP14-GFP localized to the same post-Golgi vesicle population. Both GODZ-FLAG and HIP14-GFP proteins were also evident in vesicles located just below the plasma membrane (Fig. 3C).
FIGURE 3.
Subcellular localization of GODZ and HIP14 proteins. Shown is the immunofluorescence staining of GODZ-FLAG and HIP14-GFP fusion proteins in transiently expressing COS-7 cells. A, Golgi localization of GODZ. The merged image is shown. B, presence of GODZ-FLAG and HIP14-GFP in Golgi and post-Golgi vesicles. Images are 4× magnifications of the boxed areas in A. C, subplasma membrane location of post-Golgi GODZ-FLAG and HIP14-GFP in cells stained with phalloidin to mark the plasma membrane ruffles. Images are 4× magnifications of the boxed areas in A. Note the evident subplasma membrane localization of both GODZ and HIP14 proteins in this region of normal cells.
DISCUSSION
Our evidence given here indicates that heterologously expressed GODZ is a Ca2+ transporter. GODZ-mediated Ca2+ transport exhibits many properties characteristic of other known Ca2+ channels when expressed in X. laevis oocytes (17). GODZ-mediated Ca2+ transport is driven by the electrochemical gradient for Ca2+. There is no evidence for coupling of Ca2+ uptake to other ions or to metabolic energy. Although GODZ-mediated Ca2+ transport is electrogenic and voltage-dependent, stringent determinations of single channel activity by patch-clamp studies are required to determine whether it is a facilitated transporter or a typical channel (17). GODZ-mediated Ca2+ uptake also demonstrates the property of substrate saturation. Under the conditions used here, the affinity constant Km is on the order of 170 μm. This value is considerably above the normal intracellular Ca2+ concentrations of ∼0.1 μm. However the initial transport rates were not determined, and the Km value obtained for uptake experiments over 60 min may deviate from those measured with initial kinetics. Again, this has also been observed with other Ca2+ transport studies using oocytes (17). Like other Ca2+ transporters, GODZ shows higher activity at a pH of 7.40 relative to an acidic pH of 6.0 (17). GODZ mediates the transport of a number of divalent cations in addition to Ca2+, but it does not transport Mg2+. Also similar to many Ca2+ channels is the observation that GODZ-mediated Ca2+ transport is inhibited by a number of other divalent metal cations such as La3+. In summary, the GODZ-mediated Ca2+ transport observed here was voltage-dependent, saturable, pH-sensitive, and inhibited by a number of alkali earth metals.
In addition, our evidence indicates that GODZ-mediated Ca2+ transport is modulated by palmitoylation. The DHHC domain of GODZ palmitoylates a number of substrates, including GODZ itself, through a process of autoacylation (3, 6, 7). Many cation transporters such as Na+, K+, and Ca2+ channels are known to be activated by palmitoylation (19–24). Alternatively, palmitoylation has been implicated in sorting and trafficking of other transport and receptor proteins (27–29). Our studies show that DHHC activity and palmitoylation are required for maximal Ca2+ transport mediated by GODZ. This conclusion is supported by the observation that 2-bromopalmitate, an inhibitor of palmitoyl acyltransferase, diminished wild-type GODZ-mediated Ca2+ transport by ∼80%. The surface expression of GODZ was similar before and after 3 h of incubation with 2-bromopalmitate. In addition, expression of GODZ-DHHS demonstrated diminished transport without changes in surface GODZ-DHHS protein. Of note is the observation that palmitoylation is not necessary for basal transport, as ∼20% of Ca2+ transport remained even with maximal 2-bromopalmitate inhibition or expression of acyltransferase-inactivated GODZ-DHHS. The two together were not additive, leaving ∼20% residual Ca2+ transport that presumably is the basal transport in the absence of any palmitoylation. In addition, the GODZ-V61R mutant, which did not transport Ca2+ but retained palmitoyl acyltransferase activity, restored GODZ-DHHS-mediated Ca2+ transport. These observations support the notion that GODZ-mediated Ca2+ transport is influenced by its innate palmitoyl acyltransferase. In accordance with this notion, overexpression with the independent palmitoyl acyltransferase HIP14 did not stimulate wild-type GODZ-mediated Ca2+ transport, suggesting that heterologously expressed GODZ is normally fully acylated by its innate palmitoyl acyltransferase in expressing oocytes. However, overexpression of HIP14 restored GODZ-DHHS-mediated Ca2+ transport to values observed for wild-type GODZ. This overexpressed HIP14-rescued GODZ-mediated Ca2+ transport was sensitive to 2-bromopalmitate inhibition. The HIP14ΔDHHC truncation mutant was not effective in restoring GODZ-DHHS-mediated Ca2+ transport. These data strongly establish a regulatory role for palmitoylation in GODZ-mediated transport. The evidence is that regulation of GODZ-mediated transport occurs at the depalmitoylation step rather than the palmitoylation step, i.e. GODZ is normally fully palmitoylated in expressing oocytes. This is in keeping with the notion that acylation of cysteine-containing proteins is spontaneous and driven by local acyl-CoA concentrations (2). Palmitate cycling is normally regulated at the depalmitoylation step by acyl-protein thioesterases (1, 2). Further studies are required to determine whether this model is also applicable to mammalian cells.
These conclusions are reminiscent of our observations with HIP14 (12). Using a number of independent approaches, we showed that HIP14 also possesses innate protein acyltransferase activity, mediating the transport of Mg2+ cations. HIP14 and GODZ are distinctive transporters; HIP14 does not support Ca2+ transport, and GODZ does not mediate Mg2+ uptake. However, they both possess a cytoplasmic DHHC motif. Similar to GODZ-mediated Ca2+ transport, HIP14-elicited Mg2+ uptake is inhibited by ∼50% with 2-bromopalmitate. Expression of mutant HIP14ΔDHHC, a truncated form lacking the DHHC motif, supported ∼50% Mg2+ transport activity relative to wild-type HIP14. Coexpression of wild-type GODZ with the HIP14ΔDHHC mutant restored Mg2+ transport to levels observed with wild-type HIP14. Accordingly, although HIP14 and GODZ are unique cationic transporters, they are apparently able to cross-palmitoylate each other and influence the respective channel functions.
GODZ and HIP14 proteins are located in the same subcellular regions. Both are predominately Golgi proteins (2, 7). However, a small amount of GODZ and HIP14 proteins is found in post-Golgi vesicles (3, 4, 12). Our images show that GODZ and HIP14 are present in the cytoplasmic and subplasma membrane vesicles of normal cells. We have shown previously that magnesium depletion results in an increase in the HIP14 transcript and protein in mammalian cells (12). Moreover, there is an evident trafficking of HIP14 protein to the subplasma membrane region. As HIP14 is a Mg2+ transporter, the increase in HIP14 trafficking to the subplasma membrane might be an appropriate response in getting the protein to its active site. In light of these observations, GODZ might also be regulated by yet-to-be identified influences that affect transcription/translation mechanisms and post-translational subcellular protein distribution.
The presence of GODZ and HIP14 proteins in the Golgi complex and post-Golgi vesicles likely has functional implications. As with other acyltransferases, they may function in the Golgi to regulate protein retention and release and trafficking between endosomes and the Golgi (1). However, significant amounts of GODZ and HIP14 proteins are also found in post-Golgi vesicles located within the cytoplasmic and subplasma membrane regions of the cell (3, 5, 6, 11, 12, 14). It is thought that post-Golgi acyltransferases may also be involved with protein sorting, scaffolding, and membrane recycling (1, 2). The subcellular site for palmitoylation of membrane proteins has remained elusive. It is not a priori that acyltransferases act at one site; palmitoylation/depalmitoylation might regulate proteins along the full axis from the endoplasmic reticulum through the Golgi complex to the plasma membrane (27–30). As with the enzymatic motif, the function of the channel sequence is unclear. Intracellular organelles contain variable amounts of Ca2+ and Mg2+ that are important in signaling and catalysis, so it is likely there are dedicated Ca2+ and Mg2+ transporters for each step. Why the combined functions of acyltransferase and cation channels? We speculate that the channel protein portion traffics to the post-Golgi and subplasma membrane regions, where acyltransferase provides an important if not an essential biochemical function. In this scenario, the channel sequence plays the role of a chaperone for GODZ and HIP14 acyltransferases to move from the Golgi to post-Golgi vesicles. Further studies are required to clarify the relation of the separate functions of transport and acyltransferase activities.
SERZ-β (Sertoli cell gene with zinc finger domain-β) or SERZ1 (DHHC-7 or ZDHHC-7) is a closely related paralog of GODZ, with 61% amino acid similarity between the two protein acyltransferases (31). The predicted secondary structure of SERZ-β is similar to that of GODZ, with four TMDs and a DHHC motif between TMD2 and TMD3. As with GODZ, the SERZ-β transcript is widely distributed among tissues, with GODZ mRNA being relatively higher in brain and SERZ-β mRNA higher in kidney (31). Fang et al. (7) showed that SERZ-β and GODZ palmitoylate γ2-subunit-containing GABAA. As SERZ-β and GODZ exhibit similar expression patterns and substrate specificity, these workers suggested possible in vivo functional redundancy (7). Moreover, Fang et al. demonstrated that SERZ-β forms heteromultimers with GODZ after coexpression in heterologous cells. The common properties of GODZ and SERZ-β suggest that they might have common functions, including the ability to mediate Ca2+ transport.
In summary, we have shown that GODZ mediates Ca2+ transport when expressed in Xenopus oocytes. Furthermore, palmitoylation/depalmitoylation modulates GODZ-mediated Ca2+ transport function. The role of these transporters in Ca2+ physiology is unknown, and the function of the innate acyltransferase deserves further efforts by researchers in the palmitoylation field.
Supplementary Material
Acknowledgments
We are grateful to Dr. Berhard Lüscher for GODZ-FLAG and GODZ-DHHS-FLAG and Drs. Michael Hayden and Alaa El-Husseini for the HIP14-GFP and HIP14ΔDHHC-GFP constructs. We acknowledge the BioImaging Facility at the University of British Columbia for the oocyte images.
This work was supported by Canadian Institutes of Health Research Grant MOP-53288 (to G. A. Q.) and by studentships from the Canadian Institutes of Health Research and the Michael Smith Foundation for Health Research (to R. M. H.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
- GABAA
- γ-aminobutyric acid type A
- GFP
- green fluorescent protein
- biotin-BMCC
- 1-biotinamido-4-(4′-(maleimidoethyl)cyclohexanecarboxamido)butane
- TMD
- transmembrane domain.
REFERENCES
- 1.Linder M. E., Deschenes R. J. (2007) Nat. Rev. Mol. Cell Biol. 8, 74–84 [DOI] [PubMed] [Google Scholar]
- 2.Greaves J., Chamberlain L. H. (2007) J. Cell Biol. 176, 249–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uemura T., Mori H., Mishina M. (2002) Biochem. Biophys. Res. Commun. 296, 492–496 [DOI] [PubMed] [Google Scholar]
- 4.Keller C. A., Yuan X., Panzanelli P., Martin M. L., Alldred M., Sassoè-Pognetto M., Lüscher B. (2004) J. Neurosci. 24, 5881–5891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rathenberg J., Kittler J. T., Moss S. J. (2004) Mol. Cell. Neurosci. 26, 251–257 [DOI] [PubMed] [Google Scholar]
- 6.Hayashi T., Rumbaugh G., Huganir R. L. (2005) Neuron 47, 709–723 [DOI] [PubMed] [Google Scholar]
- 7.Fang C., Deng L., Keller C. A., Fukata M., Fukata Y., Chen G., Lüscher B. (2006) J. Neurosci. 26, 12758–12768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukata M., Fukata Y., Adesnik H., Nicoll R. A., Bredt D. S. (2004) Neuron 44, 987–996 [DOI] [PubMed] [Google Scholar]
- 9.Chen Z. W., Olsen R. W. (2007) J. Neurochem. 100, 279–294 [DOI] [PubMed] [Google Scholar]
- 10.Kanaani J., Diacovo M. J., El-Husseini Ael-D., Bredt D. S., Baekkeskov S. (2004) J. Cell Sci. 117, 2001–2013 [DOI] [PubMed] [Google Scholar]
- 11.El-Husseini A. E., Craven S. E., Chetkovich D. M., Firestein B. L., Schnell E., Aoki C., Bredt D. S. (2000) J. Cell Biol. 148, 159–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goytain A., Hines R. M., Quamme G. A. (2008) J. Biol. Chem. 283, 33365–33374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singaraja R. R., Hadano S., Metzler M., Givan S., Wellington C. L., Warby S., Yanai A., Gutekunst C. A., Leavitt B. R., Yi H., Fichter K., Gan L., McCutcheon K., Chopra V., Michel J., Hersch S. M., Ikeda J. E., Hayden M. R. (2002) Hum. Mol. Genet. 11, 2815–2828 [DOI] [PubMed] [Google Scholar]
- 14.Huang K., Yanai A., Kang R., Arstikaitis P., Singaraja R. R., Metzler M., Mullard A., Haigh B., Gauthier-Campbell C., Gutekunst C. A., Hayden M. R., El-Husseini A. (2004) Neuron 44, 977–986 [DOI] [PubMed] [Google Scholar]
- 15.Yanai A., Huang K., Kang R., Singaraja R. R., Arstikaitis P., Gan L., Orban P. C., Mullard A., Cowan C. M., Raymond L. A., Drisdel R. C., Green W. N., Ravikumar B., Rubinsztein D. C., El-Husseini A., Hayden M. R. (2006) Nat. Neurosci. 9, 824–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goytain A., Hines R. M., El-Husseini A., Quamme G. A. (2007) J. Biol. Chem. 282, 8060–8068 [DOI] [PubMed] [Google Scholar]
- 17.Peng J. B., Chen X. Z., Berger U. V., Vassilev P. M., Tsukaguchi H., Brown E. M., Hediger M. A. (1999) J. Biol. Chem. 274, 22739–22746 [DOI] [PubMed] [Google Scholar]
- 18.Kang R., Wan J., Arstikaitis P., Takahashi H., Huang K., Bailey A. O., Thompson J. X., Roth A. F., Drisdel R. C., Mastro R., Green W. N., Yates J. R., 3rd, Davis N. G., El-Husseini A. (2008) Nature 456, 904–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmidt J. W., Catterall W. A. (1987) J. Biol. Chem. 262, 13713–13723 [PubMed] [Google Scholar]
- 20.Qin N., Platano D., Olcese R., Costantin J. L., Stefani E., Birnbaumer L. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 4690–4695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chien A. J., Carr K. M., Shirokov R. E., Rios E., Hosey M. M. (1996) J. Biol. Chem. 271, 26465–26468 [DOI] [PubMed] [Google Scholar]
- 22.Gubitosi-Klug R. A., Mancuso D. J., Gross R. W. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 5964–5968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan A. W., Owens S., Tung C., Stanley E. F. (2007) Cell Calcium 42, 419–425 [DOI] [PubMed] [Google Scholar]
- 24.Mies F., Spriet C., Héliot L., Sariban-Sohraby S. (2007) J. Biol. Chem. 282, 18339–18347 [DOI] [PubMed] [Google Scholar]
- 25.Roth A. F., Wan J., Bailey A. O., Sun B., Kuchar J. A., Green W. N., Phinney B. S., Yates J. R., 3rd, Davis N. G. (2006) Cell 125, 1003–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hou H., John Peter A. T., Meiringer C., Subramanian K., Ungermann C. (2009) Traffic 10, 1061–1073 [DOI] [PubMed] [Google Scholar]
- 27.Fernández-Hernando C., Fukata M., Bernatchez P. N., Fukata Y., Lin M. I., Bredt D. S., Sessa W. C. (2006) J. Cell Biol. 174, 369–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Varner A. S., Ducker C. E., Xia Z., Zhuang Y., De Vos M. L., Smith C. D. (2003) Biochem. J. 373, 91–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petäjä-Repo U. E., Hogue M., Leskelä T. T., Markkanen P. M., Tuusa J. T., Bouvier M. (2006) J. Biol. Chem. 281, 15780–15789 [DOI] [PubMed] [Google Scholar]
- 30.Greaves J., Salaun C., Fukata Y., Fukata M., Chamberlain L. H. (2008) J. Biol. Chem. 283, 25014–25026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chaudhary J., Skinner M. K. (2002) Endocrinology 143, 426–435 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.