Abstract
N-Glycans attached to the ectodomains of plasma membrane pattern recognition receptors constitute likely initial contact sites between plant cells and invading pathogens. To assess the role of N-glycans in receptor-mediated immune responses, we investigated the functionality of Arabidopsis receptor kinases EFR and FLS2, sensing bacterial translation elongation factor Tu (elf18) and flagellin (flg22), respectively, in N-glycosylation mutants. As revealed by binding and responses to elf18 or flg22, both receptors tolerated immature N-glycans induced by mutations in various Golgi modification steps. EFR was specifically impaired by loss-of-function mutations in STT3A, a subunit of the endoplasmic reticulum resident oligosaccharyltransferase complex. FLS2 tolerated mild underglycosylation occurring in stt3a but was sensitive to severe underglycosylation induced by tunicamycin treatment. EFR accumulation was significantly reduced when synthesized without N-glycans but to lesser extent when underglycosylated in stt3a or mutated in single amino acid positions. Interestingly, EFRN143Q lacking a single conserved N-glycosylation site from the EFR ectodomain accumulated to reduced levels and lost the ability to bind its ligand and to mediate elf18-elicited oxidative burst. However, EFR-YFP protein localization and peptide:N-glycosidase F digestion assays support that both EFR produced in stt3a and EFRN143Q in wild type cells correctly targeted to the plasma membrane via the Golgi apparatus. These results indicate that a single N-glycan plays a critical role for receptor abundance and ligand recognition during plant-pathogen interactions at the cell surface.
Keywords: Glycoprotein, Pathogen-associated Molecular Pattern (PAMP), Plant, Plasma Membrane, Receptor Structure-Function, EF-Tu, EFR, FLS2, N-Glycosylation, Flagellin
Introduction
The glycosylation of asparagine residues (N-glycosylation) is an essential, highly conserved co-translational modification of secreted proteins occurring in all eukaryotic cells. The oligosaccharyltransferase complex in the endoplasmic reticulum (ER)3 controls transfer of N-glycans from dolichol-pyrophosphate-linked lipid anchors to nascent polypeptide chains. N-Glycans attached to polypeptides within the ER lumen monitor correct folding of both soluble and membrane-bound proteins. Only successfully folded proteins are exported from the ER; therefore, N-glycans are crucial for vesicle-mediated distribution of glycoproteins within the secretory system (e.g. to the plasma membrane and vacuole). Prevention of N-glycosylation by tunicamycin induces the so-called unfolded protein response, a cellular stress response, which initiates programmed cell death (1–3). We and others have shown previously that aberrant N-glycosylation within the ER, as well as altered N-glycan modification in the Golgi apparatus, can affect abiotic stress tolerance (4, 5) and plant development in Arabidopsis (6–8). This work aimed at investigating to what extent biotic stress responses were also affected by altered protein N-glycosylation.
Several conserved N-glycosylation consensus sites (NX(S/T) motifs) have been identified in the leucine-rich repeat (LRR)-containing ectodomains of cell-surface receptors that recognize microbe-associated molecular patterns (MAMPs) in both animals and plants (9, 10). These so-called pattern recognition receptors (PRRs) sense MAMPs and activate a first line of defense, referred to as MAMP-triggered immunity (11, 12). In Arabidopsis, the receptor kinases FLS2 and EFR recognize bacterial flagellin and EF-Tu through their elicitor-active MAMPs flg22 and elf18, respectively. Upon activation, they stimulate multitude immune responses, including the apoplastic generation of reactive oxygen species (ROS), the so-called oxidative burst, and also seedling growth arrest (10, 13). These reactions require function of the co-receptor kinase BAK1 that forms an inducible, heterodimeric complex with FLS2, which, however, is dispensable for flg22 binding (14, 15). We noticed that the detected molecular masses of these cell-surface receptors or the cognate binding sites are much larger compared with their predicted masses, e.g. ∼110 kDa for EFR and ∼130 kDa for FLS2 (16–18). Such significant mass increases indicate substantial post-translational modifications, as would occur by the addition of glycan chains. Indeed, the ectodomains of EFR, FLS2, and BAK1 are composed of 21, 28, and 5 LRR repeats, respectively, many of them containing conserved NX(S/T) glycosylation motifs (16, 17, 19, 20).
Evidence for receptor kinase glycosylation is limited in plants but was previously described for the tomato LRR receptor protein Cf-9, the Solanum ovule receptor kinase ScORK17, and the Medicago LysM domain-containing receptor kinase nodulation factor perception (21–23). This leads to the conclusion that plant receptor kinases are subject to N-glycosylation, which in turn could be essential for the function of PRRs in triggering plant immunity. We therefore investigated the contribution of N-glycosylation to EFR and FLS2 function by monitoring efl18 and flg22 responses in a collection of mutants, which are defective in various steps of N-glycosylation in the ER and Golgi resident N-glycan modifications. EFR function was specifically sensitive to interference with core glycosylation in the ER. FLS2 and BAK1 were less affected, although both were also clearly subject to N-glycosylation.
EXPERIMENTAL PROCEDURES
A. thaliana Mutants
tDNA insertion mutants (supplemental Fig. S1B) stt3a-1, stt3a-2, stt3b-1, hgl1-1, cgl1-3, fucTa, fucTb, and xylT have been described previously (4, 5, 8). Detailed characterization of the manIa-2 manIb-1 double mutant, prepared by genetic cross of the single mutants, has been published recently (43).
Growth Conditions and Bioassays
Arabidopsis seedlings were grown under sterile conditions in liquid MS medium supplemented with Nitch vitamins and 1% sucrose. Seedling growth inhibition upon 100 nm elf18 or flg22 treatment was assessed as reported previously (17). Two-week-old seedlings grown in liquid medium were used for biochemical and reverse transcription-coupled PCR analysis. For interference with N-glycosylation, 10–30 μm tunicamycin was added to 2-week-old liquid-grown seedlings and incubated overnight. Plants used for bacterial infections were grown on soil under short day conditions for 4–5 weeks. Arabidopsis wild type and mutant plants used for transient transfections were grown under sterile conditions on solidified medium for 4–6 weeks prior to preparation of leaf protoplasts. Nicotiana benthamiana plants were grown on soil under long day conditions for 3–4 weeks. Oxidative burst of transformed N. benthamiana leaves triggered by 1 μm elf18 was measured as described in Zipfel et al. (16).
Bioinformatics
Amino acid alignment of EFR (At5g20480) with EFR-like sequences (At3g47090, At3g47110, At3g47570, and At3g47580) was done with ClustalW allowing for identification of highly conserved N-glycosylation motifs (NX(S/T)).
Cloned Constructs
All oligonucleotides used in this study were purchased from Sigma. The genomic region of EFR (bp 1–3181) was PCR-amplified with the primer pair 5′-GGG GAC AAG TTT GTA CAA AAA AGC AGG CTT AAT GAA GCT GTC CTT TTC A-3′ and 5′-GGG GAC CAC TTT GTA CAA GAA AGC TGG GTA CAT AGT ATG CAT GTC CGT ATT TA-3′ and inserted behind the 35S cauliflower mosaic virus promoter into the pXCSG-mYFP vector by Gateway cloning (Invitrogen). Mutated variants were generated by PCR-based methods using Phusion High Fidelity DNA polymerase (Finnzymes) with primer pairs 5′-CCG TCT AGT CTT TCT CAG TGC TCT AGA CTG TCG-3′ and 5′-CGA CAG TCT AGA GCA CTG AGA AAG ACT AGA CGG-3′ (N143Q), 5′-CC AAA ACA CTT GCC CAG ATC TCA AGC CTT G-3′ and 5′-C AAG GCT TGA GAT CTG GGC AAG TGT TTT GG-3′ (N288Q), 5′-CCA TGA GAA GGT AAG TTC TGA AGA GCT TCA TAG TGC-3′ and 5′-GCA CTA TGA AGC TCT TCA GAA CTT ACC TTC TCA TGG-3′ (Y702S), introducing the respective EFR mutations in pXCSG-mYFP vectors. All changes were verified by sequencing.
Antibodies and Peptides
Antisera α-FLS2, α-EFR, and α-BAK1 were used as described (24, 25).4 The α-green fluorescent protein antibody, alkaline phosphatase-conjugated α-rabbit, and α-mouse antibodies were purchased from Roche Applied Science and Sigma. Elf18 and flg22 peptides were synthesized as described previously (16, 17). Radioactively labeled elicitor-active peptide variants elf26-125I-Tyr and 125I-Tyr-flg22 were obtained from Bio-trend.
Protein Extraction and Binding Assays
Protein extraction and immunoblot analyses were essentially done as described previously (24). Chemical cross-linking and binding assays of elf26-125I-Tyr and 125I-Tyr-flg22 were performed according to Chinchilla et al. (18) and Zipfel et al. (16). Briefly, ground aerial parts of 2-week old seedlings were resuspended in specific elf26- and flg22-binding buffers and incubated with elf26-125I-Tyr or 125I-Tyr-flg22 on ice for 15 or 25 min, respectively, either alone or in the presence of excess unlabeled elf18 or flg22 peptides. Cross-linking was achieved by addition of 10 μl of 25 mm ethylene glycol bis(succinimidylsuccinate) (Pierce), and samples were separated on SDS gels, and radioactivity was visualized using a PhosphorImager (Fuji FLA-7000). Competitive binding assays of transiently transformed N. benthamiana leaf samples were performed as described previously (16), except that radioactivity retained on filters was determined by scintillation counting.
Bacterial Infections
Bacterial infections were performed as described in Zipfel et al. (26). Briefly, Pseudomonas syringae pv. tomato DC3000 bacteria (PtoDC3000) were sprayed onto the leaf surface at 0.5 × 108 colony-forming units/ml. Leaves harvested 3 days post-inoculation were surface-sterilized; two leaves each from five plants were pooled; bacteria were extracted by grinding in 10 mm MgCl2, and several dilutions were plated on medium containing appropriate antibiotics. Results of two independent experiments were combined, and statistical analysis (analysis of variance and subsequent post hoc test by Tukey's Honestly Significantly Different) was done using Systat software.
Transient Transformations
Transgene expression in N. benthamiana was achieved by transient transformation (27). Agrobacterium strains carrying binary vectors to express the respective YFP fusion constructs (35S::EFR-YFP, 35S::EFRN143Q-YFP, 35S::EFRN288Q-YFP, 35S::EFRY702S-YFP, 35S::FLS2) or the 19K silencing suppressor construct (28) were grown overnight in YEB medium with the appropriate antibiotics. Cultures were harvested by centrifugation, and cells were resuspended in 10 mm MES, pH 5.6, 10 mm MgCl2, 150 mm acetosyringone, adjusted to 0.8 A600, mixed with 1/10th of 19K culture, and incubated for 4 h at room temperature. N. benthamiana leaves were syringe-infiltrated with agrobacteria, and plants were cultivated for 3 days. In some experiments, tunicamycin (10–30 μm) was infiltrated 3 days later. Transient transfection of Arabidopsis protoplasts was achieved by the polyethylene glycol method using 30 μg of plasmid DNA (total) and dark cultivation in the absence or presence of 10 μm tunicamycin at 21–25 °C for 1–2 days (8). PNGase F digestions of protein extracts were conducted as described in Frank et al. (8).
Confocal Microscopy
Transiently transfected Arabidopsis protoplasts were imaged as described previously (8).
RESULTS
Correct N-Glycosylation Is Specifically Required for EFR Function
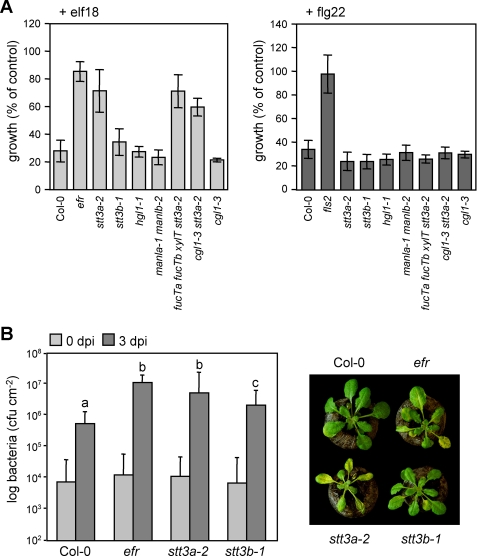
To determine a role for N-glycosylation in plant immunity, a collection of mutants, defective in various steps of N-glycosylation in the ER and N-glycan modification in the Golgi apparatus (supplemental Fig. 1), was subjected to elf18- and flg22-triggered seedling growth arrest. Out of all mutants, only staurosporin and temperature-sensitive 3a (stt3a-2) was strongly insensitive to MAMP treatment. Insensitivity of stt3a-2 was specific for elf18 (and epistatic to other glycosylation mutants tested), and its response to flg22 was similar to wild type (Fig. 1A). This was also true for the oxidative burst, an immediate early MAMP response, because elicitation with elf18 failed to trigger ROS production in the stt3a-2 mutant (supplemental Fig. 2). A causal-effect relationship between mutations in STT3A and elf18-specific insensitivity was established, because the same phenotype was observed with the stt3a-1 allele (supplemental Fig. 3A).
FIGURE 1.
ArabidopsisN-glycosylation mutants display different immune reactions. A, seedling growth arrest in response to MAMP treatment. Growth reduction upon 100 nm elf18 or flg22 treatment is shown as percentage of control growth (without the peptides). Similar results were obtained in three independent experiments. Bars represent means ± S.D. (n = 6). B, pathogen infection. Plants were spray-inoculated with PtoDC3000. Bacterial inoculation and growth were determined at 0 and 3 days post infection, respectively, and photographs were taken at 4 dpi. Bars represent means ± S.D. of two biological independent experiments each of six replicates (p < 0.0001 indicated by letters).
The elf18 insensitivity conferred enhanced susceptibility to stt3a mutants upon infection by the phytopathogenic bacterium P. syringae (Fig. 1B). Mutant plants suffered a higher degree of bacterial colonization than wild type and developed disease symptoms comparable with those determined for efr mutants. Taken together, this suggested that mutations in STT3A likely affect elf18/EFR-mediated immunity. STT3A encodes a subunit of the oligosaccharyltransferase complex responsible for the transfer of N-glycans onto nascent proteins in the ER (4). Although mutants of the paralogous gene STT3B responded like wild type to both MAMPs, immunity to bacterial infection was also significantly compromised in stt3b, albeit to a lesser extent than in stt3a (Fig. 1, A and B).
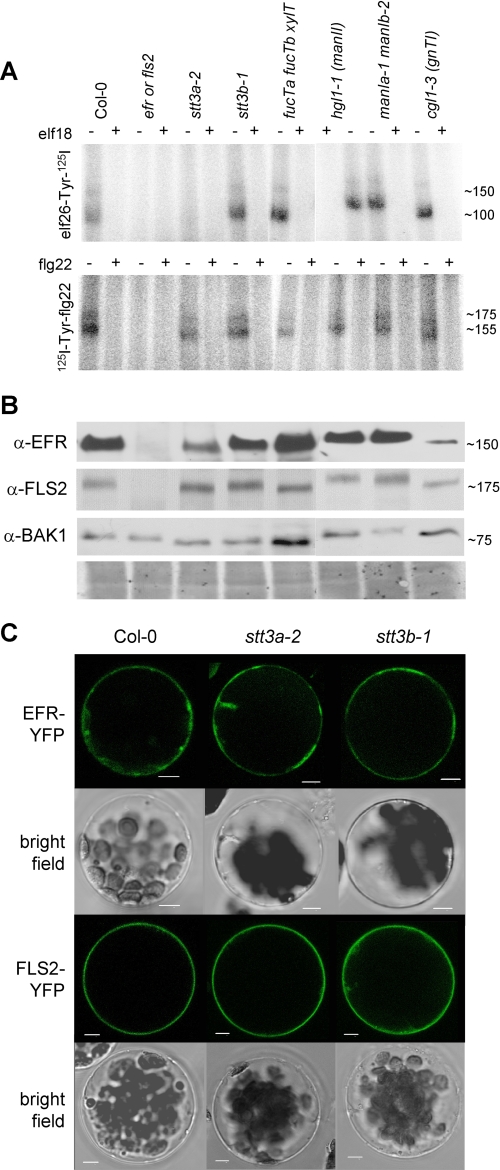
Because EFR and FLS2 (with co-receptor BAK1) share downstream components (14, 16), the elf18-insensitive but flg22-responsive phenotype of the stt3a mutant plants is indicative of a specific EFR defect but intact downstream MAMP signaling pathway. To monitor receptor functions, we conducted cross-linking assays using radiolabeled elf26, an active elf variant, and flg22 peptides (14, 18). Cross-linking was performed in vitro to allow ligand binding to most subcellular compartments. Specificity of the assay was confirmed by competition with an excess of unlabeled ligand and the use of efr and fls2 receptor mutants. As described previously, elf26 and flg22 cross-linking revealed two specific bands, ∼150/100 kDa for elf26 binding and ∼175/155 kDa for flg22 binding (Fig. 2A). The upper band corresponds to intact receptors and the lower band supposedly to a degradation product known from incubations of whole seedling extracts (14, 18). We detected specific cross-linking of both ligands in most mutants, but none for elf26 in stt3a or its mutant combinations (Fig. 2A; supplemental Fig. 3B and Fig. 4). This corresponds to above described elf18 insensitivity of stt3a mutant plants. Mutation of STT3A did not alter transcript accumulation of EFR and FLS2 (supplemental Fig. 3C). Immunoblot analysis revealed a significant decrease of EFR protein in stt3a-2. However, EFR protein levels were substantially higher than in cgl1–3 that showed strong elf26 binding (Fig. 2B). Thus, the amount of EFR accumulating in cgl1–3 mutants appeared sufficient to confer ligand binding and response. FLS2 and BAK1 protein levels remained unchanged.
FIGURE 2.
Ligand binding, protein accumulation, and localization of PRRs in selected N-glycosylation mutants. A, equal amounts of ground seedling material were incubated with elf26-Tyr-125I or 125I-Tyr-flg22 in the absence (−) or presence (+) of 10 μm elf18 or flg22 peptide, respectively. Similar results were obtained in at least three independent experiments. B, accumulation of EFR, FLS2, and BAK1 proteins. Equal amounts of the lines indicated in A were loaded for immunoblotting and revealed with specific antibodies (α-FLS2, α-EFR, or α-BAK1). As controls, efr mutants were included for elf26 binding and EFR and BAK1 accumulation, and fls2 mutants were included for flg22 binding and FLS2 accumulation. Representative Coomassie staining is shown as loading reference below. C, EFR-YFP and FLS2-YFP were expressed in protoplasts of Col-0 wild type and indicated glycosylation mutants. Confocal and bright field images of representative transfected protoplasts are shown. Scale bars, 5 μm.
Accordingly, localization of EFR at the plasma membrane was confirmed using EFR fused to the YFP expressed in Arabidopsis wild type and mutant protoplasts (Fig. 2C). The fusion proteins labeled similar structures in wild type, stt3a-2, and stt3b-1, suggesting that stt3a is not impaired in localization of EFR (Fig. 2C) but in function of the EFR protein itself. Besides the plasma membrane, EFR-YFP also labeled internal structures reminiscent of the endomembrane system. Co-expression of EFR-YFP with marker constructs mOFP-ER and SiaT-mRFP, respectively (8), confirmed that EFR partially localizes to the ER and to the Golgi apparatus in wild type protoplasts. Labeling of Golgi stacks was less obvious in protoplasts isolated from stt3a, stt3b, or efr mutants (supplemental Fig. 5, A, B, and E).
The occurrence of functional immune signaling and elf26/flg22 cross-linking to EFR and FLS2 in all N-glycan modification mutants may indicate that ER-type N-glycans on these receptors are not further modified in the Golgi apparatus. This was assessed by electrophoretic mobility of the PRR proteins. In general, all receptors accumulated to approximately wild type molecular masses. However, subtle mobility shifts were observed in N-glycan modification mutants, hinting at slightly altered receptor masses. It appeared that manIa manIb and manII mutants, lacking Golgi-localized α-mannosidase I and α-mannosidase II, respectively, displayed detectable molecular mass increases of elf26- and flg22-binding sites (Fig. 2A). This resembled increased masses of EFR, FLS2, and BAK1 proteins on immunoblots (Fig. 2B), likely caused by impaired trimming of terminal mannoses. Despite these alterations, wild type-like elf18 and flg22 responses were maintained. Thus, subtle modifications within individual oligosaccharide chains are likely tolerated for PRR function.
Interference with N-Glycosylation in the ER Alters Appearance of PRRs
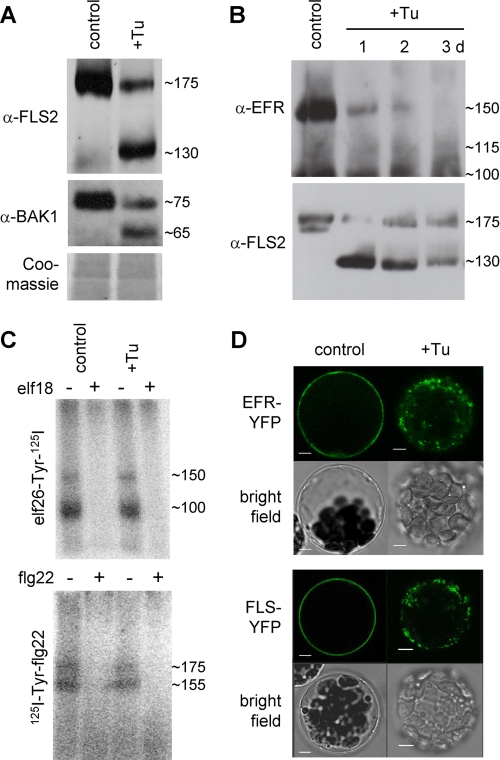
For FLS2, STT3A loss-of-function did not cause any functional abnormality, neither in flg22 binding nor response (Figs. 1A and 2A). We hypothesized that if there are functional redundancies among individual N-glycan chains in the FLS2 ectodomain, they could mask the effect of mild underglycosylation caused by stt3a-2. To address the impact of severe underglycosylation on the synthesis and function of FLS2, we examined the consequences of tunicamycin treatment and mutation of Defective Glycosylation 1 (DGL1) on physicochemical and functional properties of FLS2. Tunicamycin inhibits biosynthesis of dolichol-linked oligosaccharides, whereas mutations in DGL1 impair function of an essential oligosaccharyltransferase subunit (6). Immunoblots of tunicamycin-treated wild type plants revealed two bands for FLS2, at ∼175 and ∼130 kDa (Fig. 3A). Considering that plant N-glycans of secreted glycoproteins have an average mass of ∼2 kDa and that 21 NX(S/T) motifs are found in the LRR domain of FLS2, a shift of ∼40 kDa likely results from complete loss of its N-glycan decoration. This indicates that nearly all predicted sites are occupied (supplemental Fig. 6). In addition, two BAK1-specific bands at ∼75 and ∼65 kDa were detected in the presence of tunicamycin. The shift of ∼10 kDa also infers the use of nearly all predicted sites in BAK1 (Fig. 3A; supplemental Fig. 6). In contrast to FLS2, nonglycosylated EFR bands ∼115 kDa were hardly detectable during a tunicamycin time course (Fig. 3B). Importantly, the sizes of the newly appearing, high mobility bands are consistent with molecular masses expected for the unglycosylated PRR polypeptides (18, 20).
FIGURE 3.
Effect of interference with receptor N-glycosylation on PRR ligand binding. A, untreated (control) and tunicamycin-treated (+Tu) Col-0 wild type plants were analyzed by immunoblotting with specific antibodies (α-FLS2, α-EFR, or α-BAK1). Coomassie staining is shown for equal loading. B, time course of tunicamycin treatment (10 μg/ml for 3 days). C, cross-linking with elf18 and flg22 peptides was performed with untreated (control) or tunicamycin-treated (Tu) Col-0 seedlings. D, effect of 8–10-h tunicamycin incubation on localization of EFR- and FLS2-YFP in Col-0 wild type protoplasts. Scale bars, 5 μm.
To check for flg22 and elf26 binding activity, we tested tunicamycin-treated wild type cells. Cross-linking of flg22 or efl26 did not reveal any new bands around 130 or 115 kDa, respectively (Fig. 3C), indicating that nonglycosylated PRRs are unable to mediate ligand binding. Major size shifts corresponding to complete loss of N-glycans observed for endogenous EFR and FLS2 (Fig. 3B) were also detected for transiently expressed EFR-YFP and FLS2-YFP in N. benthamiana during a tunicamycin time course (supplemental Fig. 7). Similar to FLS2 and BAK1, the size difference of ∼35 kDa implicates full coverage of glycosylation sites in EFR. When comparing relative intensities of bands appearing upon tunicamycin treatment, EFR has higher turnover rates than FLS2, and de novo synthesized nonglycosylated EFR polypeptides seem less stable than those of FLS2. This supports our observation that EFR function is affected by underglycosylation in stt3a, which is tolerated by FLS2. Interestingly, both FLS2-YFP and EFR-YFP failed to accumulate at the plasma membrane in tunicamycin-treated protoplasts (Fig. 3D and supplemental Fig. 5C). Instead, the PRR-YFP fusions accumulated predominantly in intracellular compartments, demonstrating that severe underglycosylation impairs correct targeting of both PRRs. The localization of nonglycosylated PRR-YFP polypeptides is currently unclear, because little overlap with the ER marker and none with the Golgi marker were found (supplemental Fig. 5C).
Interference by tunicamycin treatment demonstrated that FLS2 is highly glycosylated, which is possibly important for flg22 binding and plasma membrane localization. Mutants affected in DGL1, a key component of the oligosaccharyltransferase complex in the ER, suffer post-germinative growth arrest and are thus seedling lethal (6). Immunoblot analysis with very limited dgl1-1 material did not reveal any detectable band of a known molecular mass for FLS2 (supplemental Fig. 8A), suggesting that PRR accumulation is impaired when in planta N-glycosylation is absent. However, for Defender against Death dad1 and dad2 single mutants, we found wild type-like elf18- and flg22-mediated MAMP responses and ligand binding, which suggest that these oligosaccharyltransferase subunits are not involved in glycosylation (supplemental Fig. 8B).
EFR Function Is Sensitive to Mutation of a Conserved NX(S/T) Glycosylation Motif
To test whether loss of N-glycosylation is the direct cause of dysfunctional EFR, point mutations that eliminate individual N-glycosylation motifs were introduced into the EFR coding sequence. For this purpose, a bioinformatics approach was used to identify conserved N-glycosylation sites in EFR (At5g20480) upon alignment with four EFR-like sequences (At3g47090, At3g47110, At3g47570, and At3g47580) in the Arabidopsis genome. Out of 16 NX(S/T) motifs in the LRR ectodomain, 10 were found to be highly conserved (supplemental Fig. 6), and out of these, four (Asn-143, Asn-191, Asn-288, and Asn-590) were absolutely conserved. Point mutations resulting in Asn-to-Gln amino acid replacement were introduced into EFR NX(S/T) motifs at Asn-143 and Asn-288 that appear to be exposed at the convex side of the horseshoe structure (supplemental Fig. 6). Correct targeting of the resulting EFRN143Q and EFRN288Q glycosylation variants fused to YFP was confirmed in Arabidopsis protoplasts. Similar predominant plasma membrane labeling and partial overlap with the ER marker was observed in Col-0 wild type and efr mutant cells (supplemental Fig. 5, A, D, and E).
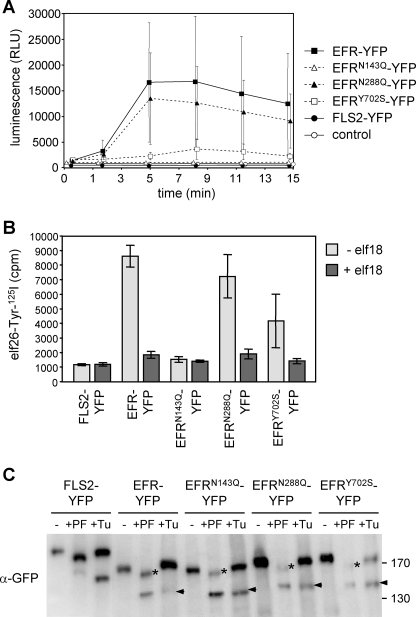
To test receptor function, all EFR-YFP variants were transiently expressed in N. benthamiana, known to lack an endogenous elf18 perception system (14). FLS2-YFP was used as a negative control. Additionally, EFRY702S, carrying a mutation in the cytoplasmic domain, was included in our analyses. Expression of EFR-YFP and EFRN288Q-YFP specifically mediated elf18-triggered ROS production. Compared with these responses, ROS release was strongly reduced upon expression of EFRY702S-YFP and completely absent from EFRN143Q-YFP and FLS2-YFP (Fig. 4A).
FIGURE 4.
Analysis of EFR mutant variants. EFR-YFP and derived mutant variations were expressed in N. benthamiana by transient transformation. A, EFR function was tested by elf18-triggered oxidative burst (control = not transformed). Data points are means ± S.D. (n = 15). Similar results were obtained in two independent experiments. RLU, relative light units. B, quantitative elf18-ligand binding. Bars represent means ± S.D. (n = 3). C, samples for PNGase F incubation (−/+PF) were taken at day 3 after transformation. Tunicamycin (+Tu, 10 μm) was infiltrated and harvested 5 h later. Note that PNGase F-resistant bands (+PF, asterisks) are indicative of post-Golgi transport of the chimeric glycoprotein-YFP fusions. Also, compared with wild type EFR-YFP, all mutant EFR variants are apparently more stable in the ER (+Tu, arrows). The immunoblot was revealed with green fluorescent protein antibodies (α-green fluorescent protein (α-GFP)).
Quantitative assays revealed potent and specific binding of elf26 ligand to transiently expressed EFR-YFP, EFRN288Q-YFP, and to lesser extent of EFRY702S-YFP but not to EFRN143Q-YFP or FLS2-YFP (Fig. 4B). Similar results were obtained by cross-linking analysis (supplemental Fig. 9A). This demonstrates that elf26-ligand binding is in accordance with elf18-elicited ROS production. When transcript levels of PRR-YFP in N. benthamiana were determined by reverse transcription-coupled PCR, all YFP variants showed comparable expression (supplemental Fig. 9C). However, substantial differences in EFR-YFP protein levels were observed. Accumulation of FLS2-YFP, EFR-YFP, and EFRN288Q-YFP was clearly detectable, whereas EFRN143Q-YFP and EFRY702S-YFP accumulated at much lower levels (supplemental Fig. 9B). This suggests that mutation of Asn-143 and Tyr-702 affects EFR protein abundance. Nevertheless, the low level of EFRY702S-YFP was sufficient to confer wild type-like elf18 binding. By contrast, a similar level of EFRN143Q-YFP did not confer elf18 binding activity and physiological elf18 responses on N. benthamiana cells (supplemental Fig. 9A). It is therefore possible that interference with N-glycosylation at position Asn-143 contributes to both EFR stability and ligand recognition.
The above-described results established that lack of a single N-glycan can impair the function of EFR. To investigate to what extent EFR-YFP and EFRN143Q-YFP protein fusions accumulate in the ER or migrate through the Golgi apparatus, we conducted peptide:N-glycosidase F (PNGase F) digests and compared them with tunicamycin-treated samples. PNGase F can only release ER-type N-glycans lacking core α1,3-fucoses, and these residues are not added until arrival in the Golgi cisternae. Upon PNGase F treatment, FLS2-YFP and EFR-YFP proteins similarly showed a shift to reduced size (Fig. 4C, +PF, asterisks). For EFR and all EFR mutant variants, deglycosylated polypeptides appeared after tunicamycin treatment to various extents (Fig. 4C, +Tu, arrows). However, no complete loss of N-glycans was recorded, which would be the case if the fusion proteins were completely retained in the ER. Importantly, EFR mutant variant N143Q obviously also passed the Golgi apparatus, which indicates that elf18 binding at the plasma membrane is abrogated. Besides unit-length bands, indicative of PRR-YFP fusions on their way to or at the plasma membrane, additional bands of higher molecular mass were sometimes detected (supplemental Fig. 7 and Fig. 9B). These low mobility bands may correspond to immature EFR forms carrying high mannose-type N-glycan chains that occur during processing in the ER. Conceivably, mutant EFR variants need extended time to accomplish folding in N. benthamiana, which matches their subcellular localization in this heterologous system (supplemental Fig. 9D).
DISCUSSION
PRRs such as EFR, FLS2, and the co-receptor BAK1 carry multiple putative N-glycosylation sites in their ectodomains. Evidently, glycosylation of receptor ectodomains contributes to correct structure and function. In particular, glycosylation patterns are conserved between plant receptors, especially NX(S/T) glycosylation motifs located in the α-helical parts of the LRR domains facing the convex, and thus the outer side of the horseshoe structure seems to be essential (21). In this study, we investigated whether alteration of N-glycosylation in the ER and N-glycan modification in the Golgi apparatus affect PRR function and thus plant immunity.
We found that probably all putative glycosylation sites of EFR, FLS2, and BAK1 are used in planta. Although glycosylation of EFR, FLS2 and BAK1 is evident, the identity of individual N-glycan chains at different positions (i.e. high mannose type versus complex type) remains to be determined. Nonglycosylated EFR polypeptides appear to be rather unstable, whereas nonglycosylated FLS2 and BAK1 polypeptides accumulate to significant levels (Fig. 3 and supplemental Fig. 7). This demonstrated that basic N-glycosylation is especially critical for EFR. The most prominent roles of protein N-glycosylation are support of correct folding and protection from degradation. Inhibition of N-glycosylation interferes with native conformation needed to pass ER-mediated quality control (ERQC) and leads to protein degradation (29). Prominent ER localization of underglycosylated EFR protein is indicative of ERQC-mediated retention. This is further supported by two recent studies reporting that loss-of-function mutations in calreticulin (CRT3) and UDP-glucose:glycoprotein glucosyltransferase (EBS1/PSL2), encoding key enzymes of the ERQC, lead to elf18 but not flg22 insensitivity (25, 30, 31). However, we now show that in stt3a underglycosylation of EFR-YFP still results in plasma membrane labeling, which indicates that correct targeting occurs in the absence of correct glycosylation. Successful folding in the ER and migration through the Golgi apparatus are supported by PNGase F treatment of the EFR and all mutant variants, including EFRN143Q (Fig. 4C). This further indicates that amino acid substitution abolishing a single N-glycosylation motif in EFR impairs receptor function at the plasma membrane. By contrast, nonglycosylated EFR- and FLS2-YFP were exclusively detected in endomembrane structures demonstrating that N-glycosylation is important for folding and subsequent transport of the two receptors to the cell surface (Fig. 2C and supplemental Fig. 5). Thus, for some PRRs N-glycosylation seems to be essential for stability and function (21, 32), whereas others are less affected (33). A specific mutant variant of the Arabidopsis brassinosteroid receptor BRI1, another BAK1 interacting kinase, also shows ERQC-mediated retention. Importantly, in this PRR mutant, N-glycosylation is unaffected but cannot prevent degradation of the misfolded polypeptide (34).
Like FLS2 and BAK1, nonglycosylated forms of the Medicago truncatula nodulation factor perception protein involved in Nod factor perception accumulated to significant levels (23). N-Glycans are typically exposed on the protein surface and form flexible hydrated branches (35). They are therefore thought to contribute to the conformational stability of receptor ectodomains likely facilitating association with other molecules. The ectodomain of mammalian Toll-like receptor 3 forms a horseshoe-shaped structure composed of 23 LRRs, of which 11 carry N-linked glycans that significantly contribute to its molecular mass (35, 36). Whereas glycosylation of Toll-like receptor 3 appears to have no effect on protein accumulation and ligand binding, it is important for activation of downstream signaling pathways (37, 38). Mutation of glycosylation sites in the tomato Cf-9 receptor did not affect protein stability but compromised its function to trigger avirulence factor 9-mediated programmed cell death (21). There is evidence that recognition of avirulence factor 9 ligand by the Cf-9 receptor is dependent on glycosylation; however, this remains to be further tested. Our results revealed that underglycosylated EFR and nonglycosylated FLS2 are not able to form functional ligand-binding sites. Therefore, LRR glycosylation of these receptors is required for stably binding their elf18 and flg22 peptide ligands, an essential function for plant immunity.
Similar to EFR, FLS2, and BAK1, all but one of the predicted glycosylation sites of the Cf-9 ectodomain are used (21). Mutational analysis revealed that most of the glycosylation sites contribute to Cf-9 function. In particular, four that are exposed at the convex side of the LRR horseshoe were shown to be essential. In this study, we identified critical N-glycosylation sites that affect EFR stability and function. Mutation of two absolutely conserved NX(S/T) motifs in exposed positions at the outer surface of the LRR horseshoe, Asn-143 and Asn-288, caused detectable ER retention of EFR-YFP and therefore reduced the amount of mutant EFR variants traveling through the Golgi apparatus before accumulating at the plasma membrane (Fig. 4C and supplemental Fig. 9D). Importantly, abolishment of N-linked glycosylation at Asn-143 was sufficient to impair binding of elf18 ligand and thereby EFR-mediated immune responses (supplemental Fig. 9A). This again indicates that correct glycosylation is essential for EFR function. Because the elf18-binding site of the EFR ectodomain has not been identified yet, it remains unclear whether glycosylation of Asn-143 stabilizes LRR conformation or facilitates ligand binding. A region within the FLS2 ectodomain (LRR-(9–15)) has been suggested to serve as the flg22-binding site (39), but whether N-glycosylation within this region is involved is still to be addressed.
The main focus of this study was to investigate EFR and FLS2 function in a collection of N-glycosylation mutants. Apparently, extensive but subtle N-glycan modifications occurring during passage through the Golgi apparatus are not essential for function of these two PRR proteins. This contrasts our findings for the membrane-bound β-glucanase KORRIGAN that is involved in abiotic stress tolerance of Arabidopsis (5). Surprisingly, stt3a was the only mutant identified to exhibit clearly impaired MAMP responses with specificity to elf18 (Fig. 1). Our data show that EFR abundance is decreased in stt3a, and more importantly that ligand binding is abolished. This can explain the elf18 insensitivity and, accordingly, enhanced susceptibility to bacterial infection. STT3A acts in concert with STT3B as catalytic subunits of the oligosaccharyltransferase complex, controlling frequency and accuracy of N-glycan transfer to polypeptides in the ER lumen (4). Detailed studies in mammalian cells recently showed that STT3A and STT3B differ in preferential use of N-glycosylation sites. STT3A seems to cover motifs in the center of nascent polypeptide chains (like EFRN143), whereas STT3B covers motifs at the N- and C-terminal ends (40). Thus, profound effects of stt3a alleles on EFR function, contrasted by more or less wild type responses in the stt3b-1 mutant, are feasible. A candidate PRR, as affected in stt3b mutant, for that probably N-glycosylation at the N- or C-terminal end matters, still needs to be identified. However, it remains to be shown whether the genetically redundant STT3A and STT3B subunits function similarly in plants.
The fact that Arabidopsis MAMP receptors EFR and FLS2 are differentially affected by altered N-glycosylation in the ER is surprising considering that both receptors group into the same subfamily of receptor kinases (41), stimulate a largely overlapping set of MAMP responses requiring BAK1 function (14, 16), and that both LRR ectodomains are similarly highly glycosylated. A possible explanation could be given on evolutionary terms. EFR is apparently a recently achieved receptor only occurring in Brassicaceae, whereas flg22 perception was found in most plant species (reviewed in Ref. 42). In this respect, EFR might not yet be optimally adapted. As our study shows, N-glycosylation is essential for MAMP-triggered immunity by differentially affecting accumulation and ligand binding of cell surface PRRs. Fine regulation of PRR activities might be important to prevent responses that are potentially harmful to the plant, and PRRs are thus likely co-evolving together with their respective pathogen-derived ligands. In this respect, N-glycosylation evidently contributes to ligand-binding affinity and maintenance of specific MAMP receptors.
Supplementary Material
Acknowledgments
The skilled technical assistance of Olessja Becker and Kerstin Fischer (Münster, Germany) is gratefully acknowledged. We thank Patrick Gallois (Manchester, United Kngdom) for dad1 and dad2 seeds and Patrice Lerouge (Rouen, France) for dgl1-1 seeds. Maik Böhmer (previously Cologne, Germany), Madlen Vetter, Petra Köchner, Renier van der Hoorn (Cologne, Germany), and Thomas Boller (Basel, Switzerland) provided materials and technical help. Judith Scharte (Münster, Germany) contributed critical comments to the final manuscript.
This work was supported by Deutsche Forschungsgemeinschaft Grants SFB670 (to S. R.) and SCHA541/11 (to A. v. S.) and United States Department of Agriculture Grants 2006-34402-17121 and 2008-34402-19195 (to H. K.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S9.
B. Schulze, T. Mentzel, A. Jehle, K. Mueller, S. Beeler, T. Boller, G. Felix, and D. Chinchilla, submitted for publication.
- ER
- endoplasmic reticulum
- EFR
- Arabidopsis receptor-like kinase-sensing EF-Tu
- EF-Tu
- bacterial translation elongation factor Tu
- elf
- bacterial elongation factor ligand
- ERQC
- ER quality control
- flg
- bacterial flagellin ligand
- LRR
- leucine-rich repeat
- MAMP
- microbe-associated molecular pattern
- PNGase F
- peptide:N-glycosidase F
- PRR
- pattern recognition receptor
- MES
- 4-morpholineethanesulfonic acid
- ROS
- reactive oxygen species
- YFP
- yellow fluorescent protein.
REFERENCES
- 1.Elbein A. D. (1987) Annu. Rev. Biochem. 56, 497–534 [DOI] [PubMed] [Google Scholar]
- 2.Martínez I. M., Chrispeels M. J. (2003) Plant Cell 15, 561–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iwata Y., Koizumi N. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 5280–5285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koiwa H., Li F., McCully M. G., Mendoza I., Koizumi N., Manabe Y., Nakagawa Y., Zhu J., Rus A., Pardo J. M., Bressan R. A., Hasegawa P. M. (2003) Plant Cell 15, 2273–2284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang J. S., Frank J., Kang C. H., Kajiura H., Vikram M., Ueda A., Kim S., Bahk J. D., Triplett B., Fujiyama K., Lee S. Y., von Schaewen A., Koiwa H. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 5933–5938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lerouxel O., Mouille G., Andème-Onzighi C., Bruyant M. P., Séveno M., Loutelier-Bourhis C., Driouich A., Höfte H., Lerouge P. (2005) Plant J. 42, 455–468 [DOI] [PubMed] [Google Scholar]
- 7.von Schaewen A., Frank J., Koiwa H. (2008) Plant Signal. Behav. 3, 871–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frank J., Kaulfürst-Soboll H., Rips S., Koiwa H., von Schaewen A. (2008) Plant Physiol. 148, 1354–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akira S., Uematsu S., Takeuchi O. (2006) Cell 124, 783–801 [DOI] [PubMed] [Google Scholar]
- 10.Zipfel C. (2008) Curr. Opin. Immunol. 20, 10–16 [DOI] [PubMed] [Google Scholar]
- 11.Jones J. D., Dangl J. L. (2006) Nature 444, 323–329 [DOI] [PubMed] [Google Scholar]
- 12.Chisholm S. T., Coaker G., Day B., Staskawicz B. J. (2006) Cell 124, 803–814 [DOI] [PubMed] [Google Scholar]
- 13.Bittel P., Robatzek S. (2007) Curr. Opin. Plant Biol. 10, 335–341 [DOI] [PubMed] [Google Scholar]
- 14.Chinchilla D., Zipfel C., Robatzek S., Kemmerling B., Nürnberger T., Jones J. D., Felix G., Boller T. (2007) Nature 448, 497–500 [DOI] [PubMed] [Google Scholar]
- 15.Heese A., Hann D. R., Gimenez-Ibanez S., Jones A. M., He K., Li J., Schroeder J. I., Peck S. C., Rathjen J. P. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 12217–12222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zipfel C., Kunze G., Chinchilla D., Caniard A., Jones J. D., Boller T., Felix G. (2006) Cell 125, 749–760 [DOI] [PubMed] [Google Scholar]
- 17.Gómez-Gómez L., Boller T. (2000) Mol. Cell 5, 1003–1011 [DOI] [PubMed] [Google Scholar]
- 18.Chinchilla D., Bauer Z., Regenass M., Boller T., Felix G. (2006) Plant Cell 18, 465–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robatzek S., Bittel P., Chinchilla D., Köchner P., Felix G., Shiu S. H., Boller T. (2007) Plant Mol. Biol. 64, 539–547 [DOI] [PubMed] [Google Scholar]
- 20.Li J., Wen J., Lease K. A., Doke J. T., Tax F. E., Walker J. C. (2002) Cell 110, 213–222 [DOI] [PubMed] [Google Scholar]
- 21.van der Hoorn R. A., Wulff B. B., Rivas S., Durrant M. C., van der Ploeg A., de Wit P. J., Jones J. D. (2005) Plant Cell 17, 1000–10015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Germain H., Gray-Mitsumune M., Lafleur E., Matton D. P. (2008) Planta 228, 851–862 [DOI] [PubMed] [Google Scholar]
- 23.Mulder L., Lefebvre B., Cullimore J., Imberty A. (2006) Glycobiology 16, 801–809 [DOI] [PubMed] [Google Scholar]
- 24.Göhre V., Spallek T., Häweker H., Mersmann S., Mentzel T., Boller T., de Torres M., Mansfield J. W., Robatzek S. (2008) Curr. Biol. 18, 1824–1832 [DOI] [PubMed] [Google Scholar]
- 25.Saijo Y., Tintor N., Lu X., Rauf P., Pajerowska-Mukhtar K., Häweker H., Dong X., Robatzek S., Schulze-Lefert P. (2009) EMBO J. 28, 3439–3449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zipfel C., Robatzek S., Navarro L., Oakeley E. J., Jones J. D., Felix G., Boller T. (2004) Nature 428, 764–767 [DOI] [PubMed] [Google Scholar]
- 27.Schöb H., Kunz C., Meins F., Jr. (1997) Mol. Gen. Genet. 256, 581–585 [DOI] [PubMed] [Google Scholar]
- 28.Voinnet O., Lederer C., Baulcombe D. C. (2000) Cell 103, 157–167 [DOI] [PubMed] [Google Scholar]
- 29.Helenius A., Aebi M. (2004) Annu. Rev. Biochem. 73, 1019–1049 [DOI] [PubMed] [Google Scholar]
- 30.Li J., Zhao-Hui C., Batoux M., Nekrasov V., Roux M., Chinchilla D., Zipfel C., Jones J. D. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 15973–15978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nekrasov V., Li J., Batoux M., Roux M., Chu Z. H., Lacombe S., Rougon A., Bittel P., Kiss-Papp M., Chinchilla D., van Esse H. P., Jorda L., Schwessinger B., Nicaise V., Thomma B. P., Molina A., Jones J. D., Zipfel C. (2009) EMBO J. 28, 3428–3438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu J., Brownlie R., Liu Q., Babiuk L. A., Potter A., Mutwiri G. K. (2009) Mol. Immunol. 46, 978–990 [DOI] [PubMed] [Google Scholar]
- 33.Kamikubo Y., Dellas C., Loskutoff D. J., Quigley J. P., Ruggeri Z. M. (2008) Biochem. J. 410, 595–604 [DOI] [PubMed] [Google Scholar]
- 34.Hong Z., Jin H., Tzfira T., Li J. (2008) Plant Cell 20, 3418–3429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bell J. K., Botos I., Hall P. R., Askins J., Shiloach J., Segal D. M., Davies D. R. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 10976–10980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choe J., Kelker M. S., Wilson I. A. (2005) Science 309, 581–585 [DOI] [PubMed] [Google Scholar]
- 37.Bell J. K., Askins J., Hall P. R., Davies D. R., Segal D. M. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 8792–8797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun J., Duffy K. E., Ranjith-Kumar C. T., Xiong J., Lamb R. J., Santos J., Masarapu H., Cunningham M., Holzenburg A., Sarisky R. T., Mbow M. L., Kao C. (2006) J. Biol. Chem. 281, 11144–11151 [DOI] [PubMed] [Google Scholar]
- 39.Dunning F. M., Sun W., Jansen K. L., Helft L., Bent A. F. (2007) Plant Cell 19, 3297–3313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruiz-Canada C., Kelleher D. J., Gilmore R. (2009) Cell 136, 272–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shiu S. H., Bleecker A. B. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 10763–10768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boller T., Felix G. (2009) Annu. Rev. Plant Biol. 60, 379–406 [DOI] [PubMed] [Google Scholar]
- 43.Kajiura H., Koiwa H., Nakazawa Y., Okazawa A., Kobayashi A., Seki T., Fujiyama K. (2010) Glycobiology 20, 235–247 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.