Abstract
Recent genome-wide transcriptome studies suggest the presence of numerous bidirectional overlapping coding gene pairs in mammalian genomes. Various antisense RNAs are reported as non-coding RNAs that regulate the expression of sense RNA. However, it is still unclear whether the expression of bidirectional overlapping coding genes are regulated by the opposite strand gene transcript acting as a non-coding RNA. Bop1 and Scx are a pair of bidirectional overlapping coding genes related to cellular proliferation and differentiation, respectively. Scx gene is localized in the intron 3 region of the Bop1 gene. The expression of these genes is reciprocally regulated by estrogen (E2) in the mouse uterus. In situ hybridization indicated that both genes are expressed in the uterine endometrial epithelial cells and that the antisense RNA of Scx (Bop1 intronic RNA) accumulates as a stable RNA in these cells. The existence of Bop1 intronic RNA was confirmed by reverse transcription-PCR and was increased after E2 treatment, coinciding with a decrease in Scx mRNA. Murine myoblasts expressing doxycycline-inducible endogenous Bop1 gene showed an increase in Bop1 intronic RNA and a simultaneous decrease in Scx mRNA. Murine fibroblasts expressing Scx mRNA from an exogenous Scx mini-gene indicated that the accumulation of Bop1 intronic RNA impairs the Scx gene expression in a trans-acting manner, which resulted in the reduction of the Scx mRNA level. This study demonstrates a novel example of hormone-stimulated intronic non-coding RNA down-regulating the expression of an opposing strand-overlapping coding gene.
Keywords: DNA/Transcription, Gene/Regulation, Hormones/Steroid, Organisms/Mammal, Organisms/Mouse, RNA, Non-coding RNA
Introduction
Genome-wide transcriptome studies suggest that about 15–25% of mammalian genes are arranged such that they overlap one another on opposite DNA strands, giving rise to pairs of sense and antisense RNAs (1–6). Several groups have classified the bidirectional overlapping gene pairs (1, 5), although a precise mechanism of the regulation is not well understood. For instance, Numata et al. (5) suggested six structural categories as follows: category 1, one transcription unit is completely overlapped within an exon of the transcription unit on the opposite strand; category 2, exonic regions overlap in convergent orientation; category 3, exonic regions overlap in divergent orientation; category 4, one transcript unit is completely overlapped within an intron of the transcription unit on the opposite strand; category 5, transcription units overlap in convergent orientation, but exonic regions do not overlap; category 6, transcription units overlap in divergent orientation, but exonic regions do not overlap. Studies focused on the exonic overlapping gene pairs (categories 1, 2 and 3) have suggested that double strand RNA can be derived from sense-antisense mRNAs (7, 8); however, little is known regarding intronic overlapping gene pairs that are included in categories 4, 5, and 6.
Antisense non-coding RNAs have the ability to regulate the expression of sense RNA through an epigenetic mechanism, as exemplified by X chromosome inactivation by Xist (9–11) or genome imprinting by Air (12–14). Recently, genome-wide studies suggest the presence of numerous bidirectional overlapping coding gene pairs (1). Whether the expressions of bidirectional overlapping coding genes can be regulated by an opposite strand gene transcript acting like a non-coding RNA is still unclear.
Bop1 and Scx are a pair of bidirectional overlapping coding genes located on mouse chromosome 15. The Scx gene consists of two exons and is embedded in the intron 3 region of the Bop1 gene (Fig. 1); therefore, based on the categorization defined above, this gene pair falls into category 4. Bop1 is a component of the nucleolar ribonucleoprotein complex, is involved in 5 S and 28 S ribosomal RNA maturation (15), and is necessary for the biogenesis of 60 S ribosomal subunit (16). It is also involved in cell proliferation because reports show that a dominant negative mutation of Bop1 prevents cell proliferation (17, 18). On the other hand, Scx is a basic helix-loop-helix type transcription factor that regulates the function of differentiated cells, such as osteoblastic cells (19), chondrogenic cells (20), tendon (21), and Sertoli cells (22). Thus, this chromosome locus is occupied by genes involved in both cell proliferation and differentiation. Studies using the Scx transgenic or knock-out mice predicted that the expression of Scx and Bop1 genes may affect each other (21, 23, 24); however, any correlation of regulation between Bop1 and Scx gene expression has not been described.
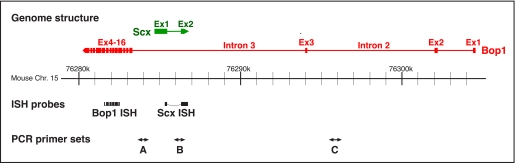
FIGURE 1.
Schematic illustration of the Bop1 and Scx genes. The location of the Bop1 (red) and Scx (green) genes on mouse chromosome 15 is illustrated with their exon-intron structure. The boxes and lines indicate exonic and intronic regions, respectively. The arrowheads depict the direction of gene transcription. The positions of the probes for ISH are indicated as black boxes (Bop1 ISH and Scx ISH). The positions of PCR-amplified element are indicated as A, B, and C. Primer sets A and B are located on Bop1 intron 3. Set A does not overlap, and set B overlaps the Scx gene. Primer set C is located on Bop1 intron 2. Ex1, Ex2, Ex3, and Ex4–16 indicate exons 1, 2, 3, and 4–16, respectively.
Estrogen (E2)2 triggers the initiation of the proliferation of endometrial epithelial cells in the mammalian uterus (25, 26). Our laboratory has examined the effect of E2 responsiveness in the mouse uterus (27–29). Previously, we reported that E2 induces the expression of cell proliferation-related genes similar to different growth factors in the ovariectomized (OVX) mouse uterus (29). In our microarray gene profile obtained from the E2-treated OVX mouse uterus, we found that the Bop1 mRNA, a proliferation-related factor, is induced, whereas the level of Scx mRNA, a differentiating cell factor, is decreased simultaneously. In this report, we demonstrate a mechanism of reciprocal regulation of Bop1 and Scx gene expression.
EXPERIMENTAL PROCEDURES
Plasmid Construction
The pTRE-c-Myc plasmid was constructed as follows. pCMV-SPORT6-mouse 3962047 plasmid that contains full-length mouse c-Myc cDNA (ATCC, Manassas, VA) was cut with EcoRI and XhoI, and the excised fragment (1.4 kb) was cloned into pCR2.1 vector (Invitrogen) at EcoRI and XhoI sites (pCR2.1-c-Myc). The XbaI fragment (1.4 kb) from pCR2.1-c-Myc plasmid was cloned into pTRE vector (Clontech) at the XbaI site. The direction of the inserted fragment was confirmed by digestion with EcoRI and EcoRV. pTRE-mBop1-cDNA plasmid was constructed as follows. pCMV-SPORT6-mouse 4193123 plasmid that contains full-length mouse Bop1 cDNA (ATCC) was cut with SacII and XbaI, and the excised fragment (2.5 kb) was cloned into pTRE vector at the SacII and XbaI site. CMV-Scx-cDNA plasmid was constructed as follows. pCMV-SPORT6.1-mouse 30138970 plasmid that contains full-length mouse Scx cDNA (ATCC) was cut with EcoRV and NotI, and the excised fragment (1.1 kb) was cloned into pcDNA3 vector (Invitrogen) at the EcoRV and NotI site. To construct the CMV-Scx-gDNA(+1) and CMV-Scx-gDNA(−250) plasmids, SKA3SH8Z11NA1 plasmid (30) that contains ∼11 kb of mouse Scx genomic DNA was used (kindly given by Dr. A. Perez, Center for Functional Genomics University at Albany). The DNA fragment (+1 to +2392) and DNA fragment (−250 to +2392), respectively, of Scx gene were cloned into the pcDNA3 vector. All plasmids were confirmed by sequencing (NIEHS sequencing core).
Animals and Treatments
All animals were handled according to National Institutes of Health guidelines and in compliance with an NIEHS, National Institutes of Health-approved animal protocol. OVX C57BL/6 mice were purchased from Charles River Laboratories (Raleigh, NC). Groups of animals (six mice per group) were treated with sesame oil vehicle (Sigma-Aldrich) or with 1 μg of E2 (Steraloids, Newport, RI), either dissolved in 100 μl of sesame oil and injected subcutaneously (6-, 12-, and 24-h groups) or dissolved in 100 μl of normal saline and injected intraperitoneally (0.5-, 1-, and 2-h groups) before necropsy. In some cases, animals (four mice per group) were treated with 45 μg of ICI 182,780 (ICI; Zeneca Pharmaceuticals, Cheshire, UK) that was dissolved in 50 μl of dimethyl sulfoxide and injected intraperitoneally, 30 min before E2 injection. Animals were euthanized at the indicated times using CO2, and uteri were collected and stored at −80 °C until use.
In Situ Hybridization
Uterine tissue was fixed in 4% paraformaldehyde and then embedded in paraffin before sectioning. Probes that corresponded to the mouse Bop1 and Scx genes were amplified by PCR using the following primers: Bop1 gene (AK079627), 5′-CCA TGC CGA GTC TTA CAA CCC ACC-3′ (nucleotides 913–936) and 5′-AGC AGC AAC ACG GCA TCA TCC ATG GC-3′ (reverse complement to nucleotides 1431–1406); Scx gene (BC062161), 5′-ACA CCC AGC CCA AAC AGA TCT GCA C-3′ (nucleotides 615–639) and 5′-CCA GGT AGA GAG CCA GCA TGG AAA GTC-3′ (reverse complement to nucleotides 960–934). A 570-bp length of plant-derived sequence (57.9% of guanine-cytosine content) was used for negative control (GENOSTAFF, Tokyo Japan). Probes (cRNA) labeled with digoxigenin were hybridized to the uterus tissue sections, and this procedure was followed by treatment with anti-digoxigenin alkaline phosphatase-conjugated antibodies and visualization by 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium (GENOSTAFF).
Establishment of Stable Transformants
C2C12 mouse myoblasts (ATCC) were maintained in Dulbecco's modified Eagle's medium (DMEM; Invitrogen) with 10% fetal bovine serum (FBS; Gemini Bio-products, West Sacramento, CA), 1000 units/ml penicillin, and 1000 μg/ml streptomycin. Cells were cultured at 37 °C under a 5% CO2, 95% air atmosphere. Tet-On-C2C12 was established as follows. C2C12 cells were seeded in a 60-mm diameter dish for transfection. Transfection was performed by using FuGENE 6 (Roche Diagnostics) according to the manufacturer's protocol. pTet-On vector (Clontech) was transfected into the C2C12 cells. The cells were switched to fresh medium with 400 μg/ml G418 at 24 h after transfection. The medium was changed every 4 days for 3 weeks. To establish the Tet-On-Myc-C2C12 and Tet-On-mBop1cDNA-C2C12, Tet-On-C2C12 cells were transfected with pTK-Hyg (Clontech) and pTRE-c-Myc or pTRE-mBop1-cDNA plasmids. To select for stable cell lines, the cells were cultured with 300 μg/ml G418 and 300 μg/ml hygromycin for 2 weeks. BALB/3T3 clone A31 mouse fibroblasts (ATCC) were maintained in DMEM with 10% newborn calf serum (Invitrogen), penicillin, and streptomycin. To establish the CMV-Scx-gDNA-BALB/3T3 and CMV-Scx-cDNA-BALB/3T3 cells, CMV-Scx-gDNA(+1), CMV-Scx-gDNA(−250), or CMV-Scx-cDNA plasmids, respectively, were transfected to BALB/3T3 cells. The cells were cultured with 400 μg/ml G418 for 3 weeks to select the stable cell line.
Cell Culture and Treatments
Tet-On-Myc-C2C12 and Tet-On-mBop1cDNA-C2C12 cells were maintained in DMEM with 10% FBS, penicillin, streptomycin, 300 μg/ml G418, and 200 μg/ml hygromycin. Tet-On-Myc-C2C12 cells (1.2 × 106 cells) were plated into 100-mm diameter dishes. Two days after, cells (90–95% confluent) were switched into DMEM with 1% FBS, penicillin, streptomycin, G418, and hygromycin that was subsequently changed every 2 days. Five days after (time 0), the cells were switched to DMEM with 10% FBS, 1 mg/ml doxycyclin, penicillin, streptomycin, G418, and hygromycin. The cells were harvested to collect RNA at 2 or 6 h after changing the medium. Transformed BALB/3T3 cell lines were maintained in DMEM with 10% newborn calf serum, penicillin, streptomycin, and G418. The cells (1.7 × 105 cells/well for CMV-Scx-gDNA(+250), 1.7 × 105 cells/well for CMV-Scx-gDNA(+1), and 1.9 × 105 cells/well for CMV-Scx-cDNA) were plated in a 6-well plate. The cells were cultured in the DMEM with 10% newborn calf serum for 24 h and then switched into the DMEM with 1% horse serum (Invitrogen) for 3 days (low serum medium condition) to maintain quiescent cells. To induce the proliferation of the cells, three wells were switched to DMEM with 10% FBS (high serum medium). The other three wells were replaced with fresh low serum medium. The cells were harvested 6 h after changing the medium for RNA extraction.
RNA Extraction and Reverse Transcription
Frozen uterine tissue was pulverized, and total RNA was extracted by using TRIzol reagent (Invitrogen) according to the manufacturer's protocol. Total RNA of the cells was extracted by using a Qiagen RNeasy kit (Qiagen, Valencia CA) according to the manufacturer's instructions. Extracted RNA was treated with TURBO DNase (Ambion) to remove the genomic DNA contamination. To make the cDNA samples, total RNA (1 μg) was incubated in 20-μl reaction mixes containing 2 μl of 10× RT buffer (200 mm Tris-HCl (pH 8.4), 500 mm KCl) (Invitrogen), 100 ng of random hexamer, 0.5 mm dNTPs, 5 mm MgCl2, 10 mm dithiothreitol, 40 units of RNaseOUT (Invitrogen), 50 units of SuperScript II reverse transcriptase (Invitrogen) at 42 °C for 50 min.
Quantitative PCR
Real-time PCR was performed by using the primer sets as described in Table 1. To amplify the mRNA, forward and reverse primers were designed on the different exonic sequences except the primers for Bop1 introns. The primers of ScxQ-F and ScxQ-R are localized on the exons 1 and 2, respectively, of the Scx gene. The Bop1Q-F primer is localized on the junction of exons 3 and 4 of the Bop1 gene, and the Bop1Q-R primer is localized on the Bop1 exon 5. The primer mix of Classic II 18 S Standards (Ambion) was used for 18 S ribosomal RNA (18 S). For cDNA amplification, 1–10 ng of cDNA were combined with 25 μl of a mixture containing Power SYBR Green PCR master mix (Applied Biosystems, Foster City, CA) and 160 nm forward and reverse primers for Scx (ScxQ), Bop1 (Bop1Q), c-Myc, and Bop1 introns A, B, and C or 26 nm primer mix for 18 S. Samples were analyzed in triplicate. Amplification was carried out as follows: 50 °C for 1 min, 95 °C for 10 min, and 40 cycles of 95 °C for 15 s and 60 °C for 1min. To determine the cycle time for each gene, the ABI PRISM 7900 sequence detection system (Applied Biosystems) and SDS 2.1 analysis software (Applied Biosystems) were used. We used the mathematical model described by Pfaffl (31) to get the relative expression levels. 18 S was used as an internal control for all reactions.
TABLE 1.
The primer sets used for real-time PCR and conventional PCR
| Name of primer set | Sequence of primer (forward) | Sequence of primer (reverse) |
|---|---|---|
| ScxQ | 5′-CGTCTTTCTGTCACGGTCTTTGCTC-3′ | 5′-CTTTCTTCCACAGCGGTCGTGC-3′ |
| Bop1Q | 5′-CAGCTCTGATGAGGAGGACATTCGGAAC-3′ | 5′-CAACCTGCTCATCAGTTAGCCG-3′ |
| Bop1 intron A | 5′-CATTTCTGGGCTGGTGTGGACAG-3′ | 5′-GTGGGTAGTTTACAAGGCAGGAACCAT-3′ |
| Bop1 intron B | 5′-CGTCTTTCTGTCACGGTCTTTGCTC-3′ | 5′-AAATACAACGGCTTTCCCCAATCC-3′ |
| Bop1 intron C | 5′-TCCGTCCCTCGTCACACATTTG-3′ | 5′-CCTGCTTTTTCTGCTGAACATCCTC-3′ |
| c-Myc | 5′-ACCCCTCAGTGGTCTTTCCCTACCC-3′ | 5′-GTTTGCCTCTTCTCCACAGACACCACATC-3′ |
| Scx | 5′-CTTCACTGCGCTGCGCACACTCATCC-3′ | 5′-GCTCTCCGTGACTCTTCAGTGGCATCC-3′ |
| Bop1 | 5′-CAGCTCTGATGAGGAGGACATTCGGAAC-3′ | 5′-TCACAGGGTGGATCATGATGTCACCGC-3′ |
PCR
PCR was performed using the primer sets as described in Table 1. The PCR of Scx was run for 26 or 32 cycles (95 °C for 30 s, 66 °C for 50 s, and 72 °C for 2 min), the PCR of Bop1 was run for 22 cycles (95 °C for 30 s, 66 °C for 50 s, and 72 °C for 2 min), the PCR of 18 S rRNA was run for 14 cycles (95 °C for 30 s, 64 °C for 50 s, and 72 °C for 4 min), and the PCR of Bop1 introns (A, B, and C) was run for 28 cycles (95 °C for 30 s, 64 °C for 50 s and 72 °C for 2 min) using Platinum Taq DNA polymerase (Invitrogen). Aliquots (6 μl) of PCR products were resolved on 2% (w/v) agarose gels stained with ethidium bromide and viewed using the UVP, LLC bioimaging system.
Statistical Analysis
Statistical analysis was performed with two-tailed paired t tests, and p < 0.05 was considered statistically significant.
RESULTS
The Expression of Bop1 and Scx Genes Is Reciprocally Regulated
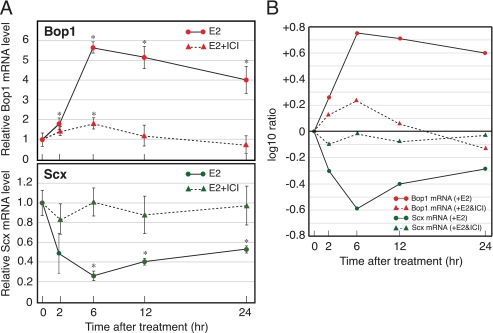
OVX adult female mice were treated with E2 at time 0, and total RNA was isolated at the times indicated (Fig. 2). Relative amounts of Bop1 and Scx mRNAs were quantified by real-time PCR and normalized with the amount of 18 S as an internal control. As shown in Fig. 2 by the solid lines, E2 stimulated a 6-fold peak of increase (+0.64 as log 10 ratio) in Bop1 mRNA following a 6-h exposure, and the mRNA level was gradually reduced until 24 h. In contrast, the level of Scx mRNA was decreased ∼80% (−0.59 as log 10 ratio) at 6 h after E2 treatment and gradually recovered by 24 h. These results indicated that the expression of Bop1 and Scx mRNAs was reciprocally regulated by E2 in the OVX mouse uterus. Additionally, we determined the effect of E2-mediated transcriptional activation on the Bop1 and Scx gene expression using ICI, an estrogen receptor (ER) antagonist that prevents ER-E2-mediated transcription (32). As shown in Fig. 2 by the dashed lines, ICI attenuated the Bop1 gene activation while also attenuating the down-regulation of the Scx gene expression. These results suggested that E2-activated transcription of the Bop1 gene may be coordinately related to the Scx gene down-regulation.
FIGURE 2.
Expression of the Bop1 and Scx genes in the E2-treated OVX mouse uterus. Animals (six or four mice per group) were administered E2 with (dashed line) or without (solid line) ICI, and uterine total RNA was prepared at the times indicated. A, the mRNA levels of Bop1 (upper panel) and Scx (lower panel) gene were quantified by real-time PCR as described under “Experimental Procedures.” Results were normalized to the level of 18 S in each sample. The values are the means ± S.D. *, p < 0.01 compared with time 0. B, the result expressed as the log 10 ratio for changes relative to time 0 to compare the changes between Bop1 and Scx mRNA levels.
Antisense RNA for the Scx Gene Is Present in the OVX Mouse Uterus
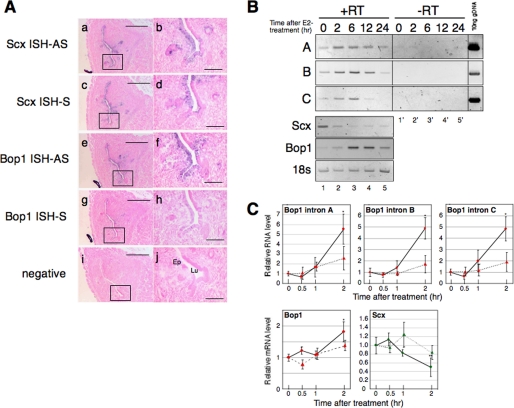
In situ hybridization (ISH) was performed using the OVX mouse uterus to localize which uterine cells express the Scx and Bop1 transcripts. The signals for the antisense probes of Scx and Bop1 transcripts were detected in the endometrial epithelial cells (Fig. 3A, panels a, b, e, and f). Interestingly, the signal for the sense probe of the Scx transcript was also detected in the endometrial epithelial cells (Fig. 3A, panels c and d). These results suggest that the antisense RNA for the Scx gene is present in the uterine cells that express Scx and Bop1 mRNAs. To confirm the existence of the antisense RNA for the Scx gene, RT-PCR was performed using RNA extracted from OVX mouse uteri. As depicted in Fig. 1, primer set A amplifies a region of intron 3 of the Bop1 gene that does not overlap with the Scx gene. Primer set B amplifies a region of intron 3 that overlaps with the Scx gene, and primer set C amplifies the intron 2 region of the Bop1 gene. We used templates that were synthesized with or without reverse transcriptase (Fig. 3B, +RT and −RT, respectively) to verify negligible amounts of genomic DNA contamination. No appreciable signal was detected in the −RT samples, demonstrating that the amplified fragments from the +RT samples were derived from RNA and not genomic DNA contamination (Fig. 3B). The size of the amplified fragment is identical to the amplicon from mouse genomic DNA, suggesting that the antisense RNA for the Scx gene, which is derived from the Bop1 intron, is present in the mouse uterus. The amount of Bop1 intron 3- and 2-derived RNA was increased at 2 h after E2 treatment, which is prior to the accumulation of Bop1 mRNA (Fig. 3, B and C). Scx mRNA was decreased at 2 h after E2 treatment, which coincides with the accumulation of Bop1 intron-derived RNA.
FIGURE 3.
Antisense RNA for the Scx gene transcript exists in the OVX mouse uterus. A, ISH results from serial sections of the OVX mouse uterus. ISH was performed using an antisense probe (AS) for Scx cDNA (panels a and b), a sense probe (S) for Scx cDNA (panels c and d), an antisense probe for Bop1 cDNA (panels e and f), and a sense probe for Bop1 cDNA (panels g and h). The position of the probes is indicated in Fig. 1. A nonspecific plant sequence was used as the negative control (panels i and j) as described under “Experimental Procedures.” The purple signal shows specific hybridization of the indicated probes. The boxes in panels a, c, e, g, and i are increased in magnification correspond to panels b, d, f, h, and j, respectively. Ep indicates endometrial epithelial cells, and Lu indicates lumen. Scale bars = 200 μm in panels a, c, e, g, and i and 50 μm in b, d, f, h, and j. B, total uterine RNA was prepared from the E2-treated mice at the indicated times. A representative RT-PCR result of various primer sets (A, B, C, Scx, Bop1, and 18 S) using the identical samples is shown. A, B, and C indicate the Bop1 intronic elements in Fig. 1. The samples of lanes 1–5 were reverse-transcribed from RNA extracted from E2-treated uteri at the indicated times (+RT). The samples of lanes 1′–5′ were not reverse-transcribed using the same RNA of 1–5 (−RT). Ten ng of genomic DNA were used as the positive control for the intronic RNA. C, E2 was administered to mice (six mice per group) with (dashed line) or without (solid line) ICI, and uterine total RNA was prepared at the times indicated. Real-time PCR was performed, and the results were normalized to the level of 18 S in each sample. The values are the means ± S.D. *, p < 0.01 when compared with time 0, **, p < 0.02 when compared with time 0.
The Induction of the Endogenous Bop1 Gene Correlates with the Down-regulation of the Scx mRNA Level in Tet-On-Myc-C2C12 Cells
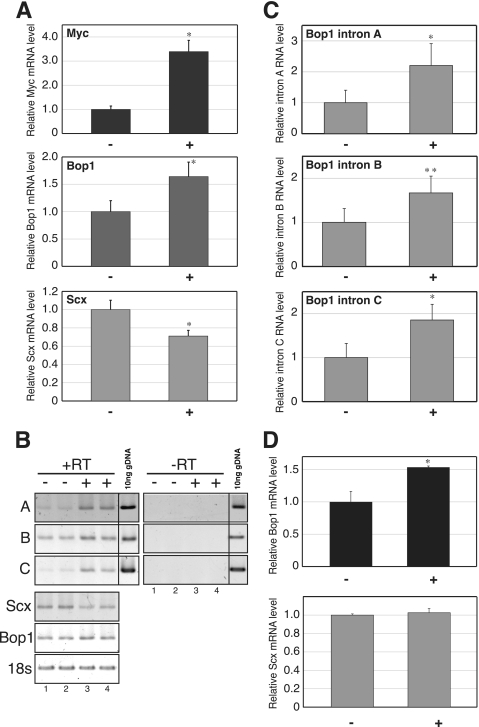
To examine the effect of Bop1 gene activation on the Scx gene down-regulation in an estrogen-independent manner, we established an in vitro model using C2C12 myoblasts, a murine cell line reported to express endogenous Scx mRNA (33). Several reports indicate that the Bop1 gene expression is induced by c-Myc (34). To activate endogenous Bop1 gene expression, we established a Tet-On-Myc C2C12 cell line that contains a tetracycline (Tet)-mediated transcription activator (TetR) expression vector and a TetR binding element fused to a c-Myc expression vector. Cells were stimulated by a Tet derivative compound, doxycycline, and harvested 6 h after stimulation. Fig. 4A shows that c-Myc mRNA was stimulated by the treatment (3.4-fold) and that Bop1 mRNA was also increased by 1.6-fold (+0.21 as log 10 ratio). In contrast, Scx mRNA was decreased 30% (−0.12 as log 10 ratio) 6 h after treatment. Samples were harvested 2 h after treatment to evaluate the expression level of Bop1 intron-derived RNA in this system. As shown in Fig. 4, B and C, Bop1 intron-derived RNA is increased by 2 h after treatment.
FIGURE 4.
The down-regulation of Scx mRNA is correlated with the induction of endogenous Bop1 gene expression. A, Tet-On-Myc-C2C12 cells were treated with (+) or without (−) doxycycline, and total RNA was prepared 6 h after treatment. Real-time PCR was performed, and the results were normalized to the level of 18 S in each sample. The values are the means ± S.D. for four independent replicates. *, p < 0.01 when compared with (−). B, a representative RT-PCR result of various primer sets using the identical samples is shown. The positions of PCR-amplified Bop1 intronic elements A, B, and C are indicated in Fig. 1. The samples of lanes 1–4 were reverse-transcribed (+RT) from the RNA that was extracted from cells treated with (+) doxycycline (2 h) without (−) doxycycline. The samples of lanes 1′–4′ were not reverse-transcribed (−RT) using the same RNA as lanes 1–4. Ten ng of genomic DNA were used for positive control of intronic RNA. C, total RNA was prepared at 2 h after with (+) or without (−) doxycycline treatment. D, Tet-On-mBop1cDNA-C2C12 cells were treated with (+) or without (−) doxycycline, and total RNA was prepared 6 h after treatment. Real-time PCR was performed, and the results were normalized to the level of 18 S in each sample. The values are the means ± S.D. for four independent replicates. *, p < 0.01 when compared with (−), **, p < 0.05 when compared with (−).
Additionally, a Tet-On-mBop1cDNA C2C12 cell line that expresses the mature Bop1 mRNA, which does not contain intronic sequences, was established to evaluate the involvement of Bop1 intron-derived RNA in the down-regulation of the Scx mRNA level. Fig. 4D shows that Bop1 mRNA was induced by doxycycline treatment after 6 h (1.5-fold); however, the level of Scx mRNA did not change after Bop1 mRNA accumulation, suggesting the need for Bop1 intronic RNA and not mRNA in Scx gene down-regulation.
Down-regulation of Scx mRNA Occurs via a Trans-acting Mechanism
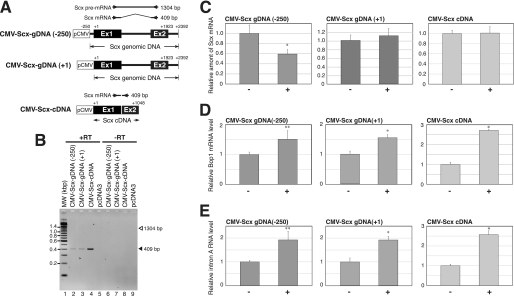
The findings above suggested that the accumulation of Bop1 intron-derived RNA is related to the reduction of the Scx mRNA level. Therefore, we examined whether the Bop1 intronic RNA leads to the down-regulation of the Scx gene in a trans-acting manner. The murine fibroblast BALB/3T3 cells were used for this experiment because these cells do not express the endogenous Scx mRNA and can be induced to express endogenous Bop1 by serum stimulation (15). We established the BALB/3T3 cell lines that express Scx mRNA from the CMV promoter fused to the Scx genomic DNA that contains +1 to +2309 sequence of the Scx gene (CMV-Scx-gDNA(+1)), −250 to +2039 sequence of the Scx gene (CMV-Scx-gDNA(−250)), or full-length Scx cDNA (CMV-Scx-cDNA) (Fig. 5A). Initially, we examined the transcripts expressed from the Scx genomic DNA and Scx cDNA by RT-PCR. The 409-bp amplicon corresponding to mature Scx mRNA was detected as the major transcript in these cell lines. The amplicon corresponding to premature Scx mRNA (1304 bp) was undetectable in the CMV-Scx-gDNA cell lines. No amplicon was detected in the empty pcDNA3 transfected cell line or in the −RT samples, suggesting that this transcript is expressed from the transfected gene (Fig. 5B). The cells were cultured in low serum medium for 3 days to reduce the endogenous Bop1 expression level. Bop1 was induced in these cells by changing to high serum medium, and the cells were harvested 6 h after changing the medium for RNA extraction. As shown in Fig. 5C, Scx mRNA decreased 40% 6 h after treatment in the CMV-Scx-gDNA(−250) cell line, whereas the level of Scx mRNA did not change in the CMV-Scx-gDNA(+1) and CMV-Scx-cDNA cell lines. The premature Scx mRNA was not detected in these cells with or without treatment (data not shown). The levels of Bop1 mRNA and Bop1 intron-derived RNA (primer set A) increased 6 h after changing to high serum medium in this system (Fig. 5, D and E). Taken together, these results suggest that Bop1 intronic RNA impairs the Scx gene transcription and acts in a trans-acting manner.
FIGURE 5.
Scx mRNA expressed from the exogenous Scx genomic DNA is reduced by increasing the endogenous Bop1 gene expression. A, schematic representation of CMV-Scx-gDNA(+1), CMV-Scx-gDNA(−250), and CMV-Scx-cDNA is shown. The position of PCR primers used for the amplification of Scx mRNAs is indicated. The expected size of the PCR amplicons is shown to the right of each primer pair. Ex1 and Ex2 indicate exons 1 and 2, respectively. B, a representative RT-PCR result of Scx transcript is shown. The samples were prepared from BALB/3T3 cell lines containing CMV-Scx-gDNA(+1), CMV-Scx-gDNA(−250), CMV-Scx-cDNA, or pcDNA3 (as a negative control). The samples in lanes 2–5 were reverse-transcribed (+RT) from the RNA, and the samples of lanes 6–9 were not reverse-transcribed (−RT) using the same RNA of 2–5. MW, molecular weight markers. C, total RNA was prepared from treated (high serum medium; +) or untreated (low serum medium; −) CMV-Scx-gDNA(+1), CMV-Scx-gDNA(−250), and CMV-Scx-cDNA BALB/3T3 cells as described under “Experimental Procedures.” A representative real-time PCR result of Scx mRNA is shown. D, a representative real-time PCR result of Bop1 mRNA is shown. E, a representative real-time PCR result of Bop1 intronic RNA (primer set A) is shown. The RNA level was normalized to the level of 18 S in each sample. The values are the means ± S.D. for three independent replicates. *, p < 0.01 when compared with (−), **, p < 0.05 when compared with (−).
DISCUSSION
Although gene profiling studies both in vivo and in vitro show that hormones stimulate and repress gene expression, the mechanisms for estrogen-mediated gene down-regulation are not extensively studied and are poorly understood. Down-regulation of gene expression related to the arrangement of overlapping genes on opposing strands, as a potential mechanism, is thought to be due to transcriptional interference, RNA masking, and/or RNA interference (35, 36). In the transcriptional interference model, because transcription by RNA polymerase II involves both large protein complexes and the unwinding of duplex DNA, it is unlikely that two overlapping transcriptional units could be transcribed concomitantly. Thus, for example, the transcript from the initiator element, which is in the first intron of the eIF2α gene on the opposing strand, decreases eIF2α transcription (37, 38). In the RNA-masking mechanism, formation of RNA duplexes between sense and antisense transcripts occurs and then inhibits the binding of trans-acting factors to the regulatory features of the transcripts. This form of steric inhibition could affect steps involved in mRNA splicing, transport, polyadenylation, translation, and degradation. As an example of this mechanism, the TRα gene encodes TRα1 as well as an alternatively spliced product, TRα2. The RevErbAα gene, which is transcribed from the opposite direction from the same locus, overlaps the TRα2 coding region. The ratio of TRα1 and TRα2 expression level is modified by the RevErbAα gene post-transcriptionally (39). In the RNA interference mechanism, formation of RNA duplexes leads to gene silencing via RNA interference pathways. Double strand RNA is potentially cleaved by the Dicer enzyme into 21–23-nucleotide duplexes (small interfering RNA). These fragments may target the specific destruction of homologous RNA (40) or silence the gene expression epigenetically (41). In this report, we show that the level of Scx mRNA expressed from the exogenous Scx genomic DNA, which contains the Scx gene promoter (CMV-Scx-gDNA(−250)), reduced as Bop1 intron-derived RNA accumulated. The Scx mRNA levels expressed from genomic DNA and cDNA without the Scx gene promoter (CMV-Scx-gDNA(+1) and CMV-Scx-cDNA) were not affected by Bop1 intronic RNA accumulation. This Scx gene promoter region, between −250 to +1, harbors two TATA boxes that are involved in Scx gene transcription initiation (supplemental Fig. S1). This result suggests that the reduction of the Scx mRNA level is due at least in part to the inhibition of Scx gene transcription. Additional detail of the antisense RNA-mediated Scx gene transcription repression will need to be assessed in future studies.
We attempted to identify a negative estrogen-responsive element in the 5′-flanking region (−7 kb) of Scx. Examination by reporter analysis did not reveal negative regulatory elements in this region; however, a potential ER-E2-mediated positive regulatory element was found (supplemental Fig. S2). ER-E2-mediated enhancement of the initiation of Scx gene transcription might be inhibited by Bop1 intronic RNA, although further investigation is needed to confirm this hypothesis.
Two different Scx knock-out mouse lines are reported, and both indicate the importance of intron 3 of the Bop1 gene with regard to Scx gene expression (21, 23). Both knock-out mice were targeted in a similar genomic position, namely a Neo cassette was inserted into exon 1 of the Scx gene to disrupt the expression of Scx mRNA (23) or an FRT-Neo cassette was inserted into the intron of the Scx gene flanking the first exon, allowing removal of the Scx exon 1 using the cre-loxP system (21). Retention of the Neo cassette in this genomic region in both mice lead to embryonic lethality due to the inhibition of cell proliferation between embryonic day 6.0 and embryonic day 6.5 (23), which indicates that this genomic region may be important for correct Bop1 expression. On the other hand, the deletion of Scx exon 1 (about 500 bp) is not embryonic lethal (21). Our ISH and RT-PCR analyses showed that the Bop1 intronic RNA corresponding to this region exists stably, and our in vitro studies showed that the accumulation of Bop1 intronic RNA impairs the Scx gene expression in a trans-acting manner, causing the reduction of the mRNA level. Bop1 intronic RNA, overlapping with the Scx gene, may be a coordinate means by which a mitogenic stimulus such as estrogen invokes an increase in a proliferation gene (Bop1) while simultaneously suppressing a differentiation gene (Scx) to facilitate the biological action. Further evaluation of other gene pairs will be needed to determine the broadness of this type of regulation and possible application to the hormonal responsiveness in this and other tissues.
Supplementary Material
Acknowledgments
We thank Dr. A. V. Perez for the generous gift of a plasmid. We thank Dr. K. Adelman for helpful comments and Dr. K. Burns and Dr. C. Srimaroeng for critical reading of the manuscript. We thank Y. Noguchi for assistance with the ISH experiment and other members of the Korach laboratory for helpful discussion.
This work was supported, in whole or in part, by National Institutes of Health Grant Z01ES70065 (to K. S. K.) through the Division of Intramural Research of the NIEHS.

The on-line version of this article (available at http://www.jbc.org) contains supplemental “Experimental Procedures” and Figs. S1 and S2.
- E2
- estrogen
- ER
- estrogen receptor
- ICI
- ICI182,780
- OVX
- ovariectomized
- 18 S
- 18 S ribosomal RNA
- ISH
- in situ hybridization
- Tet
- tetracycline
- DMEM
- Dulbecco's modified Eagle's medium
- FBS
- fetal bovine serum
- CMV
- cytomegalovirus
- RT-PCR
- reverse transcription-PCR
- +RT
- with reverse transcriptase
- −RT
- without reverse transcriptase.
REFERENCES
- 1.Kiyosawa H., Yamanaka I., Osato N., Kondo S., Hayashizaki Y.RIKEN Genome Exploration Research Group, Genome Science Laboratory Members (2003) Genome Res. 13, 1324–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen J., Sun M., Kent W. J., Huang X., Xie H., Wang W., Zhou G., Shi R. Z., Rowley J. D. (2004) Nucleic Acids Res. 32, 4812–4820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katayama S, Tomaru Y., Kasukawa T., Waki K., Nakanishi M., Nakamura M., Nishida H., Yap C. C., Suzuki M., Kawai J., Suzuki H., Carninci P., Hayashizaki Y., Wells C., Frith M., Ravasi T., Pang K. C., Hallinan J., Mattick J., Hume D. A., Lipovich L., Batalov S., Engström P. G., Mizuno Y., Faghihi M. A., Sandelin A., Chalk A. M., Mottagui-Tabar S., Liang Z., Lenhard B., Wahlestedt C. (2005) Science 309, 1564–1566 [DOI] [PubMed] [Google Scholar]
- 4.Galante P. A., Vidal D. O., de Souza J. E., Camargo A. A., de Souza S. J. (2007) Genome Biol. 8, R40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Numata K., Okada Y., Saito R., Kiyosawa H., Kanai A., Tomita M. (2007) Gene 392, 134–141 [DOI] [PubMed] [Google Scholar]
- 6.Okada Y., Tashiro C., Numata K., Watanabe K., Nakaoka H., Yamamoto N., Okubo K., Ikeda R., Saito R., Kanai A., Abe K., Tomita M., Kiyosawa H. (2008) Hum. Mol. Genet. 17, 1631–1640 [DOI] [PubMed] [Google Scholar]
- 7.Werner A. (2005) RNA Biol. 2, 53–62 [DOI] [PubMed] [Google Scholar]
- 8.Faghihi M. A., Wahlestedt C. (2006) Genome Biol. 7, R38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marahrens Y., Panning B., Dausman J., Strauss W., Jaenisch R. (1997) Genes Dev. 11, 156–166 [DOI] [PubMed] [Google Scholar]
- 10.Sheardown S. A., Duthie S. M., Johnston C. M., Newall A. E., Formstone E. J., Arkell R. M., Nesterova T. B., Alghisi G. C., Rastan S., Brockdorff N. (1997) Cell 91, 99–107 [DOI] [PubMed] [Google Scholar]
- 11.Heard E., Rougeulle C., Arnaud D., Avner P., Allis C. D., Spector D. L. (2001) Cell 107, 727–738 [DOI] [PubMed] [Google Scholar]
- 12.Lyle R., Watanabe D., te Vruchte D., Lerchner W., Smrzka O. W., Wutz A., Schageman J., Hahner L., Davies C., Barlow D. P. (2000) Nat. Genet. 25, 19–21 [DOI] [PubMed] [Google Scholar]
- 13.Sleutels F., Zwart R., Barlow D. P. (2002) Nature 415, 810–813 [DOI] [PubMed] [Google Scholar]
- 14.Seidl C. I., Stricker S. H., Barlow D. P. (2006) EMBO J. 25, 3565–3575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strezoska Z., Pestov D. G., Lau L. F. (2000) Mol. Cell. Biol. 20, 5516–5528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lapik Y. R., Fernandes C. J., Lau L. F., Pestov D. G. (2004) Mol. Cell 15, 17–29 [DOI] [PubMed] [Google Scholar]
- 17.Pestov D. G., Grzeszkiewicz T. M., Lau L. F. (1998) Oncogene 17, 3187–3197 [DOI] [PubMed] [Google Scholar]
- 18.Strezoska Z., Pestov D. G., Lau L. F. (2002) J. Biol. Chem. 277, 29617–29625 [DOI] [PubMed] [Google Scholar]
- 19.Liu Y., Nifuji A., Tamura M., Wozney J. M., Olson E. N., Noda M. (1997) J. Cell. Biochem. 67, 66–74 [DOI] [PubMed] [Google Scholar]
- 20.Kawa-uchi T., Nifuji A., Mataga N., Olson E. N., Bonaventure J., Shinomiya K., Liu Y., Noda M. (1998) J. Cell. Biochem. 70, 468–477 [DOI] [PubMed] [Google Scholar]
- 21.Murchison N. D., Price B. A., Conner D. A., Keene D. R., Olson E. N., Tabin C. J., Schweitzer R. (2007) Development 134, 2697–2708 [DOI] [PubMed] [Google Scholar]
- 22.Muir T., Sadler-Riggleman I., Skinner M. K. (2005) Mol. Endocrinol. 19, 2164–2174 [DOI] [PubMed] [Google Scholar]
- 23.Brown D., Wagner D., Li X., Richardson J. A., Olson E. N. (1999) Development 126, 4317–4329 [DOI] [PubMed] [Google Scholar]
- 24.Pryce B. A., Brent A. E., Murchison N. D., Tabin C. J., Schweitzer R. (2007) Dev. Dyn. 236, 1677–1682 [DOI] [PubMed] [Google Scholar]
- 25.Pollard J. W., Pacey J., Cheng S. V., Jordan E. G. (1987) Cell Tissue Res. 249, 533–540 [DOI] [PubMed] [Google Scholar]
- 26.Cooke P. S., Buchanan D. L., Young P., Setiawan T., Brody J., Korach K. S., Taylor J., Lubahn D. B., Cunha G. R. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 6535–6540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hewitt S. C., Deroo B. J., Hansen K., Collins J., Grissom S., Afshari C. A., Korach K. S. (2003) Mol. Endocrinol. 17, 2070–2083 [DOI] [PubMed] [Google Scholar]
- 28.Deroo B. J., Hewitt S. C., Peddada S. D., Korach K. S. (2004) Endocrinology 145, 5485–5492 [DOI] [PubMed] [Google Scholar]
- 29.Hewitt S. C., Collins J., Grissom S., Deroo B., Korach K. S. (2005) Mol. Endocrinol. 19, 657–668 [DOI] [PubMed] [Google Scholar]
- 30.Perez A. V., Perrine M., Brainard N., Vogel K. G. (2003) Mech. Dev. 120, 1153–1163 [DOI] [PubMed] [Google Scholar]
- 31.Pfaffl M. W. (2001) Nucleic Acids Res. 29, e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hyder S. M., Chiappetta C., Murthy L., Stancel G. M. (1997) Cancer Res. 57, 2547–2549 [PubMed] [Google Scholar]
- 33.Liu Y., Watanabe H., Nifuji A., Yamada Y., Olson E. N., Noda M. (1997) J. Biol. Chem. 272, 29880–29885 [DOI] [PubMed] [Google Scholar]
- 34.Schlosser I., Hölzel M., Mürnseer M., Burtscher H., Weidle U. H., Eick D. (2003) Nucleic Acids Res. 31, 6148–6156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lavorgna G., Dahary D., Lehner B., Sorek R., Sanderson C. M., Casari G. (2004) Trends Biochem. Sci. 29, 88–94 [DOI] [PubMed] [Google Scholar]
- 36.Munroe S. H., Zhu J. (2006) Cell. Mol. Life Sci. 63, 2102–2118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silverman T. A., Noguchi M., Safer B. (1992) J. Biol. Chem. 267, 9738–9742 [PubMed] [Google Scholar]
- 38.Noguchi M., Miyamoto S., Silverman T. A., Safer B. (1994) J. Biol. Chem. 269, 29161–29167 [PubMed] [Google Scholar]
- 39.Hastings M. L., Ingle H. A., Lazar M. A., Munroe S. H. (2000) J. Biol. Chem. 275, 11507–11513 [DOI] [PubMed] [Google Scholar]
- 40.Jakymiw A., Pauley K. M., Li S., Ikeda K., Lian S., Eystathioy T., Satoh M., Fritzler M. J., Chan E. K. (2007) J. Cell Sci. 120, 1317–1323 [DOI] [PubMed] [Google Scholar]
- 41.Matzke M. A., Birchler J. A. (2005) Nat. Rev. Genet. 6, 24–35 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.