Abstract
The γ-aminobutyric acid type A (GABAA) receptors play a pivotal role in fast synaptic inhibition in the central nervous system. One of the key factors for determining synaptic strength is the number of receptors on the postsynaptic membrane, which is maintained by the balance between cell surface insertion and endocytosis of the receptors. In this study, we investigated whether phospholipase C-related but catalytically inactive protein (PRIP) is involved in insulin-induced GABAA receptor insertion. Insulin potentiated the GABA-induced Cl− current (IGABA) by about 30% in wild-type neurons, but not in PRIP1 and PRIP2 double-knock-out (DKO) neurons, suggesting that PRIP is involved in insulin-induced potentiation. The phosphorylation level of the GABAA receptor β-subunit was increased by about 30% in the wild-type neurons but not in the mutant neurons, which were similar to the changes observed in IGABA. We also revealed that PRIP recruited active Akt to the GABAA receptors by forming a ternary complex under insulin stimulation. The disruption of the binding between PRIP and the GABAA receptor β-subunit by PRIP interference peptide attenuated the insulin potentiation of IGABA. Taken together, these results suggest that PRIP is involved in insulin-induced GABAA receptor insertion by recruiting active Akt to the receptor complex.
Keywords: Membrane/Trafficking, Neurobiology/Neuroscience, Phosphorylation, Receptors/Neurotransmitters, Akt/PKB, Insulin, GABA
Introduction
The γ-aminobutyric acid (GABA)4 type A (GABAA) receptors are GABA-gated chloride channels that mediate the majority of fast synaptic inhibition in the central nervous system (1–5). The perturbation of GABA-GABAA receptors-mediated neurotransmission causes several central nervous system disorders including motor coordination, anxiety, insomnia, schizophrenia, and epilepsy. Additionally, GABAA receptors are important therapeutic drug targets for sedative, anxiolytic, anticonvulsant, and hypnotic agents (1–5). Therefore, it is important to uncover how synaptic strength is regulated in GABAergic transmission. The GABAA receptors are heteropentamers composed of a combination of 18 GABAA receptor subunits, which are divided into seven subunit classes (α1–6, β1–3, γ1–3, δ, ϵ1–3, θ, and π) based on their sequence homology (1–5). Each receptor subunit has a similar structure with a large N-terminal extracellular region, which is the binding site for GABA and psychoactive drugs such as benzodiazepines, followed by four hydrophobic transmembrane domains (TM1–4) with a large intracellular loop region between TM3 and 4. This intracellular loop region is a target for protein-protein interactions, phosphorylation, ubiquitination, and palmitoylation, which control receptor trafficking, stability, and clustering on the synaptic membrane (1–5). Regulation of the number of receptors on the postsynaptic membrane is one of the key factors for determining synaptic strength, which is maintained by a balance between the insertion and endocytosis of receptors to/from the cell surface. Recently, it was reported that the dephosphorylation of the GABAA receptor β- or γ2-subunit triggers endocytosis by facilitating the binding to the μ2-subunit of adaptor protein 2 (AP2) complex, a critical component of clathrin-dependent endocytosis (6–9). On the other hand, it was reported that insulin stimulates GABAA receptor insertion into the cell surface membrane via Akt-mediated phosphorylation of the GABAA receptor β-subunit (10–14).
We previously identified a new inositol 1,4,5-trisphosphate-binding protein from rat brain lysate by affinity column chromatography (15). Our subsequent studies on the characterization of the protein revealed that 1) it has a domain organization similar to δ-type phospholipase C (PLC) but has no PLC activity, which is the reason for its name, PRIP (PLC-related but catalytically inactive protein) (16, 17). 2) PRIP has two isoforms, PRIP1 and 2, which are expressed mainly in the brain and ubiquitous organs, respectively (18–20). 3) PRIP knock-out mice are less sensitive to benzodiazepine-type drugs, such as diazepam, suggesting that the cell surface expression of γ-subunit-containing GABAA receptors is diminished in these mutant mice (21, 22). 4) PRIP facilitates GABAA receptor-associated protein (GABARAP) mediated cell surface expression of γ2-subunit-containing GABAA receptors by acting as a bridging molecule between GABARAP and receptors (22–24). 5) PRIP regulates the phosphorylation level of the GABAA receptor β-subunit by binding to protein phosphatases (25–27). 6) PRIP is involved in clathrin-dependent constitutive endocytosis of GABAA receptors (28). We also have reported that PRIP modulates brain-derived neurotrophic factor (BDNF)-induced GABAA receptor endocytosis through the regulation of the receptor phosphorylation level (29). These results suggest that PRIP regulates GABAA receptor function through receptor trafficking, phosphorylation, and endocytosis (30, 31).
In this study, we investigated whether PRIP is involved in insulin-induced GABAA receptor insertion. Insulin potentiated the GABA-induced Cl− current (IGABA) by about 30% in wild-type (WT) hippocampal neurons but not in neurons derived from PRIP1 and PRIP2 double knock-out (DKO) mice. The phosphorylation level of the β-subunit was increased by about 30% in the WT neurons but not in the DKO neurons, which was similar to the changes observed in IGABA. Using an immunoprecipitation assay and a glutathione S-transferase (GST) pull-down assay using brain lysate together with a HEK293 reconstitution system we revealed that PRIP recruited active Akt to GABAA receptors. The disruption of the binding between PRIP and the β-subunit by PRIP interference peptide attenuated the insulin-potentiated IGABA. Interestingly, pretreatment with brefeldin A (BFA), an inhibitor of anterograde trafficking from the ER to the Golgi (32, 33) decreased IGABA under insulin treatment. Collectively, these results suggest that PRIP plays an important role in insulin-induced GABAA receptor insertion by recruiting active Akt to the receptor complex.
EXPERIMENTAL PROCEDURES
Chemicals, Plasmids, and Animals
Insulin and okadaic acid were obtained from Wako. Wortmannin, BFA, and crosstide were purchased from Sigma. The PRIP1-(553–565) peptide and its scrambled peptide were described previously (29). Anti-PRIP1 and anti-PRIP2 polyclonal antibodies were described previously (20, 21). Anti-Akt polyclonal antibody, antiphospho-Akt (Thr-308 or Ser-473) polyclonal antibodies, and anti-insulin receptor β-subunit monoclonal antibody (clone 4B8) were purchased from Cell Signaling. Anti-GABAA receptor α1-subunit and anti-GABAA receptor γ2-subunit polyclonal antibodies were obtained from Alpha Diagnostic International. Anti-GABAA receptor β2/3-subunit monoclonal antibody (clone 62–3G1) and anti-N-ethylmaleimide-sensitive factor (NSF) polyclonal antibody were from Upstate. Anti-GST polyclonal antibody was purchased from Santa Cruz Biotechnology. Horseradish peroxidase-conjugated anti-rabbit and anti-mouse IgG were obtained from GE Healthcare. [32P]Orthophosphate (5.55 GBq/ml) and [γ-32P]ATP (185 MBq/ml, specific activity: 111 TBq/mmol) were purchased from PerkinElmer. Construction of the mammalian expression vectors for the Myc- and FLAG-tagged GABAA receptor subunit (α1, β2, and γ2S) was described previously (34). Briefly, the Myc or FLAG tag was introduced between amino acids 4 and 5 of the mature form of each receptor subunit. For the mammalian GST fusion protein expression vector pcDNA3.1(-)/GST1, GST was amplified using primers M-81, 5′-AAA AAG CTA GCC ACC ATG TCC CCT ATA CTA GG-3′ (underlining denotes the NheI site) and M-82, 5′-AAA AAC TCG AGA TCG ATA CCG TCG ACC TCG A-3′ (underlining denotes the XhoI site) and pGST4 as a template. The PCR products were digested using NheI/XhoI and cloned into the same sites of pcDNA3.1(-). The rat PRIP1 (rPRIP1) was amplified using primers M-85, 5′-AAA AAC TCG AGC ATG GCT GAG GGC GCG GCT A-3′ (underlining denotes the XhoI site) and M-86, 5′-AAA AAA AGC TTT CAC AAC TTC CCG TTC TCT TC-3′ (underlining denotes the HindIII site) and pcMT31 (16) as a template. The PCR products were digested using XhoI/HindIII and cloned into the same sites of pcDNA3.1(-)/GST1 to produce pcDNA3.1(-)/GST1-rPRIP1. The PRIP1 expression plasmid pSG5/rPRIP1 was described previously (16). The mammalian expression vector for Akt pECE/Akt was kindly provided by Dr. U. Kikkawa (Kobe University, Japan) (35). The generation of the DKO mice was described previously (22, 29). The handling of the mice and all procedures were approved by the Animal Care Committee of Kyushu University, according to the guidelines of the Japanese Council on Animal Care.
Electrophysiology
Electrophysiological measurements were performed in acutely isolated hippocampal CA1 pyramidal neurons using the conventional whole cell patch-clamp technique. The acutely dissociated neurons were prepared from postnatal day 10–14 WT or DKO mice, as described previously (36). All recordings were performed under voltage clamp conditions at a holding potential of −50 mV and a patch-clamp amplifier (EPC-7plus, HEKA Instruments Inc). All experiments were performed at a room temperature of 22–25 °C. The ionic composition of patch pipette solution containing 80 mm KCl, 70 mm potassium methanesulfonate, 4 mm ATP-Mg, 2 mm EGTA, 1 mm MgCl2, 10 mm HEPES, and adjusted pH to 7.2 with Tris-base. Extracellular solution containing 150 mm NaCl, 2.5 mm KCl, 2 mm CaCl2, 1 mm MgCl2, 10 mm HEPES, and 10 mm glucose. The pH was adjusted to 7.4 with Tris-base. Reagents dissolved in extracellular solution were applied by using the Y-tube perfusion system, which allows rapid exchange of the solution surrounding a cell (37, 38). All data are expressed as the means ± S.D.
Cell Culture and Transfection
HEK293 cells were grown in Dulbecoo's modified Eagle's medium (DMEM) containing 10% fetal bovine serum supplemented with 100 units/ml penicillin and 0.1 mg/ml streptomycin. The cells were maintained at 37 °C in a humidified 5% CO2 incubator. Plasmid transfection was performed using the calcium phosphate method as described elsewhere (39) or Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. Briefly, 1.5 μg of each GABAA receptor subunit (α1, β2, and γ2S) with or without 2.5 μg of pSG5/rPRIP1 and/or pECE/Akt were transfected into 7.5 × 105 cells. For the GST pull-down assay, 1.0 μg of pcDNA3.1(-)/GST1 or pcDNA3.1(-)/GST1-rPRIP1 was transfected with 2.5 μg of pECE/Akt. After 48 h of incubation, the cells were used for each experiment. Cortical neurons were prepared from postnatal day 0 (P0) WT or DKO mice, as described previously (21, 29) and were cultured for 14–18 days in vitro (DIV) before the experiments.
Immunoprecipitation, GST Pull-down, and Western Blotting
Cell lysates were prepared from cortical neurons or plasmid-transfected HEK293 cells using ice-cold lysis buffer containing 50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 5 mm EDTA, 1 mm EGTA, 1% Triton X-100, phosphatase inhibitors (50 mm NaF, 10 mm Na4P2O7, 20 mm β-glycerophosphate, and 1 mm Na3VO4), and protease inhibitors (1 mm phenylmethylsulfonyl fluoride, 100 μm (p-amidinophenyl)methanesulfonyl fluoride hydrochloride, 10 μg/ml leupeptin, 10 μg/ml pepstatin A, and 3.4 μg/ml aprotinin). The mouse whole brain lysates of WT or DKO mice were also prepared using the same buffer. In the case of co-precipitation of NSF, 0.5 mm ATP was added to the lysis buffer. The lysates were subjected to immunoprecipitation using the indicated antibodies. For the GST pull-down assay, 20 μl of glutathione-SepharoseTM 4B (GE Healthcare) were added to cell lysates expressing the GST fusion protein. The immunocomplex was washed five times with 1 ml of ice-cold lysis buffer containing phosphatase inhibitors. The lysates and immunocomplexes were subjected to SDS-polyacrylamide gel electrophoresis (PAGE) and then transferred onto polyvinylidene fluoride membrane. Western blotting was performed using the indicated antibodies, and signals were detected using the ECL plus Western blotting detection system (GE Healthcare) and LAS3000 mini (Fuji Film).
32P Labeling of Cultured Neurons
Cultured cortical neurons (DIV 14–18) were incubated with 1 ml of phosphate-free DMEM for 1 h and then labeled with 7.4 MBq/ml of [32P]orthophosphate for 4 h at 37 °C. The neurons were stimulated with 500 nm insulin for the indicated times at 37 °C. The cells were then washed twice with ice-cold phosphate-buffered saline and extracted with 500 μl of ice-cold lysis buffer containing phosphatase inhibitors and protease inhibitors. The cell lysates were subjected to immunoprecipitation using an anti-GABAA receptor β2/3-subunit monoclonal antibody. The immunocomplexes were washed five times with 1 ml of ice-cold lysis buffer containing phosphatase inhibitors and subjected to SDS-PAGE. Phosphorylated proteins were detected by autoradiography using a Bio-Image analyzer BAS2500 (Fuji Film).
Akt Kinase Assay
The Akt kinase assay was described previously (40). Briefly, immunocomplexes created using an anti-Akt polyclonal antibody were washed once with ice-cold 1 ml of Akt kinase assay buffer (50 mm Tris-HCl, pH 7.5, 10 mm MgCl2, and 1 mm dithiothreitol) and then resuspended in 30 μl of the same buffer containing 100 μm peptide substrate, crosstide, and 10 μm [γ-32P]ATP (37 kBq/reaction). After incubation for 30 min at 30 °C, the reaction was stopped by adding 10 μl of 300 mm H3PO4. The reaction products were spotted onto peptide binding paper (Whatman P81 cation exchange paper) and then washed three times with 75 mm H3PO4 to remove nonspecific radioactivity. After drying, the paper was subjected to liquid scintillation counting. Data are expressed as means ± S.D.
RESULTS
Insulin Potentiates GABA-induced Cl− Current and Phosphorylation of the GABAA Receptor β-Subunit in WT but Not DKO Neurons
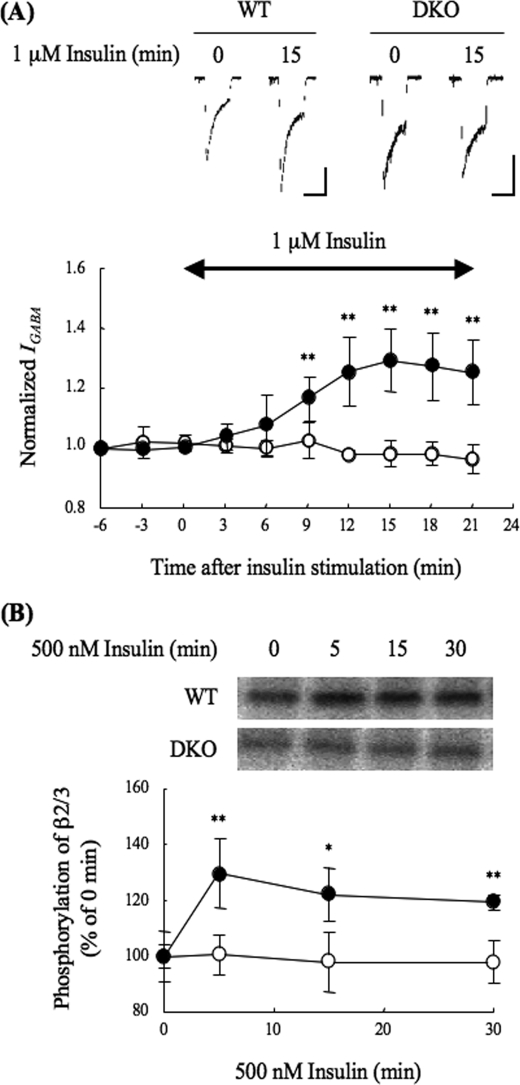
In this study, we investigated whether PRIP is involved in insulin-induced GABAA receptor insertion. We first investigated the effect of insulin on IGABA using acutely isolated hippocampal CA1 neurons from either WT or DKO mice. IGABA was increased maximally by 30% during 15 min of 1 μm insulin stimulation in WT neurons (Fig. 1A), which is consistent with previous reports by other groups (10–13). In the DKO neurons, however, no insulin effect was observed (Fig. 1A).
FIGURE 1.
Electrophysiological analysis of IGABA in insulin-stimulated hippocampal CA1 neurons and phosphorylation of the GABAA receptor β2/3-subunit. A, effect of insulin on IGABA. Electrophysiological experiments were performed using acutely prepared hippocampal CA1 neurons from WT (closed circles, n = 5) or DKO (open circles, n = 5) mice. GABA (3 μm) was applied for 15 s (3-min interval), and whole cell currents were recorded. Insulin (1 μm) was applied for the time period indicated by the double-headed arrow in graph. Upper panel shows representative GABA-induced current traces at 0 min or 15 min after insulin stimulation of WT or DKO neurons. Vertical and horizontal scales show 200 pA and 15 s, respectively. The graph shows the amplitude of IGABA normalized to that seen without insulin. All data are represented as means ± S.D. Significance was determined using the Student's t test (**, p < 0.01, compared with the results from DKO). B, phosphorylation of the β-subunit in response to insulin stimulation. The cultured cortical neurons (DIV. 14–18) of the WT or DKO mice were metabolically labeled with [32P]orthophosphates for 4 h. The neurons were stimulated with 500 nm insulin for the indicated time, and then the cell lysates were subjected to immunoprecipitation using an anti-GABAA receptor β2/3-subunit antibody. The immunocomplexes were separated by SDS-PAGE and then subjected to autoradiography. Phosphorylated bands were detected using BAS2500. The autoradiograph represents one of four independent experiments. The other experiments gave similar results. The graph shows quantitative data concerning the phosphorylation of the GABAA receptor β2/3-subunit of WT (closed circles) or DKO (open circles) neurons. As mentioned above, 32P incorporation was analyzed, because the phosphospecific antibody currently available recognizes the di-phosphorylated β3-subunit at both Ser-408 and Ser-409 (29, 41), and insulin causes a single phosphorylation at Ser-410 or Ser-409 of β2- or β3-subunit, respectively (11, 12). Data are represented as means ± S.D. (n = 4). Significance was determined using the Student's t test (*, p < 0.05; **, p < 0.01, compared with the results from DKO).
We next investigated insulin-induced phosphorylation of the GABAA receptor β-subunit using cortical neurons from each genotype because insulin-induced membrane insertion of GABAA receptors is accompanied by the phosphorylation of the β-subunit (11, 12). For this purpose, cultured neurons were metabolically labeled with [32P]orthophosphate for 4 h and then stimulated with 500 nm insulin for 5, 15, or 30 min. The GABAA receptor β-subunits were precipitated using an anti-GABAA receptor β2/3-subunit antibody, followed by separation by SDS-PAGE and autoradiography. As shown in Fig. 1B, phosphorylation of the GABAA receptor β-subunit was increased by about 30% after 5 min of insulin stimulation and continued for 30 min in WT, but no such increase in phosphorylation was observed in the DKO neurons. These results suggest that PRIP participates in the insulin-induced phosphorylation of the GABAA receptor β-subunit, leading to the insulin-induced potentiation of IGABA.
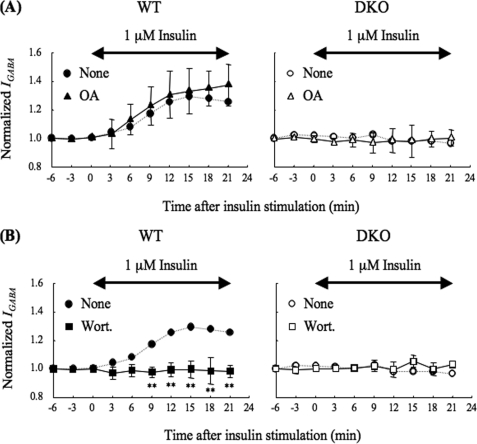
Two possible explanations for the low phosphorylation of the β-subunit observed in DKO neurons are higher activity of phosphatases or lower activity of kinases. We previously reported that PRIP participates in the regulation of the phosphorylation level of the GABAA receptor β-subunit by acting as a scaffolding protein for protein phosphatases (PP1 and PP2A) (27, 29). So, we investigated the effects of protein phosphatase inhibitors on the insulin-induced potentiation of IGABA. We pretreated neurons with 10 μm okadaic acid for 15 min, which inhibits both PP1 and PP2A at this concentration (42) and then measured the effect of insulin on IGABA. Pretreatment with okadaic acid had no effect on IGABA in the presence of insulin stimulation with DKO neurons (Fig. 2A, right panel). If higher phosphatase activity is responsible for the low phosphorylation of the β-subunit, okadaic acid would have increased IGABA. WT neurons exhibited a marginal increase of IGABA insulin potentiation, but the effect was not significant (Fig. 2A, left panel). These results indicate that the PRIP deficiency caused the failure of kinase(s) action, rather than the regulation of phosphatases.
FIGURE 2.
Effect of okadaic acid or wortmannin on the insulin-potentiation of IGABA. A, effect of okadaic acid on the insulin potentiation of IGABA. Neurons from WT (left panel, closed triangles, n = 8) or DKO (right panel, open triangles, n = 3) mice were pretreated with 10 μm okadaic acid, an inhibitor of the protein phosphatases PP1 and PP2A (42), for 15 min and throughout the experiment. The experiment was performed as shown in Fig. 1A except for the okadaic acid treatment. All data are represented as means ± S.D. The IGABA from WT (left panel, closed circles, dashed line) or DKO (right panel, open circles, dashed line) mice without okadaic acid (none), which were taken from Fig. 1A, are also shown as references. B, effect of wortmannin on the insulin potentiation of IGABA. Neurons from WT (left panel, closed squares, n = 6) or DKO (right panel, open squares, n = 3) mice were pretreated with 100 nm of wortmannin, a potent PI 3-kinase inhibitor (45), for 15 min and throughout the experiment. The experiments were performed as shown in Fig. 1A except for the wortmannin treatment. All data are represented as means ± S.D. The IGABA from WT (left panel, closed circles, dashed line) or DKO (right panel, open circles, dashed line) mice without wortmannin (none), which were taken from those shown in Fig. 1A, are also shown as references. Double-headed arrows indicate the period of insulin stimulation. Significance was determined using the Student's t test (**, p < 0.01 from the results obtained in the absence of the drug). none, no addition; OA, okadaic acid; Wort, wortmannin.
It is well known that insulin activates the phosphatidylinositol 3-kinase (PI 3-kinase)-Akt signaling pathway (43, 44) and the Akt-mediated phosphorylation of the GABAA receptor β-subunit, and the potentiation of miniature inhibitory postsynaptic currents (mIPSCs) is also reported to require the process (11, 12). We pretreated neurons with 100 nm wortmannin, a potent PI 3-kinase inhibitor (45) for 15 min prior to insulin stimulation. Consistent with previous reports (12, 13), pretreatment with wortmannin completely blocked the insulin potentiation of IGABA in WT neurons (Fig. 2B, left panel), while wortmannin had no effect on the IGABA in DKO neurons (Fig. 2B, right panel), confirming that the PI 3-kinase signaling pathway was required in our experiments.
Akt Activation following PI 3-Kinase Activation in Response to Insulin Stimulation
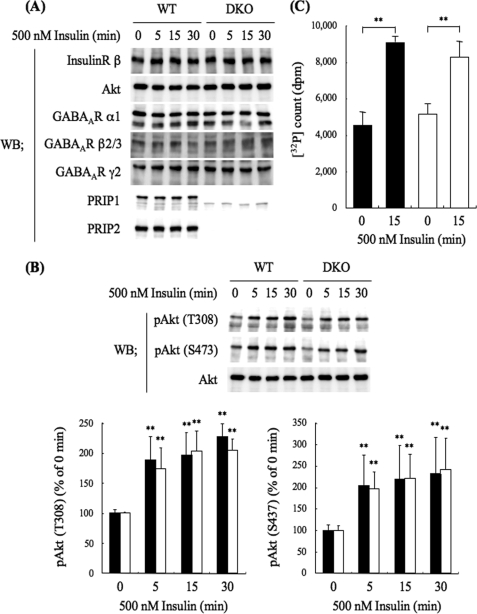
To find reasonable explanations for the failure of the phosphorylation of the GABAA receptor β-subunit in response to insulin stimulation observed in the DKO neurons, we investigated whether PRIP deficiency impaired the insulin signaling pathway. For this purpose, we first examined the expression level of molecules involved in insulin signaling. Cortical neurons from WT or DKO mice were cultured until 14–18 DIV. After serum starvation for 4 h, the neurons were stimulated with 500 nm insulin for 5, 15, or 30 min, and cell lysates were prepared, followed by Western blotting using relevant antibodies. As shown in Fig. 3A, PRIP deficiency had no effect on the expression levels of the insulin receptor β-subunit, Akt, or several GABAA receptor subunits, and insulin stimulation for up to 30 min also had no effect on the expression of these molecules.
FIGURE 3.
PRIP deficiency caused little changes in the expression level of molecules possibly involved in insulin signaling. A, Western blotting analysis of the expression level of insulin signaling molecules. WT or DKO cortical neurons were cultured for 14–18 days and then stimulated with 500 nm insulin for the indicated time. The cell lysates were analyzed by Western blotting using the indicated antibodies shown on the left. The blot shown is a typical result from six experiments. B, Western blotting analysis of Akt activation. The WT or DKO cortical cell lysates were prepared in the same way as described above and analyzed by Western blotting using antiphospho-Akt antibodies. The blot and graph shown are a typical result and the summary of seven experiments, respectively. The densities of phospho-Akt at Thr-308 (left panel) and Ser-473 (right panel) relative to the total amount of Akt are shown. The filled and open columns represent the results obtained for WT and DKO mice, respectively. C, Akt kinase activity assayed in vitro. The cell lysates of WT (filled columns) or DKO (open columns) neurons stimulated with 500 nm insulin for 15 min were subjected to immunoprecipitation using an anti-Akt antibody. The immunocomplexes were subjected to an Akt kinase assay using crosstide as a substrate and [γ-32P]ATP. Data are represented as means ± S.D. (n = 3). Significance was determined by Student's t test (*, p < 0.05; **, p < 0.01, compared with the result before insulin stimulation), but no difference was detected between WT and DKO.
We then examined Akt activation in response to insulin stimulation by monitoring the Akt phosphorylation at the Thr-308 and Ser-473 residues using antiphospho-Akt antibodies. As shown in Fig. 3B, the phosphorylation of Akt, an index of Akt activation was increased at 5 min stimulation and sustained for 30 min, which did not differ between the WT and DKO neurons. Wortmannin completely blocked the increase in phospho-Akt (results not shown). The activity of Akt was biochemically assayed: immunoprecipitates of anti-Akt antibody attached to WT or DKO neurons stimulated with insulin for 15 min were subjected to an Akt kinase assay in vitro using crosstide as a substrate and [γ-32P]ATP. As shown in Fig. 3C, the immunoprecipitates from the neurons stimulated with insulin exhibited an ∼2-fold increase of 32P radioactivity incorporation, and there was no significant difference between the genotypes. The results indicate that Akt kinase activation, which is probably responsible for insulin-induced phosphorylation of the GABAA receptor β-subunit (11, 12), was not impaired by PRIP deficiency.
PRIP Facilitates Complex Formation between GABAA Receptors and Akt
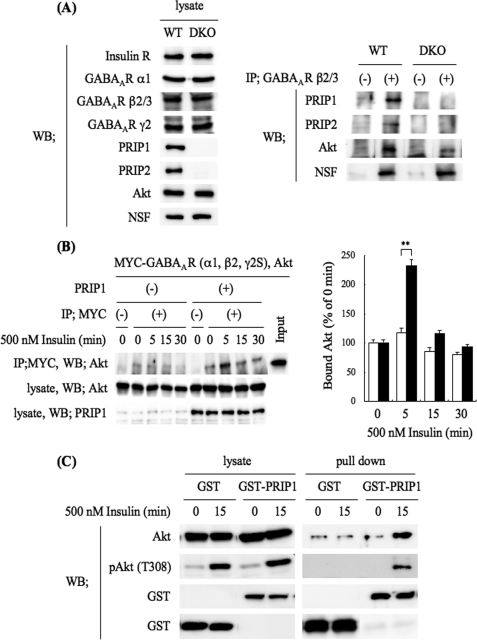
Because PRIP deficiency had no effect on insulin-induced Akt activation but caused the impairment of the insulin-induced phosphorylation of the GABAA receptor β-subunit, we hypothesized that PRIP might function as a scaffolding molecule that makes Akt more accessible to the GABAA receptor β-subunit. To examine this possibility, we performed a co-immunoprecipitation assay using brain lysates. The brain lysates prepared from WT or DKO mice were immunoprecipitated using an anti-GABAA receptor β2/3-subunit and then analyzed by Western blotting using anti-PRIP1, anti-PRIP2, and anti-Akt antibodies. Assessment by Western blotting of the amount of immunoprecipitated GABAA receptor β2/3-subunits was not possible because the corresponding bands overlapped with that for the immunoglobulin heavy chain used for the immunoprecipitation. However, we confirmed in advance that the antibody we used was able to precipitate the GABAA receptor β-subunit using a HEK293 reconstitution system with GABAA receptor subunits in combination with the [35S]methionine pulse-chase technique (results not shown). Consistent with our previous reports (27, 28), PRIP1 and -2 in the WT brain lysates were co-immunoprecipitated with the GABAA receptor β-subunit (Fig. 4A, right panel). There were no corresponding bands for PRIP1 or 2 in the immunocomplexes produced from the DKO brain lysates (Fig. 4A, right panel). The amount of Akt co-precipitated with the GABAA receptor β2/3-subunit was much greater in the WT lysates than in the DKO lysates (Fig. 4A, right panel), indicating that PRIP promotes complex formation between the β2/3-subunit and Akt. It is noteworthy that PRIP deficiency caused no effect on the direct binding between GABAA receptor β2/3-subunit and NSF, one of the β-subunit-binding proteins (46) (Fig. 4A, right panel). We next investigated whether insulin affects this complex formation, using a cultured reconstitution system. We exogenously expressed Myc-tagged GABAA receptor subunits (α1, β2, and γ2S) and Akt, with or without PRIP1 in HEK293 cells, which intrinsically contain trace amounts of PRIP1 and 2. After insulin stimulation for 5, 15, or 30 min, the cell lysates were subjected to immunoprecipitation using an anti-Myc antibody, followed by Western blotting for Akt. The amount of immunoprecipitated GABAA receptors was not apparent for the same reason as mentioned above. A small amount of Akt was seen in fractions co-precipitated with GABAA receptors in the nonstimulated cells. Insulin stimulation only increased the amount of Akt co-precipitated with GABAA receptors in the PRIP-expressing cells (Fig. 4B, the left and right panels show typical blots and a summary of multiple experiments, respectively), suggesting that PRIP facilitates complex formation between the GABAA receptor and Akt under insulin stimulation.
FIGURE 4.
Complex formation among GABAA receptor, PRIP, and Akt. A, GABAA receptors were immunoprecipitated using an anti-GABAA receptor β2/3-subunit antibody from WT or DKO brain lysates. The cell lysates (left panel) and immunoprecipitates (right panel) were analyzed by Western blotting using the indicated antibodies shown on the left. The blots shown are from one of three independent experiments. The other experiments gave similar results. B, Myc-tagged GABAA receptor subunits (α1, β2, and γ2S) and Akt with or without PRIP1 were exogenously expressed in HEK293 cells. After stimulation with 500 nm insulin for the indicated time, the cell lysates were subjected to immunoprecipitation using an anti-Myc antibody. The immunocomplexes were separated by SDS-PAGE and then analyzed by Western blotting using an anti-Akt antibody. The cell lysates were also analyzed by Western blotting using the indicated antibodies. The blots shown are one of three independent experiments. The other experiments gave similar results. The graph shows quantitative data concerning the Akt co-precipitated with GABAA receptors in PRIP expressing (filled columns) or control (open columns) cells. Significance was determined using the Student's t test (**, p < 0.01 from the control cells without exogenous PRIP1). C, HEK293 cells were transfected with Akt and GST-PRIP1 (or GST) expression plasmids. After stimulation with 500 nm insulin for 15 min, GST fusion proteins were precipitated with glutathione-SepharoseTM 4B. The protein complexes were separated by SDS-PAGE and then analyzed by Western blotting using an anti-Akt antibody, an antiphospho-Akt (Thr-308), and an anti-GST polyclonal antibody. The cell lysates were also analyzed by Western blotting using the indicated antibodies. The blots shown are one of three independent experiments. The other experiments gave similar results.
We next examined the direct binding between PRIP1 and Akt using an in vivo GST pull-down assay. Genes for GST or GST-rat(r)PRIP1 were transfected with Akt into HEK293 cells. After insulin stimulation for 15 min, GST alone or GST-rPRIP1 was precipitated from the cell lysates using glutathione-conjugated beads, followed by Western blotting for Akt and phospho-Akt. As shown in Fig. 4C, GST-rPRIP1, but not GST, bound to Akt when the cells were stimulated with insulin. Taken together, these results suggest that PRIP recruits phosphorylated (active) Akt to GABAA receptors by forming a ternary complex under insulin stimulation. Thus, PRIP might be implicated in Akt-dependent phosphorylation of GABAA receptors, leading to their insertion into the cell surface membrane.
PRIP1-(553–565) Peptide Attenuates Insulin Potentiation of IGABA
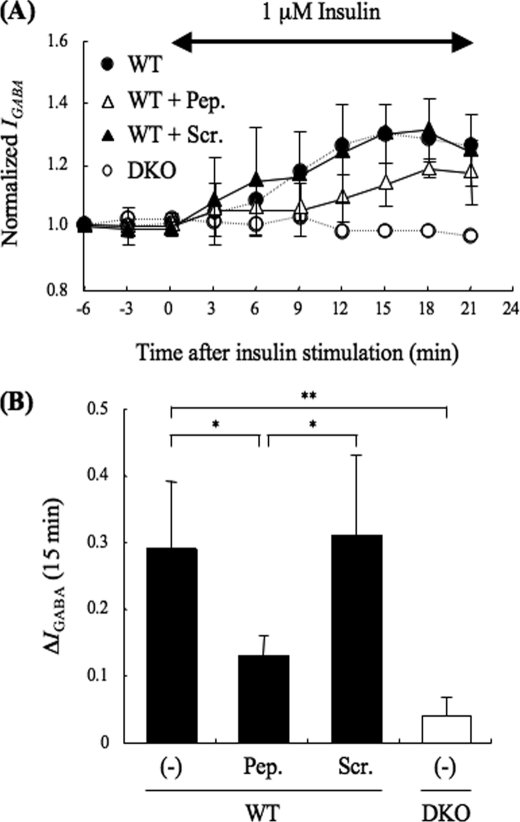
We next investigated whether such complex formation is important for the insulin potentiation of IGABA. To address this issue, PRIP1-(553–565) peptide at 3 μg/ml, which reduces the binding between PRIP1 and GABAA receptor β-subunit in cultured cells (29), was applied into WT neurons through a patch pipette, and then IGABA was measured in the presence of insulin. As shown in Fig. 5, the PRIP1-(553–565) peptide but not the control peptide (scrambled peptide of PRIP1-(553–565)) partially attenuated the insulin potentiation of IGABA, indicating that the association between the β-subunit and PRIP is important for making Akt accessible to the receptor β-subunit, resulting in the potentiation of IGABA.
FIGURE 5.
Effect of PRIP1-(553–565) peptide on insulin potentiation of IGABA in hippocampal CA1 neurons. A, PRIP1-(553–565) peptide (3 μg/ml) (open triangles, n = 3), which diminishes the binding between PRIP and the GABAA receptor β-subunit (29), or its scramble peptide (3 μg/ml) (closed triangles, n = 3) were introduced using a patch pipette. The experiment was performed in the same way as that shown in Fig. 1A. A double-headed arrow indicates the time period of insulin application. Data are represented by the means ± S.D.. The IGABA from WT (closed circles, dashed line) or DKO (open circles, dashed line) mice without the peptide, which were taken from those shown in Fig. 1A, are also shown as references. B, graph shows the potentiation of IGABA at 15 min after insulin stimulation in WT (filled columns) or DKO (open column) neurons with or without the indicated peptides (Pep., PRIP1-(553–565 peptides); Scr., PRIP1-(553–565) scramble peptides; (−), no peptides). Data are represented as means ± S.D. Significance was determined using the Student's t test (*, p < 0.05; **, p < 0.01, between indicated two columns).
BFA Reverses the Effect of Insulin on IGABA
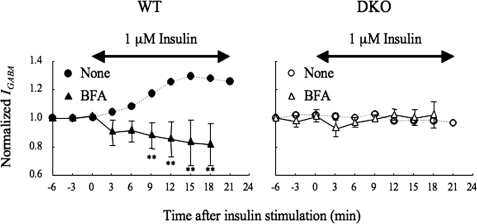
Insulin triggers the activation of Akt, leading to the phosphorylation of GABAA receptors, which are resistant to internalization, by inhibiting its association with AP2 complex (6–9). Therefore, the apparent potentiation of IGABA observed in WT neurons could have resulted from the inhibition of insulin-induced internalization rather than insulin-induced facilitation of GABAA receptor insertion. To examine this possibility, we pretreated WT neurons with 5 μg/ml BFA, an inhibitor of anterograde trafficking from the ER to the Golgi (32, 33) for 15 min and measured the effect of insulin on IGABA. If the assumption is correct, BFA would have had little effect; however, BFA caused further decreases in IGABA below the control level after insulin treatment (Fig. 6, left panel). This effect was not observed under nonstimulated conditions (time: −6 to 0 min) (Fig. 6, left panel). BFA had no effect on IGABA in the DKO neurons (Fig. 6, right panel). The results suggest that insulin induces both GABAA receptor insertion and subsequent endocytosis of the GABAA receptor and that this insertion mainly occurs in WT neurons. Additionally, the result indicates that PRIP is an important molecule in the mechanism that allows insulin to execute its effects on GABAA receptor trafficking.
FIGURE 6.
Effect of BFA on insulin potentiation of IGABA. Neurons from either WT (left panel, closed triangles, n = 3) or DKO (right panel, open triangles, n = 3) were pretreated with 5 μg/ml of BFA, which inhibits anterograde trafficking from the ER to the Golgi apparatus (32, 33) for 15 min and throughout the experiment. The experiment was performed in the same way as that described for Fig. 1A. All data are represented as means ± S.D. The IGABA from either WT or DKO without BFA, which were taken from those shown in Fig. 1A, are also shown as references. Significance was determined using the Student's t test (**, p < 0.01, from the results obtained in the absence of the drug). Double-headed arrows indicate the time period of insulin stimulation. none, no drug; BFA, brefeldin A.
DISCUSSION
It has been reported that insulin triggers rapid translocation of functional GABAA receptors from the intracellular pool to the cell surface membrane, thus increasing the amplitude of GABAA receptor-mediated mIPSCs (10). The underlying molecular mechanisms have been proposed as follows: insulin elicits tyrosine phosphorylation at residues Tyr-372 and Tyr-379 of the β2-subunit of GABAA receptors by unknown kinase(s), and these phosphotyrosines are then recognized by the SH2 domain of p85 (13), a regulatory subunit of PI 3-kinase. The PI 3-kinase bound to GABAA receptors produces phosphatidylinositol 3,4,5-trisphosphate, an upstream activator of serine/threonine kinase Akt around the receptors, leading to the phosphorylation of the intracellular loop region of the β-subunits (Ser-409 in β1, Ser-410 in β2, or Ser-409 in β3), which is essential for the membrane insertion of GABAA receptors (11, 12).
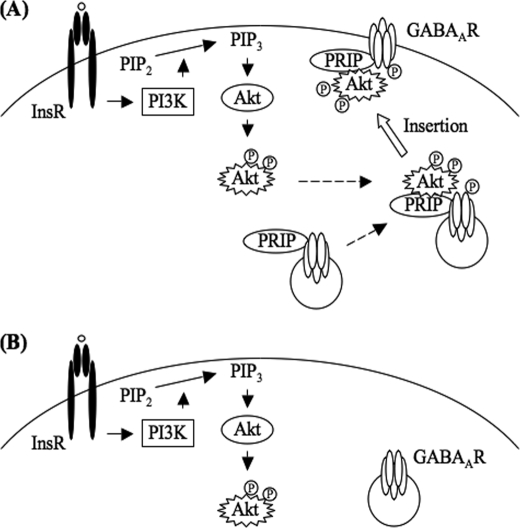
The current study was motivated by the finding that no effect of insulin on the potentiation of IGABA was seen in neurons derived from PRIP-deficient mice, indicating that PRIP is involved in process(es) triggered by insulin stimulation. Therefore, our studies exploring the possible mechanisms in which PRIP is implicated have continued to examine each step involved in known insulin signaling pathways (43, 44). PRIP deficiency neither perturbs protein expression profiles including those of insulin receptors and Akt nor impairs the activation of Akt in whole cell extract, as assessed by the phosphorylation of residues Thr-308 and Ser-473 and in vitro enzymatic activity using synthetic peptide substrate. However, the phosphorylation of the GABAA receptor β-subunit in neurons from DKO mice was not augmented by insulin stimulation. This phenomenon is probably attributed to the fact that Akt is not accessible to GABAA receptor β-subunits in the absence of PRIP. Based on the observations, we propose that PRIP functions as a scaffolding protein that presents the active form of Akt to GABAA receptors, enabling insulin signaling to potentiate IGABA (Fig. 7).
FIGURE 7.
Schematic representation of the role of PRIP in insulin-induced membrane insertion of GABAA receptors. A, insulin stimulation induces Akt activation in a PI 3-kinase-dependent manner. Subsequent phosphorylation of the β-subunits of GABAA receptors by Akt is facilitated by PRIP through the ternary complex formation with activated Akt and β-subunit, which triggers an enhancement of the insertion of GABAA receptors into the postsynaptic membrane. B, absence of PRIP fails in making activated Akt accessible to β-subunit. Arrows indicate the signaling pathways to activate downstream target. Dashed arrows indicate the complex formation. White arrow indicates membrane insertion of GABAA receptor. InsR, insulin receptor; GABAAR, GABAA receptor, PI3K, PI 3-kinase; PIP2, phosphatidylinositol 4,5-bisphosphate; PIP3, phosphatidylinositol 3,4,5-trisphosphate, PRIP, PLC-related but catalytically inactive protein. Circled P indicates the phosphorylation.
We previously reported that PRIP is involved in the regulation of BDNF-induced endocytosis of GABAA receptors (29). In this case, PKC, which directly associates with the GABAA receptor β-subunit, is activated by BDNF stimulation and triggers the phosphorylation of the Ser-408 and Ser-409 residues of the β3-subunit. These residues are subsequently dephosphorylated by PP2A, which is recruited to the vicinity of the receptors via PRIP (29, 41). Thus, BDNF stimulation triggers transient phosphorylation of the β-subunit, followed by long-lasting dephosphorylation. The μ2-subunit of AP2 complex specifically binds to the dephosphorylated form of the β-subunit, leading to clathrin-mediated endocytosis of the GABAA receptor (6–8). Correspondingly, a transient increase and a subsequent long-lasting decrease in IGABA is observed (29, 41). On the other hand, in DKO neurons, BDNF caused a gradual increase in the phosphorylation of the β-subunit and therefore of IGABA, which lasted for the full 30-min examination period (29). Taken together, these results indicate that different extracellular stimuli evoke phosphorylation of the β-subunit of the GABAA receptor at the same residues via different kinases, the level of which is regulated by the balance of activity between kinase(s) and phosphatase(s) especially in the vicinity of GABAA receptors but not inside cells. Therefore, the time courses of the phosphorylation level appear to be dependent on the type of stimuli involved. In either case, PRIP through direct association with the GABAA receptor β-subunit, plays an important role in recruiting proteins including the active form of Akt (this study) and protein phosphatases (PP1 and PP2A) (27, 29), which regulate the phosphorylation of the GABAA receptor β-subunit, leading to the regulation the number of receptors on the cell surface membrane. In fact, we observed a further decrease of insulin-mediated IGABA below the control level in the BFA-treated WT but not DKO neurons. This observation suggests that insulin elicits both the insertion and subsequent endocytosis of GABAA receptors and that the balance shifts to membrane insertion in insulin-stimulated WT neurons. The result also suggests that PRIP is involved in both insulin-induced membrane insertion and endocytosis of GABAA receptors. Other scaffolding molecules such as receptor for activated C kinase-1 (RACK-1) for protein kinase C (47, 48), protein kinase A-anchoring protein (AKAP) 79/150 for cAMP-dependent protein kinase A (PKA) (49), and PRIP (27, 29) have been reported to determine the specificity of the specific kinase(s) or phosphatase(s) recruited to the vicinity of GABAA receptors. We still do not know the exact molecular mechanisms by which different stimuli regulate the recruitment of kinase(s) and phosphatase(s) to the vicinity of GABAA receptors. The phosphorylation state of the scaffolding molecules may be one of the pathways that regulates the interaction among these molecules. Additionally, the molecular mechanisms by which phosphorylation of β-subunit triggers the membrane insertion of GABAA receptors remains largely unknown.
Is there any physiological or pathological relevance of the insulin-induced membrane insertion of GABAA receptors and the involvement of PRIP? It is reported that oxygen-glucose deprivation (OGD), an ischemia-like challenge, decreases the number of cell surface GABAA receptors and thereby leads to excitotoxic cell death in cultured hippocampal neurons. Insulin treatment counteracts the OGD-induced diminishment of the number of cell surface GABAA receptors and thus prevents ischemic cell death (14). Additionally, it is reported that insulin-induced cell surface expression of GABAA receptors leads to membrane hyperpolarization in islet α cells, thereby suppressing glucagon secretion (12), suggesting its involvement in diabetic pathogenesis. Another example is that interleukin-1β (IL-1β) increases in the cell surface expression of GABAA receptors depend on the PI 3-kinase-Akt signaling pathway (50). Patients with sepsis-associated encephalopathy (SAE), a neurological complication in sepsis, have higher plasma levels of IL-1β, therefore this may contribute to the cognitive dysfunction observed in SAE by altering GABAergic synaptic strength (50). It is possible that PRIP is implicated in such neuronal dysfunction and pathogenesis through the recruitment of active Akt to GABAA receptors, suggesting that PRIP could a therapeutic target.
In conclusion, we showed here that PRIP is implicated in the insulin-induced membrane insertion of GABAA receptors as it recruits active Akt to the vicinity of GABAA receptors. The subsequent complex formation may serve as the molecular basis for the efficient phosphorylation of GABAA receptors through Akt and receptor insertion into the cell surface membrane. Therefore, PRIP is a key factor in the control of the plasticity of GABAergic transmission.
Acknowledgments
We thank Dr. U. Kikkawa (Kobe University, Japan) for kindly donating the mammalian expression vector for Akt, pECE/Akt. We thank all of the laboratory members for their critical discussion and reading the manuscript.
This work was supported by grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (to M. F., T. K., and M. H.), the Cooperative Study Program of the National Institute for Physiological Sciences (to T. K., J. N., and M. H.), the Japan Diabetes Foundation (to T. K.), and the Pharmacological Research Foundation, Tokyo (to T. K.).
- GABA
- γ-aminobutyric acid
- AP2
- adaptor protein 2
- BDNF
- brain-derived neurotrophic factor
- DIV
- days in vitro
- DKO
- PRIP1 and PRIP2 double knockout
- GABAA receptor
- γ-aminobutyric acid type A receptor
- GABARAP
- GABAA receptor-associated protein
- GST
- glutathione S-transferase
- IGABA
- GABA-induced Cl− current
- PP
- protein phosphatase
- PRIP
- phospholipase C-related but catalytically inactive protein
- WT
- wild-type
- NSF
- N-ethylmaleimide-sensitive factor
- PI
- phosphatidylinositol.
REFERENCES
- 1.Moss S. J., Smart T. G. (2001) Nat. Rev. Neurosci. 2, 240–250 [DOI] [PubMed] [Google Scholar]
- 2.Lüscher B., Keller C. A. (2004) Pharmacol. Ther. 102, 195–221 [DOI] [PubMed] [Google Scholar]
- 3.Vicini S., Ortinski P. (2004) Pharmacol. Ther. 103, 109–120 [DOI] [PubMed] [Google Scholar]
- 4.Michels G., Moss S. J. (2007) Crit. Rev. Biochem. Mol. Biol. 42, 3–14 [DOI] [PubMed] [Google Scholar]
- 5.Jacob T. C., Moss S. J., Jurd R. (2008) Nat. Rev. Neurosci. 9, 331–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kittler J. T., Chen G., Honing S., Bogdanov Y., McAinsh K., Arancibia-Carcamo I. L., Jovanovic J. N., Pangalos M. N., Haucke V., Yan Z., Moss S. J. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 14871–14876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen G., Kittler J. T., Moss S. J., Yan Z. (2006) J. Neurosci. 26, 2513–2521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith K. R., McAinsh K., Chen G., Arancibia-Carcamo I. L., Haucke V., Yan Z., Moss S. J., Kittler J. T. (2008) Neuropharmacology 55, 844–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kittler J. T., Chen G., Kukhtina V., Vahedi-Faridi A., Gu Z., Tretter V., Smith K. R., McAinsh K., Arancibia-Carcamo I. L., Saenger W., Haucke V., Yan Z., Moss S. J. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 3616–3621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wan Q., Xiong Z. G., Man H. Y., Ackerley C. A., Braunton J., Lu W. Y., Becker L. E., MacDonald J. F., Wang Y. T. (1997) Nature 388, 686–690 [DOI] [PubMed] [Google Scholar]
- 11.Wang Q., Liu L., Pei L., Ju W., Ahmadian G., Lu J., Wang Y., Liu F., Wang Y. T. (2003) Neuron. 38, 915–928 [DOI] [PubMed] [Google Scholar]
- 12.Xu E., Kumar M., Zhang Y., Ju W., Obata T., Zhang N., Liu S., Wendt A., Deng S., Ebina Y., Wheeler M. B., Braun M., Wang Q. (2006) Cell Metab. 3, 47–58 [DOI] [PubMed] [Google Scholar]
- 13.Vetiska S. M., Ahmadian G., Ju W., Liu L., Wymann M. P., Wang Y. T. (2007) Neuropharmacology 52, 146–155 [DOI] [PubMed] [Google Scholar]
- 14.Mielke J. G., Wang Y. T. (2005) J. Neurochem. 92, 103–113 [DOI] [PubMed] [Google Scholar]
- 15.Kanematsu T., Takeya H., Watanabe Y., Ozaki S., Yoshida M., Koga T., Iwanaga S., Hirata M. (1992) J. Biol. Chem. 267, 6518–6525 [PubMed] [Google Scholar]
- 16.Kanematsu T., Misumi Y., Watanabe Y., Ozaki S., Koga T., Iwanaga S., Ikehara Y., Hirata M. (1996) Biochem. J. 313, 319–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanematsu T., Yoshimura K., Hidaka K., Takeuchi H., Katan M., Hirata M. (2000) Eur. J. Biochem. 267, 2731–2737 [DOI] [PubMed] [Google Scholar]
- 18.Matsuda M., Kanematsu T., Takeuchi H., Kukita T., Hirata M. (1998) Neurosci. Lett. 257, 97–100 [DOI] [PubMed] [Google Scholar]
- 19.Uji A., Matsuda M., Kukita T., Maeda K., Kanematsu T., Hirata M. (2002) Life Sci. 72, 443–453 [DOI] [PubMed] [Google Scholar]
- 20.Otsuki M., Fukami K., Kohno T., Yokota J., Takenawa T. (1999) Biochem. Biophys. Res. Commun. 266, 97–103 [DOI] [PubMed] [Google Scholar]
- 21.Kanematsu T., Jang I. S., Yamaguchi T., Nagahama H., Yoshimura K., Hidaka K., Matsuda M., Takeuchi H., Misumi Y., Nakayama K., Yamamoto T., Akaike N., Hirata M., Nakayama K. (2002) EMBO. J. 21, 1004–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mizokami A., Kanematsu T., Ishibashi H., Yamaguchi T., Tanida I., Takenaka K., Nakayama K. I., Fukami K., Takenawa T., Kominami E., Moss S. J., Yamamoto T., Nabekura J., Hirata M. (2007) J. Neurosci. 27, 1692–1701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coyle J. E., Nikolov D. B. (2003) Neuroscientist 9, 205–216 [DOI] [PubMed] [Google Scholar]
- 24.Chen Z. W., Olsen R. W. (2007) J. Neurochem. 100, 279–294 [DOI] [PubMed] [Google Scholar]
- 25.Yoshimura K., Takeuchi H., Sato O., Hidaka K., Doira N., Terunuma M., Harada K., Ogawa Y., Ito Y., Kanematsu T., Hirata M. (2001) J. Biol. Chem. 276, 17908–17913 [DOI] [PubMed] [Google Scholar]
- 26.Yanagihori S., Terunuma M., Koyano K., Kanematsu T., Ho Ryu S., Hirata M. (2006) Adv. Enzyme. Regul. 46, 203–222 [DOI] [PubMed] [Google Scholar]
- 27.Terunuma M., Jang I. S., Ha S. H., Kittler J. T., Kanematsu T., Jovanovic J. N., Nakayama K. I., Akaike N., Ryu S. H., Moss S. J., Hirata M. (2004) J. Neurosci. 24, 7074–7084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanematsu T., Fujii M., Mizokami A., Kittler J. T., Nabekura J., Moss S. J., Hirata M. (2007) J. Neurochem. 101, 898–905 [DOI] [PubMed] [Google Scholar]
- 29.Kanematsu T., Yasunaga A., Mizoguchi Y., Kuratani A., Kittler J. T., Jovanovic J. N., Takenaka K., Nakayama K. I., Fukami K., Takenawa T., Moss S. J., Nabekura J., Hirata M. (2006) J. Biol. Chem. 281, 22180–22189 [DOI] [PubMed] [Google Scholar]
- 30.Kanematsu T., Takeuchi H., Terunuma M., Hirata M. (2005) Mol. Cells 20, 305–314 [PubMed] [Google Scholar]
- 31.Kanematsu T., Mizokami A., Watanabe K., Hirata M. (2007) J. Pharmacol. Sci. 104, 285–292 [DOI] [PubMed] [Google Scholar]
- 32.Klausner R. D., Donaldson J. G., Lippincott-Schwartz J. (1992) J. Cell Biol. 116, 1071–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chardin P., McCormick F. (1999) Cell 97, 153–155 [DOI] [PubMed] [Google Scholar]
- 34.Connolly C. N., Krishek B. J., McDonald B. J., Smart T. G., Moss S. J. (1996) J. Biol. Chem. 271, 89–96 [DOI] [PubMed] [Google Scholar]
- 35.Konishi H., Matsuzaki H., Tanaka M., Ono Y., Tokunaga C., Kuroda S., Kikkawa U. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 7639–7643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mizoguchi Y., Ishibashi H., Nabekura J. (2003) J. Physiol. 548, 703–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kakazu Y., Akaike N., Komiyama S., Nabekura J. (1999) J. Neurosci. 19, 2843–2851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nabekura J., Ueno T., Okabe A., Furuta A., Iwaki T., Shimizu-Okabe C., Fukuda A., Akaike N. (2002) J. Neurosci. 22, 4412–4417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fujii M., York J. D. (2005) J. Biol. Chem. 280, 1156–1164 [DOI] [PubMed] [Google Scholar]
- 40.Wang Q., Somwar R., Bilan P. J., Liu Z., Jin J., Woodgett J. R., Klip A. (1999) Mol. Cell. Biol. 19, 4008–4018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jovanovic J. N., Thomas P., Kittler J. T., Smart T. G., Moss S. J. (2004) J. Neurosci. 24, 522–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Favre B., Turowski P., Hemmings B. A. (1997) J. Biol. Chem. 272, 13856–13863 [DOI] [PubMed] [Google Scholar]
- 43.Alessi D. R., Downes C. P. (1998) Biochim. Biophys. Acta. 1436, 151–164 [DOI] [PubMed] [Google Scholar]
- 44.van der Heide L. P., Ramakers G. M., Smidt M. P. (2006) Prog. Neurobiol. 79, 205–221 [DOI] [PubMed] [Google Scholar]
- 45.Kong D., Yamori T. (2008) Cancer Sci. 99, 1734–1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goto H., Terunuma M., Kanematsu T., Misumi Y., Moss S. J., Hirata M. (2005) Mol. Cell Neurosci. 30, 197–206 [DOI] [PubMed] [Google Scholar]
- 47.Brandon N. J., Uren J. M., Kittler J. T., Wang H., Olsen R., Parker P. J., Moss S. J. (1999) J. Neurosci. 19, 9228–9234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brandon N. J., Jovanovic J. N., Smart T. G., Moss S. J. (2002) J. Neurosci. 22, 6353–6361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brandon N. J., Jovanovic J. N., Colledge M., Kittler J. T., Brandon J. M., Scott J. D., Moss S. J. (2003) Mol. Cell Neurosci. 22, 87–97 [DOI] [PubMed] [Google Scholar]
- 50.Serantes R., Arnalich F., Figueroa M., Salinas M., Andrés-Mateos E., Codoceo R., Renart J., Matute C., Cavada C., Cuadrado A., Montiel C. (2006) J. Biol. Chem. 281, 14632–14643 [DOI] [PubMed] [Google Scholar]