Abstract
The tumor suppressor p53 is a transcription factor that regulates cell cycle, DNA repair, senescence, and apoptosis in response to DNA damage. Phosphorylation of p53 at Ser-46 is indispensable for the commitment to apoptotic cell death. A previous study has shown that upon exposure to genotoxic stress, DYRK2 translocates into the nucleus and phosphorylates p53 at Ser-46, thereby inducing apoptosis. However, less is known about mechanisms responsible for intracellular control of DYRK2. Here we show the functional nuclear localization signal at N-terminal domain of DYRK2. Under normal conditions, nuclear and not cytoplasmic DYRK2 is ubiquitinated by MDM2, resulting in its constitutive degradation. In the presence of proteasome inhibitors, we detected a stable complex of DYRK2 with MDM2 at the nucleus. Upon exposure to genotoxic stress, ATM phosphorylates DYRK2 at Thr-33 and Ser-369, which enables DYRK2 to escape from degradation by dissociation from MDM2 and to induce the kinase activity toward p53 at Ser-46 in the nucleus. These findings indicate that ATM controls stability and pro-apoptotic function of DYRK2 in response to DNA damage.
Keywords: Cell/Apoptosis, DNA/Damage, Phosphorylation/Serine/Threonine, Signal Transduction/Protein Kinases, Protein Degradation, Ubiquitination, ATM, DYRK2, MDM2, p53
Introduction
Dual-specificity tyrosine-regulated kinases (DYRKs)3 are a novel subfamily of protein kinases that catalyze their autophosphorylation on tyrosine residues and the phosphorylation of serine/threonine residues on exogenous substrates (1–3). DYRK2 shares a conserved kinase domain and adjacent N-terminal DH box but does not contain a C-terminal PEST (the proline-, glutamic acid-, serine- and threonine-rich) domain. DYRK2 is presumed to be involved in regulating key developmental and cellular processes such as neurogenesis, cell proliferation, cytokinesis, and cellular differentiation. Recent findings have shown that DYRK1A and DYRK2 phosphorylate NFATc, which regulates calcium signaling, to lead NFATc inactivation by its cytoplasmic sequestration (4, 5).
Upon exposure to genotoxic stress, p53 is stabilized and activated by phosphorylation at Ser-15 and Ser-20 to regulate a cell cycle checkpoint and DNA repair. In case of the lesion for irreparable DNA damage, p53 induces apoptotic cell death by a mechanism in which an additional phosphorylation increases the binding affinity of p53 to promoters of pro-apoptotic genes, such as p53AIP1. In this context previous studies have established the mechanism in which p53 transactivates p53AIP1 by its additional phosphorylation at Ser-46; thereby, this phosphorylation is essential for p53-dependent apoptosis (6, 7). We recently demonstrated that DYRK2 is a novel Ser-46 kinase (8–10). Ataxia telangiectasia mutated (ATM) was involved in DYRK2 activation and Ser-46 phosphorylation. Furthermore, DYRK2 accumulated in the nucleus after DNA damage. Significantly, DYRK2 phosphorylation of Ser-46 was associated with the induction of apoptosis. These findings provide a novel signaling mechanism in which phosphorylation of p53 at Ser-46 by DYRK2 regulates apoptotic cell death in response to DNA damage. Although certain insights are, thus, available regarding the signals that activate p53-mediated apoptosis, less is known about the mechanisms responsible for the intracellular regulation of DYRK2 in response to genotoxic stress.
The present findings provide a model in which ATM phosphorylates and activates DYRK2 to induce apoptotic cell death. ATM phosphorylates DYRK2 at Thr-33 and Ser-369. Intriguingly, nuclear DYRK2 is constitutively degraded by MDM2-mediated ubiquitination, and phosphorylated DYRK2 is dissociated from MDM2, resulting in the nuclear accumulation of DYRK2 in response to DNA damage. These findings collectively support an essential role for ATM in the intracellular control of DYRK2.
MATERIALS AND METHODS
Cell Culture and Induction of DNA Damage
U2OS cells, which express p53 wild type, were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 100 units/ml penicillin, 100 μg/ml streptomycin, and 2 mm l-glutamine. 293T, HCT116, and GM 5849 (11) cells were grown in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum and supplements. Cells were treated with 2 μg/ml adriamycin (Sigma), 10 μm etoposide (Sigma), MG-132 (carbobenzoxy-l-leucyl-l-leucyl-l-leucinal; Nacalai Tesque), or cycloheximide (Merck).
Plasmids
DYRK2 cDNA was amplified by PCR using the Expand high fidelity plus PCR system (Roche Applied Science) from the HL-60 cDNA library and cloned into the pcDNA3-FLAG vector and pEGFP-C1 vector as previously described (8). Site-directed mutagenesis was performed by PCR (12–14) and verified by DNA sequencing.
Cell Transfections
Plasmid DNA was transfected by using FuGENE 6 (Roche Applied Science) or by calcium phosphate co-precipitation (15–17). Oligo siRNAs were purchased from Invitrogen or Qiagen. Transfection of siRNAs was performed with Lipofectamine 2000 or Lipofectamine RNAi MAX (Invitrogen).
Immunoblot, Immunoprecipitation, and Immunohistochemistry Analyses
Cells were washed with chilled phosphate-buffered saline and resuspended in lysis buffer (18, 19). Cell extracts were centrifuged for 5 min at 4 °C. The supernatants were separated by SDS-PAGE and transferred to nitrocellulose membranes. The membranes were incubated anti-phospho-Ser/Thr ATM/ATR substrate (anti-phospho-SQ/TQ; Cell Signaling Technology), anti-phospho-p53(Ser-15) (Cell Signaling Technology), mouse anti-phospho-p53(Ser-46) (provided by Dr. Yoichi Taya), anti-FLAG (Sigma), anti-GFP (Nacalai Tesque), anti-tubulin (Sigma), anti-lamin B1 (Santa Cruz Biotechnology), anti-ATM (Santa Cruz Biotechnology), anti-DYRK2 (Santa Cruz Biotechnology), anti-His (Santa Cruz Biotechnology), anti-MDM2 (Merck), or anti-GST (Nacalai Tesque). Immune complexes were incubated with secondary antibodies and visualized by chemiluminescence (PerkinElmer Life Sciences). For immunoprecipitation, lysates were incubated with anti-FLAG-agarose (Sigma) for 2 h at 4 °C, and then the beads were washed 3 times with lysis buffer and boiled for 5 min. Co-precipitates were eluted with FLAG peptide (Sigma) as needed. Immunofluorescence analyses were performed as described elsewhere (20, 21).
In Vitro Kinase Assays
For immunoprecipitation of ATM, lysates were incubated with anti-ATM (Merck) for 2 h at 4 °C followed by 1 h of incubation with protein A-Sepharose beads (GE Healthcare). The immunoprecipitates were washed 3 times with lysis buffer and then with kinase buffer (20 mm HEPES, pH 7.0, 10 mm MgCl2, 10 mm MnCl2, 0.1 mm Na3VO4, and 2 mm dithiothreitol). As a substrate, recombinant GST-p53 or His-DYRK2 was obtained from Santa Cruz Biotechnology or Millipore, respectively. Immunoprecipitates were incubated in kinase buffer with substrates and ATP for 20 min at 30 °C. Samples were boiled for 5 min and analyzed by SDS-PAGE.
In Vitro Ubiquitination Assays
In vitro ubiquitination assays were performed by incubating with His-DYRK2 (Millipore), GST-ubiquitin (Merck), rabbit E1 (Merck), GST-UbcH5b (Merck), and GST-MDM2 (Abnova) in ubiquitination buffer (50 mm Tris-HCl, pH 7.6, 2 mm ATP, 5 mm MgCl2, 2 mm dithiothreitol, 30 mm creatine phosphate, and 0.05 mg/ml creatine phosphokinase). The reactions were incubated at 30 °C for 30 min. The reaction products were eluted using MagExtractor-His-tag (Toyobo).
Apoptosis Assays
Cells were cultured poly-d-lysine coated 4-well chamber slides (BD Biosciences). Apoptosis was detected by TUNEL assays using DeadEnd Fluorometric TUNEL system (Promega). For cells expressing GFP-tagged proteins, Cy5-dUTP (GE Healthcare) was used for the detection of the DNA strand breaks (22, 23).
Cell Fractionation Assays
Cell fractionation assays were performed as described with minor modification (24, 25).
RESULTS
N-terminal Region of DYRK2 Contains Potential Nuclear Localization Signals
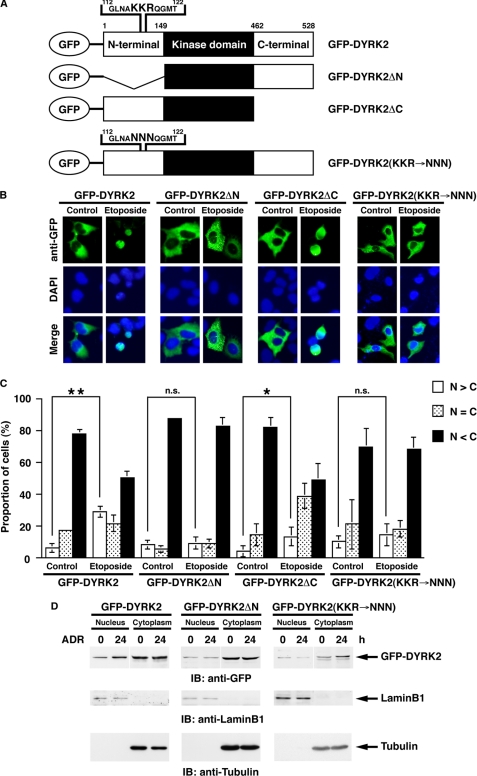
A recent study has shown that DYRK2 is targeted to the nucleus after genotoxic stress exposure (8); however, a molecular mechanism of nuclear translocation remains unclear. In this regard, the finding that nuclear accumulation of DYRK2 is independent of its kinase activity indicates the possibility that there are some potential nuclear localization signals (NLSs) in DYRK2. Indeed, there are several potential NLS sequences that are conformed from the clusters of basic amino acids in both N-terminal and C-terminal regions (Fig. 1A). To determine whether the N-terminal and/or C-terminal domains containing these amino acid sequences are required for nuclear translocation of DYRK2, we constructed N-terminal or C-terminal deletion mutants of DYRK2 (DYRK2ΔN or DYRK2ΔC, respectively) (Fig. 1A). U2OS cells were transfected with GFP-DYRK2 or deletion mutants, then treated with DNA-damaging agent etoposide for 24 h. Immunostaining with anti-GFP revealed that, as reported previously, DYRK2 is predominantly expressed in the cytoplasm in control cells and moves into the nucleus after genotoxic stress (Fig. 1, B and C). Comparable translocation was observed in cells transfected with DYRK2ΔC (Fig. 1, B and C). In contrast, DYRK2ΔN was dominantly retained in the cytoplasm even after etoposide stimulation (Fig. 1, B and C), suggesting that N-terminal region of DYRK2 is required for its nuclear targeting in response to DNA damage. To confirm the impairment of DYRK2ΔN on nuclear accumulation after genotoxic stress, we performed subcellular fractionation assays. The results demonstrated that full-length DYRK2 moved into the nucleus after another DNA-damaging agent adriamycin (ADR) exposure (Fig. 1D). In contrast, DYRK2ΔN remained in the cytoplasm even in damaged cells (Fig. 1D), indicating that the N-terminal region including potential NLS sequence is essential for nuclear accumulation of DYRK2. To further determine whether the putative NLS motif at the N terminus is required for nuclear localization of DYRK2, mutations were introduced into the core NLS motif (GFP-DYRK2(KKR→NNN) Fig. 1A). Immunofluorescence analysis revealed that GFP-DYRK2(KKR→NNN) was dominantly expressed in the cytoplasm and that there was no significant nuclear translocation after etoposide stimulation (Fig. 1, B and C). Similar results were obtained from subcellular fractionation assays (Fig. 1D). These findings suggest that this NLS motif is functional for the nuclear targeting of DYRK2 in response to DNA damage.
FIGURE 1.
The N-terminal domain of DYRK2 is critical for its nuclear targeting in the apoptotic response to DNA damage. A, shown is the domain structure of GFP-tagged human DYRK2 and the deletion mutants. Potential nuclear localization sequences are shown in enlargement. Amino acids KKR indicate the core NLS motif. The putative NLS mutant was constructed in which the core NLS motif KKR were substituted for NNN (GFP-DYRK2(KKR→NNN)). B, U2OS cells were transfected with GFP-DYRK2, the N-terminal deletion mutant (GFP-DYRK2ΔN), the C-terminal deletion mutant (GFP-DYRK2ΔC), or GFP-DYRK2(KKR→NNN) and then treated with etoposide for 24 h. Cells were stained with anti-GFP. The nuclei were stained with DAPI. C, U2OS cells were transfected as in B. The localization of DYRK2 in each cell was scored according to whether it was higher in the nucleus (open bars), evenly distributed between the nucleus and the cytoplasm (dotted bars), or higher in the cytoplasm (closed bars). Results are the mean ± S.D. of values obtained from five fields of 30–100 cells in each of three independent experiments. Statistical analysis was performed with Student's t test. Double asterisks, single asterisk, and n.s. indicate p < 0.01, p < 0.05, and not significant, respectively. D, 293T cells were transfected with GFP-DYRK2, GFP-DYRK2ΔN, or GFP-DYRK2(KKR→NNN) and then treated with ADR for 24 h. Nuclear and cytoplasmic lysates were analyzed by immunoblotting (IB) with anti-GFP (top panel), anti-lamin B1 (middle panel), or anti-tubulin (bottom panel).
ATM Regulates Nuclear Targeting of DYRK2 in Response to DNA Damage
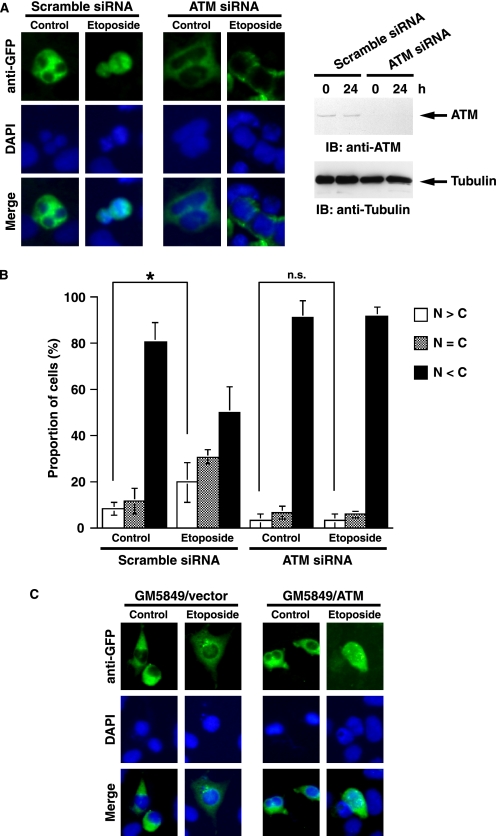
A recent study has also demonstrated that ATM activates nuclear DYRK2 after genotoxic stress (8). In this context, it is plausible that ATM is involved in nuclear targeting of DYRK2. To address this hypothesis, GFP-DYRK2 was co-transfected together with scramble siRNA or ATM siRNA in U2OS cells that were left untreated or treated with etoposide. Analysis of immunofluorescent staining demonstrated that ATM was required for nuclear accumulation of DYRK2 after DNA damage (Fig. 2, A and B). Similar results were obtained in 293T cells (data not shown). To confirm these results, we performed similar experiments using A-T cells, which are GM 5849/pEBS7 (A-T fibroblast with empty vector only) and GM 5849/pEBS7-YZ5 (A-T fibroblast complemented with ATM cDNA) (11). As shown for ATM-depleted U2OS cells, DYRK2 was retained in the cytoplasm after etoposide stimulation in GM 5849/pEBS7 cells (Fig. 2C). By contrast, in GM 5849/pEBS7-YZ5 cells, DYRK2 accumulated in the nucleus upon exposure to etoposide, clearly indicating that ATM is required for nuclear localization of DYRK2 in response to DNA damage. Taken together with previous findings, these data indicate that ATM controls nuclear localization and activation of DYRK2 in response to DNA damage.
FIGURE 2.
ATM is required for nuclear accumulation of DYRK2 in response to genotoxic stress. A, U2OS cells were co-transfected with GFP-DYRK2 and scramble siRNA or ATM-specific siRNA (20) and then treated with etoposide for 24 h. Cells were stained with anti-GFP (left). The nuclei were stained with DAPI (left). Cell lysates were subjected to immunoblot analysis (IB) with anti-ATM (right, upper panel) or anti-tubulin (right, lower panel). B, U2OS cells were transfected as in A. The localization of DYRK2 was scored according to whether it was higher in the nucleus (open bars), evenly distributed between the nucleus and the cytoplasm (dotted bars), or higher in the cytoplasm (closed bars). Results are the mean ± S.D. of values obtained from 5 fields of 30–100 cells in each of three independent experiments. Statistical analysis was performed with Student's t test. The single asterisk and n.s. indicate p < 0.05 and not significant, respectively. C, GM 5849/pEBS7 (vector) or GM 5849/pEBS7-YZ5 (ATM) cells were transfected with GFP-DYRK2 and left untreated or treated with 10 μm etoposide for 24 h. Cells were stained with anti-GFP. The nuclei were stained with DAPI.
ATM Phosphorylates DYRK2
The findings demonstrating ATM-dependent regulation of DYRK2 led us to determine whether ATM phosphorylates DYRK2. ATM belongs to the phosphatidylinositol 3-kinase superfamily and preferably phosphorylates Ser/Thr adjacent to Gln (SQ or TQ). Upon exposure to DNA double strand breaks, ATM is rapidly activated to phosphorylate numerous substrates including p53 at Ser-15 (26). To assess the kinetics of ATM activation in response to DNA damage, anti-ATM immunoprecipitates were subjected to in vitro kinase assays with GST-p53 as a substrate. As expected, ATM was activated after early periods of DNA damage (Fig. 3A). Intriguingly, activity of ATM was sustained at least 24 h post-ADR stimulation, indicating the continuous activation of ATM in response to DNA damage. Given that ATM is a nuclear kinase, ATM phosphorylation of DYRK2 could be detected with delayed kinetics after DNA damage, as nuclear targeting and activation of DYRK2 occur at a later period after genotoxic stress (8). To examine whether ATM phosphorylates DYRK2 in vitro, we performed in vitro kinase assays using His-DYRK2 as a substrate. Immunoblot analysis with anti-phospho-Ser/Thr ATM/ATR substrate (anti-phospho-SQ/TQ) revealed that there was no significant phosphorylation of DYRK2 by ATM in unstressed cells (Fig. 3B). By contrast, activation of ATM by treatment with ADR for 24 h was associated with modest phosphorylation of DYRK2 (Fig. 3B), indicating the possibility that ATM phosphorylates DYRK2 in cells upon exposure to genotoxic stress. To establish whether ATM is involved in DYRK2 phosphorylation, we performed similar experiments using transfected A-T cells. Immunoblot analysis with anti-SQ/TQ revealed that DYRK2 was phosphorylated in GM 5849/pEBS7-YZ5, but not GM 5849/pEBS7 cells, after treatment with ADR (Fig. 3C). This finding confirms ATM phosphorylation of DYRK2 in response to DNA damage. In this context, there are two SQ/TQ sequences in DYRK2 as potential phosphorylation sites by ATM (Fig. 3D). To determine phosphorylation sites, two potential amino acid residues were individually mutated to Ala (Fig. 3D). We also constructed the mutant that both residues are replaced with Ala (Fig. 3D). 293T cells were transfected with FLAG-DYRK2 wild type (wt) or mutants followed by stimulation with ADR for 24 h. Immunoblot analysis of anti-FLAG immunoprecipitates with anti-phospho-SQ/TQ demonstrated that DYRK2 was phosphorylated on SQ/TQ sites at 24 h post-stimulation (Fig. 3E). Moreover, phosphorylation of the T33A, S369A, or T33A/S369A double mutant was substantially impaired after genotoxic stress (Fig. 3E). These findings clearly indicate that ATM phosphorylates DYRK2 at Thr-33 and Ser-369 after exposure to DNA damage. To examine whether ATM phosphorylation of DYRK2 affects its kinase activity, we monitored phosphorylation levels of p53 at Ser-46, which is the only clarified DYRK2 substrate in cells (8). As expected from earlier work (8), Ser-46 was heavily phosphorylated in DYRK2 wt-expressed cells after ADR stimulation (Fig. 3E). Slightly less significant, but substantial phosphorylation of Ser-46 was observed in cells transfected with the DYRK2 T33A mutant. By sharp contrast, Ser-46 phosphorylation was attenuated in cells ectopically expressed with the DYRK2 S369A or the DYRK2 T33A/S369A mutant (Fig. 3E). These findings, thus, clearly demonstrate that the DYRK2 T33A mutant does not have much effect, whereas the DYRK2 S369A mutant substantially reduces the levels of phosphorylated Ser-46 of p53 either as a single or double mutant. Of note, it is conceivable that residual Ser-46 phosphorylation in the presence of mutant form of DYRK2 could be due to activation of endogenous DYRK2 in cells. To explore this possibility, 293T cells were transduced with the GFP-DYRK2 T33A/S369A mutant, which is resistant to the silencing by the DYRK2 siRNA (designated GFP-rDYRK2 T33A/S369A). Cells were then transfected with scramble siRNA or DYRK2 siRNA followed by treatment with ADR. Upon exposure to ADR, ectopic expression of the GFP-rDYRK2 T33A/S369A mutant was associated with substantial induction of p53 phosphorylation at Ser-46 (Fig. 3F). In contrast, the silencing of endogenous DYRK2 markedly attenuated Ser-46 phosphorylation, suggesting that the GFP-rDYRK2 T33A/S369A mutant has little if any activity on Ser-46 phosphorylation after DNA damage in cells. Taken together, these results demonstrate that ATM-mediated phosphorylation of Ser-369 is crucial for DYRK2 activation in response to DNA damage.
FIGURE 3.
ATM phosphorylates DYRK2. A, 293T cells were left untreated or treated with ADR for indicated times. Lysates were immunoprecipitated with anti-ATM. The immunoprecipitates were incubated with GST-tagged p53 and ATP. Reaction products were analyzed by immunoblotting (IB) with anti-phospho-p53(Ser-15) (top panel), anti-phospho-Ser/Thr ATM/ATR substrate (anti-phospho-(SQ/TQ); second panel), anti-ATM (third panel), or anti-GST (bottom panel). B, 293T cells were left untreated or treated with ADR for 24 h. Lysates were immunoprecipitated (IP) with rabbit anti-ATM or rabbit normal IgG. Immunoprecipitates were incubated with His-tagged DYRK2 and ATP. Reaction products were analyzed by immunoblotting with anti-phospho-(SQ/TQ) (upper panel), anti-ATM (middle panel), or anti-His (lower panel). C, GM 5849/pEBS7-YZ5 (ATM) or GM 5849/pEBS7 (vector) cells were transfected with FLAG vector or FLAG-DYRK2 followed by treatment with 1 μg/ml ADR for 24 h. Anti-FLAG immunoprecipitates were subjected to immunoblot analysis with anti-phospho-(SQ/TQ) (top panel) or anti-FLAG (second panel). Cell lysates were analyzed by immunoblotting with anti-ATM (third panel) or anti-tubulin (bottom panel). D, sequence alignment of DYRK2. Two SQ/TQ motifs of DYRK2 were replaced by AQ. E, 293T cells were transfected with FLAG vector, FLAG-DYRK2 wild type, or various AQ mutants and then treated with ADR for 24 h. Lysates were immunoprecipitated with anti-FLAG-agarose. The immunoprecipitates were analyzed by immunoblotting with anti-phospho-SQ/TQ (top panel) or anti-FLAG (second panel). Whole cell lysates were subjected to immunoblot analysis with anti-phospho-p53(Ser-46) (third panel), anti-p53 (fourth panel), or anti-lamin B1 (bottom panel). F, 293T cells were co-transfected with scramble siRNA or DYRK2-specific siRNA (8) and the GFP-DYRK2 T33A/S369A mutant, which is resistant for DYRK2 siRNA (designated GFP-rDYRK2 T33A/S369A). Cell lysates were analyzed by immunoblotting with anti-phospho-p53(Ser-46) (top panel), anti-p53 (second panel), anti-GFP (third panel), anti-DYRK2 (fourth panel), or anti-tubulin (bottom panel).
ATM Phosphorylation of DYRK2 Contributes to the Execution of p53-dependent Cell Death
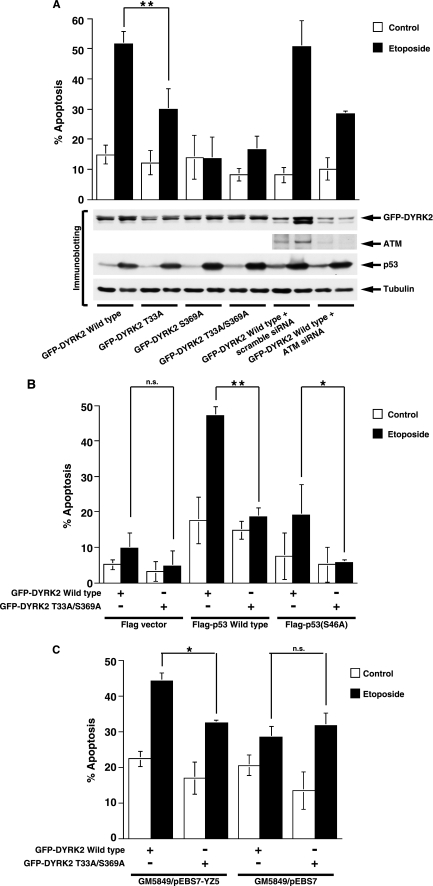
To define the functional significance of DYRK2 phosphorylation by ATM, we focused on DYRK2-mediated apoptosis induction. Given the previous findings that DYRK2 induces p53-dependent apoptosis by phosphorylating p53 at Ser-46 (8), U2OS cells, which express wild type p53, were transfected with wild type or various mutants of GFP-DYRK2 plasmids. After etoposide stimulation, apoptotic cells were monitored by TUNEL assays. As a control, GFP-DYRK2 wt was co-transfected together with scramble siRNA or ATM siRNA. In concert with the level of Ser-46 phosphorylation by DYRK2, introduction of DYRK2 wt enhanced DNA damage-induced apoptosis (Fig. 4A). Less significant, but substantial induction of apoptosis was also observed in cells ectopically expressed with the DYRK2 T33A mutant (Fig. 4A). In contrast, expression with the DYRK2 S369A mutant or the DYRK2 T33A/S369A mutant was associated with much less apoptosis induction compared with that with DYRK2 wt (Fig. 4A). Similar results were obtained in HCT116 cells (data not shown). These results demonstrated that ATM-mediated phosphorylation of Ser-369 is crucial for DYRK2-mediated apoptosis in response to DNA damage. Intriguingly, the finding that knocking down ATM attenuated induction of apoptosis after etoposide stimulation further supports a mechanism in which phosphorylation of DYRK2 by ATM is required for DNA damage-induced apoptosis (Fig. 4A). To confirm whether the activation of DYRK2 by ATM augments apoptosis in a p53-dependent fashion, HCT116/p53−/− cells were co-transfected with GFP-DYRK2 wt or the GFP-DYRK2 T33A/S369A mutant and FLAG vector, FLAG-p53 wt, or the FLAG-p53 S46A mutant in which Ser-46 is substituted with Ala. Cells were then treated with or without etoposide for 24 h. The analysis of TUNEL assays demonstrated that treatment of cells with etoposide induced apoptotic cell death (Fig. 4B). Importantly, co-expression of p53 wt and DYRK2 wt significantly enhanced apoptosis elicited by etoposide. By contrast, ectopic expression of the DYRK2 T33A/S369A mutant together with p53 wt attenuated apoptosis induction (Fig. 4B). Moreover, there was little if any augmentation of apoptosis in p53 S46A-expressing cells (Fig. 4B). Similar results were obtained in SaOS2 cells (data not shown). To confirm these findings in A-T cells, GFP-DYRK2 wt or the GFP-DYRK2 T33A/S369A mutant were transfected into cells in the absence or presence of etoposide. Apoptotic induction was significantly attenuated in ATM-complemented A-T cells ectopically expressed with the DYRK2 T33A/S369A mutant compared with that with DYRK2 wt (Fig. 4C). By sharp contrast, there was no remarkable difference on apoptosis induction between DYRK2 wt and the DYRK2 T33A/S369A mutant in A-T cells (Fig. 4C). These findings provide a model in which, upon exposure to genotoxic stress, ATM activates DYRK2 by phosphorylation, resulting in the exertion of p53 phosphorylation at Ser-46. This process renders cells more sensitive to p53-mediated apoptosis in a Ser-46 phosphorylation-dependent manner.
FIGURE 4.
ATM regulates DYRK2-mediated induction of apoptosis in response to DNA damage. A, U2OS cells were transfected with GFP-DYRK2 or its mutants and then treated with etoposide for 24 h. The percentages of apoptotic cells were quantified by TUNEL assays. The results are represented as the percentage of TUNEL-positive cells out of a total of GFP-positive cells. Values indicate the mean ± S.D. from three independent experiments. Cells were also analyzed by immunoblotting with indicated antibodies. Statistical analysis was performed with Student's t test. Double asterisks indicate p < 0.01. B, HCT116/p53−/− cells were transfected with FLAG vector, FLAG-p53, or the FLAG-p53 S46A mutant and GFP-DYRK2 or the GFP-DYRK2 mutant and then treated with etoposide for 24 h. Apoptotic cells were analyzed by TUNEL assays as described above. Double asterisks, the single asterisk, and n.s. indicate p < 0.01, p < 0.05, and not significant, respectively. C, GM 5849/pEBS7 or GM 5849/pEBS7-YZ5 cells were transfected with GFP-DYRK2 wild type or the T33A/S369A mutant followed by treatment with 20 μm etoposide for 24 h. The percentages of apoptotic cells were quantified by TUNEL assays. The results are represented as the percentage of TUNEL-positive cells out of a total of GFP-positive cells. Values indicate the mean ± S.D. from three independent experiments. The single asterisk and n.s. indicate p < 0.05 and not significant, respectively.
Constitutive Degradation of DYRK2 in the Nucleus
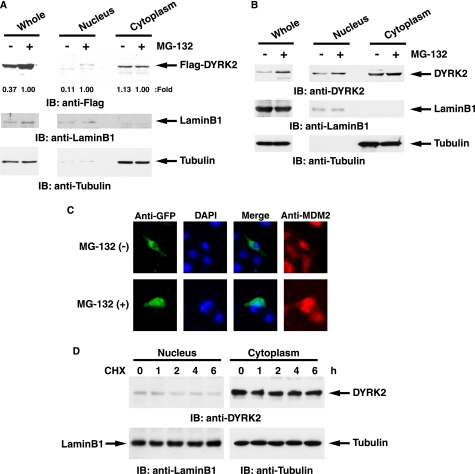
Previous studies demonstrated that knock down of ATM attenuates DYRK2 expression at a post-translational mechanism (8). Moreover, expression of DYRK2 was increased in response to DNA damage. In this regard, it is conceivable that, upon exposure to genotoxic agents, ATM-dependent phosphorylation of DYRK2 protects its degradation, such as the ubiquitination and proteasomal system. To examine this possibility, 293T cells transfected with FLAG-DYRK2 wt were left untreated or treated with a proteasome inhibitor MG-132. Immunoblot analysis of whole cell lysates with anti-FLAG revealed that MG-132 treatment elevated DYRK2 expression (Fig. 5A). Intriguingly, up-regulation of DYRK2 was observed in the nucleus but not in the cytoplasm (Fig. 5A). To further obtain convincing evidence in the physiological condition, we assessed the expression status of endogenous DYRK2 with or without MG-132. Subcellular fractionation assays revealed that endogenous DYRK2 was stabilized in the nucleus but not in the cytoplasm (Fig. 5B). As a result, analysis of whole cell lysates indicated that total DYRK2 expression was increased in the presence of MG-132 (Fig. 5B). To confirm these findings, we performed the immunostaining experiments. U2OS cells were left untreated or treated with MG-132. DYRK2 was exclusively expressed in the cytoplasm (Fig. 5C). In contrast, inhibition of proteasomal degradation was associated with substantial nuclear accumulation of DYRK2 (Fig. 5C). Nevertheless, it is still conceivable that DYRK2 expression is regulated by its half-life stability and is independent of proteasome-mediated degradation. To exclude this possibility, we examined the half-life of endogenous DYRK2 in the nucleus as well as in the cytoplasm. 293T cells were treated with an inhibitor of protein synthesis, cycloheximide. Subcellular fractionation assays demonstrated that cytoplasmic DYRK2 was constitutively stable at least 6 h after cycloheximide treatment (Fig. 5D). Intriguingly, nuclear DYRK2 was slightly decreased but was, however, substantially expressed even after inhibition of protein synthesis (Fig. 5D). Taken together, these data demonstrate that nuclear DYRK2 is constitutively degraded by the proteasome-dependent pathway.
FIGURE 5.
Nuclear DYRK2 is constitutively degraded by the ubiquitin-proteasome machinery. A, 293T cells ectopically expressing FLAG-DYRK2 were treated with 2 μm MG-132 or DMSO. Whole cell, nuclear, and cytoplasmic lysates were analyzed by immunoblot analysis (IB)with anti-FLAG (upper panel), anti-lamin B1 (middle panel), or anti-tubulin (lower panel). A ratio of FLAG-DYRK2 expression in each fraction was determined by the densitometric analysis using the ImageJ program. The expression in each of the MG-132-treated cells was defined as 1.00. B, 293T cells were treated with 2 μm MG-132 or DMSO. Whole cell, nuclear, and cytoplasmic lysates were analyzed by immunoblot analysis with anti-DYRK2 (upper panel), anti-lamin B1 (middle panel), or anti-tubulin (lower panel). C, U2OS cells were transfected with GFP-DYRK2 and left untreated or treated with 2 μm MG-132 for 4 h. Cells were stained with anti-GFP or anti-MDM2. The nuclei were stained with DAPI. D, 293T cells were treated with 40 μg/ml cycloheximide (CHX) for the indicated times. Nuclear and cytoplasmic lysates were analyzed by immunoblotting with anti-DYRK2 (upper panel), anti-lamin B1 (lower left panel), or anti-tubulin (lower right panel).
ATM Phosphorylation of DYRK2 Blocks Its Ubiquitination-dependent Degradation by MDM2 after DNA Damage
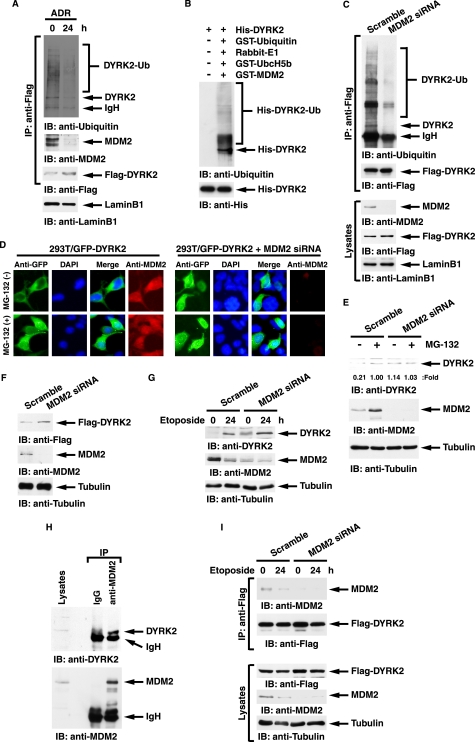
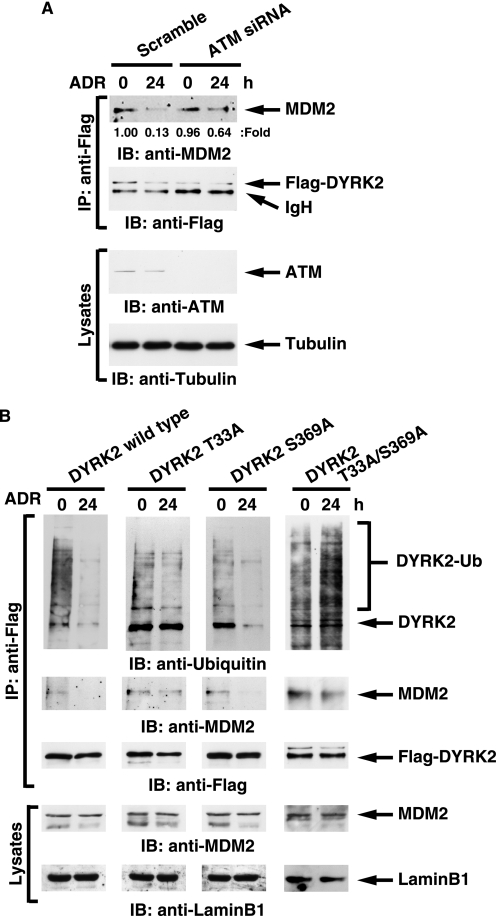
A recent study showed that E3 ubiquitin ligase MDM2 controls the p53-signaling pathway including HIPK2 (27). To determine whether degradation of DYRK2 is regulated by MDM2 and DNA damage abrogates MDM2-mediated proteolysis, FLAG-DYRK2 was ectopically expressed in 293T cells in the presence of MG-132. Analysis of anti-FLAG immunoprecipitates with anti-ubiquitin demonstrated that DYRK2 is polyubiquitinated, and this modification is completely abolished after ADR (Fig. 6A). Furthermore, the findings that DYRK2 interacted with MDM2 in unstressed cells and that the binding was abrogated after ADR treatment provide the possibility that an E3 ubiquitin ligase for DYRK2 is MDM2 (Fig. 6A). To address this possibility in vitro, purified DYRK2 was incubated with ubiquitin, E1, E2, and MDM2. Analysis with anti-ubiquitin demonstrated that DYRK2 is ubiquitinated by MDM2 in vitro (Fig. 6B). To confirm this in cells, 293T cells expressed with FLAG-DYRK2 were transfected with scramble siRNA or MDM2 siRNA followed by treatment with MG-132. Analysis of anti-FLAG immunoprecipitates with anti-ubiquitin from nuclear lysates demonstrated that the polyubiquitination of DYRK2 was impaired in cells depleted with MDM2 (Fig. 6C). To prove whether degradation of nuclear DYRK2 is MDM2 dependence, 293T cells transfected with GFP-DYRK2 were left untreated or treated with MG-132. DYRK2 was exclusively stained in the cytoplasm (Fig. 6D). However, inhibition of proteasome enabled DYRK2 to be also expressed in the nucleus (Fig. 6D). Importantly, depletion of MDM2 conferred nuclear localization of DYRK2 even in the absence of the proteasome inhibitor (Fig. 6D). To further assess the effect of MDM2 depletion on endogenous DYRK2 expression, 293T cells were transfected with scramble siRNA or MDM2 siRNA followed by treatment with MG-132. As expected, expression of endogenous DYRK2 increased after MG-132 treatment (Fig. 6E). Significantly, silencing of MDM2 allowed endogenous DYRK2 to be stabilized even in the absence of proteasome inhibitors, again suggesting that DYRK2 expression is controlled by MDM2 (Fig. 6E). Similarly, the finding that MDM2 depletion increased expression of exogenous DYRK2 in the absence of proteasome inhibitors confirms the involvement of MDM2 in DYRK2 expression (Fig. 6F). In addition, we have examined the role for MDM2 in DYRK2 expression before and after genotoxic stress. As shown for ADR, treatment of cells with etoposide, another DNA-damaging agent, also induced expression of DYRK2 (Fig. 6G, left two lanes). Importantly, knocking down MDM2 retrieved DYRK2 expression to a significant level even in unstressed condition (Fig. 6G, first lane versus third lane). These data support a model in which constitutive degradation of DYRK2 is, at least in part, in a MDM2-dependent manner and in which DNA damage abrogates MDM2-mediated degradation of DYRK2. To establish endogenous interaction between DYRK2 and MDM2, 293T cells were treated with MG-132. Lysates were immunoprecipitated with anti-MDM2 followed by immunoblotting with anti-DYRK2. The results demonstrated a stable complex of MDM2 with DYRK2 in cells in the presence of proteasome inhibitor (Fig. 6H). To further establish dissociation of this complex after genotoxic stress, 293T cells were transfected with FLAG-DYRK2. As similarly shown in Fig. 6A, DYRK2 formed a complex with MDM2 in unstressed cells, and the complex formation was diminished after etoposide treatment (Fig. 6I). As a control, there was little if any DYRK2·MDM2 complex in cells silenced for MDM2 (Fig. 6I). To further define the role for ATM in the MDM2-dependent DYRK2 degradation, 293T cells were co-transfected with FLAG-DYRK2 and scramble siRNA or ATM siRNA. As expected, the DYRK2·MDM2 association was completely abrogated after DNA damage in control cells (Fig. 7A). By contrast, DYRK2 retained the binding to MDM2 even after ADR treatment in ATM-deficient cells (Fig. 7A). To determine whether ATM phosphorylation of DYRK2 is involved in the dissociation from MDM2, 293T cells were transfected with DYRK2 wt, the DYRK2 T33A mutant, the DYRK2 S369A mutant, or the DYRK2 T33A/S369A mutant in the presence of MG-132. Analysis of anti-FLAG immunoprecipitates with anti-ubiquitin demonstrated that the DYRK2 T33A mutant and the T33A/S369A mutant, but not wild type or the S369A mutant, remained polyubiquitinated even after ADR stimulation (Fig. 7B). In concert with this result, the DYRK2 T33A mutant and the T33A/S369A mutant abrogated dissociation from MDM2 after DNA damage (Fig. 7B). These results provide a model in which ATM phosphorylation of DYRK2 at Thr-33 is, at least in part, associated with abrogation of MDM2 binding in the nucleus and thereby of ubiquitin-proteasomal degradation.
FIGURE 6.
DYRK2 degradation is associated with MDM2-mediated polyubiquitination. A, 293T cells expressing FLAG-DYRK2 were treated with ADR for 24 h in the presence of 1 μm MG-132. Cell lysates were immunoprecipitated (IP) with anti-FLAG-agarose. The immunoprecipitates were analyzed by immunoblotting (IB) with anti-ubiquitin (top panel), anti-MDM2 (second panel), or anti-FLAG (third panel). Lysates were also analyzed by immunoblotting with anti-lamin B1 (bottom panel). B, in vitro ubiquitination assays were performed and analyzed by immunoblotting with anti-ubiquitin (upper panel) or anti-His (lower panel). C, 293T cells ectopically expressing FLAG-DYRK2 were transfected with scramble siRNA or MDM2 siRNA followed by treatment with MG-132. Nuclear lysates were immunoprecipitated with anti-FLAG-agarose. The immunoprecipitates were analyzed by immunoblotting with anti-ubiquitin (top panel) or anti-FLAG (second panel). Lysates were also analyzed by immunoblotting with anti-MDM2 (third panel), anti-FLAG (fourth panel), or anti-lamin B1 (bottom panel). D, 293T cells were transfected with GFP-DYRK2 and/or MDM2 siRNA. The cells were left untreated or treated with 2 μm MG-132 for 4 h. Cells were stained with anti-GFP or anti-MDM2. The nuclei were stained with DAPI. E, 293T cells were transfected with scramble siRNA or MDM2 siRNA followed by treatment with MG-132. Cell lysates were subjected to immunoblot analysis with anti-DYRK2 (upper panel), anti-MDM2 (middle panel), or anti-tubulin (lower panel). A ratio of DYRK2 expression was determined by the densitometric analysis using the ImageJ program. The expression in MG-132-treated cells transfected with scramble siRNA was defined as 1.00. F, 293T cells ectopically expressed with FLAG-DYRK2 were transfected with scramble siRNA or MDM2 siRNA. Lysates were analyzed by immunoblotting with the indicated antibodies. G, 293T cells were transfected with scramble siRNA or MDM2 siRNA and then treated with 20 μm etoposide for 24 h. Lysates were analyzed by immunoblotting with the indicated antibodies. H, 293T cells were treated with MG-132. Cell lysates were immunoprecipitated with normal IgG or anti-MDM2 followed by immunoblot analysis with anti-DYRK2 (upper panel) or anti-MDM2 (lower panel). I, 293T cells expressing FLAG-DYRK2 were treated with 20 μm etoposide for 24 h. Cell lysates were immunoprecipitated with anti-FLAG-agarose. The immunoprecipitates were analyzed by immunoblotting with anti-MDM2 (top panel) or anti-FLAG (second panel). Lysates were also analyzed by immunoblotting with anti-FLAG (third panel), anti-MDM2 (fourth panel), or anti-tubulin (bottom panel).
FIGURE 7.
ATM is required for the escape from the degradation of DYRK2. A, 293T cells ectopically expressed with FLAG-DYRK2 were transfected with scramble siRNA or ATM siRNA and then left untreated or treated with ADR for 24 h in the presence of MG-132. Lysates were immunoprecipitated with anti-FLAG-agarose. Immunoprecipitates (IP) were subjected to immunoblot analysis (IB) with anti-MDM2 (top panel) or anti-FLAG (second panel). Lysates were also subjected to immunoblot analysis with anti-ATM (third panel) or anti-tubulin (bottom panel). A ratio of MDM2 bound to DYRK2 was determined by the densitometric analysis using ImageJ program. The expression in unstressed cells transfected with scramble siRNA was defined as 1.00. B, 293T cells were transfected with FLAG-DYRK2 or the AQ mutants and then treated with ADR for 24 h in the presence of MG-132. Nuclear lysates were immunoprecipitated with anti-FLAG-agarose. Immunoprecipitates were subjected to immunoblot analysis with anti-ubiquitin (top panel), anti-MDM2 (second panel), or anti-FLAG (third panel). Lysates were also analyzed by immunoblotting with anti-MDM2 (fourth panel) or anti-lamin B1 (bottom panel).
DISCUSSION
DYRK2 is a requisite kinase for the induction of apoptosis by phosphorylating p53 at Ser-46 in response to DNA damage. Previous studies demonstrate that DYRK2 translocates into the nucleus where it phosphorylates p53 to initiate the apoptotic pathway (8). Moreover, transfected DYRK2 induces p53-dependent apoptosis, whereas its depletion renders cells more resistant to p53-mediated apoptosis in a Ser-46 phosphorylation dependent manner. Intriguingly, ATM is necessary for stabilization and activation of DYRK2 after genotoxic stress. Indeed, expression of DYRK2 increases after DNA damage, whereas that is significantly reduced in cells silenced for ATM (8). In this regard, the present study demonstrated that ATM phosphorylates DYRK2 at Thr-33 and Ser-369. Thr-33 phosphorylation was required for stabilization of DYRK2 because there was little if any increase after ADR exposure with the T33A mutant, at least in the overexpressing conditions (Fig. 3E). Given the finding that the potential NLS was localized near Thr-33 at the N-terminal region, it is conceivable that Thr-33 phosphorylation affects the NLS to trigger nuclear entrapment and stabilization of DYRK2. ATM-dependent stabilization of DYRK2 is also supported by the findings that ATM abrogated MDM2-mediated degradation of DYRK2 in the nucleus. The present data showed that nuclear, but not cytoplasmic, DYRK2 is constitutively degraded by MDM2-mediated polyubiquitination and subsequent proteasomal system (Fig. 8). Upon exposure to DNA damage, ATM phosphorylation of Thr-33 enabled DYRK2 to protect against MDM2-mediated degradation, possibly by dissociation of DYRK2 from MDM2 (Fig. 7B). Domains responsible for the binding of DYRK2 to MDM2 are presently unclear. Importantly, however, expression of DYRK2 with a T33A mutation abrogated disruption of binding to MDM2 and resulted in its destabilization at least in the overexpressing conditions. These findings indicate that ATM-dependent phosphorylation induces nuclear accumulation of DYRK2 by dissociating from MDM2 after genotoxic stress (Fig. 8). Taken together, it is less likely that cytoplasmic DYRK2 moves into the nucleus in response to DNA damage. Indeed, we observed relative increments of nuclear DYRK2 after ADR exposure but still found its substantial expression in the cytoplasm (Fig. 1C). Instead, DYRK2 theoretically localizes in both the nucleus and the cytoplasm; however, constitutive degradation of nuclear DYRK2 in control cells seems predominantly expressed in the cytoplasm. After ADR stimulation, abrogation of MDM2 access to DYRK2 renders cells its substantial nuclear appearance. Previous studies have demonstrated that MDM2 is degraded by itself in response to genotoxic stress (28, 29). In this context it is also conceivable that the degradation of nuclear DYRK2 is, at least in part, attenuated by self-clearance of MDM2 after DNA damage. Until now, we cannot exclude the possibility that DYRK2 shuttles between the nucleus and the cytoplasm. Indeed, our study clarified the potential NLS of DYRK2 at the N-terminal region. However, it remains obscure whether this NLS is functional even in cells in the unstressed condition. This possibility is now under investigation.
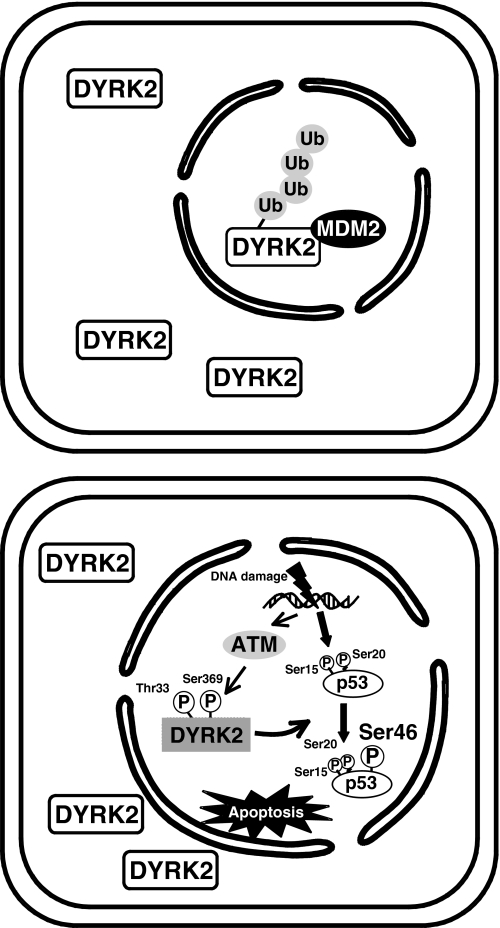
FIGURE 8.
A proposal model for ATM-mediated phosphorylation of DYRK2 in the apoptotic response to DNA damage. Under normal conditions, nuclear DYRK2 is targeted to ubiquitination (Ub) by MDM2 and subsequent degradation by proteasome (upper panel). Upon exposure to genotoxic stress, ATM phosphorylates DYRK2 at Thr-33 and Ser-369. Phosphorylated DYRK2 is resistant from the degradation and is capable for phosphorylation of p53 at Ser-46, thereby inducing apoptosis (lower panel).
Our previous studies demonstrated that ATM is involved in DYRK2 activation in response to DNA damage, whereas a precise mechanism remains unclear (8). The present study clarified that ATM phosphorylates DYRK2 at Thr-33 and Ser-369 upon exposure to genotoxic stress. Unfortunately, we are unable to show the direct phosphorylation of DYRK2 by ATM in vitro as, to our knowledge, purified active ATM protein is not available. Importantly, however, the findings that expression of DYRK2 with a S369A mutation significantly attenuated not only phosphorylation of p53 at Ser-46 but also ADR-induced apoptosis at least in the overexpressing conditions indicate the indispensable phosphorylation on Ser-369 for DYRK2 activation. In this context the present results that induction of apoptosis was attenuated by ectopically expressing the T33A mutant (Fig. 4A) suggest at least in part prerequisite for nuclear accumulation of DYRK2 on DNA damage-induced apoptosis. As shown previously, DYRK2-induced apoptosis was mediated by p53 phosphorylation at Ser-46. Moreover, given the findings that ATM controls DYRK2 activity, ATM could function as a pro-apoptotic kinase in response to genotoxic stress. In concert with this model, DNA damage-induced apoptosis was substantially abrogated in cells silenced for ATM (Fig. 4A). A proapoptotic role for ATM is currently controversial. Nevertheless, under our experimental conditions, ATM affects apoptotic execution by controlling DYRK2 expression and activation after DNA damage. Intriguingly, sustained exposure with DNA lesion is required for nuclear accumulation of DYRK2 after phosphorylation by ATM, mainly due to dissociation from MDM2. However, it remains uncertain why it takes considerably longer periods as p53 is rapidly stabilized after genotoxic stress. Obviously, further studies are needed to address these issues.
Acknowledgments
We thank Y. Taya and S. Mizutani for providing anti-phospho-p53(Ser-46) antibodies and GM 5849 cells, respectively.
This work was supported by grants from the Ministry of Education, Science, and Culture of Japan (to K. Y. and Y. M.), Uehara Memorial Foundation (to K. Y.), Senri Life Science Foundation (to K. Y.), Takeda Science Foundation (to K. Y.), and Kowa Life Science Foundation (to K. Y.).
- DYRK2
- dual-specificity tyrosine-regulated kinase 2
- ATM
- ataxia telangiectasia mutated
- MG-132
- carbobenzoxy-l-leucyl-l-leucyl-l-leucinal
- NLS
- nuclear localization signal
- ADR
- adriamycin
- wt
- wild type
- siRNA
- small interfering RNA
- GFP
- green fluorescent protein
- GST
- glutathione S-transferase
- TUNEL
- terminal dUTP nick-end labeling
- DAPI
- 4′,6-diamidino-2-phenylindole
- ATR
- ATM- and Rad3-related.
REFERENCES
- 1.Becker W., Joost H. G. (1999) Prog. Nucleic Acid Res. Mol. Biol. 62, 1–17 [DOI] [PubMed] [Google Scholar]
- 2.Campbell L. E., Proud C. G. (2002) FEBS Lett. 510, 31–36 [DOI] [PubMed] [Google Scholar]
- 3.Himpel S., Panzer P., Eirmbter K., Czajkowska H., Sayed M., Packman L. C., Blundell T., Kentrup H., Grötzinger J., Joost H. G., Becker W. (2001) Biochem. J. 359, 497–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arron J. R., Winslow M. M., Polleri A., Chang C. P., Wu H., Gao X., Neilson J. R., Chen L., Heit J. J., Kim S. K., Yamasaki N., Miyakawa T., Francke U., Graef I. A., Crabtree G. R. (2006) Nature 441, 595–600 [DOI] [PubMed] [Google Scholar]
- 5.Gwack Y., Sharma S., Nardone J., Tanasa B., Iuga A., Srikanth S., Okamura H., Bolton D., Feske S., Hogan P. G., Rao A. (2006) Nature 441, 646–650 [DOI] [PubMed] [Google Scholar]
- 6.Oda K., Arakawa H., Tanaka T., Matsuda K., Tanikawa C., Mori T., Nishimori H., Tamai K., Tokino T., Nakamura Y., Taya Y. (2000) Cell 102, 849–862 [DOI] [PubMed] [Google Scholar]
- 7.Okamura S., Arakawa H., Tanaka T., Nakanishi H., Ng C. C., Taya Y., Monden M., Nakamura Y. (2001) Mol. Cell 8, 85–94 [DOI] [PubMed] [Google Scholar]
- 8.Taira N., Nihira K., Yamaguchi T., Miki Y., Yoshida K. (2007) Mol. Cell 25, 725–738 [DOI] [PubMed] [Google Scholar]
- 9.Yoshida K. (2008) Trends Mol. Med. 14, 305–313 [DOI] [PubMed] [Google Scholar]
- 10.Yoshida K. (2008) Biochem. Pharmacol. 76, 1389–1394 [DOI] [PubMed] [Google Scholar]
- 11.Peretz S., Jensen R., Baserga R., Glazer P. M. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 1676–1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshida K., Kharbanda S., Kufe D. (1999) J. Biol. Chem. 274, 34663–34668 [DOI] [PubMed] [Google Scholar]
- 13.Yoshida K., Weichselbaum R., Kharbanda S., Kufe D. (2000) Mol. Cell. Biol. 20, 5370–5380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshida K., Liu H., Miki Y. (2006) J. Biol. Chem. 281, 5734–5740 [DOI] [PubMed] [Google Scholar]
- 15.Yoshida K., Komatsu K., Wang H. G., Kufe D. (2002) Mol. Cell. Biol. 22, 3292–3300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshida K., Kufe D. (2001) Mol. Pharmacol. 60, 1431–1438 [DOI] [PubMed] [Google Scholar]
- 17.Yoshida K., Yamaguchi T., Shinagawa H., Taira N., Nakayama K. I., Miki Y. (2006) Mol. Cell. Biol. 26, 3414–3431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shinagawa H., Miki Y., Yoshida K. (2008) Antioxid. Redox Signal. 10, 939–949 [DOI] [PubMed] [Google Scholar]
- 19.Nihira K., Taira N., Miki Y., Yoshida K. (2008) Oncogene 27, 7285–7295 [DOI] [PubMed] [Google Scholar]
- 20.Yoshida K., Wang H. G., Miki Y., Kufe D. (2003) EMBO J. 22, 1431–1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoshida K., Yamaguchi T., Natsume T., Kufe D., Miki Y. (2005) Nat. Cell Biol. 7, 278–285 [DOI] [PubMed] [Google Scholar]
- 22.Liu H., Lu Z. G., Miki Y., Yoshida K. (2007) Mol. Cell. Biol. 27, 8480–8491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimura J., Nguyen S. T., Liu H., Taira N., Miki Y., Yoshida K. (2008) Nucleic Acids Res. 36, 5250–5259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamaguchi T., Kimura J., Miki Y., Yoshida K. (2007) J. Biol. Chem. 282, 33943–33948 [DOI] [PubMed] [Google Scholar]
- 25.Yamaguchi T., Miki Y., Yoshida K. (2007) Cell. Signal. 19, 2088–2097 [DOI] [PubMed] [Google Scholar]
- 26.Khanna K. K., Keating K. E., Kozlov S., Scott S., Gatei M., Hobson K., Taya Y., Gabrielli B., Chan D., Lees-Miller S. P., Lavin M. F. (1998) Nat. Genet. 20, 398–400 [DOI] [PubMed] [Google Scholar]
- 27.Rinaldo C., Prodosmo A., Mancini F., Iacovelli S., Sacchi A., Moretti F., Soddu S. (2007) Mol. Cell 25, 739–750 [DOI] [PubMed] [Google Scholar]
- 28.Fang S., Jensen J. P., Ludwig R. L., Vousden K. H., Weissman A. M. (2000) J. Biol. Chem. 275, 8945–8951 [DOI] [PubMed] [Google Scholar]
- 29.Honda R., Yasuda H. (2000) Oncogene 19, 1473–1476 [DOI] [PubMed] [Google Scholar]