Abstract
Oligosaccharyltransferase (OST) catalyzes the transfer of an oligosaccharide from a lipid donor to an asparagine residue in nascent polypeptide chains. In the bacterium Campylobacter jejuni, a single-subunit membrane protein, PglB, catalyzes N-glycosylation. We report the 2.8 Å resolution crystal structure of the C-terminal globular domain of PglB and its comparison with the previously determined structure from the archaeon Pyrococcus AglB. The two distantly related oligosaccharyltransferases share unexpected structural similarity beyond that expected from the sequence comparison. The common architecture of the putative catalytic sites revealed a new catalytic motif in PglB. Site-directed mutagenesis analyses confirmed the contribution of this motif to the catalytic function. Bacterial PglB and archaeal AglB constitute a protein family of the catalytic subunit of OST along with STT3 from eukaryotes. A structure-aided multiple sequence alignment of the STT3/PglB/AglB protein family revealed three types of OST catalytic centers. This novel classification will provide a useful framework for understanding the enzymatic properties of the OST enzymes from Eukarya, Archaea, and Bacteria.
Keywords: Glycosylation, Methods/Site-Directed Mutagenesis, Methods/X-ray Crystallography, Protein/Structure, Enzyme Catalysis, AglB, PglB, STT3, Oligosaccharyltransferase
Introduction
Protein N-glycosylation is an important posttranslational modification that occurs in all domains of life (1). The enzyme that creates the oligosaccharide-asparagine linkage is oligosaccharyltransferase (OST).5 OST catalyzes the en bloc transfer of a preassembled oligosaccharide from a lipid carrier to asparagine residues in the glycosylation consensus (Asn-X-Thr/Ser, where X represents any amino acid except for Pro) of polypeptide chains (2–4). OST is a multisubunit membrane protein complex in higher eukaryotes. Yeast (Saccharomyces cerevisiae) OST consists of eight different subunits: Ost1p, Ost2p, Ost3p/Ost6p, Ost4p, Wbp1, Swp1, Stt3p, and Ost5p (5), where Ost3p and Ost6p are paralogs that are present in two distinct OST isoforms (6). The cryoelectron microscopy structure of the digitonin-solubilized OST complex from yeast provided the relative arrangement of Ost1p, Wbp1, and Stt3p on the lumenal side of the complex (7, 8).
Stt3p is the catalytic subunit of the yeast OST enzyme (9). The vertebrate, insect, and plant equivalents are the two paralog proteins, STT3A and STT3B, which define distinct OST isoforms (10, 11). In lower eukaryotes, such as trypanosomatids, OST is a single-polypeptide membrane protein (3), and these single-subunit OST proteins consist of STT3 (staurosporine and temperature sensitivity 3) alone. In fact, the STT3s from Trypanosoma cruzi, Trypanosoma brucei, and Leishmania major can each function as an OST enzyme when transferred into stt3-deficient yeast cells (12–15). The prokaryotic OST is also a single-polypeptide protein. The STT3 homologs, PglB (protein glycosylation B) and AglB (archaeal glycosylation B), comprise the bacterial OST and the archaeal OST, respectively (16–20). Multiple STT3/PglB/AglB paralogs also exist in some single-subunit OSTs. The trypanosomatids L. major and T. brucei contain four and three STT3 paralogs, respectively. In contrast, their related species, the trypanosomatid T. cruzi, contains one STT3 species. Similarly, the archaeon Pyrococcus furiosus contains two copies of AglB, but the bacterium Campylobacter jejuni contains one PglB species. Thus, the existence of multiple OST isoforms containing or consisting of different STT3/PglB/AglB paralogs raises the interesting question of the functional differences between these OST isoforms in an organism. The OST isoforms containing STT3A and STT3B in mammalian cells have different enzymatic properties and play complementary roles in the cotranslational and posttranslational N-glycosylation of proteins (21). The STT3 paralogs of T. brucei have distinct glycan donor specificities (13).
The roles of the subunits other than STT3/PglB/AglB in the multisubunit OST are not still clear. Ost1p/Ribophorin I was proposed to regulate the delivery of a set of proteins to the catalytic center of STT3 (22, 23). The Swp1-Wbp1-Ost2p subcomplex was suggested to bear the second regulatory binding site for the selection of glucosylated oligosaccharide donors (24). Structural information has greatly assisted in the elucidation of the molecular functions of these subunits. The crystal structure of the lumenal domain of yeast Ost6p (166 residues) revealed a thioredoxin-like fold (25). Indeed, yeast Ost6p has disulfide oxidoreductase activity and may prevent the nascent polypeptide from forming disulfide bonds during the cotranslational N-glycosylation (25).
The primary sequences of the STT3/PglB/AglB proteins share a common architecture. A multispan transmembrane region exists in the N-terminal half. The membrane topologies of yeast Stt3p and mouse STT3A were experimentally deduced (26). These eukaryotic STT3s have 11 transmembrane helices and an overall Ncytoplasm-Clumen orientation. The C-terminal half of the primary sequence forms a globular domain bearing a well conserved five-residue motif, WWDYG. The side chain carboxylate group of the central aspartate in the WWDYG motif probably functions as a catalytic base for the OST reaction (9, 16, 17). The entire primary sequences of eukaryotic STT3 are highly homologous across eukaryotes, including animals, plants, fungi, and protists, indicating the essential biological role of N-glycosylation in eukaryotic cells. By contrast, archaeal AglB shows remarkable sequence diversity, which may reflect a wider range of oligosaccharide structures than those found in eukaryotic glycoproteins (20, 27). N-Glycosylation of extracellular proteins, as well as O-glycosylation, facilitates the adaptation of archaeal organisms to the extreme environments where they thrive (28). Few bacteria have the protein N-glycosylation system. Until recently, N-glycosylation was reported exclusively in Campylobacterales, among bacteria, and the best characterized species is the human enteropathogenic bacterium, Campylobacter jejuni (29). N-Glycosylation is important for the virulence of this organism by increasing its adherence to and invasion of host cells. Recently, a comparative genomic analysis of two ϵ-proteobacterial species from a deep sea vent added new PglB sequences belonging to orders other than Campylobacterales (30). The multiple sequence alignment revealed a moderate level of sequence conservation (supplemental Fig. S1).
A meaningful multiple sequence alignment of STT3, PglB, and AglB across the three domains of life is virtually impossible, except for the vicinity of the WWDYG motif, due to the very low sequence homology among them. In such cases, reference to three-dimensional structures frequently helps to create a more reliable alignment. We previously reported the crystal structure of the C-terminal globular domain of P. furiosus AglB protein (PF0156, a longer paralog of the two AglBs) (31, 32). The crystal structure revealed the putative catalytic site by identifying a local structure consisting of the WWDYG motif and a long, kinked helix adjacent to the motif. We found a pair of Asp and Lys residues spaced three residues apart (DXXK, where X can be any residue) that is conserved in yeast Stt3p, the two Pyrococcus AglB paralogs, and Campylobacter PglB and named it the “DK” motif. The identification of the new motif enabled the extension of the alignment from the vicinity of the WWDYG motif to 100-residue segments, including the WWDYG motif and its flanking regions. This alignment seems valid between eukaryotic STT3 and archaeal AglB, because in vivo mutational studies indicated that the Asp and Lys residues in the motif were catalytically important in yeast Stt3p and in L. major STT3–1 (14, 31). The multiple sequence alignment using the recently published bacterial PglB sequences (supplemental Fig. S1), however, raised the possibility of an improper alignment of the DXXK sequence for Campylobacter PglB. To answer this question, we have determined the crystal structure of the C-terminal globular domain of Campylobacter PglB in the present study, and compared it with the previously determined structure of Pyrococcus AglB. Due to their high structural similarity, the structure-based sequence alignment yielded an accurate sequence alignment between Campylobacter PglB and Pyrococcus AglB. In fact, the counterpart of the DXXK sequence was found to be the MXXI sequence in the bacterial PglB. This finding provided the novel classification of the catalytic center of OST and unexpected insights into the evolutionary relationship between the OSTs from the three domains of life.
EXPERIMENTAL PROCEDURES
Crystallization of the Globular Domain of C. jejuni PglB
The cloning, expression, purification, and crystallization of the C-terminal soluble, globular domain (sPglB) of C. jejuni PglB protein (Q5HTX9_CAMJR) will be published elsewhere. Briefly, the expression plasmid encoding the C-terminal soluble domain (residues 428–713) was constructed by inserting a PCR product from genomic DNA (ATCC700819D) into the pGEX-6P-1 vector (GE Healthcare). The GST-fused sPglB protein was expressed by the addition of isopropyl-β-d-thiogalactopyranoside at 310 K in the Escherichia coli BL21(DE3)pLysS strain (Novagen) in selenomethionine core medium (Wako). The cells were disrupted by sonication. After centrifugation, the GST-sPglB protein in the supernatant was adsorbed to glutathione-Sepharose 4B resin (GE Healthcare) and was cleaved by GST-fused 3C protease on the resin. The sPglB was eluted, concentrated, and subjected to reductive methylation of the lysine residues, as described (33). The methylated sPglB protein was purified by gel filtration, using a Superdex75 column (GE Healthcare) and then by anion exchange chromatography, using a Resource Q column (GE Healthcare). The protease cleavage yielded N-terminally extended sPglB, containing five extra residues (Gly-Pro-Leu-Gly-Ser), and the reductive lysine methylation resulted in the dimethylation of the ϵ-amino group of all 24 lysine residues and the N-terminal α-amino group. Selenomethionyl derivative, methylated sPglB crystals grew from a hanging drop with a 1:1 volume ratio (total volume, 1 μl) of the protein stock solution (10 mg ml−1, 10 mm Tris-HCl, pH 8.0) and the reservoir solution (0.1 m sodium cacodylate, pH 6.5, 18% polyethylene glycol 8000, 0.2 m calcium acetate) at 293 K.
Data Collection and Structure Determination
The sPglB crystals were cryoprotected by soaking in 0.1 m MES, pH 6.5, 18% polyethylene glycol 8000, and 0.2 m calcium acetate and then in 0.1 m MES, pH 6.5, 30% polyethylene glycol 8000, and 0.2 m calcium acetate. The diffraction data were recorded at BL-38B1 and BL-44XU, SPring-8 (Harima, Japan). The data were processed with HKL-2000 (34) up to a resolution of 2.8 Å and yielded the space group P64. The crystal contained two molecules in the asymmetric unit cell (52.4% solvent, VM = 2.58 Å3 Da−1). A site search for the selenium atoms with autoSHARP (35) found 12 of 16 selenium sites in an asymmetric unit. The six selenium sites in each molecule were used for phasing. Phases were further improved by the density modification method, using DM and Solomon from the CCP4 package (36), with a mean figure of merit value of 0.86. Refinement was performed using the program CNS (37), and manual rebuilding was carried out with the program COOT (38). Translation/libration/screw (TLS) refinement was performed using the program REFMAC5 in the CCP4 package at the final round. The TLS groups were determined by the TLS Motion Determination server (available on the Washington University Web site). The final R factors obtained were Rwork/Rfree 24.5/26.0%. The data collection and refinement statistics are summarized in Table 1. In chain A, the N-terminal five extra residues as well as residues 428–435, 515–518, and 583–589 were disordered. Chain B displayed a higher degree of disorder than chain A. The two structures in the asymmetric unit are similar, with a Cα root mean square deviation value of 0.55 Å over 210 residues. Chain A was used for further analysis, and the figures were generated with the PyMOL version 1.1 (available on the World Wide Web). Superposition of the two structures was performed by the program GASH (available on the Protein Data Bank Japan Web site) (39).
TABLE 1.
Data collection and refinement statistics
| Se-Peak | Se-Edge | Se-Remote1 | Se-Remote2 | |
|---|---|---|---|---|
| Data collection | ||||
| X-ray source | BL-38B1 | BL-38B1 | BL-38B1 | BL-44XU |
| Wavelength (Å) | 0.97881 | 0.97909 | 0.94000 | 1.1000 |
| Space group | P64 | P64 | P64 | P64 |
| Unit cell dimensions (Å) | ||||
| a = b | 115.118 | 115.131 | 115.126 | 115.258 |
| c | 88.777 | 88.903 | 88.854 | 88.883 |
| Resolution (Å) | 30-3.0 | 30-3.2 | 30-3.2 | 30-2.8 |
| Outer shell (Å) | 3.11-3.0 | 3.31-3.2 | 3.31-3.2 | 2.9-2.8 |
| Mosaicity (degrees) | 0.193 | 0.234 | 0.209 | 0.206 |
| No. of reflections | ||||
| Observed | 200,768 | 83,950 | 85,597 | 63,435 |
| Unique | 13,487 | 11,117 | 11,112 | 16,601 |
| Completeness (%) | 99.7 (99.5)a | 99.7 (99.5) | 99.6 (99.7) | 99.9 (100) |
| Redundancy | 14.9 (14.9) | 7.6 (7.6) | 7.7 (7.8) | 3.8 (3.8) |
| 〈I〉/〈σI〉 | 11.2 (4.58) | 10.3 (3.38) | 10.8 (4.09) | 9.3 (3.37) |
| R-sym (%)b | 13.3 (47.0) | 12.3 (42.2) | 12.6 (35.9) | 8.5 (50.3) |
| Phasing (by autoSHARP) | ||||
| No. of selenium sites (found/all) | 12/16 | |||
| Phasing power | ||||
| Anomalous | 0.914 | 0.672 | 0.087 | 0.118 |
| Dispersive (centric/acentric) | 0.550/0.718 | 0.551/0.794 | 0.568/0.723 | |
| Overall figure of merit (initial/after DM) | 0.416/0.860 | |||
| Refinement | ||||
| Resolution (Å) | 20-2.8 | |||
| No. of reflections (work/test) | 15,279/1,276 | |||
| R-work/R-free (%)c | 24.51/26.00 | |||
| 〈B〉 (Å2) | 33.28 | |||
| Root mean square deviation | ||||
| Bonds (Å) | 0.002 | |||
| Angles (degrees) | 0.335 | |||
| Ramachandran plot analysis (%) | ||||
| Most favored | 77.7 | |||
| Additional allowed | 19.6 | |||
| Generously allowed | 2.6 | |||
| Disallowed | 0.0 | |||
a The highest resolution shell is shown in parentheses.
b R-sym = Σ|Ii − 〈Ii〉|/ΣIi, where Ii is the observed intensity, and 〈Ii〉 is the average intensity obtained from multiple observations of symmetry-related reflections.
c R-work = Σhkl‖Fo| − |Fc‖/Σhkl|Fo|. 7.7% of the reflections were excluded for R-free calculation.
Multiple Sequence Alignment
The amino acid sequences of the oligosaccharyltransferases (STT3/AglB/PglB) were retrieved from the InterPro 17.0 data base (available on the World Wide Web). The family IPR003674 contains 278 sequences, of which 154 sequences belong to Eukarya, 84 to Archaea, and 40 to Bacteria. The multiple sequence alignment was performed with the program MAFFT, version 6 (available on the World Wide Web) (40).
Preparation of the E. coli Membrane Fractions Containing the Full-length PglB
A codon-optimized DNA sequence of C. jejuni PglB (residues 1–713, Q5HTX9_CAMJR) for expression in E. coli cells was obtained from GenScript in the pUC57 plasmid. The entire coding sequence was subcloned into pET-41b(+) (Novagen) between the NdeI and SalI sites, with a C-terminal His8 tag. The PglB variants were generated using a KOD plus mutagenesis kit (TOYOBO). PglB and its variants were expressed in the E. coli BL21GOLD(DE3) strain (Novagen). The transformed E. coli cells were grown at 310 K in LB medium, supplemented with 30 mg/liter kanamycin. When the A600 reached 0.6, isopropyl-β-d-thiogalactopyranoside was added to a final concentration of 0.5 mm. After a 4-h induction at 310 K, the cells were harvested by centrifugation. The cell pellets from a 150-ml LB culture were resuspended in 20 ml of 50 mm Tris-HCl buffer, pH 7.4, containing 150 mm NaCl, 2 mm MgCl2, and 1 mm phenylmethylsulfonyl fluoride, supplemented with complete protease inhibitor mixture (Roche Applied Science) according to the manufacturer's instructions. After cell disruption by sonication, the lysate was centrifuged at 5,700 × g for 10 min. The supernatant was transferred into eight polycarbonate tubes in 3-ml aliquots and was ultracentrifuged, using a Beckman Coulter Optima TLX ultracentrifuge equipped with a TLA 110 rotor, at 100,000 × g and at 4 °C for 1 h. The supernatant was discarded, and the pellets were kept at −80 °C until use. Each pellet was dissolved in 5 ml of Triton buffer (20 mm Tris-HCl, pH 7.4, 150 mm NaCl, and 1% Triton X-100), incubated for 1 h on ice, and then divided into two polycarbonate tubes. After ultracentrifugation at 100,000 × g, the supernatant was collected as the membrane fraction. The amount of the PglB protein in the membrane fractions was quantified by SDS-PAGE and Western blotting with anti-His tag antibodies (mouse penta-His antibody, Qiagen) and goat anti-mouse IgG antibodies labeled with fluorescent IRDye 800CW (LI-COR). The fluorescence image was recorded with an Odyssey infrared imaging system (LI-COR) and was quantified using the Odyssey software, version 3.0.
Preparation of Lipid-linked Oligosaccharide from C. jejuni Cells
The glycerol stock (JCM catalog number 2013) of C. jejuni subsp. jejuni strain was obtained from the Microbe Division/Japan Collection of Microorganisms of the RIKEN Bioresource Center (Saitama, Japan). A microaerophilic atmosphere was set up in a 7-liter rectangular jar, with an AnaeroPack-MicroAero sachet (Mitsubishi Gas Chemical, Tokyo), which works as an oxygen absorber-CO2 generator for microaerophilic cultivation. The frozen stock cells were streaked onto a CCDA plate (blood-free Campylobacter medium, Kanto Chemical, Tokyo) and incubated at 37 °C for 3 days in the rectangular jar. Some colonies were inoculated into LB medium and incubated with gentle shaking in the rectangular jar at 37 °C for 3 days. The cells were collected by centrifugation. Lipid-linked oligosaccharides were extracted twice from 0.5 g of the cell pellets with 20 ml of chloroform/methanol/water (10:20:3 (v/v/v), solvent A), using a Polytron homogenizer (PT1200E, Kinematica). The combined extracts were concentrated under a nitrogen gas stream. The dried residue was dissolved in 2 ml of solvent A and was stored at −20 °C.
Oligosaccharyltransferase Assay
The OST assay was performed by the PAGE method (41). A 5-μl aliquot of lipid-linked oligosaccharides in solvent A was transferred to a 1.5-ml plastic tube and dried in a SpeedVac concentrator (ThermoSavant). Eight microliters of buffer (50 mm Tris-HCl, pH 7.5, containing 1 mm dithiothreitol, 10 mm MnCl2, and 0.02% Tween 20) were added, and the assay tube was sonicated in a bath-type sonicator (120 watts) for 5 min. One microliter of 30 μm carboxytetramethylrhodamine-peptide solution and 3 μl of the E. coli membrane fraction containing the full-length PglB or its mutants were added. The acceptor peptide sequence (carboxytetramethylrhodamine-Ala-Asp-Gln-Asn-Ala-Thr-Tyr-Lys-COOH, 8 residues; the glycosylation consensus is underlined) was an optimized sequence for PglB (42). The reaction mixture (total 12 μl) was incubated for 2 h at 37 °C. The reaction was stopped by the addition of 2.4 μl of 5× SDS sample buffer. The fluorescence image of the SDS-polyacrylamide gel was recorded with an LAS-3000 multicolor image analyzer (Fuji Film) and was quantified using the ImageGauge software (Fuji Film).
RESULTS AND DISCUSSION
Overall Structure of the C-terminal Globular Domain of PglB
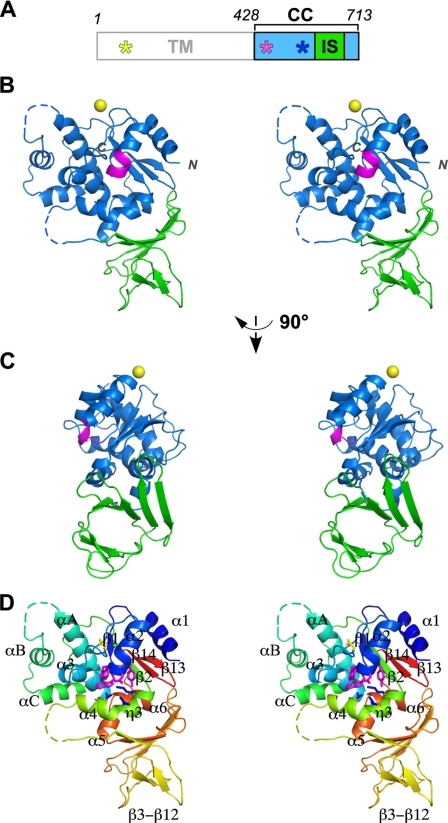
The C. jejuni oligosaccharyltransferase is a single-subunit membrane protein called PglB. It consists of 11 deduced transmembrane helices in the N-terminal half (427 residues) and a globular domain in the C-terminal half (286 residues) of the primary sequence (Fig. 1A). The C-terminal globular domain was expressed as a fusion protein in E. coli and was purified after the removal of the GST tag. The crystal structure of the C-terminal domain of C. jejuni PglB was determined at 2.8 Å resolution (Fig. 1). A typical electron density is shown in supplemental Fig. S2. The overall structure can be described as one central core domain (CC domain; blue) with an inserted β-structure (IS domain; green). A cation (yellow sphere) binds to the CC domain.
FIGURE 1.
Crystal structure of the C-terminal globular domain of PglB. A, domain structure of C. jejuni PglB. TM, transmembrane domain; CC, central core domain, residues 428–592 plus 675–713; IS, insertion domain, residues 593–674. The positions of the XXD motif, WWDYG motif, and MI motif are indicated by yellow, magenta, and blue asterisks, respectively. B, stereo view of the overall structure of the C-terminal domain of PglB (residues 436–713). Dashed lines indicate the residues that were not included in the final model because of incomplete electron density. The WWDYG motif is colored magenta. The bound metal cation is shown as a yellow sphere. The side-chain carboxylate group of Glu451 and the backbone carbonyl group of Thr557 are involved in the coordination. This metal ion is not directly involved in the catalysis, because it is far away from the catalytic site. Note that the position of the bound metal ion in PglB is different from that in the previously determined structure of AglB. C, different view from B. D, same view as B but with the secondary structure elements defined in supplemental Fig. S4. The side chains of the WWDYG motif (magenta), MI motif (blue), and the conserved Asp (yellow; see supplemental Fig. S1) were drawn.
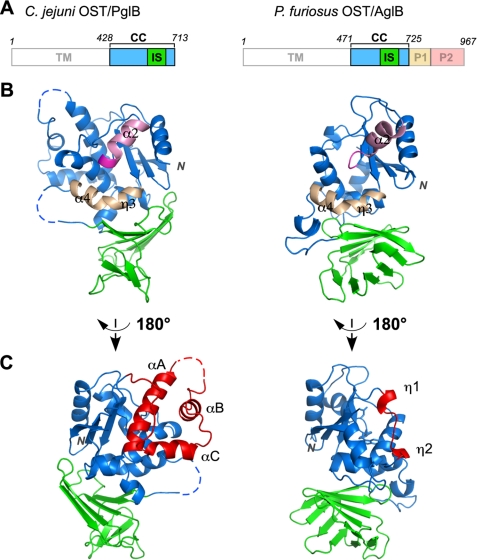
A comparison with the previously determined structure of the archaeal oligosaccharyltransferase, P. furiosus AglB (31), revealed a striking level of folding similarity, despite their low sequence homology. The C-terminal globular domain (497 residues) of the AglB protein is much longer than that (286 residues) of the PglB protein (Fig. 2A). The AglB protein consists of four structural domains, CC, IS, peripheral domain 1 (P1), and peripheral domain 2 (P2). The P1 and P2 domains are both β-sheet-rich domains and encircle the CC domain. The counterparts of the P1 and P2 regions in AglB are missing in the PglB protein. Interestingly, the C terminus of the PglB protein exactly corresponds to the domain boundary between CC and P1 of AglB. The structural superposition of the CC + IS portion resulted in a Cα root mean square deviation value of 3.0 Å, despite sequence identity as low as 13%, over the 167 aligned residues (supplemental Fig. S3). The high overall similarity of the CC + IS structure facilitated the structure-aided sequence alignment (supplemental Fig. S4). The short stretch containing two successive 310 helices, η1 and η2, of AglB is replaced by a long sequence containing three α-helices, αA, αB, and αC, in PglB (Fig. 2C, red). This long sequence forms a lining structure on one side of the CC domain in PglB, and its shorter counterpart is covered by the P1 and P2 domains in AglB.
FIGURE 2.
Comparison of the structures of PglB and AglB. A, domain structures of C. jejuni PglB and P. furiosus AglB. PglB and AglB are the catalytic subunits of the bacterial and archaeal oligosaccharyltransferases, respectively. P1 and P2 are additional domains of AglB. B, overall structures of the C-terminal domains of PglB (left) and AglB (right). The P1 and P2 domains of AglB were omitted for clarity. The WWDYG motif is colored magenta. The helix following the WWDYG motif is colored pink. The long, kinked helix that constitutes the active site of OST is colored light brown. C, different view from B. The portion of the CC domain of PglB that lacks a counterpart in the structure of AglB is colored red (left). The short stretch located at the corresponding position of AglB is also colored red (right). This segment is not solvent-exposed and is actually covered by the P1 and P2 domains in AglB.
Comparison of the IS Domains
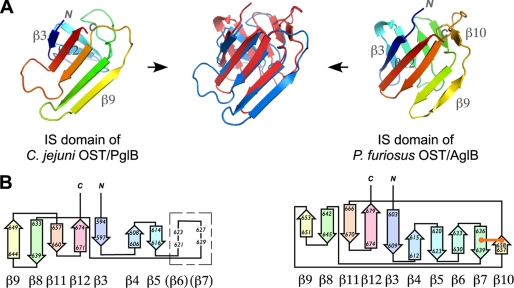
The IS domain of PglB is clearly the counterpart of the IS domain of AglB (Fig. 2). The Cα root mean square deviation is 2.4 Å over 68 residues (Fig. 3A). In a previous report, we described the topology of the IS domain of Pyrococcus AglB as a 10-stranded antiparallel β-barrel structure (31). The reanalysis using the PDBsum server (available on the EMBL-European Bioinformatics Institute Web site), however, demonstrated that the IS domain is a “distorted barrel,” because hydrogen bonds are not formed between the edge strands. Thus, we now describe the IS domain of Pyrococcus AglB as a barrel-like structure. The IS domain of PglB is regarded as a more distorted barrel, because there are two gaps in the β-β contacts. We describe the IS domain of Campylobacter PglB as a β-sandwich structure. Fig. 3B shows the topology diagram of the IS domains of the two structures. The IS domain of PglB contains seven β-strands, but we infer two additional strands in the region with high temperature factors, considering the overall structural similarity between PglB and AglB. We thus describe the IS domain of PglB as a nine-stranded structure consisting of almost antiparallel strands but with one parallel orientation. The AglB IS structure contains an extra, 10th strand, which is connected to the neighboring strand by a disulfide bond. The contact area between the CC and IS domains is about 2,000 Å2 in the PglB structure and 1,500 Å2 in the AglB structure. The wide contacts suggest that the relative orientation of the two domains is fixed in solution as well as in the crystal.
FIGURE 3.
Structural comparison of the IS domains of PglB and AglB. A, the overlaid structures (blue, PglB; red, AglB) are placed in between the IS structures of PglB (left) and AglB (right). B, topology diagram of the IS domains, generated by the PDBsum server. The location of the two putative β-strands suggested by structural similarity between PglB and AglB in a region with high temperature factors is shown in a box. The disulfide bond is shown as an orange line.
It is reasonable to assume that the IS domain has some important functions, considering the conservation of the IS domain across the two domains of life. A similarity search based on secondary structure matching (available on the EMBL-European Bioinformatics Institute Web site) for the PDB and SCOP data bases generated several β-barrel/sandwich-containing proteins. Some of them are sugar-binding proteins, but most of the sugar-binding sites are found in structures other than β-barrel/sandwich structures. One interesting example is the N-terminal three tandem β-barrel domains of the cation-independent mannose 6-phosphate receptor. The crystal structure revealed that the third barrel domain has a binding site for a mannose 6-phosphate molecule (43). In the three-dimensional structure, the IS domain is far away from the putative catalytic center in the CC domain (Fig. 2). We postulate that the IS domain recognizes the distal end sugar residues of the lipid-linked oligosaccharide substrates for the selection of in vivo oligosaccharide donors in Bacteria and Archaea. It would be interesting to determine whether a similar β-barrel/sandwich structure is present in the eukaryotic STT3 protein. Secondary structure predictions, however, did not predict any repetitive short strands, but rather helices, in the corresponding region in the eukaryotic STT3 proteins, such as those from yeast and Trypanosoma. We suggest that a different helical structure of the STT3 protein or other subunit(s) performs the same function in the eukaryotic OST. The structure determination of a eukaryotic STT3 will clarify this point.
Comparison of the Putative Catalytic Sites
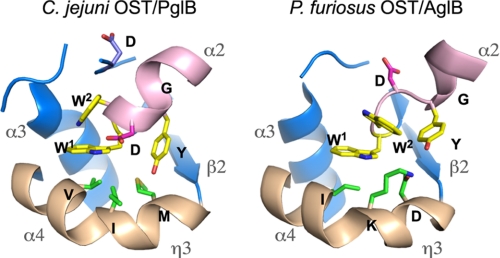
We focused our attention on the catalytic center of PglB and compared it with that of AglB. The conserved WWDYG motif (magenta) is located at the N terminus of the helix α2 (pink) and is close to the kinked helix η3 + α4 (light brown), as shown in Figs. 1 and 2. The putative catalytic site is formed between the WWDYG motif and the kinked helix (Fig. 4). The side chains of the Trp1 residue and the Tyr residue of the WWDYG motif occupy the same positions with similar side chain orientations in the two structures, but the side chains of the Trp2 and Asp residues protrude in opposite directions. The N-terminal part of the WWDYG motif in AglB adopts a rare left-handed helical conformation, which is apparently stabilized by the neighboring molecule in the crystals. By contrast, the corresponding part forms a typical right-handed helix in PglB. This mirror image relationship of the two conformations results in the opposite directions of the side chains of the Trp2 and Asp residues (Fig. 4) and consequently the different orientations of the helix α2 (Fig. 2B). Without the crystal packing effect, the directions of the side chains of the Trp2 and Asp residues in AglB would be the same as those found in PglB. This consideration implies that the correct configuration of the catalytic residues is that found in the PglB crystal. Alternatively, the two conformations of the WWDYG motif in the two structures reflect some plasticity of the active site structure, which enables large conformational changes during the OST reaction cycle.
FIGURE 4.
Close-up views of the putative catalytic sites of PglB and AglB. The side chains of the two Trp residues and one Tyr residue of the WWDYG motif are colored yellow, and that of the Asp residue is shown in red. The helix α2 following the WWDYG motif is colored pink. The long, kinked helix, η3 + α4, containing the DK or MI motif is colored light brown. The side chains of the DK and MI motifs are green. The side chain of the Asp residue conserved in the PglB protein family (Asp475) is also shown in the left panel.
A pair of Asp and Lys residues is present on the kinked helix in the AglB structure (Fig. 4). Instead of these residues, Met and Ile residues occupy the same positions in PglB. This finding prompted us to reexamine the multiple sequence alignment of the STT3/PglB/AglB protein family in more detail. We confirmed that the DXXK sequence is widely conserved in Eukarya and a portion of Archaea (supplemental Fig. S5). This motif was proved to be catalytically important by in vivo mutational studies; the substitution of the Asp and Lys residues in the motif each led to a lethal phenotype in yeast Stt3p and L. major STT3-1 (14, 31). The archaeal domain can be divided into two major phyla, Crenarchaeota and Euryarchaeota. All of the AglB proteins from Crenarchaeota and the class Thermococci (including Pyrococcus) of Euryarchaeota contain the typical DK motif. The DXXK sequence may be extended to DXXKXXX(M/I). The AglB proteins of other classes of Euryarchaeota possess a variant of the original DK motif, DXXMXXX(K/I). We refer to this as the “DM” motif. The three residues that define the motifs face the same side of the kinked helix, and thus the side chains come into close proximity (Fig. 4). This situation makes the second and third positions in the patterns interchangeable, and thus the DK and DM motifs are regarded as being functionally identical in OST catalysis. Some AglBs seem to contain a relaxed version, DXXMX(5,13)K, where X(m,n) denotes that at least m and at most n residues of any type may occur at this position, but the significance is not clear. There is the third set of classes in Euryarchaeota, in which the AglBs do not contain either the DK motif or the DM motif. We found that this archaeal group AglB possesses a pattern similar to that found in the bacterial PglB, MXX(K/I)XXXW. The archaeal and bacterial patterns can be combined into MXXIXXX(I/V/W), which we call the “MI” motif. Since the chemical properties of the side chains of the MI motif are very different from those of the DK/DM motif, the functional commonality is currently unclear. In summary, a careful sequence alignment of the STT3/PglB/AglB protein family revealed three types of conserved motifs on the kinked helix in the putative active site by reference to the two crystal structures of PglB and AglB.
Apart from the WWDYG motif, the sequence alignment of the PglB protein family indicates two strictly conserved residues, a conserved Asp and a conserved Gln (supplemental Fig. S1). The Asp residue is located near the catalytic site (Fig. 4), and the Gln residue is situated on the opposite face of the molecule. It is conceivable that the conserved Asp residue is involved in the catalysis, but the direction of the side chain is opposite to the catalytic site. Future studies are needed to uncover possible roles of these conserved residues in the structure and function of PglB.
Another Catalytic Motif in the Transmembrane Region
STT3/PglB/AglB is a new member of the glycosyltransferase GT-C superfamily (44). The members of the GT-C clan (CL0111 in the Pfam data base, available on the World Wide Web) are diverse glycosyltransferases that possess 8–13 predicted transmembrane helices and a DXD signature in the first lumenal/extracellular loop. This motif is also found in a mannosyltransferase, which catalyzes the O-mannosylation of proteins using d-mannose-P-dolichol as a sugar donor. Therefore, the role of this motif is estimated to be the binding to dolichyl-(pyro)phosphate via a bound metal ion, and thus it is indirectly involved in the catalysis. An EXD sequence is found in STT3 and a subset of AglB, belonging to Crenarchaeota (supplemental Fig. S6). A DXD sequence exists in another subset of AglB, belonging to the class Thermococci. The conservation of the first Asp is not strict in the remainder of the AglB proteins and all of the PglB proteins, and thus the signature pattern must be relaxed to XXD, but the Asp at the third position is absolutely conserved in all STT3/PglB/AglB proteins. The importance of the diacidic motifs was shown by in vivo mutational studies of the EXD motif in yeast Stt3p and L. major STT3–1; the substitution of the Asp residue in the motif led to a lethal phenotype in yeast Stt3p, and that of the Glu and Asp residues each had the same lethal effect in L. major STT3–1 (14).
Three Types of OST Catalytic Centers
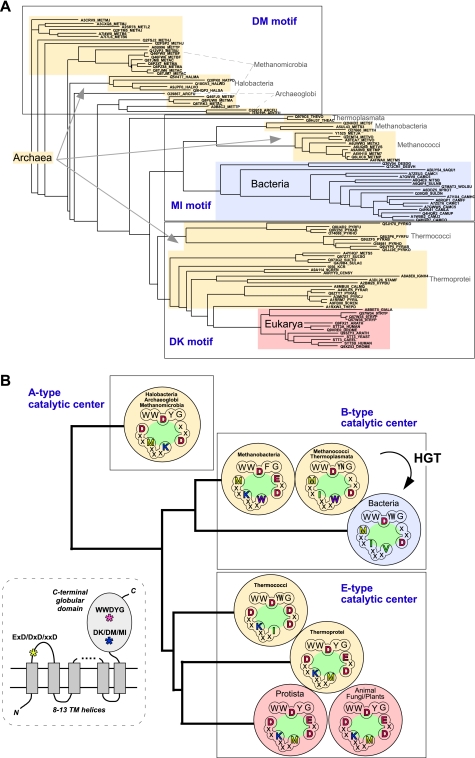
Fig. 5A shows a phylogenetic tree constructed from the full-length sequences of the STT3/PglB/AglB proteins. The multiple sequence alignment is difficult to interpret on a residue-by-residue basis due to the very low sequence homology, but the construction of a phylogenetic tree using the overall similarities is significant. Unexpectedly, the entries from the archaeal domain reside on different branches of the phylogenetic tree, as shown in the three boxes in Fig. 5A. This seemingly complicated situation can be simplified by identifying all of the entries in each box with the same type of DK/DM/MI motif. The clear grouping on the phylogenetic tree, based on the full-length sequences, confirms the significance of the conservation of the DK/DM/MI motif. We assumed that the catalytic center of the OST enzyme consists of the three short motifs. First, the catalytic motif, WWDYG, is highly conserved, but a variation is seen at the fourth position; Tyr is replaced by Trp, Asn, or Phe in some organisms (supplemental Fig. S5). Second, the DK, DM, or MI motif resides on the kinked helix in the active site. Third, the EXD, DXD, or XXD motif exists in the loop region that connects the first and second transmembrane helices. We classified the catalytic centers of the OST enzymes into three types (Fig. 5B). The E-type catalytic center consists of the WWD(Y/W)G, DK, and (E/D)XD motifs. The B-type catalytic center consists of the WWD(Y/W/N/F)G, MI, and XXD motifs. Finally, the A-type catalytic center consists of the WWDYG, DM, and XXD motifs. Eukaryotic STT3 exclusively has the E-type catalytic center, whereas the bacterial PglB contains the B-type only. Archaeal AglB is divided into three groups, bearing either the A-, B-, or E-type catalytic center. The E-type catalytic center must have a very ancient origin, before the divergence of Archaea and Eukarya, because the E-type exists in both the archaeal and eukaryal domains. In contrast, the B-type catalytic center in Bacteria probably originated from horizontal gene transfer from the B-type Archaea, considering the limited distribution of the N-glycosylation system in the bacterial domain.
FIGURE 5.
Three types of catalytic centers in the oligosaccharyltransferases. A, phylogenetic tree of the STT3/PglB/AglB proteins, based on the full-length sequences. The multiple-sequence alignment was performed by the program MAFFT, and the results were rendered by the program ATV (available on the Phylosoft Web site). The STT3/PglB/AglB entries in the phylogenetic tree can be classified into the three groups in the boxes, according to their type of DK/DM/MI motif. B, the catalytic centers (green oval) can be grouped into three types, assuming that the catalytic center of the OST enzyme is composed of the amino acid residues that define the three conserved motifs, WWDYG, DK/DM/MI, and EXD/DXD/XXD. The distribution of the three types is superimposed on the phylogenetic tree of STT3/PglB/AglB. Note that multiple STT3/PglB/AglB paralogs in an organism contain the same type of catalytic center, in principle. Eukaryotic STT3 and bacterial PglB exclusively have the E-type and B-type catalytic centers, respectively. Archaeal AglB is divided into three groups, which have either the A-, B-, or E-type catalytic center. Bacteria probably acquired the pglB gene by horizontal gene transfer (HGT) from the archaeal B-type. The inset shows the overall topology of the STT3/PglB/AglB proteins and the locations of the three catalytic motifs.
Site-directed Mutagenesis to Analyze Amino Acid Importance in the Catalytic Motifs
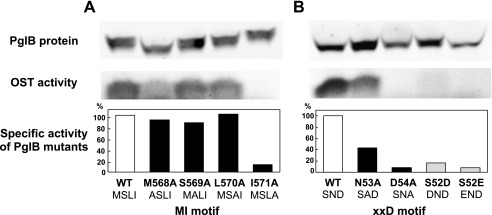
We used site-directed mutagenesis to address the role of the newly identified MI motif. Because the C-terminal globular domain alone has no catalytic activity, the full-length PglB protein was expressed in recombinant E. coli membrane fractions and used for an in vitro oligosaccharide transfer assay. We mutated four consecutive residues in the MI motif (Fig. 6A). The three alanine mutations, including the Met residue, had nearly the same specific activities as the wild type PglB, but the alanine mutation of the Ile residue led to substantially reduced activity.
FIGURE 6.
Effect of amino acid substitutions on the oligosaccharide transfer activity of C. jejuni PglB. A, mutations of the MI motif (residues Met568-Ser569-Leu570-Ile571); B, mutations of the XXD motif (Ser52-Asn53-Asp54). The full-length PglB, with the wild-type sequence and its mutated sequences, was expressed in E. coli, and the Triton X-100-solubilized membrane fractions were prepared. The amount of PglB in the membrane fractions was quantified by a Western blot analysis, using an anti-His tag antibody. It was essential to use a secondary antibody labeled with an infrared fluorescent dye for the accurate quantification and a wide linear dynamic range. The oligosaccharide transfer assay was performed by the PAGE method (41). The assay solution was the mixture of the membrane fraction containing PglB, crude lipid-linked oligosaccharide donors (cLLO) extracted from C. jejuni cells, and an acceptor peptide containing the Asp-Gln-Asn-Ala-Thr sequence and a fluorescent dye, TAMRA. The fluorescence image of the SDS-polyacrylamide gel was recorded and quantified. The specific activity was calculated as the percentage of the wild-type PglB.
We also tested the alanine mutations of the XXD motif (Fig. 6B), which resides in the first loop in the transmembrane region (inset of Fig. 5). The substitution of the second residue in the XXD motif, Asn53, retained moderate activity, but the mutation of the Asp residue resulted in nearly complete loss of the activity. Together with the inactive mutations in the WWDYG motif reported previously (16, 17), the three catalytic motifs all contribute to the constitution of the B-type catalytic center of PglB. Finally, we converted the XXD motif to the DXD and EXD motifs, unique to the E-type catalytic center. These changes led to the substantial loss of the oligosaccharide transfer activity (Fig. 6B). This implies that intimate coordination between the three catalytic motifs is necessary to constitute the functional catalytic centers of OST.
Comparative Structure Biology of OST in the Three Domains of Life
The comparative biology viewpoint (Fig. 5) provides some interesting ideas for future experiments to elucidate the catalytic mechanism of OST. (i) Two crystal structures of the C-terminal globular domain of OST are now available: one from Campylobacter jejuni PglB and the other from Pyrococcus furiosus AglB. As a eukaryotic model, the Pyrococcus structure is more suitable than the Campylobacter structure, because the former has the E-type catalytic center. (ii) Some archaea, such as Halobacteria, contain the A-type catalytic center. The structure determination is desired because no crystal structure of this type is available. (iii) Some genomes encode more than two STT3/PglB/AglB paralogs. With few exceptions, the paralogs in an organism have the same type of catalytic center. It would be interesting to investigate why a certain organism with multiple OSTs does not have those with different types of catalytic centers.
Conclusions
The structural comparison of two distantly related catalytic subunits of the oligosaccharyltransferases, bacterial PglB and archaeal AglB, highlighted the common architecture of the catalytic center, an inserted β-structure domain, and new, conserved catalytic motifs, beyond sequence comparison. The discovery of the new motifs was impossible without the identification of the equivalent positions in the catalytic center by reference to the structures, because the amino acid residues found at the equivalent positions have vastly different chemical properties. The site-directed mutagenesis study demonstrated the involvement of the newly identified MI motif in the oligosaccharide transfer reaction catalyzed by PglB as well as the DK motif in the eukaryotic STT3. We propose that the new DK/DM/MI motifs, in addition to the known WWDYG and EXD/DXD/XXD motifs, compose the three distinct types of catalytic centers of the OST enzyme (Fig. 5B). This novel classification will provide a useful framework for understanding the common and distinct enzymatic properties of the OST enzymes from Eukarya, Archaea, and Bacteria.
Supplementary Material
Acknowledgments
We thank Masumi Otsu (Laboratory for Technical Support, Medical Institute of Bioregulation, Kyushu University) for the DNA sequencing and Drs. Kaori Sasaki and Katsumi Maenaka for assistance in synchrotron data collection. We thank the staff at beamlines BL-38B1 and BL-44XU of SPring-8 and at beamlines BL-5A and AR-NW12A of the Photon Factory.
The experiments at SPring-8 were approved by the Japan Synchrotron Radiation Research Institute as Proposal 2007A1204. The experiments at the Photon Factory were approved by the High Energy Accelerator Research Organization (KEK) as Proposals 2007G208 and 2009G021.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S6.
The atomic coordinates and structure factors (code 3AAG) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
- OST
- oligosaccharyltransferase
- CC
- central core
- GST
- glutathione S-transferase
- IS
- insertion
- sPglB
- soluble domain of PglB
- TLS
- translation/libration/screw
- MES
- 4-morpholineethanesulfonic acid.
REFERENCES
- 1.Spiro R. G. (2002) Glycobiology 12, 43R–56R [DOI] [PubMed] [Google Scholar]
- 2.Yan A., Lennarz W. J. (2005) J. Biol. Chem. 280, 3121–3124 [DOI] [PubMed] [Google Scholar]
- 3.Kelleher D. J., Gilmore R. (2006) Glycobiology 16, 47R–62R [DOI] [PubMed] [Google Scholar]
- 4.Gavel Y., von Heijne G. (1990) Protein Eng. 3, 433–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knauer R., Lehle L. (1999) Biochim. Biophys. Acta 1426, 259–273 [DOI] [PubMed] [Google Scholar]
- 6.Schwarz M., Knauer R., Lehle L. (2005) FEBS Lett. 579, 6564–6568 [DOI] [PubMed] [Google Scholar]
- 7.Li H., Chavan M., Schindelin H., Lennarz W. J., Li H. (2008) Structure 16, 432–440 [DOI] [PubMed] [Google Scholar]
- 8.Chavan M., Chen Z., Li G., Schindelin H., Lennarz W. J., Li H. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 8947–8952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan Q., Lennarz W. J. (2002) J. Biol. Chem. 277, 47692–47700 [DOI] [PubMed] [Google Scholar]
- 10.Koiwa H., Li F., McCully M. G., Mendoza I., Koizumi N., Manabe Y., Nakagawa Y., Zhu J., Rus A., Pardo J. M., Bressan R. A., Hasegawa P. M. (2003) Plant Cell 15, 2273–2284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelleher D. J., Karaoglu D., Mandon E. C., Gilmore R. (2003) Mol. Cell 12, 101–111 [DOI] [PubMed] [Google Scholar]
- 12.Nasab F. P., Schulz B. L., Gamarro F., Parodi A. J., Aebi M. (2008) Mol. Biol. Cell 19, 3758–3768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Izquierdo L., Schulz B. L., Rodrigues J. A., Güther M. L., Procter J. B., Barton G. J., Aebi M., Ferguson M. A. (2009) EMBO J. 28, 2650–2661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hese K., Otto C., Routier F. H., Lehle L. (2009) Glycobiology 19, 160–171 [DOI] [PubMed] [Google Scholar]
- 15.Castro O., Movsichoff F., Parodi A. J. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 14756–14760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wacker M., Linton D., Hitchen P. G., Nita-Lazar M., Haslam S. M., North S. J., Panico M., Morris H. R., Dell A., Wren B. W., Aebi M. (2002) Science 298, 1790–1793 [DOI] [PubMed] [Google Scholar]
- 17.Glover K. J., Weerapana E., Numao S., Imperiali B. (2005) Chem. Biol. 12, 1311–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feldman M. F., Wacker M., Hernandez M., Hitchen P. G., Marolda C. L., Kowarik M., Morris H. R., Dell A., Valvano M. A., Aebi M. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 3016–3021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaban B., Voisin S., Kelly J., Logan S. M., Jarrell K. F. (2006) Mol. Microbiol. 61, 259–268 [DOI] [PubMed] [Google Scholar]
- 20.Abu-Qarn M., Eichler J. (2007) Archaea 2, 73–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruiz-Canada C., Kelleher D. J., Gilmore R. (2009) Cell 136, 272–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson C. M., Roebuck Q., High S. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 9534–9539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson C. M., High S. (2007) J. Cell Sci. 120, 648–657 [DOI] [PubMed] [Google Scholar]
- 24.Kelleher D. J., Banerjee S., Cura A. J., Samuelson J., Gilmore R. (2007) J. Cell Biol. 177, 29–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schulz B. L., Stirnimann C. U., Grimshaw J. P., Brozzo M. S., Fritsch F., Mohorko E., Capitani G., Glockshuber R., Grütter M. G., Aebi M. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 11061–11066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim H., von Heijne G., Nilsson I. (2005) J. Biol. Chem. 280, 20261–20267 [DOI] [PubMed] [Google Scholar]
- 27.Voisin S., Houliston R. S., Kelly J., Brisson J. R., Watson D., Bardy S. L., Jarrell K. F., Logan S. M. (2005) J. Biol. Chem. 280, 16586–16593 [DOI] [PubMed] [Google Scholar]
- 28.Eichler J. (2003) Microbiology 149, 3347–3351 [DOI] [PubMed] [Google Scholar]
- 29.Szymanski C. M., Wren B. W. (2005) Nat. Rev. Microbiol. 3, 225–237 [DOI] [PubMed] [Google Scholar]
- 30.Nakagawa S., Takaki Y., Shimamura S., Reysenbach A. L., Takai K., Horikoshi K. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 12146–12150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Igura M., Maita N., Kamishikiryo J., Yamada M., Obita T., Maenaka K., Kohda D. (2008) EMBO J. 27, 234–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Igura M., Maita N., Obita T., Kamishikiryo J., Maenaka K., Kohda D. (2007) Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 63, 798–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walter T. S., Meier C., Assenberg R., Au K. F., Ren J., Verma A., Nettleship J. E., Owens R. J., Stuart D. I., Grimes J. M. (2006) Structure 14, 1617–1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Otwinowski Z., Minor W. (1997) Methods Enzymol. 276, 307–326 [DOI] [PubMed] [Google Scholar]
- 35.Vonrhein C., Blanc E., Roversi P., Bricogne G. (2007) Methods Mol. Biol. 364, 215–230 [DOI] [PubMed] [Google Scholar]
- 36.Collaborative Computational Project 4 (1994) Acta Crystallogr. D Biol. Crystallogr. 50, 760–76315299374 [Google Scholar]
- 37.Brünger A. T., Adams P. D., Clore G. M., DeLano W. L., Gros P., Grosse-Kunstleve R. W., Jiang J. S., Kuszewski J., Nilges M., Pannu N. S., Read R. J., Rice L. M., Simonson T., Warren G. L. (1998) Acta Crystallogr. D Biol. Crystallogr. 54, 905–921 [DOI] [PubMed] [Google Scholar]
- 38.Emsley P., Cowtan K. (2004) Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 [DOI] [PubMed] [Google Scholar]
- 39.Standley D. M., Toh H., Nakamura H. (2005) BMC Bioinformatics 6, 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Katoh K., Misawa K., Kuma K., Miyata T. (2002) Nucleic Acids Res. 30, 3059–3066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kohda D., Yamada M., Igura M., Kamishikiryo J., Maenaka K. (2007) Glycobiology 17, 1175–1182 [DOI] [PubMed] [Google Scholar]
- 42.Chen M. M., Glover K. J., Imperiali B. (2007) Biochemistry 46, 5579–5585 [DOI] [PubMed] [Google Scholar]
- 43.Olson L. J., Dahms N. M., Kim J. J. (2004) J. Biol. Chem. 279, 34000–34009 [DOI] [PubMed] [Google Scholar]
- 44.Liu J., Mushegian A. (2003) Protein Sci. 12, 1418–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.