Abstract
Neuroactive steroids are potent modulators of γ-aminobutyric acid type A receptors (GABAARs), and their behavioral effects are generally viewed in terms of altered inhibitory synaptic transmission. Here we report that, at concentrations known to occur in vivo, neuroactive steroids specifically enhance a tonic inhibitory conductance in central neurons that is mediated by extrasynaptic δ subunit-containing GABAARs. The neurosteroid-induced augmentation of this tonic conductance decreases neuronal excitability. Fluctuations in the circulating concentrations of endogenous neuroactive steroids have been implicated in the genesis of premenstrual syndrome, postpartum depression, and other anxiety disorders. Recognition that δ subunit-containing GABAARs responsible for a tonic conductance are a preferential target for neuroactive steroids may lead to novel pharmacological approaches for the treatment of these common conditions.
Keywords: hippocampus, cerebellum, neurosteroids, inhibitory postsynaptic currents, δ knockout mice
GABAARs (γ-aminobutyric acid type A receptors) are pentameric proteins that form Cl--permeable ion channels activated by the neurotransmitter GABA. To date, 19 mammalian GABAA subunit isoforms have been identified, and these assemble to produce the dozen or so different receptor subtypes most frequently found in the brain (1). The most potent positive endogenous modulators of GABAAR function are the 3α-hydroxy ring A-reduced pregnane steroids, that have sedative-hypnotic, anticonvulsant, and anxiolytic effects (2–4). Severe mood disorders that can occur during the menstrual cycle and after pregnancy are suggested to involve alterations in the function of synaptic GABAARs (2, 3, 5) triggered by rapid decreases in the concentrations of these progesterone-derived neuroactive steroids (6).
Recently, it has become apparent that distinct GABAARs participate in two types of inhibitory control. Transient activation of synaptic GABAARs is responsible for conventional phasic inhibition, whereas the continuous activation of extrasynaptic GABAARs can generate a form of tonic inhibition (7–14). GABAARs containing the δ subunit are restricted to extrasynaptic locations (15) and have an unusually high affinity for GABA (16, 17), making them likely mediators of the tonic GABAA conductance recorded in both cerebellar (7, 8) and dentate gyrus granule cells (DGGC) (10, 11). In mice lacking the δ subunit of the GABAAR, the effects of neuroactive steroids are greatly reduced (18). Moreover, recent reports (17, 19, 20) have raised the possibility that the steroid sensitivity of δ subunit-containing GABAARs may be much higher than previously thought (21). In light of these findings, and the possible involvement of δ subunit-containing receptors in generating tonic conductances (8–11), we recorded from wild-type and δ-/- mice, and examined the effects of the naturally occurring neuroactive steroid 3α,21-dihydroxy-5α-pregnan-20-one (allotetrahydrodeoxycorticosterone, THDOC) on the tonic GABAAR-mediated conductance present in DGGC and cerebellar granule cells (CGC), two cell populations known to be rich in the δ subunit (22, 23).
Materials and Methods
Slice Preparation and Electrophysiology. We used 110 male mice (30–181 days old): 40 C57BL/6J mice, 32 δ-/- mice, and 38 wild-type littermates. The δ-/- mice were bred at the University of California, Los Angeles, Division of Laboratory Animal Medicine. Heterozygous (δ+/-) breeding pairs (C57BL/6J × 129Sv/SvJ) were donated by G. Homanics (Univ. of Pittsburgh, Pittsburgh), and δ+/- breeding pairs (C57BL/6J) were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were anesthetized with halothane according to a protocol approved by the University of California, Los Angeles, Chancellor's Animal Research Committee. The brains were removed and placed in ice-cold artificial cerebrospinal fluid containing 126 mM NaCl, 2.5 mM KCl, 2 mM CaCl2, 1–2 mM MgCl2, 1.25 mM NaH2PO4, 26 mM NaHCO3, and 10–25 mM D-glucose (pH 7.3–7.4 when bubbled with 95% O2 and 5% CO2).
Whole Cell Recordings. Recordings were made from visually identified neurons at 33–35°C in coronal hippocampal slices (350 μm) at Vh = -60 mV with an artificial cerebrospinal fluid containing 3–5 mM kynurenic acid and the anti-GABA transporter 1 (GAT-1) GABA transporter blocker NO-711 (10 μM) (11) and in parasagittal cerebellar slices (150–250 μm) at 20–22°C at Vh = -70 mV. Electrodes (5–8 MΩ) were filled with 140 mM CsCl, 1 mM MgCl2, 10 mM Hepes, and 4 mM Na-ATP (pH 7.25, 280–290 mosmol). D-AP5, SR95531, and THDOC (Sigma) were added to the external solution as indicated.
Extracellular Field Recordings. In artificial cerebrospinal fluid containing 5 μM GABA, field potentials simultaneously recorded in the dentate gyrus molecular layer and in the striatum radiatum of the CA1 were evoked (paired pulses 20 ms apart, 0.05 Hz) by stimulating the medial perforant path and the Schaffer collateral/commissural pathway, respectively. Bipolar electrodes delivered a constant current stimulus (A365, WPI Instruments, Waltham, MA). At a stimulus width (W) of 60 μs, the intensity was increased until a threshold response was collected over a 10-min stable baseline. The W was then varied (PG4000, Neurodata, Cygnus Technologies, Scottsdale, AZ) to create stimulus-response curves by delivering two stimulation trials (10 stimuli each) with W from 20 to 240 μs (20- and 40-μs increments). After the control trial, THDOC (10 nM) was perfused for 20 min before generating a second pair of stimulus–response curves.
Data Analysis. Low-pass filtered (3 kHz) recordings were digitized at 20 kHz. Field recordings were filtered between 0.10 and 3 KHz. In-house software was used to detect and analyze synaptic currents; further analysis was performed by using AXOGRAPH 4.6 (Axon Instruments) or IGOR PRO 3.14 (WaveMetrics, Lake Oswego, OR). The decay of averaged inhibitory postsynaptic currents (IPSCs) was fit with a double exponential: I(t) = A1*(exp(-t/τ1) + A2*exp(-t/τ2), where A1 and A2 are the fast and slow component amplitudes, and τ1 and τ2 are their respective time constants. The weighted decay time constant (τw) was calculated as the IPSC integral divided by its peak. The tonic GABAAR-mediated current was defined as the current blocked by SR95531 (8) and measured as described (10). To account for cell-to-cell variability, the tonic current was expressed as a conductance normalized to membrane capacitance (pS/pF). Whole cell capacitance was not affected by genotype.
Stimulus–response curves were fit to a Boltzman equation of the form f(W) = (MAX/(1 + exp((W - W50)/k)) + MAX), where W is stimulus width, MAX is the maximum response relative to the response elicited by the largest stimulus width (240 μs) under control conditions, k is a slope factor, and W50 is the stimulus width that elicits 50% of MAX (ORIGIN 6.1; OriginLab, Northampton, MA).
Differences were considered significant at P < 0.05, as determined by Student's t test (when measures were normally distributed) or the Mann–Whitney U test or Wilcoxon matchedpair test (when distributions were not normal).
Results
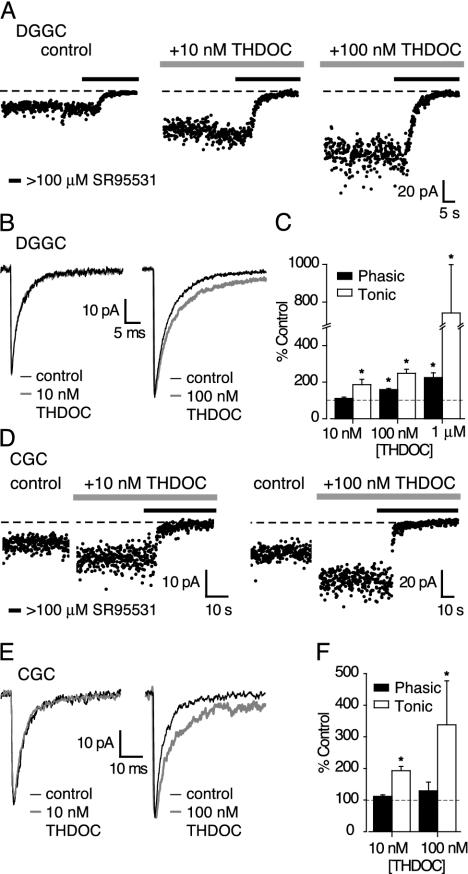
Brain and plasma neurosteroid concentrations reflect the animal's physiological state (24, 25). For THDOC, recent estimates of the basal plasma concentration in male rats range from ≈5 to 8 nM (26–28), to nearly 20 nM after acute swim stress (26). As shown in Fig. 1, concentrations of THDOC as low as 10 nM significantly potentiated the tonic conductance in both DGGCs and CGCs. Adult male mice were used to eliminate developmental and/or gender bias. In both DGGCs (Fig. 1 A and C) and CGCs (Fig. 1 D and F) the tonic conductance was nearly doubled by 10 nM THDOC (43.8 ± 10.8 pS/pF to 86.2 ± 11.2 pS/pF, n = 6 DGGCs; and 49.7 ± 7.5 pS/pF to 94.5 ± 14.2 pS/pF, n = 5 CGCs; both P < 0.05 paired t test). At this low concentration, THDOC failed to affect the 10–90% rise times (RT), peak amplitudes, or decay kinetics (τ1, τ2, A1, A2) of spontaneous IPSCs (sIPSCs) in either DGGCs or CGCs (Fig. 1 B and E). Accordingly, in 10 nM THDOC, the average charge transfer through individual sIPSCs was unaffected in both DGGCs (Fig. 1C; 282 ± 14 fC in control versus 313 ± 24 fC in THDOC; n = 6, P > 0.05 paired test) and CGCs (Fig. 1F; 122 ± 14 fC versus 134 ± 9 fC; n = 3, P > 0.05 paired t test). Likewise, the 10–90% RT (385 ± 49 μs, 379 ± 39 μs), peak amplitudes (43.6 ± 4.5 pA, 49.5 ± 2.9 pA), and decay kinetics of sIPSCs (τw 4.2 ± 0.3 ms, 4.2 ± 0.4 ms) recorded in DGGCs in the absence of NO-711 were unaffected by 10 nM THDOC (n = 4), indicating that NO-711 (either directly, or by elevating the ambient GABA) was not responsible for masking any effects of the neurosteroid on IPSCs.
Fig. 1.
Selective modulation by THDOC of a tonic GABAAR-mediated conductance. (A) Current values were averaged over 10-ms epochs at 100-ms intervals, under control conditions and in the presence of 10 and 100 nM THDOC. Horizontal bars indicate the application of the GABAAR antagonist SR95531 (final concentration ≥100 μM). The dotted line is the mean current after complete block of GABAARs used to calculate the magnitude of the tonic GABAAR-mediated conductance. This conductance is increased in the presence of both 10 and 100 nM THDOC, and GABAAR blockade rapidly reduces both its magnitude and variance. (B) Effects of 10 and 100 nM THDOC on averaged sIPSCs recorded in two DGGCs. (C) Concentration-dependent effects of THDOC on the tonic conductance (open bars) and average charge transfer through phasic sIPSCs (filled bars) expressed as a percentage of control values in the absence of THDOC (dashed line). Error bars denote SEM, and asterisks denote significance (P < 0.05). (D) Increase in baseline current in two CGCs by 5-min applications of 10 or 100 nM THDOC and the effect of GABAAR blockade (filled bars). (E and F) Same as B and C for CGCs.
The concentration-dependent enhancement of the tonic conductance by THDOC is shown for DGGCs in Fig. 1 A and C and for CGCs in Fig. 1 D and F. THDOC (100 nM) increased the tonic conductance from 86.7 ± 9.2 pS/pF to 132.0 ± 17.1 pS/pF in DGGCs (n = 7; P < 0.05 paired t test), and from 55.6 ± 10.6 pS/pF to 142.3 ± 33.5 pS/pF in CGCs (n = 6; P < 0.05 paired t test). This concentration of THDOC produced no change in sIPSC 10–90% RT, peak amplitude, or frequency in either DGGCs (n = 3) or CGCs (n = 6). In both cell types, there were rather variable effects on the sIPSC decay. In DGGCs, the slow component (τ2) of the decay was prolonged in two cells (Fig. 1B), and the average charge transfer significantly increased (Fig. 1C; 318 ± 59 fC to 499 ± 76; P < 0.05, paired t test). In CGCs, although the contribution of τdeacy2 to the sIPSC decay was increased in three cells (Fig. 1E), overall the average charge transfer was not significantly increased (Fig. 1F; 420 ± 78 fC vs. 580 ± 156 fC P > 0.05). However, in accord with previous data (29), a higher concentration of THDOC (1 μM) consistently prolonged the decay of sIPSCs in DGGCs, increasing the average charge transfer by 125 ± 13% (Fig. 1C; n = 5, P < 0.05 paired t test), with no effect on the 10–90% RT or peak amplitude. At 1 μM, THDOC also produced an 8-fold increase (n = 6) in the tonic conductance in DGGCs (Fig. 1C), but this concentration is 10–100 times higher than those found in the brain under various pharmacological treatments or stressful conditions (24, 25).
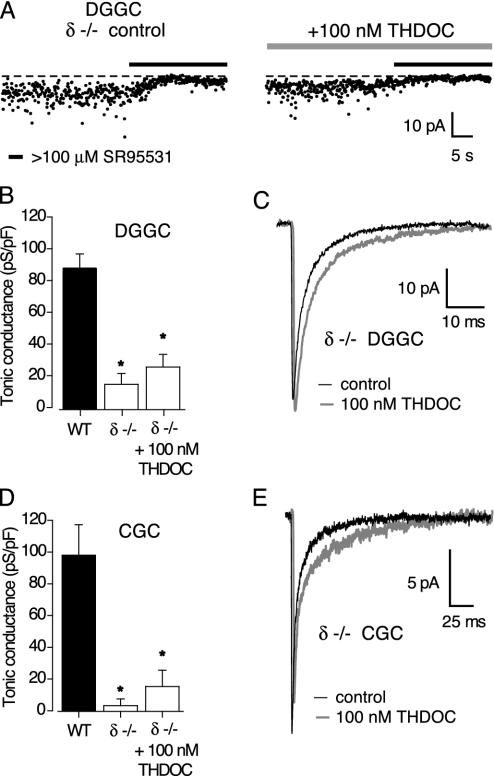
Having established that low concentrations of THDOC selectively enhanced the tonic conductance, we next investigated the subunit identity of this continuously active GABAAR population. The tonic conductance is known to be absent in CGCs of mice lacking the α6 subunit of the GABAAR (8). However, because of the close partnership between α6 and δ subunits in CGCs, in α6-/- animals these cells also lack δ subunit-containing receptors (30). Thus, to examine the specific contribution of δ subunits to the tonic conductance, we recorded GABAAR-mediated currents in DGGCs and CGCs of δ-/- mice. Our recordings clearly showed that the tonic GABAA conductance was much reduced in DGGCs and eliminated in CGCs of these mice (Fig. 2 A, B, and D). In DGGCs, the tonic conductance was reduced from 86.7 ± 9.2 pS/pF (n = 7 wild-type littermates, WT) to 15.4 ± 6.7 pS/pF in δ-/- mice (n = 7; P < 0.05). The corresponding change in CGCs was from 98.0 ± 19.1 pS/pF (n = 19) to 3.2 ± 4.3 pS/pF (n = 16, P < 0.05). Moreover, in both cell populations the properties of IPSCs were not significantly different between strains. Thus, in DGGCs the frequency of detectable IPSCs (2.1 ± 0.1 Hz in WT vs. 3.5 ± 0.4 Hz in δ-/-), their 10–90% RT (211 ± 14 μs vs. 224 ± 9 μs), peak amplitude (55.8 ± 6.0 pA vs. 48.0 ± 1.9 pA), and τw (5.7 ± 0.8 ms vs. 4.5 ± 0.5 ms) were not significantly different (P > 0.05 for all four parameters; n = 9 for WT and 5 for the δ-/-). Similarly, there was no significant difference between these measures in CGCs (frequency 0.8 ± 0.2 Hz vs. 0.6 ± 0.2 Hz; RT 961 ± 54 μs vs. 944 ± 39 μs; peak 32.3 ± 3.3 pA vs.18.5 ± 2.3 pA; τw 21.1 ± 2.9 ms vs. 17.1 ± 1.8 ms; P > 0.05; n = 19 for WT and 15 for δ-/-). A lack of effect on IPSC properties in δ-/- mice has previously been reported for CGCs (31) and ventrobasal thalamic neurons (32). Mihalek et al. (18) also observed no change in the frequency, RT, or amplitude of miniature IPSCs in DGGCs of δ-/- mice, but did note a small but significant decrease in the 90–37% decay time. Overall, there appears to be no gross alteration in the amount of vesicular GABA released in δ-/- animals. Therefore, the reduction in the tonic conductance is likely to reflect the loss of δ subunit-containing GABAARs, which under normal conditions are continuously activated by low concentrations of ambient GABA. In keeping with this idea, administration of a higher concentration of THDOC (100 nM) had no effect on the residual tonic conductance recorded in δ-/- DGGCs (15.4 ± 6.7 pS/pF in control vs. 26.0 ± 7.7 pS/pF in 100 nM THDOC; P > 0.05, unpaired t test, n = 7) or CGCs (4.3 ± 4.6 pS/pF in control vs. 15.2 ± 10.2 pS/pF in 100 nM THDOC; P > 0.05, paired t test, n = 5) (Fig. 2 B and D). It is known that THDOC at high concentrations can activate GABAARs in the absence of GABA (4, 33, 34). Its lack of effect in δ-/- mice suggests that, even at a concentration of 100 nM, the neuroactive steroid does not directly activate any of the remaining GABAARs. Finally, although the effect of 100 nM THDOC on the tonic conductance was abolished in δ-/- mice, a variable prolongation of the sIPSC decay, similar to that seen in wild-type cells, was still observed (Fig. 2 C and E).
Fig. 2.
Reduced tonic conductance in δ-/- mice. (A) Effects of GABAAR blockade on the holding current in two representative DGGCs from δ-/- mice (details as in Fig. 1 A). The tonic current is smaller than that seen in wild-type mice, and is not increased by 100 nM THDOC (see Fig. 1 A). (B) Tonic conductance in DGGCs from wild-type (filled bar) and δ-/- mice (open bars). Note the significant reduction in tonic conductance (asterisk), and the lack of effect of 100 nM THDOC, in δ-/- mice. (C) Averaged sIPSCs recorded in a DGGC from a δ-/- mouse, illustrating the effect of 100 nM THDOC. (D and E) Data from CGCs.
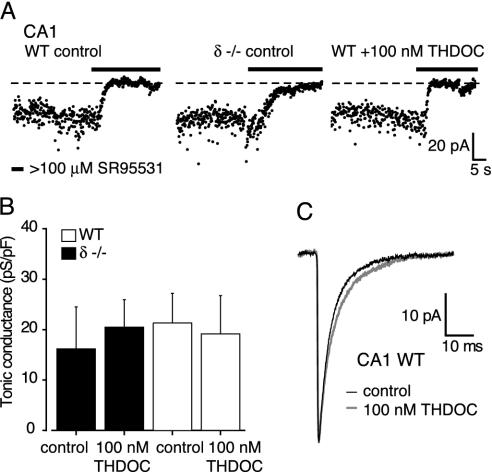
GABAARs with δ subunits are not the only receptors that can produce a tonic GABAA conductance in central neurons. A tonic conductance can be recorded in CA1 pyramidal cells (10), a neuronal population not particularly rich in the δ subunit (22, 23). However, the relatively small tonic conductance recorded in these cells (Fig. 3 A and B) was no different in wild-type and δ-/- mice (16.2 ± 8 pS/pF, n = 8, vs. 21.3 ± 5.9 pS/pF, n = 4; P > 0.05, unpaired t test), indicating that δ subunit-containing GABAARs do not contribute to this particular tonic conductance. Regardless of the exact subunit composition of the GABAARs responsible for this tonic conductance in CA1 pyramidal cells, THDOC (100 nM) was ineffective in enhancing the tonic conductance recorded in wild-type (16.2 ± 8.3 pS/pF in control vs. 20.5 ± 5.4 in 100 nM THDOC; P > 0.05 unpaired t test, n = 8 and 9) and δ-/- animals (21.3 ± 5.9 pS/pF in control vs. 19.1 ± 7.6 pS/pF in 100 nM THDOC; P > 0.05 unpaired t test, n = 4 and 4; Fig. 3 A and B). At this concentration, THDOC did not produce a significant change in the 10–90% RT (311 ± 30 μs, 361 ± 91 μs), peak amplitudes (59.9 ± 9.8 pA, 43.1 ± 15.8 pA), or decay kinetics (τw 4.3 ± 0.4 ms, 4.1 ± 1.2 ms) of sIPSCs in CA1 cells (n = 3) (Fig. 3C). These findings demonstrate that in the adult brain the likely site of action for THDOC, at concentrations reported to occur in vivo (24, 25), is a tonic conductance mediated by δ subunit-containing GABAARs.
Fig. 3.
The tonic conductance in CA1 pyramidal cells is not mediated by δ subunit-containing GABAARs and is not sensitive to 100 nM THDOC. (A) The effects of GABAAR blockade on the holding current in three representative CA1 pyramidal cells from wild-type or δ-/- mice (details as in Fig. 1 A). (B) The tonic conductances in wild-type (filled bars) and δ-/- (open bars) neurons were similar in the absence or presence of 100 nM THDOC. (C) Averaged sIPSCs recorded from a wild-type CA1 pyramidal cell in the absence and presence of 100 nM THDOC. The small prolongation of the sIPSC decay (≈25% increase in τ2) did not affect the average charge transfer (305 ± 38 fC in control; 319 ± 90 fC in THDOC; n = 3) or τw (see Materials and Methods).
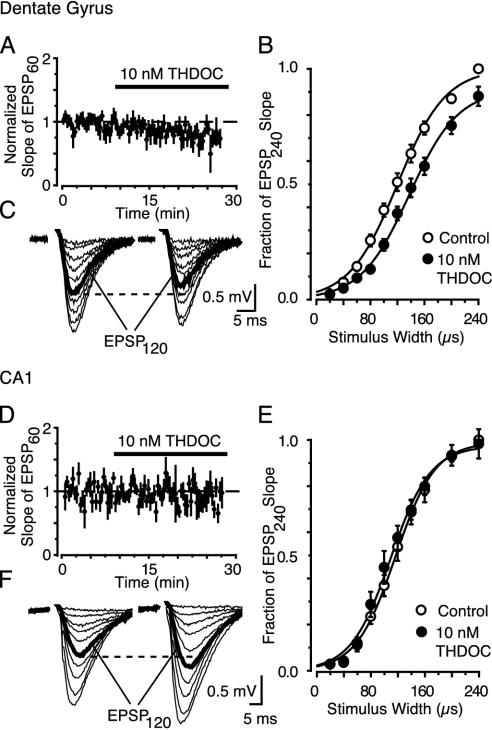
Having established that a low concentration of THDOC specifically modulates the δ subunit-mediated tonic conductance, we next measured the effect of the neurosteroid under more physiological conditions. To determine how signal integration is modulated by the THDOC-induced increase in tonic conductance, we recorded excitatory field potentials (fEPSPs) evoked before and after application of 10 nM THDOC in the presence of 5 μM GABA (35). We simultaneously obtained stimulus–response curves for DGGCs and CA1 pyramidal cells. Consistent with the THDOC modulation of the tonic conductance in DGGCs but not in CA1 pyramidal cells, THDOC (10 nM) gradually suppressed dentate fEPSPs evoked by threshold stimulation (60 μs at 0.05 Hz; Spearman rank order correlation Rs = -0.70, P < 0.05, Fig. 4A), but had no significant effect in CA1 (Rs = -0.17, P > 0.05, Fig. 4D). A much clearer effect of THDOC in the dentate gyrus was apparent with larger W stimuli, as shown for representative individual experiments (Fig. 4 C and F), and for pooled data (Fig. 4 B and E). In dentate, the average W required to evoke a half maximal response (W50) increased from 121.7 ± 6.3 μs in control to 138.7 ± 7.2 μs in THDOC (P < 0.05, n = 4). The field EPSPs recorded at the same time in the CA1 were unaffected by THDOC (average W50 119.7 ± 7.4 μs in control and 110.0 ± 10.4 μs in THDOC; P > 0.05). To examine the possibility that THDOC may have altered other channels, we repeated the dentate experiments in the presence of a specific GABAAR antagonist (SR95531; 50 μM). Epileptiform activity was avoided by adding the specific N-methyl-D-aspartate (NMDA) antagonist (RS)-CPP (25 μM). The effect of THDOC on the stimulus–response curves was completely blocked by SR95531 (average W50 123.7 ± 11.9 μs in control and 125.9 ± 14.6 μs in THDOC; P > 0.05, n = 5). This finding also demonstrates the lack of any time-dependent changes affecting stimulus–response relationships done in succession. Although THDOC does not seem to affect NMDA receptors (36), we performed additional control experiments in the presence of an NMDA antagonist. In 25 μM CPP, the effect of THDOC was similar to that seen under control conditions, and the stimulus–response curves were shifted to the right (W50 was 110.8 ± 3.3 μs in control and 124.4 ± 4.1 μs in THDOC; P < 0.05, n = 4). Therefore, as predicted by our conductance measurements, 10 nM THDOC acts on δ subunit-containing GABAARs to reduce the excitability of DGGCs.
Fig. 4.
A physiological concentration (10 nM) of THDOC reduces the fEPSP slope when δ-subunits are present. (A and D) Time vs. average fEPSP slope evoked by W = 60 μs (EPSP60). Data were pooled and normalized to the average fEPSP slope evoked during first 10 min (n = 4 slices). (B and E) fEPSPs evoked during stimulus response curves before (Left) and after (Right)10nM THDOC (20 min). The dashed line compares the two EPSP120 responses (bold, evoked by a W closest to the fitted W50). (C and F) Stimulus–response curves after 20 min of 10 nM THDOC (•) in the dentate gyrus (C) or the striatum radiatum of CA1 (F). Data (±SEM) are normalized to the slope of the EPSP240 (evoked by W = 240 μs) under control conditions (○). Lines represent the Boltzman equation fitted to the means.
Discussion
Our results demonstrate that δ subunit-containing GABAARs are activated by ambient GABA, giving rise to a tonic conductance in both DGGCs and CGCs. We also show that physiological concentrations of neurosteroids selectively enhance this conductance and thereby modulate the excitability of specific neuronal populations.
Few previous studies have described effects on GABAARs of physiologically relevant concentrations of neurosteroids. Low nanomolar concentrations of THDOC potentiate GABA responses in α1β2γ2-expressing human embryonic kidney (HEK) cells (33) and produce a small but significant increase in GABA currents of cultured hippocampal neurons (26). In the latter study, however, a 100% increase in the GABA response was seen only with 150 nM THDOC, a concentration 15 times greater than that required in our study to produce a similar effect on the tonic conductance of DGGCs and CGCs. Thus, in both CGCs and DGGCs, the tonic conductance mediated by extrasynaptic δ subunit-containing GABAARs is highly THDOC-sensitive. Recent studies on recombinant receptors show that a high sensitivity to neurosteroids is conferred by the δ subunit (17, 19, 20). Hence, the selectivity of THDOC observed in the present study likely reflects different properties of synaptic and extrasynaptic receptors that also experience different GABA concentrations. Other factors such as phosphorylation state may also play a role in the sensitivity of GABAARs to steroids (37). Theoretical considerations suggest that, under a variety of plausible conditions, GABA transporter stoichiometry determines a lower limit to the concentration of extracellular GABA in the submicromolar to low micromolar range (38, 39), in reasonable agreement with in vivo measurements (40–42). Receptors containing the δ subunit have a high affinity for GABA (16, 17) and display limited desensitization (43, 44), allowing them to be persistently activated by the low concentration of ambient GABA. Although steroids can enhance desensitization of δ subunit-containing receptors (44), such an effect did not mask the potentiation of the tonic inhibition even by relatively high concentrations of THDOC. In rat CGCs, a recent study (9) found only a modest potentiation (35%) by 100 nM THDOC of the tonic GABA-mediated conductance, labeling it as “neurosteroid insensitive.” The greater THDOC modulation seen in the present study could reflect species and/or gender differences (male mice vs. rats of both sexes), but our data agree with the emerging view that δ subunit-containing receptors are sensitive to neurosteroids, which act to increase the efficacy of GABA at these receptors (17, 19, 20).
Our recordings from δ-/- mice suggest an important role for δ subunit-containing GABAARs in mediating the tonic conductance in both DGGCs and CGCs, but the role of potential compensatory changes in other GABAAR subunits may need to be considered in the null mutants. Consistent with the loss of δ-containing receptors, tissue from δ-/- mice (18) shows reduced high-affinity [3H]muscimol binding both in the cerebellum and the forebrain (45), but in extracerebellar regions normally expressing δ subunits, α4 subunits are reduced and γ2 subunits are increased (23, 45). The cerebella of δ-/- mice show a similar increase in γ2 subunits, but no change in α6 subunits, whose partnership with δ subunits is not obligatory (46). [3H]Ro15-4513 binding to benzodiazepine sites in the cerebellum and forebrain is increased in δ-/- mice, consistent with an augmented assembly of γ2 subunits with α6 and α4 subunits (47–49). Our findings indicate that such compensatory changes in receptor composition are not capable of sustaining any THDOC-sensitive tonic conductance in DGGCs or CGCs of δ-/- mice.
Immunocytochemical studies suggest that δ subunit-containing GABAARs, although present in the extrasynaptic membrane of CGCs and DGGCs, may not be present at synapses (15, 50). The extrasynaptic location of these receptors is thought to reflect the mutual exclusion of δ and γ subunits from the receptor assembly (1) and the apparent requirement of a γ subunit for synaptic clustering of some GABAARs (51). This scenario would favor a selective action of neurosteroids on receptors normally activated in a paracrine fashion by low concentrations of ambient GABA. However, when release is triggered from multiple sites, extrasynaptic δ subunit-containing GABAARs may be activated in a phasic manner by the spillover of GABA from the synaptic cleft. This could explain the reported sensitivity of stimulus-evoked GABAAR-mediated IPSCs to 100 nM THDOC in rat CGCs (9). On the other hand, the action of higher concentrations of THDOC on the decay of sIPSCs in CGCs and DGGCs most likely reflects modulation of synaptic GABAARs lacking the δ subunit. Nevertheless, at both low and high THDOC concentrations, an increase in the GABAAR-mediated tonic conductance appears to be the principal effect of neurosteroids in both DGGCs (Fig. 1C) and CGCs (Fig. 1F). Thus, these extrasynaptic δ subunit-containing GABAARs represent a novel site of action for neurosteroids in the adult brain. Recent reports identify these receptors as selectively modulated by low concentrations of ethanol (52, 53), highlighting a possible locus for interactions between neuroactive steroids and alcohol.
In DGGCs and CGCs, tonic inhibition is mediated by δ subunit-containing GABAARs, but these receptors are not unique in generating GABA-evoked tonic conductances in central neurons. A tonic GABAAR-mediated conductance is present in CA1 pyramidal cells, which do not express δ subunits, but this conductance is not sensitive to 100 nM THDOC. In these neurons, α5 subunit-containing GABAARs are found extrasynaptically (54), but their sensitivity to neurosteroids remains to be determined. A tonic GABA-mediated conductance is present in hippocampal interneurons (14), but the subunit composition of the underlying receptors is unknown.
We have shown how neurosteroids, by enhancing a tonic conductance mediated by δ-containing GABAARs, can alter neuronal excitability. Both theoretical (55) and experimental (7–9) studies have demonstrated that a tonic conductance, as described here, produces a shunting inhibition that is capable of affecting neuronal excitability and gain control (56, 57). In the dentate gyrus and the CA1 region, we used extracellular field recordings to assay population responses without perturbing individual cell excitability. Under these conditions, a physiological concentration of THDOC selectively decreased excitability in the dentate. This effect of THDOC was not mediated by nicotinic acetylcholine receptors (58), voltage-gated Ca2+ currents (59), or σ1 receptors (60), all of which have been suggested as sites of action for 3α-hydroxy ring A-reduced pregnane steroids. The lack of an effect of physiological concentrations of THDOC on fEPSPs in the presence of SR95531 identifies GABAARs as the exclusive site of action for THDOC. Given our results from whole-cell recordings in wild-type and δ-/- animals, the effect of THDOC on cellular excitability can be attributed to an action on GABAARs containing δ subunits. By enhancing tonic inhibition, physiological concentrations of THDOC produced a reduction of the fEPSP slope of similar magnitude to that seen during long-term depression (61).
We propose that the anxiolytic, anticonvulsant, and sedative effects of neuroactive steroids may reflect actions on tonic inhibition generated by δ subunit-containing GABAARs, found not only in the cerebellum and hippocampus, but also at high levels in thalamus, striatum, and all layers of the cortex (22). Chronic in vivo administration and withdrawal of progesterone, which mimics hormonal changes seen during the menstrual cycle (6), increases the expression of α4βδ GABAARs (52). Consequently, drugs interacting with δ subunit-containing GABAARs may provide novel pharmacological treatments for a variety of conditions, such as premenstrual dysphoric syndrome and catamenial epilepsy, which have been related to alterations in the circulating levels of neuroactive steroids (2–5). The loss of this neuroactive steroid target could also explain some of the multiple neurological and psychiatric anomalies seen in patients with the 1p36 deletion syndrome, a chromosomal deletion that includes the δ subunit of the human GABAAR (62).
Acknowledgments
We thank S. G. Cull-Candy, Z. Nusser, and R. W. Olsen for comments on the manuscript. We are indebted to G. Homanics for sending us mice that originated our δ-/- colonies. This work was supported by National Institute of Neurological Disorders and Stroke Grant NS-30549 and the Coelho Endowment (to I.M.), and a Wellcome Trust Programme grant (to S. G. Cull-Candy, University College London).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: GABAA, γ-aminobutyric acid type A; GABAAR, GABAA receptor; DGGC, dentate gyrus granule cells; THDOC, 3α,21-dihydroxy-5α-pregnan-20-one; CGC, cerebellar granule cells; IPSC, inhibitory postsynaptic current; sIPSC, spontaneous IPSC; RT, rise time; fEPSP, excitatory field potentials.
References
- 1.McKernan, R. M. & Whiting, P. J. (1996) Trends Neurosci. 19, 139-143. [DOI] [PubMed] [Google Scholar]
- 2.Majewska, M. D. (1992) Prog. Neurobiol. 38, 379-395. [DOI] [PubMed] [Google Scholar]
- 3.Olsen, R. W. & Sapp, D. W. (1995) Adv. Biochem. Psychopharmacol. 48, 57-74. [PubMed] [Google Scholar]
- 4.Paul, S. M. & Purdy, R. H. (1992) FASEB J. 6, 2311-2322. [PubMed] [Google Scholar]
- 5.Smith, S. S. (2001) Cell. Mol. Life Sci. 58, 1263-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith, S. S., Gong, Q. H., Hsu, F. C., Markowitz, R. S., Ffrench-Mullen, J. M. & Li, X. (1998) Nature 392, 926-930. [DOI] [PubMed] [Google Scholar]
- 7.Brickley, S. G., Cull-Candy, S. G. & Farrant, M. (1996) J. Physiol. (London) 497, 753-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brickley, S. G., Revilla, V., Cull-Candy, S. G., Wisden, W. & Farrant, M. (2001) Nature 409, 88-92. [DOI] [PubMed] [Google Scholar]
- 9.Hamann, M., Rossi, D. J. & Attwell, D. (2002) Neuron 33, 625-633. [DOI] [PubMed] [Google Scholar]
- 10.Nusser, Z. & Mody, I. (2002) J. Neurophysiol. 87, 2624-2628. [DOI] [PubMed] [Google Scholar]
- 11.Stell, B. M. & Mody, I. (2002) J. Neurosci. 22, RC223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wall, M. J. & Usowicz, M. M. (1997) Eur. J. Neurosci. 9, 533-548. [DOI] [PubMed] [Google Scholar]
- 13.Rossi, D. J., Hamann, M. & Attwell, D. (2003) J. Physiol. 548, 97-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Semyanov, A., Walker, M. C. & Kullmann, D. M. (2003) Nat. Neurosci. 6, 484-490. [DOI] [PubMed] [Google Scholar]
- 15.Nusser, Z., Sieghart, W. & Somogyi, P. (1998) J. Neurosci. 18, 1693-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saxena, N. C. & Macdonald, R. L. (1996) Mol. Pharmacol. 49, 567-579. [PubMed] [Google Scholar]
- 17.Brown, N., Kerby, J., Bonnert, T. P., Whiting, P. J. & Wafford, K. A. (2002) Br. J. Pharmacol. 136, 965-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mihalek, R. M., Banerjee, P. K., Korpi, E. R., Quinlan, J. J., Firestone, L. L., Mi, Z. P., Lagenaur, C., Tretter, V., Sieghart, W., Anagnostaras, S. G., et al. (1999) Proc. Natl. Acad. Sci. USA 96, 12905-12910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adkins, C. E., Pillai, G. V., Kerby, J., Bonnert, T. P., Haldon, C., McKernan, R. M., Gonzalez, J. E., Oades, K., Whiting, P. J. & Simpson, P. B. (2001) J. Biol. Chem. 276, 38934-38939. [DOI] [PubMed] [Google Scholar]
- 20.Wohlfarth, K. M., Bianchi, M. T. & Macdonald, R. L. (2002) J. Neurosci. 22, 1541-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu, W. J., Wang, J. F., Krueger, K. E. & Vicini, S. (1996) J. Neurosci. 16, 6648-6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pirker, S., Schwarzer, C., Wieselthaler, A., Sieghart, W. & Sperk, G. (2000) Neuroscience 101, 815-850. [DOI] [PubMed] [Google Scholar]
- 23.Peng, Z., Hauer, B., Mihalek, R. M., Homanics, G. E., Sieghart, W., Olsen, R. W. & Houser, C. R. (2002) J. Comp. Neurol. 446, 179-197. [DOI] [PubMed] [Google Scholar]
- 24.Purdy, R. H., Morrow, A. L., Moore, P. H., Jr., & Paul, S. M. (1991) Proc. Natl. Acad. Sci. USA 88, 4553-4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corpechot, C., Young, J., Calvel, M., Wehrey, C., Veltz, J. N., Touyer, G., Mouren, M., Prasad, V. V., Banner, C., Sjovall, J., et al. (1993) Endocrinology 133, 1003-1009. [DOI] [PubMed] [Google Scholar]
- 26.Reddy, D. S. & Rogawski, M. A. (2002) J. Neurosci. 22, 3795-3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Serra, M., Pisul, M. G., Dazzi, L., Purdy, R. H. & Biggio, G. (2002) J. Psychopharmacol. 16, 133-138. [DOI] [PubMed] [Google Scholar]
- 28.Porcu, P., Sogliano, C., Cinus, M., Purdy, R. H., Biggio, G. & Concas, A. (2003) Pharmacol. Biochem. Behav. 74, 683-690. [DOI] [PubMed] [Google Scholar]
- 29.Cooper, E. J., Johnston, G. A. & Edwards, F. A. (1999) J. Physiol 521, 437-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones, A., Korpi, E. R., McKernan, R. M., Pelz, R., Nusser, Z., Mäkelä, R., Mellor, J. R., Pollard, S., Bahn, S., Stephenson, F. A., et al. (1997) J. Neurosci. 17, 1350-1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vicini, S., Losi, G. & Homanics, G. E. (2002) Neuropharmacology 43, 646-650. [DOI] [PubMed] [Google Scholar]
- 32.Porcello, D. M., Huntsman, M. M., Mihalek, R. M., Homanics, G. E. & Huguenard, J. R. (2003) J. Neurophysiol. 89, 1378-1386. [DOI] [PubMed] [Google Scholar]
- 33.Puia, G., Santi, M. R., Vicini, S., Pritchett, D. B., Purdy, R. H., Paul, S. M., Seeburg, P. H. & Costa, E. (1990) Neuron 4, 759-765. [DOI] [PubMed] [Google Scholar]
- 34.Lambert, J. J., Belelli, D., Harney, S. C., Peters, J. A. & Frenguelli, B. G. (2001) Brain Res. Brain Res. Rev. 37, 68-80. [DOI] [PubMed] [Google Scholar]
- 35.Overstreet, L. S. & Westbrook, G. L. (2001) J. Neurophysiol. 86, 596-603. [DOI] [PubMed] [Google Scholar]
- 36.Burg, M., Heinemann, U. & Schmitz, D. (1998) Eur. J. Neurosci. 10, 2880-2886. [DOI] [PubMed] [Google Scholar]
- 37.Fancsik, A., Linn, D. M. & Tasker, J. G. (2000) J. Neurosci. 20, 3067-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Attwell, D., Barbour, B. & Szatkowski, M. (1993) Neuron 11, 401-407. [DOI] [PubMed] [Google Scholar]
- 39.Wu, Y., Wang, W. & Richerson, G. B. (2003) J. Neurophysiol. 89, 2021-2034. [DOI] [PubMed] [Google Scholar]
- 40.Lerma, J., Herranz, A. S., Herreras, O., Abraira, V. & Martin del Rio, R. (1986) Brain Res. 384, 145-155. [DOI] [PubMed] [Google Scholar]
- 41.Tossman, U., Jonsson, G. & Ungerstedt, U. (1986) Acta Physiol. Scand. 127, 533-545. [DOI] [PubMed] [Google Scholar]
- 42.Kennedy, R. T., Thompson, J. E. & Vickroy, T. W. (2002) J. Neurosci. Methods 114, 39-49. [DOI] [PubMed] [Google Scholar]
- 43.Bianchi, M. T., Haas, K. F. & Macdonald, R. L. (2001) J. Neurosci. 21, 1127-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bianchi, M. T., Haas, K. F. & Macdonald, R. L. (2002) Neuropharmacology 43, 492-502. [DOI] [PubMed] [Google Scholar]
- 45.Korpi, E. R., Mihalek, R. M., Sinkkonen, S. T., Hauer, B., Hevers, W., Homanics, G. E., Sieghart, W. & Luddens, H. (2002) Neuroscience 109, 733-743. [DOI] [PubMed] [Google Scholar]
- 46.Tretter, V., Hauer, B., Nusser, Z., Mihalek, R. M., Hoger, H., Homanics, G. E., Somogyi, P. & Sieghart, W. (2001) J. Biol. Chem. 276, 10532-10538. [DOI] [PubMed] [Google Scholar]
- 47.Lüddens, H., Pritchett, D. B., Kohler, M., Killisch, I., Keinanen, K., Monyer, H., Sprengel, R. & Seeburg, P. H. (1990) Nature 346, 648-651. [DOI] [PubMed] [Google Scholar]
- 48.Wisden, W., Herb, A., Wieland, H., Keinanen, K., Lüddens, H. & Seeburg, P. H. (1991) FEBS Lett. 289, 227-230. [DOI] [PubMed] [Google Scholar]
- 49.Benke, D., Michel, C. & Möhler, H. (1997) J. Neurochem. 69, 806-814. [DOI] [PubMed] [Google Scholar]
- 50.Wei, W., Zhang, N., Peng, Z. Houser, C. R. & Mody, I. (2003) J. Neurosci., in press. [DOI] [PMC free article] [PubMed]
- 51.Crestani, F., Lorez, M., Baer, K., Essrich, C., Benke, D., Laurent, J. P., Belzung, C., Fritschy, J. M., Luscher, B. & Möhler, H. (1999) Nat. Neurosci. 2, 833-839. [DOI] [PubMed] [Google Scholar]
- 52.Sundstrom-Poromaa, I., Smith, D. H., Gong, Q. H., Sabado, T. N., Li, X., Light, A., Wiedmann, M., Williams, K. & Smith, S. S. (2002) Nat. Neurosci. 5, 721-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wallner, M., Hanchar, H. J. & Olsen, R. W. (2003) Proc. Natl. Acad. Sci. USA 100, in press. [DOI] [PMC free article] [PubMed]
- 54.Brunig, I., Scotti, E., Sidler, C. & Fritschy, J. M. (2002) J. Comp. Neurol. 443, 43-55. [DOI] [PubMed] [Google Scholar]
- 55.Gabbiani, F., Midtgaard, J. & Knöpfel, T. (1994) J. Neurophysiol. 72, 999-1009. [DOI] [PubMed] [Google Scholar]
- 56.Chance, F. S., Abbott, L. F. & Reyes, A. D. (2002) Neuron 35, 773-782. [DOI] [PubMed] [Google Scholar]
- 57.Mitchell, S. J. & Silver, R. A. (2003) Neuron 38, 433-445. [DOI] [PubMed] [Google Scholar]
- 58.Shiraishi, M., Shibuya, I., Minami, K., Uezono, Y., Okamoto, T., Yanagihara, N., Ueno, S., Ueta, Y. & Shigematsu, A. (2002) Anesth. Analg. 95, 900-906. [DOI] [PubMed] [Google Scholar]
- 59.Ffrench-Mullen, J. M., Danks, P. & Spence, K. T. (1994) J. Neurosci. 14, 1963-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maurice, T., Urani, A., Phan, V. L. & Romieu, P. (2001) Brain Res. Brain Res. Rev. 37, 116-132. [DOI] [PubMed] [Google Scholar]
- 61.Bear, M. F. & Abraham, W. C. (1996) Annu. Rev. Neurosci. 19, 437-462. [DOI] [PubMed] [Google Scholar]
- 62.Windpassinger, C., Kroisel, P. M., Wagner, K. & Petek, E. (2002) Gene 292, 25-31. [DOI] [PubMed] [Google Scholar]