Abstract
Store-operated Ca2+ entry (SOCE) due to activation of Ca2+ release-activated Ca2+ (CRAC) channels leads to sustained elevation of cytoplasmic Ca2+ and activation of lymphocytes. CRAC channels consisting of four pore-forming Orai1 subunits are activated by STIM1, an endoplasmic reticulum Ca2+ sensor that senses intracellular store depletion and migrates to plasma membrane proximal regions to mediate SOCE. One of the fundamental properties of CRAC channels is their Ca2+-dependent fast inactivation. To identify the domains of Orai1 involved in fast inactivation, we have mutated residues in the Orai1 intracellular loop linking transmembrane segment II to III. Mutation of four residues, V151SNV154, at the center of the loop (MutA) abrogated fast inactivation, leading to increased SOCE as well as higher CRAC currents. Point mutation analysis identified five key amino acids, N153VHNL157, that increased SOCE in Orai1 null murine embryonic fibroblasts. Expression or direct application of a peptide comprising the entire intracellular loop or the sequence N153VHNL157 blocked CRAC currents from both wild type (WT) and MutA Orai1. A peptide incorporating the MutA mutations had no blocking effect. Concatenated Orai1 constructs with four MutA monomers exhibited high CRAC currents lacking fast inactivation. Reintroduction of a single WT monomer (MutA-MutA-MutA-WT) was sufficient to fully restore fast inactivation, suggesting that only a single intracellular loop can block the channel. These data suggest that the intracellular loop of Orai1 acts as an inactivation particle, which is stabilized in the ion permeation pathway by the N153VHNL157 residues. These results along with recent reports support a model in which the N terminus and the selectivity filter of Orai1 as well as STIM1 act in concert to regulate the movement of the intracellular loop and evoke fast inactivation.
Keywords: Calcium, Calcium/Channels, Calcium/Imaging, Immunology/Humoral response/NF-AT, Immunology/T-cell receptor, Signal Transduction/Calcium, Ca2+ Release-activated Ca2+ Channels, Store-operated Ca2+ Entry
Introduction
In immune cells, ligand binding to plasma membrane receptors triggers Ca2+ entry through Ca2+ release-activated Ca2+ (CRAC)3 channels, which plays an important role in cell activation. The chain of events following activation of the cell surface receptors includes generation of the second messenger inositol 1,4,5-trisphosphate, opening of the inositol 1,4,5-trisphosphate receptors, depletion of endoplasmic reticulum Ca2+ stores, and translocation of STIM1, which activates CRAC channels. This last process, referred to as store-operated Ca2+ entry (SOCE), allows for sustained Ca2+ entry and is necessary for nuclear translocation of NFAT (nuclear factor of activated T cells), which enhances transcription of downstream genes (1–5).
CRAC channels comprise two key elements, Orai1 and STIM1, identified using RNA interference screens in Drosophila cells and HeLa cells (6–13). Orai1 is a four-transmembrane-containing plasma membrane protein (6, 11–12, 14), whereas STIM1, which has an EF-hand motif in its N terminus and resides in the endoplasmic reticulum, functions as a Ca2+ sensor (7, 15). Upon detection of endoplasmic reticulum Ca2+ depletion, STIM aggregates into multimers and translocates into endoplasmic reticulum structures called “puncta” proximal to the plasma membrane, where it activates Orai1 by direct interaction (15–19). Activation of Orai proteins involves their translocation into puncta and aggregation into tetramers (20, 21). These two steps, translocation and tetramerization, are a part of the activation process. Recent truncation studies have identified a minimal cytoplasmic fragment in the carboxyl terminus of STIM1 (residues 344–442), termed SOAR (STIM1 Orai-activating region) or CAD (CRAC activation domain), that interacts with the C-terminal α-helical segment of Orai proteins to open the channel independently of puncta formation (22–24).
Tight regulation of CRAC channel activation and inactivation is essential for proper cell function because elevated cytoplasmic Ca2+ concentrations ([Ca2+]) can potentially lead to apoptosis and cell death. One of the most thoroughly studied modes of inactivation of CRAC channel is its Ca2+-dependent fast inactivation (CDI) (25–27). Recent studies have identified many regions of Orai1 and STIM1 that modulate CDI. For instance, recent mutagenesis experiments have suggested that ion permeation at the selectivity filter controls CRAC channel fast inactivation and that 2-aminoethoxydeiphenyl borate, an activator of CRAC channels at low concentrations, hinders this inactivation process (28–30). Other studies have suggested involvement of residues in the N terminus of Orai1 and an inhibitory segment of STIM1 in modulating fast inactivation of Orai1 (31–33). Mullins et al. (33) showed that calmodulin binds to the N terminus of Orai1, and mutations of residues in this region that decrease calmodulin binding reduce fast inactivation. Seven acidic residues in STIM1, residing between amino acids 475 and 483, have been shown to play a role in fast inactivation. Mutation of these residues either facilitated or abolished fast inactivation (31–33). In addition, Lee et al. (32) showed that C-terminal glutamate residues regulate fast inactivation of Orai2 and Orai3, both of which show stronger inactivation than Orai1. All of these results demonstrate the involvement of N and C termini of Orai proteins in CDI. However, the role of the intracellular loop of Orai1 located between TM II and TM III in modulating the function of CRAC channels has not been determined so far.
In this study, we performed mutational analysis of residues in the intracellular loop between TM II and TM III of Orai1 to examine their role in the activation and inhibition of Orai1. We identified a mutant in the intracellular loop (MutA) that shows higher CRAC currents, resulting from loss of fast inactivation. Expression of short peptides corresponding to the intracellular loop blocked native CRAC channels, resulting in reduced SOCE. Furthermore, intracellular application of a peptide derived from this loop blocks CRAC currents from both WT and MutA Orai1. Experiments with concatenated WT and MutA Orai1 tetramers show that the presence of a single WT loop can restore fast inactivation. These data suggest that the intracellular loop of Orai1 is important for the fast inactivation process and may play the role of an inactivation particle.
EXPERIMENTAL PROCEDURES
Reagents
Thapsigargin, puromycin, and 2-aminoethoxydiphenyl borate were purchased from EMD Biochemicals. Anti-FLAG antibody (F3165) from Sigma and anti-hemagglutinin antibody (sc-7392) from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA) were used at a 1:1000 dilution for immunocytochemistry and flow cytometry analysis. Alexa Fluor 488- or Alexa Fluor 568-labeled secondary antibodies were purchased from Invitrogen and used at 1:1000 dilutions for immunocytochemistry.
Plasmids
Full-length cDNA of human Orai1 subcloned into bicistronic retroviral expression vector pMSCV-CITE-eGFP-PGK-Puro, which allows for simultaneous expression of Orai1, GFP, and a puromycin resistance gene, has been described previously (14). Some of the mutants were generated using Orai1 cDNA that expresses a FLAG tag in its extracellular loop between transmembrane segments III and IV, similar to the extracellular hemagglutinin tag described previously (14). Four amino acid-substituted mutants were generated by introducing a NotI restriction endonuclease site at the position of the amino acids that were to be changed. Two independent PCRs were performed, the first set using a 5′ primer at the beginning of Orai1 cDNA and a 3′ primer with the NotI site at positions of either MutA, MutB, or MutC clones and the second set with the 5′ primer starting from the MutA, -B, and -C sites (changed to a NotI site) and 3′ primer at the end of Orai1 cDNA. The primer sequences are mentioned in supplemental Table 1. The PCRs were digested with XhoI-NotI (set 1 fragment) and NotI-EcoRI (set 2 fragment) and ligated together with the XhoI-EcoR1 digested vector. Single-point mutants were generated using the QuikChange XL site-directed mutagenesis kit (Stratagene). For expression of fragments of the intracellular loop of Orai1 into cells, an initiation codon (Met) was added before the coding sequence for either the WT 37-mer peptide (ALMISTCILPNIEAVSNVHNLNSVKESPHERMHRHIE), MutA 37-mer peptide (ALMISTCILPNIEAAAAAHNLNSVKESPHERMHRHIE), or WT 5-mer peptide (NVHNL). Tetramers containing different monomers of WT or MutA Orai1 were cloned into pMSCV-CITE-eGFP-PGK-Puro vector. Primers were generated to exclude the termination codon from the first three monomers and to introduce a linker sequence encoding amino acids QLNQLE to allow for independent folding of each monomer. The linker residues, QLNQLE, have been previously used for construction of concatenated Orai1 (34). The order of monomers in each tetramer is as follows: 4 WT (all four WT monomers), 4 MutA (all four MutA monomers), 3MutA1WT (MutA-MutA-MutA-WT), 2MutA2WT (WT-WT-MutA-MutA), 1MutA3WT (WT-WT-MutA-WT). Primers used for mutagenesis are described in supplemental Table 1. All of the clones were verified by sequencing.
Cell Lines and Transductions
HEK293, HeLa, and Jurkat T cell lines were obtained from ATCC. Cells were cultured in Dulbecco's modified Eagle's medium (Mediatech, Hargrave, VA) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT), 10 mm HEPES, 10 mm glutamine, and 1% penicillin/streptomycin (Mediatech). Cells were transfected at 80–90% confluence using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. HeLa O+S cells were generated by transducing HeLa cells with retroviruses encoding STIM1 and Orai1 proteins. STIM1 cDNA was encoded from a plasmid co-expressing hCD25 (35, 36), and positively transduced cells were selected by using hCD25-coated magnetic beads (Invitrogen). Orai1 cDNA was encoded from a pMSCV-CITE-PGK-Puro vector, and transduced cells were selected by puromycin (1 μg/ml). Expression of full-length Orai1 and STIM1 proteins was verified by immunoblotting. For retroviral transductions, phoenix cells stably expressing gag-pol and ecotropic env (ATCC) were transfected with plasmids encoding Orai proteins to produce ecotropic, replication-incompetent retrovirus using the calcium phosphate transfection method. Virus-containing supernatant was collected at 2 and 3 days after transfection, and cells were transduced twice on day 2 and day 3 in the presence of 8 μg/ml Polybrene. Transduction efficiencies were evaluated by GFP expression using flow cytometry and Orai1 expression using immunoblotting and immunocytochemistry.
T Cell Purification
Murine CD4+ T cells were purified by magnetic bead selection (Dynal) from the lymph nodes and spleens of young (6–8-week-old) B6 wild type mice or mixed background, Orai1-null mice following the manufacturer's protocols. Purified T cells were stimulated with plate-coated anti-CD3 (1 μg/ml) and anti-CD28 (1 μg/ml) antibodies, followed by retroviral transduction. Cells were detached from the plates after 2 days of stimulation and cultured for 4 more days in medium supplemented with recombinant IL-2 (10 units/ml). Ca2+ entry in these T cells was measured after a total of 5–6 days of stimulation.
Establishment of Murine Embryonic Fibroblast (MEF) Cell Lines
Murine embryonic fibroblasts (MEFs) were isolated by dissecting Orai1−/− embryonic day 14.5 embryos. After 3–4 passages, MEFs were immortalized by stable expression of SV40 Large T antigen with retroviruses.
Single-cell Ca2+ Imaging
T Cells were loaded at 1 × 106 cells/ml with 1 μm Fura-2/AM (Molecular Probes) in culture medium for 30 min at 22–25 °C, resuspended in loading medium, and attached to poly-l-lysine-coated coverslips for 15 min. Fibroblasts and HeLa cells were grown directly on UV-sterilized coverslips and loaded with 2 μm Fura-2/AM for 45 min at 22–25 °C. For [Ca2+]i measurements, cells were mounted in a RC-20 closed bath flow chamber (Warner Instrument Corp., Hamden, CT) and analyzed on an Olympus IX51 epifluorescence microscope with Slidebook (Intelligent Imaging Innovations, Inc.) imaging software. Cells were perfused with Ca2+-free Ringer's solution, and Ca2+ stores were passively depleted with 1 μm thapsigargin. Fura-2 emission was detected at 510 nm with excitation at 340 and 380 nm, and the Fura-2 emission ratio (340/380) was acquired at every 5-s interval after subtraction of background. For each experiment, 50–100 individual cells were analyzed using OriginPro (Originlab) analysis software. [Ca2+]i was estimated from the relation [Ca2+]i = K*(R − Rmin)/(Rmax − R). K*, Rmin, and Rmax were measured in control cells. Peak Ca2+ was calculated as the maximal amount of Ca2+ accumulated in the cell after store depletion, upon reintroduction of Ca2+ containing Ringer's solution.
Measurement of CRAC Currents by Whole-cell Recording
For Figs. 2, 4, and 5, HEK293T cells were co-transfected with plasmids encoding STIM1 and WT or MutA Orai1 at a molar ratio of 1:1 using Lipofectamine 2000 (Invitrogen). Cells were used for experiments 16–24 h post-transfection. HeLa O+S cells stably expressing Orai1 and STIM1 proteins were used for experiments in Fig. 3. Cells were plated on glass bottom dishes 4–6 h before performing the experiments. Patch clamp recordings were performed using an Axopatch 200B amplifier (Molecular Devices) interfaced to a digitizer (Digidata 1320A; Axon Instruments) for stimulation and data acquisition. Currents were filtered at 1 kHz with a 4-pole Bessel filter and sampled at 5 kHz. Recording electrodes were pulled from borosilicate glass capillaries (WPI, Sarasota, FL), using a Flaming Brown pipette puller (Sutter Instrument Co.) to a final resistance of 2–7 megaohms. Stimulation, data acquisition, and analysis were performed using pCLAMP8 and SigmaPlot or Origin software. The standard extracellular Ringer solution contained 145 mm sodium aspartate, 4.5 mm KCl, 2 mm CaCl2, 10 mm d-glucose, and 10 mm Na-Hepes (pH 7.35). Some experiments were performed in the presence of 6 mm external CaCl2. High CaCl2 external solution contained 110 mm CaCl2, 10 mm d-glucose, and 5 mm HEPES (pH 7.35). The standard internal solution contained 145 mm cesium glutamate, 8 mm MgCl2, 12 mm EGTA, and 10 mm Cs-Hepes (pH 7.3). Where indicated, Orai1-(137–173) peptide (ALMISTCILPNIEAVSNVHNLNSVKESPHERMHRHIE; Genscript (Piscataway, NJ)) or MutA Orai1-(137–173) (ALMISTCILPNIEAAAAAHNLNSVKESPHERMHRHIE; Genscript) was added to the internal pipette solution at 50 μm final concentration. Purity of the peptide was >80%. Short pentapeptide (153NVHNL157; Genescript; >95% purity) was used at a concentration of 50 μm. The peptides used were acetylated at their N termini and amidated at their C termini.
FIGURE 2.
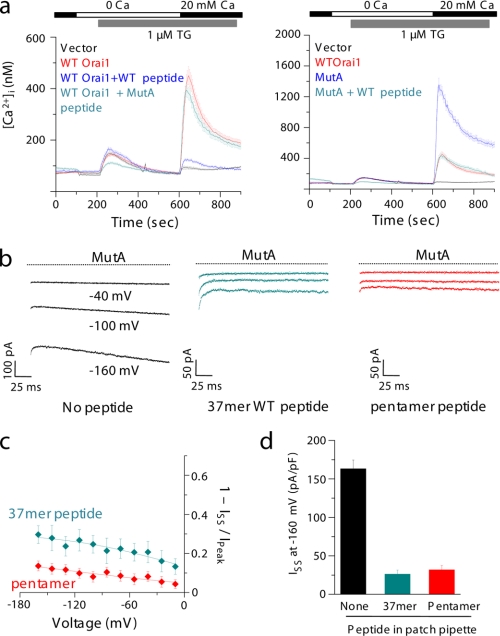
MutA Orai1 shows increased CRAC currents due to loss of fast Ca2+-dependent inactivation. a, CRAC currents in HEK293 cells expressing WT or MutA Orai1 together with STIM1 (molar ratio of 1:1). The left panel shows representative raw current traces obtained with voltage steps of 300 ms starting at −100 mV and up to 0 mV (20-mV intervals) from a holding potential of 0 mV. The right panel shows inwardly rectifying I-V curves typical of CRAC channels from cells expressing either WT (black trace) or MutA (red trace) Orai1. For these experiments, the intracellular pipette solution contained 12 mm EGTA, and the external solution contained 2 mm CaCl2. b, fast Ca2+-dependent inactivation of WT or MutA Orai1 monomers. HEK293 cells expressing WT (left, black traces) or MutA (right, red traces) Orai1 along with STIM1 (1:1 molar ratio) were perfused with extracellular solution containing 110 mm CaCl2. WT Orai1 showed strong fast inactivation at hyperpolarizing voltages, whereas MutA Orai1 showed a complete lack of fast inactivation. Two exponentials were required to fit the WT traces (red lines), yielding time constants of 3.3 ± 0.39 and 35 ± 5.4 ms. c, Ca2+ dependence (measured as apparent voltage dependence) of fast inactivation. The extent of inactivation or percentage inhibition, estimated as 1 − Iss/Ip, is plotted as a function of voltage for WT (black trace) as well as MutA (red trace) Orai1 channels. d, steady state currents from WT or MutA Orai1 channels. The data show average steady state currents from HEK293 cells expressing WT or MutA Orai1 channels (same as in b) in extracellular solution containing 110 mm CaCl2. Each bar represents an average of 10–12 cells.
FIGURE 4.
Overexpression of intracellular loop between TM II and TM III of Orai1 blocks CRAC currents from MutA Orai1. a, inhibition of MutA-mediated increase in SOCE by co-expression of WT peptide. Shown are Orai1 knock-out MEFs stably expressing either WT or MutA 37-mer peptides in the presence of WT Orai1 (left) or MutA Orai1 (right). WT but not MutA peptide blocks SOCE after thapsigargin-induced intracellular Ca2+ store depletion. Each trace represents an average from 30–50 GFP-positive cells. b, inhibition of MutA derived CRAC currents by WT peptide. Whole-cell patch clamp experiments were carried out with HEK293 cells expressing MutA Orai1 along with STIM1 (1:1 molar ratio). Currents were recorded with pipette solution containing 12 mm EGTA and 110 mm Ca2+ in the bath solution. Where indicated, a 50 μm concentration of either WT 37-mer peptide (cyan trace) or WT 5-mer peptide (red trace) was added to the pipette solution. The black traces represent currents from cells expressing MutA Orai1 in the absence of any peptide in the intracellular solution. The currents in the presence of WT 37-mer peptide are fitted by two exponentials (black lines). c, plot of percentage inhibition (1 − Iss/Ip) as a function of voltage. The extent of inactivation or percentage inhibition estimated as 1 − Iss/Ip is plotted as a function of voltage for MutA Orai1 in the presence of 37-mer (cyan trace) or 5-mer (red trace) peptide. d, steady state currents from MutA Orai1 channels in the absence or presence of peptides. Data show average steady state currents at −160 mV from HEK293 cells expressing MutA Orai1 channels (same as in b) in extracellular solution containing 110 mm CaCl2. Each bar represents an average of 4–6 cells.
FIGURE 5.
In concatenated Orai1 tetramers, a single WT intracellular loop restores fast Ca2+-dependent inactivation. a, schematic showing concatenated Orai1 tetramers with zero (4 MutA), one, two, three, or four WT inactivation particles. MutA monomers are depicted in red. b, measurement of SOCE in Orai1-null MEFs expressing concatenated tetramers with different stoichiometry of WT and MutA subunits. Store-operated Ca2+ entry was measured in Orai1-null MEFs stably expressing either empty vector or concatenated Orai1 tetramers comprising zero, one, two, or three WT inactivation particles. The bar graph (right) shows averages of peak [Ca2+]i from at least three independent experiments. Each trace represents an average from 30–50 GFP-positive cells. c, measurement of CRAC currents from HEK293 cells expressing STIM1 and various tetramers of Orai1 (molar ratio of 1:1). Whole-cell currents were recorded with pipette solution containing 12 mm EGTA and an external solution containing 110 mm Ca2+. The traces show currents obtained with WT tetramer (blue), MutA tetramer (red), and 3MutA1WT tetramer (cyan). The individual traces for WT and 3MutA1WT tetramers are fit by two exponentials (black lines). d, Ca2+-dependent fast inactivation in concatenated Orai1 tetramers. The extent of inactivation (1 − Iss/Ip) is plotted as a function of voltage for tetramers comprising four WT subunits (blue), one WT and three MutA subunits (cyan), or four MutA subunits (red). e, steady state currents from concatenated Orai1 tetramers. Data show the average (and S.E.) steady state currents at −160 mV from HEK293 cells expressing tetramers of Orai1 with either four, zero, or one WT monomer (same as in b) in extracellular solution containing 110 mm CaCl2. Each bar represents an average of 4–6 cells.
FIGURE 3.
Exogenous expression of intracellular loop between TM II and TM III of Orai1 blocks CRAC currents from WT Orai1. a, expression of peptide derived from WT intracellular loop blocks SOCE in HeLa cells stably expressing Orai1 and STIM1. Left, HeLa cells stably expressing Orai1 and STIM1 (HeLa O+S cells) were transfected with plasmids encoding 37-mer peptide corresponding to WT intracellular loop (WT peptide; red trace) or MutA intracellular loop (MutA peptide; blue trace). SOCE measured in cells transfected with the empty vector is shown in black. Expression of WT but not MutA peptide blocks SOCE after thapsigargin-induced intracellular Ca2+ store depletion. The plot shows average peak [Ca2+]i values from three independent experiments. 40–60 GFP-positive cells were selected for analysis from each experiment. b, expression of WT intracellular peptide blocks native CRAC channels in primary CD4+ T cells. Primary CD4+ T cells were transduced with retrovirus encoding either empty vector (black trace) or WT (red trace) or MutA (blue trace) peptides. Average peak [Ca2+]i values from three independent experiments are depicted in the bar graph. 50–100 GFP-positive cells were selected for analysis. c, inhibition of CRAC currents by WT peptide. Whole-cell patch clamp experiments were carried out with HeLa O+S cells. Currents were recorded with pipette solution containing 12 mm EGTA and an external solution containing 6 mm Ca2+. Residual currents measured after treatment with 100 μm 2-aminoethoxydeiphenyl borate were subtracted from the total currents, yielding inwardly rectifying CRAC currents. For peptide inhibition, currents were recorded with a 50 μm concentration of either WT 37-mer, MutA 37-mer, or WT 5-mer synthetic peptides added to the pipette solution. The black traces are from control cells in the absence of any peptide in the intracellular solution. The cyan and red traces show inhibited CRAC currents with WT 37-mer and WT 5-mer peptides, respectively, whereas the blue trace represents CRAC currents with MutA 37-mer peptide in the patch pipette. d, normalized peak currents from HeLa O+S cells in the presence of peptides in the intracellular solution. Recording conditions and solutions were same as shown for c. Each symbol represents the peak current from an individual cell 5 min after gaining access to the intracellular milieu.
Analysis of Patch Clamp Data
Unless otherwise stated, current traces were not corrected for leak currents. The time course of current decay was fitted by a biexponential function, I = I0 + A1e−t/τ1 + A2e−t/τ2, using SigmaPlot after removing the first 2 ms at the beginning of the pulse to minimize the effect of uncompensated membrane capacitance. Peak currents (Ip) were measured by extrapolating the fast component of inactivation to t = 0. Steady state currents for WT Orai1 (Iss) were measured using a current average at the end of the pulse. In the case of MutA Orai1, because the currents at the end of the pulse were larger than peak currents, Iss was measured right after peak currents, at time points between 25 and 50 ms. The extent of inactivation estimated as 1 − Iss/Ip, which corresponds to percentage inhibition, is plotted as a function of voltage for steps ranging from −160 mV to −20 mV. The extent of inactivation may have been underestimated as a result of removing the first 2 ms of recording.
RESULTS
The Intracellular Loop of Orai1 between TM II and III Inhibits CRAC Currents
To determine the functional role of the intracellular loop of Orai1 located between TM II and TM III, which contains hydrophobic residues and is well conserved throughout evolution (Fig. 1a and supplemental Fig. 1), we systematically mutated the loop residues and studied their role in store-operated Ca2+ entry. To measure the activity of individual Orai1 mutants without interference from endogenous Orai1, mutants were stably expressed in Orai1-deficient MEFs using retroviral transduction (37). SOCE was strongly reduced in Orai1-null MEFs (37) but was restored to WT levels by expression of WT Orai1 (Fig. 1, b and c). Initially, we performed alanine-scanning mutagenesis by introducing a NotI restriction enzyme site into the region between two proline residues, Pro146 and Pro164. Introduction of a NotI site generated simultaneous mutations of four amino acids to alanine. The mutants generated included MutA with mutation of residues V151SNV154 → A151AAA154, MutB with mutations of H155NLN158 → A155AAA158, and MutC with mutations of V160KES163 → A160AAA163. Expression of either MutA or MutB in Orai1-null MEFs increased peak [Ca2+]i more than 2-fold as compared with wild type Orai1, whereas MutC had no effect (Fig. 1, b and c). To identify the residues responsible for this effect, we generated a series of point mutants that replaced amino acids within the region of MutA and MutB with alanine. As seen in Fig. 1b (right) and 1c, mutations of single residues, N153A, V154A, H155A, N156A, and L157A, enhanced peak [Ca2+]i more than 2-fold, suggesting that the conserved residues N153VHNL157 play a role in CRAC channel inhibition. On the contrary, mutations of P146A, E149A, and P164A diminished Ca2+ influx, implying their positive contribution to the function of CRAC channels (Fig. 1c).
FIGURE 1.
Mutational analysis of the Orai1 intracellular loop between TM II and III. a, alignment of the intracellular loop, including part of TM II and TM III of human Orai1, -2, and -3 and Drosophila Orai (dOrai). Residues labeled in blue identify mutations tested in this loop. MutA, MutB, and MutC represent groups of four amino acids mutated to alanine (boxed residues). b, SOCE induced in Orai1-null MEFs by retroviral expression of WT and mutant Orai1 proteins. Intracellular Ca2+ stores were first depleted by a SERCA blocker, thapsigargin (TG; 1 μm), and then SOCE was measured by the addition of 20 mm external Ca2+. Each trace represents an average from 30–60 cells. The left panel shows representative Ca2+ entry traces obtained with groups of four mutants, whereas the right panel shows traces with single-point mutants of Orai1. c, peak [Ca2+]i was estimated for each mutant following store depletion. The bar graph shows the average and S.E. values from at least three independent experiments. The five amino acids, N153VHNL157, that increase SOCE upon mutation are labeled in red. Amino acid residues that abrogate SOCE upon mutation have been underlined (Pro146, Glu149, and Pro164). d, reconstitution of SOCE in Orai1-null CD4+ T cells. WT and MutA Orai1 cDNAs were expressed in Orai1-deficient primary T cells using a retroviral vector, expressing GFP from an internal ribosomal entry site (IRES). SOCE was measured using ratiometric Ca2+ imaging after intracellular store depletion using thapsigargin (left) or anti-CD3 antibodies (right). The bar graph on the far right shows average peak [Ca2+]i from three independent experiments using thapsigargin.
To observe surface expression and compare the expression levels of each of the mutant proteins, we examined cell surface targeting in HEK293 cells using immunocytochemistry and flow cytometry. Expression levels of the mutants were comparable with the WT protein as judged by surface staining and flow cytometry analysis (supplemental Figs. 2–4) (data not shown). To examine whether increased SOCE by MutA can be due to enhanced translocation of MutA into puncta, we measured the rate of translocation of GFP-tagged WT and MutA Orai1 proteins into puncta. GFP-tagged WT and MutA Orai1 proteins when co-expressed with STIM1 did not show any significant difference in their rate of translocation into puncta upon thapsigargin treatment as judged by total internal reflection fluorescence microscopy analysis (supplemental Fig. 5). These experiments suggest that increased SOCE by MutA and the individual mutants is not due to enhanced expression, increased interaction with STIM1, or facilitated translocation into puncta. Instead, these results suggest that the increase in Ca2+ entry may be due to a change in the gating properties of Orai1 channels.
To examine MutA-induced increase in SOCE in primary T cells where the function of CRAC channels are well defined, we expressed WT and MutA Orai1 into Orai1-null CD4+ T cells. These cells were activated using either thapsigargin or physiological stimulation of anti-CD3 antibody cross-linking to deplete intracellular Ca2+ stores. In Orai1-null T cells, residual Ca2+ influx was low, and expression of WT Orai1 restored the SOCE levels to those observed in WT T cells (Fig. 1d), as described previously (37). As expected, expression of MutA Orai1 increased SOCE by ∼1.5-fold (Fig. 1d). Consistent with the results from Orai1-null MEFs, these data suggest that the residues included in MutA within the intracellular loop of Orai1 may have an inhibitory role in CRAC channel function.
The Intracellular Loop of Orai1 Mediates Fast Inactivation
To investigate the mechanism of MutA-induced increase in Ca2+ entry, we carried out whole-cell patch clamp experiments with HEK293 cells overexpressing WT or MutA Orai1 together with STIM1. Overexpression of WT Orai1 with STIM1 induced large currents that showed strong inward rectification characteristic of CRAC channels (8, 38) (Fig. 2a, right, black traces). Cells expressing MutA Orai1 with STIM1 showed an almost 2-fold increase in CRAC currents in 2 mm extracellular Ca2+ (Fig. 2a, red traces) with average currents of 17 ± 3.5 pA/pF (n = 10) at −80 mV as compared with 10.5 ± 2.4 pA/pF (n = 9) for WT Orai1. The current-voltage (I-V) relationship for WT and MutA Orai1 showed similar reversal potentials at approximately +40 mV, suggesting that MutA did not alter Ca2+ selectivity of Orai1 (Fig. 2a, right). The current traces of MutA also showed reduced CDI in 2 mm extracellular Ca2+ solution as compared with WT Orai1 (Fig. 2a, left).
To investigate whether the increased CRAC currents of MutA were due to loss of CDI, we measured CRAC currents from WT and MutA channels in the presence of 110 mm extracellular Ca2+-containing solution to maximize Ca2+-dependent fast inactivation. In these experiments, CRAC currents were allowed to develop in the presence of 2 mm CaCl2-containing external solution, after which the external solution was exchanged for 110 mm CaCl2-containing solution. As seen in Fig. 2b (black traces), WT Orai1 channels exhibited strong fast inactivation at hyperpolarized voltages. The kinetics of inactivation of WT channels was fitted with a biexponential function, giving two time constants of 3.3 ± 0.39 and 35 ± 5.4 ms measured at −160 mV in extracellular solution containing 110 mm CaCl2. In the presence of 6 mm extracellular Ca2+, the time constants were 3.25 ± 1.2 and 47.5 ± 4 ms, similar to a previous report (27). Surprisingly, even in the presence of 110 mm external Ca2+, MutA channels showed minimal fast inactivation (Fig. 2b, red traces). The extent of inhibition measured as 1 − Iss/Ip shows apparent voltage dependence (possibly arising from Ca2+ dependence of fast inactivation as described in Ref. 27) for WT Orai1 but not for MutA (Fig. 2c). On average, MutA-expressing cells showed a more than 2-fold increase in steady state currents measured at −160 mV (Fig. 2d) in 110 mm external solution. A complete lack of fast inactivation in cells expressing MutA Orai1 suggests that the increased Ca2+ entry observed with MutA Orai1, was due to removal of fast inactivation and that the intracellular loop of Orai1 is a part of the fast inactivation process.
Peptides Derived from the Intracellular Loop of Orai1 Block CRAC Channel Activity
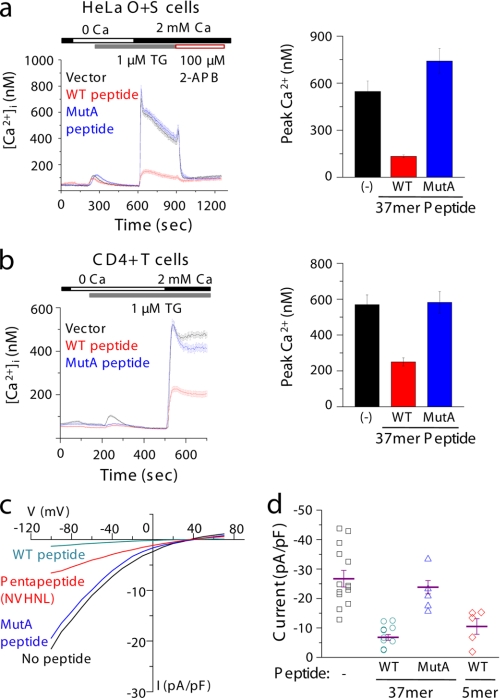
We hypothesized that the lack of fast inactivation in MutA can be due to two possibilities. First, the residues of MutA may serve as a docking site for inhibitory molecules involved in fast inactivation. Recently, negatively charged residues in STIM1 and binding of other proteins like calmodulin (CaM) have been shown to modulate fast inactivation of Orai1 (31–33). However, neither of these molecules binds to the intracellular loop of Orai1 (23, 33). In a second scenario, the intracellular loop can have a direct role in blocking the ion permeation pathway. If the latter hypothesis is correct, expression of a peptide derived from the intracellular loop should block the CRAC channels with great efficacy. To examine these possibilities, we expressed a 37-amino acid peptide corresponding to the TM II-TM III intracellular loop sequence Ala137-Glu173 (WT 37-mer peptide) and a control peptide incorporating mutations of V151SNV154 → A151AAA154, corresponding to those of MutA (MutA peptide) in HeLa cells stably expressing Orai1 and STIM1 (HeLa O+S). Intracellularly expressed peptides contained an additional Met codon at the N terminus for translation initiation. As seen in Fig. 3a, expression of a 37-mer WT peptide in HeLa O+S cells strongly decreases Orai1-mediated SOCE, whereas expression of MutA peptide does not have any effect. To examine whether expression of these peptides can block native CRAC channels, we expressed either the WT or MutA peptide in Jurkat T cells and primary mouse CD4+ T cells. Expression of the WT 37-mer peptide blocked endogenous CRAC channel mediated SOCE by more than 50% in primary CD4+ T cells (Fig. 3b) and Jurkat T cells (supplemental Fig. 6a), whereas MutA peptide had no effect. To validate the Ca2+ imaging data, whole-cell patch clamp experiments were performed with HeLa O+S cells using 50 μm synthetic WT 37-mer or MutA peptides in the intracellular pipette solution. As expected, in these cells, we observed amplified inwardly rectifying CRAC currents (Fig. 3c, black trace), which averaged 26.7 ± 2.8 pA/pF (n = 14; Fig. 3d) at −100 mV. These currents were substantially blocked in the presence of 50 μm synthetic 37-mer WT peptide (Fig. 3c, cyan trace). The currents obtained with 50 μm WT peptide averaged 6.8 ± 1.0 pA/pF, which is 4-fold lower than those obtained without any peptide (Fig. 3d, n = 7 cells). Addition of 50 μm MutA peptide in the intracellular solution had very little effect on CRAC currents (Fig. 3c, blue trace), with average peak currents of 23.8 ± 2.3 pA/pF, comparable with those obtained in the absence of any peptides in the intracellular solution (Fig. 3d, n = 5 cells).
Our previous results with individual point mutations of residues N153VHNL157 underlined the importance of these residues in inhibition of SOCE (Fig. 1c). To examine whether a peptide composed of these key residues has any effect on SOCE, we expressed the short peptide N153VHNL157 in HeLa O+S cells. This short peptide also contained an additional Met codon necessary for translation initiation. Expression of this short peptide in HeLa O+S cells resulted in a marked reduction in Ca2+ entry, suggesting that it may be able to block the ion permeation pathway as effectively as the 37-mer WT peptide (supplemental Fig. 6b). To verify whether this short peptide can block CRAC currents, we incorporated 50 μm synthetic 5-mer peptide (composed of residues N153VHNL157) into the intracellular solution and measured CRAC currents in HeLa O+S cells. As seen in Fig. 3c, the short pentapeptide significantly blocked CRAC currents, resulting in average currents of 10.5 ± 2.6 pA/pF (Fig. 3d, n = 5 cells). These data strongly support the hypothesis that the intracellular loop of Orai1 can act as an inactivation particle to block the Ca2+ ion permeation pathway.
Next we checked whether the inhibitory peptides can block the CRAC currents from MutA Orai1 as effectively as WT Orai1. To examine whether WT 37-mer peptide can block MutA-evoked increase in SOCE, we generated Orai1-null MEFs stably expressing MutA Orai1 with either the WT 37-mer peptide or the MutA peptide. Expression of WT 37-mer peptide significantly reduced SOCE in MutA-expressing cells, whereas co-expression of MutA peptide had no effect (Fig. 4a, right) (data not shown). As expected, SOCE mediated by WT Orai1 was strongly inhibited by co-expression of 37-mer peptide, but not with MutA peptide in Orai1 null MEFs (Fig. 4a, left). These data suggest that WT 37-mer peptide is able to block MutA-evoked CRAC currents in addition to the currents from WT Orai1.
To measure the effect of peptides on MutA-evoked currents, we expressed MutA Orai1 together with STIM1 in HEK293 cells and measured CRAC currents in the presence of 110 mm extracellular [Ca2+]. As seen previously, MutA Orai1 did not show any fast inactivation in the absence of peptides (Fig. 4b, black traces). Interestingly, both the long 37-mer and the short 5-mer peptides had potent inhibitory effects on MutA-evoked currents (Fig. 4b, cyan and red traces). Furthermore, blocking by both peptides did not recover fast inactivation in MutA Orai1 channels (Fig. 4b, compare with WT trace in Fig. 2c). In the experiments with the 37-mer or 5-mer WT peptides in the intracellular solution, when the membrane was held at 0 mV, we first observed development of CRAC currents as judged by a decrease in membrane resistance, and within 1 min, the resistance increased again, suggesting a block of the channels by the peptides. Subsequent application of hyperpolarizing voltage steps showed blocked currents as seen in Fig. 4b. In contrast, in the presence of MutA 37-mer peptide, the membrane resistance dropped and stayed low, and resultantly, high CRAC currents were observed at hyperpolarizing voltages. These results suggest that once the channels are open by the presence of 12 mm EGTA in the patch pipette, the free peptides can enter the open channels in a Ca2+-independent manner because the currents were blocked even when the membrane was held at a potential of 0 mV. Consequently, we did not see a voltage dependence of block by the peptides (Fig. 4c, compare with the black trace in Fig. 2c). The block with the peptides was very strong, with less than 25% residual currents in the presence of peptides in the intracellular solution (Fig. 4d). These data support the hypothesis that the intracellular loop of Orai1 functions as an inactivation particle that enters the ion permeation pathway upon channel opening.
A Single WT Intracellular Loop Restores Fast Inactivation in Orai1 Tetramers
Recent data show that active CRAC channels are tetramers of Orai1 subunits (20–21, 34). To investigate the number of WT intracellular loops necessary to restore fast inactivation, we measured store-operated Ca2+ entry and CRAC currents using concatenated Orai1 tetramers composed of 0–4 MutA monomers (schematically represented in Fig. 5a). Similar to MutA monomers, tetramers of MutA also enhanced SOCE by 3-fold as compared with WT tetramers when expressed in Orai1-null MEFs (Fig. 5b, red and blue traces and bar graph). Interestingly, expression of a tetramer with a single WT monomer and three MutA monomers (3MutA1WT tetramer) showed about 1.5-fold higher peak [Ca2+] than WT tetramers, suggesting an ∼50% reduction in MutA-induced SOCE (Fig. 5b, cyan trace and bar graph). Furthermore, expression of tetramers with two and three WT monomers restored SOCE to levels similar to that of WT tetramer (Fig. 5b, bar graph). These results suggest that the presence of only one WT intracellular loop may be sufficient to reconstitute fast inactivation in linked tetramers. To verify whether this was indeed the case, we measured CRAC currents in the presence of 110 mm extracellular Ca2+ in HEK293 cells expressing STIM1 along with either WT, MutA, or 3MutA1WT tetramers. As seen in Fig. 5c, concatenated MutA tetramers also lacked fast inactivation (red traces), whereas WT tetramers showed fast inactivation very similar to WT monomers (black traces). The kinetics of inactivation of WT tetramers was fitted with a biexponential function, giving two time constants of 3.9 ± 0.52 and 53 ± 6.7 ms measured at −160 mV in extracellular solution containing 110 mm CaCl2. These values are very close to time constants of WT monomers and previously published values (27). Furthermore, currents obtained with 3MutA1WT tetramers were almost indistinguishable from WT tetramers in terms of fast inactivation (Fig. 5c, cyan traces). The kinetics of inactivation of 3MutA1WT channels could also be fitted with a biexponential function, with time constants of 3.58 ± 0.5 and 48 ± 9 ms measured under the same conditions as WT tetramers. The extent of inactivation of WT and MutA tetramers recapitulated those observed for individual monomers (Fig. 5d, blue and red traces; compare with Fig. 2c). The presence of just one WT monomer restored a majority of inactivation in 3MutA1Wt tetramers (Fig. 5d, cyan trace). The amplitude of steady state current for 3MutA1WT tetramers was also close to that of WT tetramers (Fig. 5e). These data suggest that a single WT intracellular loop is sufficient to restore most of the Ca2+-dependent fast inactivation in Orai1 tetramers.
DISCUSSION
In many ion channels, inactivation is caused by insertion into the ion pathway of a particle formed by either an intracellular loop or either the N or C terminus of the pore-forming protein. In Na+ channels, the role of an inactivation particle has been demonstrated with channel mutants lacking inactivation and where inhibition was restored by a short peptide with a sequence similar to that of the inactivation particle (39, 40). Our results suggest that the intracellular loop between TM II and TM III of Orai1 functions as an inactivation particle that mediates fast inactivation of CRAC channels. First, mutation of only four amino acid residues in the intracellular loop abolished fast inactivation. Second, the addition of a 37-amino acid peptide derived from the same intracellular loop blocked CRAC currents generated by WT as well as MutA Orai1, the latter lacking fast inactivation (Figs. 3 and 4). In contrast, the addition of the same peptide carrying the mutations corresponding to those of MutA had no effect on CRAC currents derived from WT or MutA Orai1, suggesting that the blocking effect was specifically due to the WT peptide (Fig. 3) (data not shown). Third, similar to Na+ channels (39–41), a short peptide composed of five residues derived from the intracellular loop (N153VHNL157) blocked CRAC currents with great efficacy with less than 25% residual current in the presence of 50 μm WT peptides in the intracellular solution (Figs. 3 and 4). Finally, consistent with our hypothesis, the intracellular loop has not been identified as an interaction motif for any of the proteins known to modulate the fast inactivation of Orai1 (e.g. CaM or STIM1) (23, 31, 33). Altogether, these results strongly support the hypothesis that the intracellular loop of Orai1 acts as an inactivation particle to block the ion permeation pathway.
Our results from concatenated tetramers of Orai1 suggest that a single intracellular loop of Orai1 is sufficient to restore a majority of fast inactivation in CRAC channels (Fig. 5). This result differs from predictions derived from other models of inactivation. For instance, in the Monod-Wyman-Changeux model, one particle has almost no effect, and full inactivation requires the four particles to act in a concerted fashion (42). In a conventional Hodgkin-Huxley model, full inactivation requires the four particles, and the rate of inactivation with a single WT particle is one-quarter of that obtained with four WT subunits (43). This is clearly not the case with the CRAC channel tetramers because 1WT3MutA mimicked the effects of four WT subunits by showing recovery of ∼70% CDI (Fig. 5). Thus, in CRAC channels, one subunit may have a “dominant effect” according to the nomenclature proposed elsewhere (44). In addition, a strong block by expression of the WT peptides on MutA activity (Fig. 4) complements our data with concatenated tetramers of Orai1, where a single WT intracellular loop is sufficient to restore fast inactivation. This is also consistent with our observation that HEK293 cells showing very mild expression of MutA (as judged by co-expression of GFP from an internal ribosomal entry site) did not show a strong increase in Ca2+ influx, possibly due to interference from the intracellular loop of endogenous WT Orai1 (data not shown). To observe a robust increase in Ca2+ entry mediated by MutA Orai1, either the absence of endogenous Orai1 expression (as in Orai1-null cells) or very high expression in normal cell lines (as judged by GFP signal) was required. Together, these observations are consistent with the model in which a single loop is sufficient for fast inactivation of CRAC channels.
Fast Ca2+-dependent inactivation of CRAC channels was first reported in the early 1990s (25, 27). Recently, the N terminus of Orai1 and the cytoplasmic region of STIM1 have been shown to modulate the fast inactivation of Orai1 (32, 33). Mutations of residues 68–91 in the N terminus of Orai proteins reduce CaM binding and alter inactivation properties of CRAC currents (33). In addition, a negatively charged segment of STIM between residues 470 and 491 has been shown to be required for fast inactivation of Orai1 channels (31–33). Therefore, it is possible that CaM, the N terminus of Orai1, and the short inhibitory segment in the cytoplasmic region of STIM1 are closely related in regulation of the movement of the intracellular loop in and out of the ion permeation pathway. Further experiments of compound mutations of MutA together with the residues in the Orai1 N terminus or STIM1-inhibitory segment may allow us to determine whether the N terminus of Orai1 or STIM1 intracellular domains involve blocking by the intracellular loop. In addition to these structural elements that are involved in fast inactivation, a recent report has shown that mutations in the selectivity filter of Orai1 also affect both gating and permeation (28). Because mutations at the selectivity filter abolished fast inactivation and because the extracellular loops of Orai1 do not possess structures corresponding to the inactivation particle of other ion channels, it had been suggested that fast Ca2+-dependent inactivation was mediated by gating at the selectivity filter (28). Our data showing removal of fast inactivation in MutA suggest that the process of fast inactivation is complex and may involve both the selectivity filter and the intracellular loop. The selectivity filter and the intracellular loop may be functionally interdependent in blocking the ion permeation pathway, as has been recently shown for voltage-dependent Ca2+ channels (45, 46).
In summary, our data show that the intracellular loop linking TM II to TM III may play the role of an inactivation particle in CRAC channels, and we propose a working model whereby binding of Ca2+/CaM to the intracellular domains of Orai1 and/or STIM1 regulates the movement of the inactivation particle in a Ca2+-dependent manner. Because mutations in the intracellular loop and the selectivity filter of Orai1 abolish fast inactivation, we propose that both structures interact to block Ca2+ flux.
Supplementary Material
Acknowledgments
We thank faculty members of Department of Physiology (UCLA), especially Drs. Kenneth Philipson and Diane Papazian, for critical reading of the manuscript and Julio Vergara for suggestions on the peptide inhibition experiments.
This work was supported, in whole or in part, by National Institutes of Health Grant AI083432. This work was also supported by a grant from the Cancer Research Coordinating Committee (University of California) (to Y. G.) and a fellowship from the American Heart Association (to S. S.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table 1 and Figs. 1–6.
- CRAC
- Ca2+ release-activated Ca2+
- SOCE
- store-operated Ca2+ entry
- CDI
- Ca2+-dependent fast inactivation
- TM
- transmembrane segment
- WT
- wild type
- GFP
- green fluorescent protein
- MEF
- murine embryonic fibroblast
- Ip
- peak current
- Iss
- steady state current
- pF
- picofarad
- CaM
- calmodulin.
REFERENCES
- 1.Cahalan M. D. (2009) Nat. Cell Biol. 11, 669–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feske S. (2007) Nat. Rev. Immunol. 7, 690–702 [DOI] [PubMed] [Google Scholar]
- 3.Hogan P. G., Chen L., Nardone J., Rao A. (2003) Genes Dev. 17, 2205–2232 [DOI] [PubMed] [Google Scholar]
- 4.Lewis R. S. (2001) Annu. Rev. Immunol. 19, 497–521 [DOI] [PubMed] [Google Scholar]
- 5.Putney J. W., Jr. (1986) Cell Calcium 7, 1–12 [DOI] [PubMed] [Google Scholar]
- 6.Feske S., Gwack Y., Prakriya M., Srikanth S., Puppel S. H., Tanasa B., Hogan P. G., Lewis R. S., Daly M., Rao A. (2006) Nature 441, 179–185 [DOI] [PubMed] [Google Scholar]
- 7.Liou J., Kim M. L., Heo W. D., Jones J. T., Myers J. W., Ferrell J. E., Jr., Meyer T. (2005) Curr. Biol. 15, 1235–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peinelt C., Vig M., Koomoa D. L., Beck A., Nadler M. J., Koblan-Huberson M., Lis A., Fleig A., Penner R., Kinet J. P. (2006) Nat. Cell Biol. 8, 771–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prakriya M., Feske S., Gwack Y., Srikanth S., Rao A., Hogan P. G. (2006) Nature 443, 230–233 [DOI] [PubMed] [Google Scholar]
- 10.Roos J., DiGregorio P. J., Yeromin A. V., Ohlsen K., Lioudyno M., Zhang S., Safrina O., Kozak J. A., Wagner S. L., Cahalan M. D., Veliçelebi G., Stauderman K. A. (2005) J. Cell Biol. 169, 435–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vig M., Peinelt C., Beck A., Koomoa D. L., Rabah D., Koblan-Huberson M., Kraft S., Turner H., Fleig A., Penner R., Kinet J. P. (2006) Science 312, 1220–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeromin A. V., Zhang S. L., Jiang W., Yu Y., Safrina O., Cahalan M. D. (2006) Nature 443, 226–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang S. L., Yeromin A. V., Zhang X. H., Yu Y., Safrina O., Penna A., Roos J., Stauderman K. A., Cahalan M. D. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 9357–9362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gwack Y., Srikanth S., Feske S., Cruz-Guilloty F., Oh-hora M., Neems D. S., Hogan P. G., Rao A. (2007) J. Biol. Chem. 282, 16232–16243 [DOI] [PubMed] [Google Scholar]
- 15.Zhang S. L., Yu Y., Roos J., Kozak J. A., Deerinck T. J., Ellisman M. H., Stauderman K. A., Cahalan M. D. (2005) Nature 437, 902–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cahalan M. D., Zhang S. L., Yeromin A. V., Ohlsen K., Roos J., Stauderman K. A. (2007) Cell Calcium 42, 133–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gwack Y., Feske S., Srikanth S., Hogan P. G., Rao A. (2007) Cell Calcium 42, 145–156 [DOI] [PubMed] [Google Scholar]
- 18.Lewis R. S. (2007) Nature 446, 284–287 [DOI] [PubMed] [Google Scholar]
- 19.Putney J. W., Jr. (2007) J. Cell Sci. 120, 1959–1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Penna A., Demuro A., Yeromin A. V., Zhang S. L., Safrina O., Parker I., Cahalan M. D. (2008) Nature 456, 116–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ji W., Xu P., Li Z., Lu J., Liu L., Zhan Y., Chen Y., Hille B., Xu T., Chen L. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 13668–13673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuan J. P., Zeng W., Dorwart M. R., Choi Y. J., Worley P. F., Muallem S. (2009) Nat. Cell Biol. 11, 337–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park C. Y., Hoover P. J., Mullins F. M., Bachhawat P., Covington E. D., Raunser S., Walz T., Garcia K. C., Dolmetsch R. E., Lewis R. S. (2009) Cell 136, 876–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muik M., Fahrner M., Derler I., Schindl R., Bergsmann J., Frischauf I., Groschner K., Romanin C. (2009) J. Biol. Chem. 284, 8421–8426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoth M., Penner R. (1993) J. Physiol. 465, 359–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prakriya M., Lewis R. S. (2003) Cell Calcium 33, 311–321 [DOI] [PubMed] [Google Scholar]
- 27.Zweifach A., Lewis R. S. (1995) J. Gen. Physiol. 105, 209–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamashita M., Navarro-Borelly L., McNally B. A., Prakriya M. (2007) J. Gen. Physiol. 130, 525–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peinelt C., Lis A., Beck A., Fleig A., Penner R. (2008) J. Physiol. 586, 3061–3073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeHaven W. I., Smyth J. T., Boyles R. R., Bird G. S., Putney J. W., Jr. (2008) J. Biol. Chem. 283, 19265–19273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Derler I., Fahrner M., Muik M., Lackner B., Schindl R., Groschner K., Romanin C. (2009) J. Biol. Chem. 284, 24933–24938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee K. P., Yuan J. P., Zeng W., So I., Worley P. F., Muallem S. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 14687–14692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mullins F. M., Park C. Y., Dolmetsch R. E., Lewis R. S. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 15495–15500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mignen O., Thompson J. L., Shuttleworth T. J. (2008) J. Physiol. 586, 419–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi Y., Sawada J., Sui G., Affar el B., Whetstine J. R., Lan F., Ogawa H., Luke M. P., Nakatani Y., Shi Y. (2003) Nature 422, 735–738 [DOI] [PubMed] [Google Scholar]
- 36.Nakatani Y., Ogryzko V. (2003) Methods Enzymol. 370, 430–444 [DOI] [PubMed] [Google Scholar]
- 37.Gwack Y., Srikanth S., Oh-Hora M., Hogan P. G., Lamperti E. D., Yamashita M., Gelinas C., Neems D. S., Sasaki Y., Feske S., Prakriya M., Rajewsky K., Rao A. (2008) Mol. Cell. Biol. 28, 5209–5222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mercer J. C., Dehaven W. I., Smyth J. T., Wedel B., Boyles R. R., Bird G. S., Putney J. W., Jr. (2006) J. Biol. Chem. 281, 24979–24990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eaholtz G., Scheuer T., Catterall W. A. (1994) Neuron 12, 1041–1048 [DOI] [PubMed] [Google Scholar]
- 40.West J. W., Patton D. E., Scheuer T., Wang Y., Goldin A. L., Catterall W. A. (1992) Proc. Natl. Acad. Sci. U.S.A. 89, 10910–10914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goldin A. L. (2003) Curr. Opin. Neurobiol. 13, 284–290 [DOI] [PubMed] [Google Scholar]
- 42.Monod J., Wyman J., Changeux J. P. (1965) J. Mol. Biol. 12, 88–118 [DOI] [PubMed] [Google Scholar]
- 43.Hodgkin A. L., Huxley A. F. (1952) J. Physiol. 117, 500–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Craig T. J., Ashcroft F. M., Proks P. (2008) J. Gen. Physiol. 132, 131–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Babich O., Matveev V., Harris A. L., Shirokov R. (2007) J. Gen. Physiol. 129, 477–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Babich O., Reeves J., Shirokov R. (2007) J. Gen. Physiol. 129, 461–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.