Abstract
Purpose
Doxycycline, a broad spectrum antibiotic, has certain anti-angiogenic properties and can inhibit matrix metalloproteinases (MMPs/gelatinases). We investigated the effects of doxycycline on choroidal neovascularization (CNV), and regulation of MMP-2/-9 and pigment epithelium-derived factor (PEDF).
Methods
Doxycycline was orally administered to rats at 500, 50, 5, and 0.5 mg/kg/day, using non-treated animals as controls. Experimental CNV was induced with laser 7 days after doxycycline treatment started. At seven days post-induction, animals were euthanized, and eyes collected. RPE/choroid flat-mounts were labeled with isolectin IB4 to determine CNV lesion volumes using confocal microscopy and Volocity® software. MMP-2, MMP-9 and PEDF protein levels were determined by ELISA. MMP catalytic activity was determined in solution using fluorogenic gelatin and peptide substrates, by gelatin zymography in SDS-PAGE and by in situ DQ-gelatin zymography in RPE/choroid sections.
Results
CNV complex lesion volumes decreased with doxycycline in a dose-response relationship. A dosage of 500 mg/kg/day caused a 70% inhibition of CNV complex volume compared to control animals. Doxycycline elevated PEDF levels in plasma, and did not affect the plasma pro- and active MMP-2 and MMP-9 levels. However, the in vitro enzymatic activities of purified MMP-2 and MMP-9 declined significantly with doxycycline. MMP-2, MMP-9 and gelatinolytic activities in situ increased early in CNV lesion development. Doxycycline treatments and exogenous additions inhibited gelatinolytic activities in CNV lesions.
Conclusions
Doxycycline effectively hampered the progression of experimental CNV. The results suggest that orally administrated doxycycline can reach the choroid to attenuate proteolytic enzymes that remodel Bruch's membrane and promote the anti-angiogenic PEDF to inhibit neovascularization.
Doxycycline is a member of the tetracycline antibiotics group that is clinically useful due to its broad antimicrobial properties1, e.g., for treatment of acne, infectious diarrheas, rickettsial infections, anthrax, Lyme disease and certain spirochetal diseases2, 3. It has a long serum half-life (18 to 22 hours) and is highly lipid soluble3. It is well absorbed orally even in the presence of food, and has excellent tissue penetration, permitting good central nervous system penetration3. In addition, serum concentrations of doxycycline following oral and intravenous administration are comparable4. New uses of doxycycline are being reported. For example, doxycycline is also used in “Tet-on” and “Tet-off” tetracycline controlled transcriptional activation to regulate transgene expression in organisms and cell cultures. It can also modulate expression of certain endogenous genes5–8. More relevant to the present study, enzyme kinetic studies have indicated that doxycycline is the most potent matrix metalloproteinase (MMP) inhibitor among antimicrobial tetracyclines9, 10. Gelatinases or MMP-2 and MMP-9 belong to a family of zinc-dependent endopeptidases11. Doxycycline acts as a non-competitive inhibitor of MMPs by interacting with the zinc or calcium atoms within the structural center of these proteins required for stability12–14. These enzymes can degrade elastin, gelatin and collagen I, IV and V, and act in facilitating the breakdown of the basement membrane and extracellular matrix allowing endothelial cells to migrate during angiogenesis15.
The formation of choroidal neovascularization (CNV) complexes is the hallmark of exudative age-related macular degeneration (AMD)16, 17. It is also observed in other ocular conditions like ocular histoplasmosis syndrome, high myopia, angioid streaks, among others18. MMP-2 and MMP-9 localize to Bruch's membrane in areas of new vessel formation, with MMP-9 having the greatest expression at the margins of CNV membranes19–21. The importance of these enzymes in the progression of CNV is evident from genetic and chemical experiments with animal models. It has been demonstrated that ablation of the mmp-2, mmp-9 or double mmp-2/mmp-9 genes inhibits formation of CNV complexes induced by breakage of Bruch's membrane22. In addition, administration or overexpression of enzymatic inhibitors of MMP-2 and MMP-9 blocks experimental CNV formation23, 24. Interestingly, MMP-9 levels in serum of humans affected with CNV are significantly higher than in control healthy individuals, while MMP- 2 levels remain unchanged25.
MMP-2 and MMP-9 can target pigment epithelium-derived factor (PEDF), a potent anti-angiogenic factor, and abolish its biological actions26. There are several lines of evidence for inhibition of CNV directed by this protein. Several cell types express PEDF transcripts and the protein product is found in extracellular matrixes and in circulating plasma27–29. PEDF is secreted apicolaterally from the retinal pigment epithelium (RPE) and is found in the interphotoreceptor matrix, vitreous, aqueous humor, and cornea27, 30. Downregulation of PEDF correlates with ocular neovascular diseases; and increased expression of PEDF inhibits the formation of aberrant new vessels in animal models of CNV and pathological retinal neovascularization27, 31, 32. It is not known whether MMP-2 and MMP-9 can modulate the levels of PEDF in vivo.
The ability of doxycycline to inhibit MMPs has made it an important prospective anti-angiogenic agent in research, in particular in tumor angiogenesis. However, little is known about the effects of doxycycline on progression of ocular neovascularization. Several experimentally-induced CNV animal models have been developed33–35. We employed an imaging technique that allows three-dimensional reconstruction and precise measurement of laser-induced CNV lesions in rat choroid/RPE flatmounts, which provides excellent morphologic detail for accurate preclinical evaluation of antiangiogenic molecules36. In this study, we investigated the effects of doxycycline on a laser-induced CNV model in Brown Norway Rats using both confocal imaging and biochemical techniques.
Materials and Methods
Doxycycline administration
All animal experiments were conducted according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Eight-week old Brown-Norway rats were obtained from Charles River Laboratories (Raleigh, NC). Doxycycline was dissolved and diluted in drinking water at a concentration of 3.2 g/L for the highest dose (500 mg doxycycline per kg (rat weight) per day). Rats began receiving doxycycline-enhanced drinking water one week before laser injury. The average weight of the animals was 200 g and the doses used were 500, 50, 5 and 0.5 mg/kg/day. Doxycycline treatments were continued for 7 days after laser injury. There were no evident systemic effects in our rats after 2 weeks of oral doxycycline treatment (J.A., personal observations). Animals were euthanized and eyes were collected for RPE/choroid flatmount preparations.
Laser-induced CNV
Induction of experimental CNV, RPE/choroid flatmount preparation, visualization and quantification of lesions were performed following methods previously described36. CNV was induced by laser injury of Bruch's membrane. Lesion quantification was performed in confocal images of RPE/choroid flatmounts labeled with Alexa Fluor 568-Isolectin IB4 on endothelial cells to outline vessel formation. Quantification of CNV complex lesions was accomplished using Volocity® Classification Module. Note that Isolectin IB4 can also label microglia and perivascular cells, however these were excluded from quantification (see ref. 36). Given that removal of the neural retina for RPE/choroid flatmount preparation could potentially disrupt larger CNV complexes within the subretinal space, we examined the neural retinas from these samples. We found minimal evidence of residual elements of the CNV complex adhering to the outer retina, indicating that removal of the neural retina did not significantly disrupt the vessel complexes.
Blood sample collection and serum albumin depletion
Under deep CO2 anesthesia, the chest cavity was opened and a butterfly needle was inserted in the left ventricle, 2 ml of blood was collected using blood collection tubes with heparin (BD Vacutainer, BD Diagnostics; Oxford, UK), and centrifuged at 1000 x g for 15 min at room temperature. Serum from each animal was collected, divided into aliquots, which were stored at −80 °C. Before use, serum samples were albumin-depleted using the Qproteome Murine Albumin Depletion Kit following manufacturer's instructions (Qiagen; Valencia, CA, USA). This step removes the most abundant protein in serum and allows a more precise analysis of low-abundant proteins, such as PEDF, MMPs, etc.
Protein concentration assay
Protein concentration in soluble samples was measured using the BioRad Protein Assay Dye Reagent (BioRad, Inc.; Hercules, CA) following instructions by manufacturer. Protein concentrations were determined in triplicates with three different dilutions.
Enzyme-linked immunoabsorbent (ELISA) assay
MMP-2, MMP-9 and PEDF protein levels were quantified by a sandwich ELISA using the Quantikine total human/Rat/Mouse MMP-2, and MMP-9 kits (R&D Systems, Inc.; Minneapolis, MN, USA) and the ELISAquant PEDF kit (BioProducts MD, LLC; Middletown, MD), respectively. All of the assays were performed in triplicate per sample and according to instructions by the manufacturer. Standard curves of recombinant human MMP-2, recombinant human MMP-9 or recombinant human PEDF were used to determine the sample concentration for each protein and the average of values were normalized against the average of total protein concentration for each sample.
Gelatin zymography
Gelatin zymography was performed with the use of precast Zymogram Gelatin Gels (10% polyacrylamide containing 0.1% gelatin; Novex/Invitrogen Corp.; Carlsbad, CA) following instructions by manufacturer. The enzyme sample was denatured in SDS buffer (without reducing conditions) using Tris-Glycine SDS Sample Buffer™ (Novex/Invitrogen Corp.; Carlsbad, CA). After electrophoresis at 125 V for 90 minutes, the enzymes were renatured by incubating the gels in Zymogram Renaturing Buffer™ (Novex/Invitrogen Corp.; Carlsbad, CA) at room temperature for 30 minutes. The gels were equilibrated in Zymogram Developing Buffer™ (Novex/Invitrogen Corp.; Carlsbad, CA) for 16 hours at 37°C (to add the divalent metal cation required for enzymatic activity) followed by staining with Coomassie blue in 40% methanol and 7% acetic acid, and destaining in 40% methanol and 7% acetic acid. The protease bands appeared as clear bands against a dark background where the protease had digested the gelatin substrate. The gels were scanned as TIFF files. To assay the effect of doxycycline on gelatinolytic activities, doxycycline was added to the Zymogram Developing Buffer™ in the protocol described above.
Proteolysis solution assays
The enzymatic activities of MMP-2 and MMP-9 were measured against DQ-gelatin substrate (Molecular Probes/Invitrogen Corp.; Eugene, OR) in solution according to manufacturer's instructions. In this assay, proteolyzed DQ-gelatin emits fluorescence and product formation can be followed by fluorometry. A total of 10 ng of each purified recombinant human MMP-2 and MMP-9 (a kind gift from Dr. William G. Stettler-Stevenson) was added to Low Salt Collagenase Buffer (LSCB, 150 mM NaCl, 50 mM Tris, 5 mM CaCl2 and 1 μM ZnCl2, pH 7.5) containing 20 μg DQ-gelatin. Product formation was determined every 10 minutes for up to 60 minutes with excitation at 495 nm and emission at 530 nm using the Wallac 1420 VICTOR plate reader (Perkin Elmer, Inc.; Waltham, MA). Data were plotted and the rate per minute was calculated from the linear range of activity as a function of time using Excel (Microsoft® Office; Seattle, WA) and Prism (GraphPad Software, Inc.; La Jolla, CA).
The enzymatic activities were also measured using the fluorogenic peptide substrate Mca-PLGL-Dpa-AR-NH2 (R&D Systems, Inc.; Minneapolis, MN). In this assay, the proteolyzed substrate emits fluorescence and product formation can be followed by fluorometry. A total of 10 ng of each purified recombinant human MMP-2 and MMP-9 (a kind gift from Dr. William G. Stettler-Stevenson) was added to a final concentration of 10 μM fluorogenic substrate in a total of 100 μL reaction mixture. Product formation was determined every 10 minutes for up to 60 minutes with excitation at 320 nm and emission at 405 nm using the Wallac 1420 VICTOR plate reader (Perkin Elmer; Waltham, Massachusetts). Data were plotted using Excel (Microsoft Office).
Immunohistochemistry
Eyes were enucleated and fixed in 4% paraformaldehyde (EM Grade; Polysciences, Inc.; Warrington, PA) in phosphate-buffered saline (PBS; 9 g/L NaCl, 0.232 g/L KH2PO4, 0.703 g/L Na2HPO4 pH 7.3) for 1 hour and cryoprotected. Then, eyes were embedded in Tissue-Tek optimum cutting temperature (OCT) compound and cut into 10 μm sections. Immunohistochemical labeling was performed by incubating the sections sequentially in 5% blocking serum for 30 min at 23°C, then in anti-MMP-2 at 10 μg/ml (R&D Systems, Inc.; Minneapolis, MN) or in anti-MMP-9 at 2.5 μg/ml (Calbiochem; Madison, WI) antibodies for 16 hours at 4°C. Sections were washed and incubated in AlexaFluor 488 conjugated secondary antibodies (Invitrogen-Molecular Probes; Eugene, OR) at 6.6 μg/ml, 1 μg/ml of 4',6-diamidino-2-phenylindole (DAPI) (Invitrogen-Molecular Probes; Eugene, OR) and 10 μg/ml of isolectin IB4 conjugated with Alexa Fluor 568 (Invitrogen-Molecular Probes, Eugene, OR) for 30 minutes at 23°C. Confocal microscopy was used to visualize immunolabeling (SP2; Leica, Exton, PA). Files were imported into Photoshop and converted to PSD format for layout purposes.
In situ gelatin zymography
Gelatinolytic activity was demonstrated in unfixed cryosections (10 μm thick) using DQ-gelatin as a substrate (Invitrogen-Molecular Probes; Eugene, OR). Cryosections of rat eyes were vacuum dried for 30 min. DQ-gelatin at 0.1 mg/ml in 1% (w/v) low gelling temperature agarose (Sigma, Aldrich; St. Louis, MO) in PBS containing 1 μg/ml DAPI was prepared A total of 100 μl of the above mixture was layered on top of each section and covered with a cover slip. The agar was then gelled at 4°C for 2 min, and the sections were viewed under confocal microscopy (SP2; Leica, Exton, PA). Files containing images were imported into Photoshop and converted to TIFF format for layout purposes.
Statistical Analysis
Statistical analyses among groups were assessed using One-way analysis of variance and a Dunnett's multiple comparison test, and a p value ≤ 0.05 was considered statistically significant. Non-linear regression analyses (Dose-response - Inhibition) were performed with automatic outlier elimination and interpolation with 95% confidence interval using GraphPad Prism® 4 (La Jolla, CA).
RESULTS
Effect of Doxycycline Treatments on CNV Complex Lesion Volumes
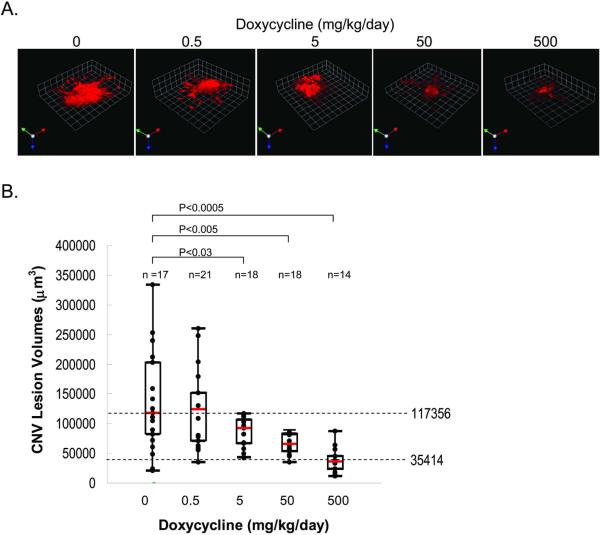
To investigate the effects of doxycycline on the formation of CNV complexes, four groups of rats were provided with different concentrations of doxycycline in their drinking water for 7 days. Then, experimental CNV was induced by laser injury to break Bruch's membranes. Oral administration of doxycycline continued for 7 more days before CNV lesion analysis. RPE/choroid flatmounts were fluorescently labeled to view CNV complexes under confocal microscopy. To measure the changes in lesion volume Volocity analysis software was used. Figure 1A shows CNV complexes labeled with Alexa568-conjugated-isolectin IB4, which was used to label newly formed vessels (red). Quantification of CNV complexes from non-treated and treated rats is summarized in figure 1B. Control rats (no doxycycline treatment) had a median CNV complex volume of 117,356 μm3. The median of CNV complex volumes decreased with doxycycline dosage in a dose-response relationship, i.e., a linear decrease with respect to logarithmic doses of doxycycline. Dose-response analyses demonstrated that the half maximally inhibitory concentration (IC50) was achieved with 23 mg/kg/day of doxycycline. Those treated with 500 mg/kg/day dosage of doxycycline showed median volumes of 35,414 μm3, indicating that the highest dosage of doxycycline caused a 70% inhibition in CNV complex volume (P< 0.0005) when compared to control rats. The results demonstrate that doxycycline inhibited experimentally-induced CNV in rats in a dose-dependent fashion.
Figure 1. Effects of doxycycline on experimental CNV.
Doxycycline-enhanced water was supplied to rats for 7 days. Then laser injury of Bruch's membrane was performed to induce CNV. At seven days post-laser, animals were euthanized and eyes were collected. RPE-choroid flatmounts were prepared and endothelial cells labeled with Alexa568-isolectin-IB4. Images were collected using confocal microscopy and imported into Volocity® for quantification of lesion volume.
(A) Representative images of isolectin-IB4 vessel staining (red) in laser lesions from animals treated with the indicated dose of doxycycline.
(B) Graph of the quantification of CNV lesion volumes of doxycycline-treated rats. Each point in this graph represents the average of three determinations of lesion volume, five animals per condition were analyzed and `n' represents the number of lesions. Depicted here is a box and whisker plot of quantification of CNV lesions from rats treated with differing doses of doxycycline (as indicated in the x-axis). The box shows the central 50% of the data. The median values are depicted with a bar in red. Horizontal dotted lines correspond to median values of no doxycycline and the lowest median in each sample set. Differences reaching statistical significance are noted by line segments between samples with the p value depicted on top (Oneway analysis of variance and a Dunnett's multiple comparison test).
Effects of doxycycline on plasma levels of MMP-2 and MMP-9
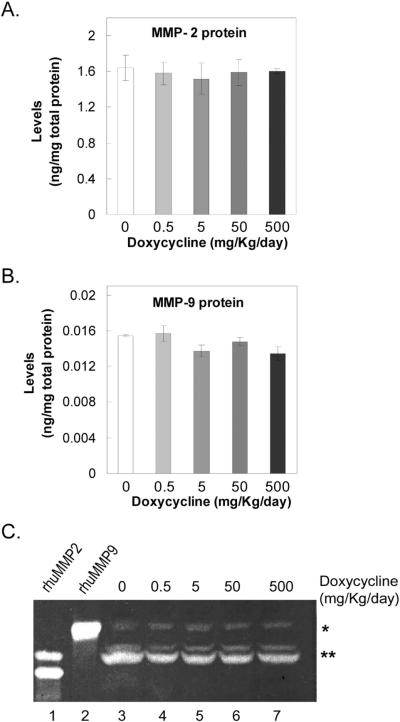
The plasma levels of MMP-2 and MMP-9 were determined in rats treated with increasing doses of doxycycline. Figures 2A and 2B show that the plasma levels of total MMP-2 and MMP-9 proteins (active and pro-enzymes) did not change significantly with doxycycline. Then the presence of MMP-2 and MMP-9 zymogens in plasma from these animals was investigated using gelatin zymography. Figure 2C shows that all the plasma samples contained zymogens with migration patterns similar to recombinant human MMP-2 and MMP-9. The intensity of the MMP-2/-9 comigrating bands relative to protein loaded per lane did not appear to change with doxycycline treatment. These results show that oral administration of doxycycline did not affect significantly the levels of active and pro-MMP-2 and -MMP-9 in plasma.
Figure 2. MMP-2 and MMP-9 in plasma of doxycycline-treated rats.
(A) and (B) graph of levels of total MMP-2 and MMP-9 (active and pro-enzymes) determined by ELISA in albumin-depleted plasma from doxycycline-treated rats. Each bar corresponds to the average of triplicate determinations per rat from four rats per condition. x-axis, doses of doxycycline; y-axis, MMP protein levels in nanograms per milligrams of total protein in the albumin-depleted plasma.
(C) Gelatin zymography of plasma from doxycycline-treated animals. Albumin-depleted plasma proteins (10 μl) were resolved by gelatin-zymography. The dose of doxycycline is indicated at the top. Purified recombinant human MMP-2 and MMP-9 (10 ng per lane; rhuMMP-2 and rhuMMP-9) are controls. The * and ** to the right show the migration position for co-migrating bands with rhuMMP-9 and rhuMMP-2, respectively. An extra zymogen is observed for rhuMMP-2 corresponding to the active enzyme of about 63-kDa. The corresponding amounts of total protein applied to each lane were: lane 1, 10 ng rhuMMP2; lane 2, 10 ng rhuMMP9; lane 3, 102 μg; lane 4, 87 μg; lane 5, 100 μg; lane 6, 76 μg; and lane 7, 86 μg.
Effects of doxycycline on the enzymatic activity of MMP-2 and MMP-9
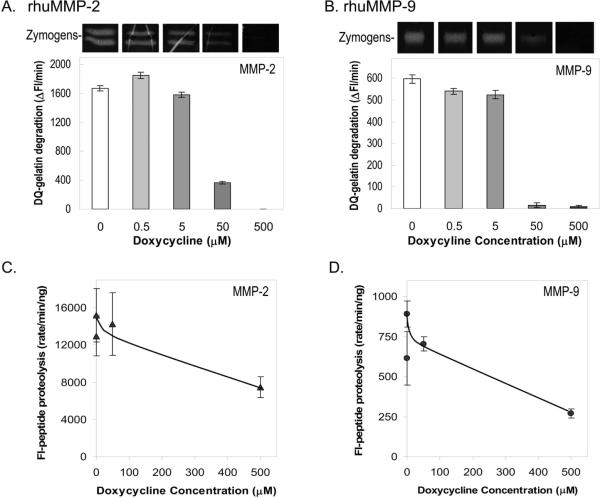
To investigate the effects of doxycycline on enzymatic MMP activities, proteolysis assays were performed with purified MMP-2 and MMP-9 enzymes in the presence of doxycycline. Using recombinant human MMP-2 and MMP-9 in gelatinolytic assays we found that doxycycline significantly inhibited the activity of these enzymes in a dose-dependent manner (Figs. 3A and 3B). In DQ-gelatin zymography, the activities of the MMP-2 and MMP-9 zymogens gradually decreased in zymography reactions with doxycycline concentrations increasing from 0 μM to 500 μM. The activities of both zymogens were abolished at the highest concentrations of doxycycline. In solution assays against DQ-gelatin substrate, the rate of product formation by recombinant MMP-2 and MMP-9 incubated in the presence of doxycycline decreased in a dose-dependent manner. Effective inhibition was observed with 50 μM and 500 μM doxycycline, with the highest concentration diminishing the MMP-2 and MMP-9 activities by 100% and 99%, respectively, when compared to controls without doxycycline. The results paralleled the decrease in intensities of the observed zymogen bands.
Figure 3. Effects of doxycycline on the enzymatic activities of MMP-2 and MMP-9.
(A) and (B) Gelatin zymography and DQ-gelatinase solution assays of rhuMMP2 and rhuMMP-9 in the presence of increasing concentrations of doxycycline, respectively. Enzymes (10 ng per lane) were applied to gelatin-containing polyacrylamide gels. Lanes containing enzymes were excised and each developed in zymography reactions with differing concentrations of doxycycline (0, 0.5, 5, 50, 500 μM). Photos of excised lanes are shown at the top of each plot. DQ-gelatinase solution assays were with 10 ng of enzyme and 20 μg DQ-gelatin substrate in 200 μl reactions. Fluoresceinated products were measured every 10 min for 60 min. Graphs depict the rate of DQ-gelatin degradation products measured as a change in fluorescence per min (Δ Fluorescence/min; y-axis) vs. concentration of doxycycline (μM; x-axis). Each bar is the average of four replicate assays.
(C) and (D) Proteolysis assays in solution were with 10 μM fluorogenic peptide substrate (Mca-PLGL-Dpa-AR- NH2) and 10 ng of rhMMP-2 or 30 ng of rhuMMP-9 in 100 μl reactions. Products were measured every 10 min for 60 min. The graphs depict the rate of proteolyzed products measured as a change in fluorescence per min per ng MMP (rate/min/ng; y-axis) vs. doxycycline concentration (μM; x-axis). Each bar is the average of four replicate assays.
Proteolysis solution assays against a fluorogenic peptide substrate for MMP-2 and MMP-9 were also performed. The rates of peptide proteolysis of both enzymes declined with doxycycline (Figs. 3C and 3D). Effective inhibition was observed with 500 μM doxycycline, which diminished the MMP-2 and MMP-9 activities by 51% and 70%, respectively, when compared to controls without doxycycline.
Altogether, these results show that doxycycline directly inhibited the enzymatic activities of the MMP-2 and MMP-9 proteins.
Effects of doxycycline on PEDF levels
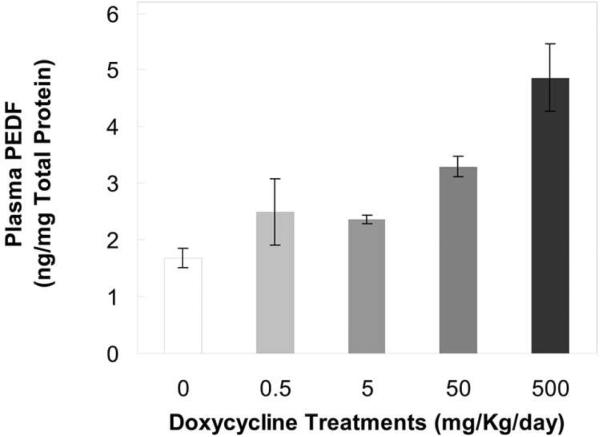
We investigated the effects of doxycycline-mediated MMP-2 and MMP-9 inhibition on plasma levels of PEDF, a previously reported substrate of these enzymes26. PEDF protein concentrations were determined in albumin-depleted plasma from animals fed with doxycycline-enhanced water for 14 days. Figure 4 shows that plasma levels of PEDF protein increased with doxycycline dosage. A 3-fold increase was observed with the highest dose of doxycycline (500 mg/kg/day) over that of controls without the inhibitor. The experiment was conducted twice and both presented similar results. These data suggest inhibition of MMP-mediated proteolysis of the anti-angiogenic factor by doxycycline.
Figure 4. PEDF levels in plasma of doxycycline-treated animals.
PEDF amounts were measured in triplicates of albumin-depleted plasma per animal with eight animals per condition. Total protein concentration in plasma was determined. PEDF levels are expressed in nanograms per milligram of total protein in serum (y-axis) and plotted as a function of doxycycline dosage (x-axis).
MMP-2 and MMP-9 at the site of CNV injury
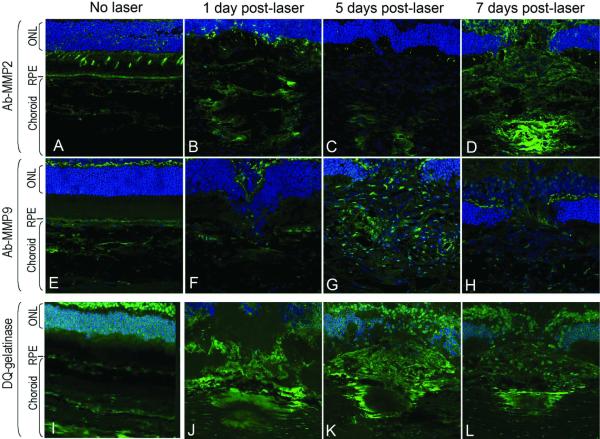
To evaluate the role of MMP-2 and MMP-9 in CNV formation, the localization patterns of these proteins were examined by immunohistochemistry. Figures 5A – 5H show the temporal and spatial distribution of MMP-2 and MMP-9 protein in cryosections of rat retina/RPE/choroid at the site of laser injury. Confocal images through CNV lesions at 1, 5 and 7 days after laser injury show MMP-2 and MMP-9 label localized in the choroid as early as 1 day post-laser injury. The signal was localized to the site of laser injury and increased with time in the area surrounding the CNV complex. Negative controls without antibody to MMP-2 or anti-MMP-9 did not show fluorescent signal (see Figs. 6A and 6G). These results demonstrate that MMP-2 and MMP-9 levels were elevated at the site of injury within 24 hours and remained above background.
Figure 5. MMP-2 and MMP-9 immunolocalization and gelatinase activity at the site of laser injury.
RPE/choroid/retina cryosections (10-μm thickness) from rats without laser injury, and 1, 5 and 7 days post-laser injury stained with anti-MMP-2 or anti-MMP-9 primary antibodies followed by highly absorbed-Alexa 488 secondary antibody (Panels A – D, Ab-MMP-2; Panels E – H, Ab-MMP-9) or subjected to in situ DQ-gelatin zymography (Panels I – L, DQ-Gelatinase). Green, immunostain or gelatinase activity; blue, DAPI. RPE, retinal pigment epithelium; ONL, outer nuclear layer.
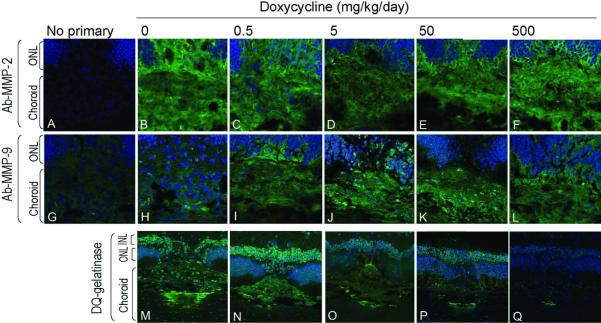
Figure 6. Local MMP-2 and MMP-9 protein and gelatinase activities in CNV lesions of doxycycline-treated rats.
RPE/choroid/retina cryosections (10-μm thickness) 7 days post-laser injury from rats treated with doxycycline (dosage as indicated at the top) stained with anti-MMP-2 (Panels A – F) or anti-MMP-9 (Panels G – L) primary antibodies (as indicated to the left) followed by highly absorbed-Alexa 488 secondary antibody (green). DAPI (blue) was used as a counterstain for nuclei. In situ DQ-gelatin zymography of cryosections of RPE/choroid/retina from doxycycline-treated rats at 7 days post-laser injury are shown in Panels M – Q (DQ-Gelatinase). Signal (green) corresponds to fluorescent product produced by the gelatinase activities. In blue, DAPI stain is shown. ONL, outer nuclear layer; INL, inner nuclear layer. Image in panel M is identical to that in figure 5L.
To determine the local activity of the MMP enzymes, in situ DQ-gelatin zymography was conducted. Gelatinase activity was investigated at 1, 5, and 7 days after laser injury in rats without doxycycline treatments. Figures 5I – 5L show cross sections through the laser lesion with gelatinase activity labeled in green and DAPI labeled nuclei in blue. The images demonstrated the presence of signal for gelatinolytic product formation in the choroid at the site of laser injury and as early as 1 day after laser injury. Gelatinase activity remained visible in lesions at 5 and 7 days post-laser. Very low signal was observed in choroid of eyes without injury. These observations demonstrate that gelatinases were active in CNV lesions in situ and that these gelatinolytic activities increased early in progression of CNV (24 hours post-laser). The observations are in agreement with previous reports showing in situ gelatinolytic activity in CNV lesions in mice after 5 days of laser injury23.
Effects of doxycycline at the site of CNV injury
Upon establishing that gelatinases were active in CNV lesions in situ, we sought to determine the effect of doxycycline on the MMP-2 and MMP-9 proteins and activities at the site of laser injury. First, we investigated the effects of oral doxycycline administration on MMP-2 and MMP-9 protein distribution in CNV lesions at 7 days post-laser. Immunohistochemistry was performed on consecutive cryosections of retina/RPE/choroid of controls and doxycycline-treated rats. Figures 6A – 6L show immunolocalization of MMP-2 and MMP-9 at the site of laser injury as a function of doxycycline dosage (0, 0.5, 5, 50, 500 mg/kg/day). The intensities of the fluorescent signals for both MMP-2 and MMP-9 localization (green) appeared relatively constant with increasing dosages of doxycycline. Negative controls without antibody to MMP-2 or anti-MMP-9 did not show fluorescent signal (Figs. 6A and 6G).
Then, we investigated the effects of oral administration of doxycycline on MMP activity at the site of laser injury in rats 7 days post-laser. In situ DQ-gelatin zymography was performed on cryosections of retina/RPE/choroid of rats treated with doxycycline. Figures 6M – 6Q (bottom row) shows a relative decrease in choroidal fluorescent signal with increasing doxycycline dosage, and the concomitant decrease in lesion size, as shown above (see Fig. 1). These observations imply a decrease in MMP/gelatinolytic activities in the choroidal lesions with increasing doses of doxycycline.
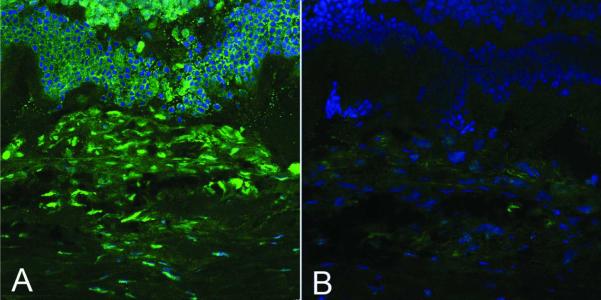
Finally, the direct effects of doxycycline on the gelatinolytic activities in situ were explored. In situ DQ-gelatin zymography was performed on two consecutive sections of retina/RPE/choroid. The sections contained CNV injury lesions at 7 days post-laser from animals without doxycycline administration. One zymography was performed in the presence of exogenous doxycycline and the other without the inhibitor as control. Figure 7 shows fluorescent signal in green for the control (no doxycycline; panel A), which markedly decreased with exogenous addition of doxycycline (500 μM) during DQ-gelatin incubation reactions (panel B). Similar doxycycline-mediated inhibitory effects were observed with sections from three different rats. The results clearly demonstrate doxycycline-mediated inhibition of gelatinase activity in situ, implying that the exogenous addition of doxycycline blocked the MMP activity at the site of laser injury.
Figure 7. Effects of exogenous doxycycline on gelatinase activities of CNV lesion 7 days post laser.
In situ DQ-gelatin zymography of CNV lesions was performed without and with exogenous addition of doxycycline. Doxycycline at 500 μM was added to one of two consecutive RPE/choroid/retina sections from a non-treated 7 days post-laser lesion sample during DQ-gelatin zymography reaction. The other consecutive section was not treated with doxycycline. Left, control received exogenous PBS without doxycycline. Right, received exogenous doxycycline in PBS. Green, signal of fluorescent product produced by the gelatinase activities. Blue, DAPI stain.
DISCUSSION
In this study we have shown that doxycycline effectively inhibits the progression of CNV, inhibits the activity of gelatinases MMP-2 and MMP-9, and increases the levels of PEDF, all of which participate positively or negatively in CNV development. These conclusions are derived from quantification of CNV lesion volumes, ELISA determinations, and immunolocalization and biochemical enzymatic reactions in vitro and in situ using an established CNV model. The results are in agreement with previous reports demonstrating the contribution of MMP-2 and MMP-9 in CNV progression: i) MMP-2 and MMP-9 distribution in newly forming CNV membranes19, 37; ii); severe inhibition of CNV in mmp2 and mmp9 deficient mice23 iii) increase of MMP-9 serum levels in patients with AMD and CNV25; and iv) inhibition of rat CNV by oral administration of the MMP-2,-9,-14 inhibitor, N-Biphenylsulfonyl-phenylaline hydroxamic acid38. They suggest that orally administrated doxycycline can reach the choroid to inhibit proteolytic enzymes that remodel Bruch's membrane, can attenuate the proteolysis of a major ocular anti-angiogenic protein PEDF and can restrain neovascularization.
Modulation of MMP-2 and MMP-9 gene expression is a plausible second level of regulation of proteins that affect extracellular remodeling at sites for new vessel formation and Bruch's membrane19. Doxycycline-mediated downregulation of mmp-2 and mmp-9 mRNA expression has been demonstrated in certain cells5–8. However, doxycycline does not appear to affect the overall MMP-2 and MMP-9 protein levels neither in plasma nor at the site of CNV injury, even when the volume of lesions diminishes with doxycycline dosage (Figs. 1 and 6). Given that doxycycline effectively prevents gelatinase activities in situ, we conclude that the dramatic effect on CNV complexes is mainly a result of direct inhibition of MMP activity at the site of injury by doxycycline. Interestingly, serum levels of patients with CNV have been shown to have a mean MMP-9 levels that are higher than control groups25, providing a relevant physiological target for doxycycline.
Several lines of evidence point to PEDF as an effective CNV inhibitor27. Heterologous overexpression of PEDF inhibits experimental CNV in many animal models. It has been demonstrated that intraperitoneal injections of PEDF protein have an effect in the retina, in particular in retinal neovascularization39, suggesting that the relative PEDF levels present in the eye may be proportional to PEDF plasma levels. Moreover, the choroid/RPE is permeable to PEDF40 allowing the plasma circulating protein to diffuse through the blood-retina barrier from the subconjunctiva through the choroid and RPE. The observed decrease in gelatinase activity in lesions may also favor an increase in PEDF levels, as MMP/gelatinolytic-mediated proteolysis directed to PEDF26 may lower. The fact that PEDF plasma levels increased significantly with doxycycline lead us to propose that, in addition to MMP inhibition, the effects of doxycycline on rat CNV progression may be mediated in part by the angiostatic properties of PEDF.
Increased expression of VEGF has been strongly implicated in the development of CNV in patients with AMD41. VEGF is the main target for CNV inhibition currently applied in the clinic16. In rats, VEGF increases at the site of injury with CNV development following laser-induced rupture of Bruch's membrane42. Heterologous VEGF overexpression in the rat RPE43, 44, and subretinal injections of VEGF protein in rabbits can induce CNV45. However, Oshima et al. (2004) described overexpression of VEGF in the RPE of rats induced heterologously with oral doxycycline at 2 mg/ml (0.625-fold the highest dose of the present study, i.e., 3.2 mg/ml) that could not develop CNV46. This result agrees with the conclusions from the present study and suggests that doxycycline also attenuates VEGF-mediated CNV induction, in addition to inducing transcription of heterologous genes under the control of a tet-inducible promoter. It is not clear whether doxycycline affects expression of VEGF mRNA and/or attenuates VEGF actions at posttranscriptional levels. However, we found that doxycycline added to the media of monkey RPE cells at concentrations of 0.5 μM and 5 μM did not change the levels of secreted VEGF protein, but decreased VEGF production when added to the media at concentrations of 50 μM without a change in VEGF/PEDF ratio (Supplementary Figs. S1 and S2, and Supplementary Methods). It has been reported that VEGF can modulate MMP-2 and MMP-9. Hoffman et al. (2006) have demonstrated that VEGF induces MMP-2 and MMP-9 mRNA expression in RPE cells47. In addition, Hollborn et al. (2007) have reported that exogenous VEGF not only upregulates MMP-9 gene expression in human RPE cells, but it induces MMP-9 protein secretion to the culturing media48. They also reported that exogenous MMP-9, but not MMP-2, can cause an upregulation of VEGF gene expression and VEGF protein secretion from RPE cells. It has been proposed that MMPs can regulate extracellular VEGF bioavailability through intramolecular processing by cleaving matrix-bound isoforms of VEGF and releasing soluble active fragments49, i.e., extracellular matrix proteins bound to VEGF are proteolyzed by MMPs to release soluble bioactive VEGF. In contrast, the main VEGF antagonist in the eye PEDF is proteolyzed by MMP-2 and MMP-9, which can abolish PEDF's anti-angiogenic properties26. These observations imply that doxycycline-mediated inhibition of MMP/gelatinases may impair the positive feedback between MMP-9 and VEGF in RPE, and increase PEDF bioavailability. In this way doxycycline can shift the balance between pro-and anti-angiogenic factors to inhibit the progression of CNV. It is worth noting that other angiogenic regulators, singularly or in combination with VEGF could be affected. Previous reports have described the anti-angiogenic effects in the reduction of CNV by antibiotic-related drugs (e.g., rapamycin50, polyaminosterols51) as well as the non-steroidal anti-inflammatory drug, nepafenac52, which may affect multiple or alternative angiogenic regulators.
In summary, our study shows that orally administered doxycycline, an antibiotic with MMP-inhibitory properties, is able to significantly inhibit CNV complex development in a dose-response fashion. The present results lead us to propose a possible mechanism of doxycycline's inhibition of CNV through biochemical modulation of MMPs that affects the VEGF/PEDF balance.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Dr. William G. Stettler-Stevenson for generously providing purified recombinant MMP-2 and MMP-9 enzyme proteins; Dr. Luigi Notari and Miss Sara Moghaddam-Taaheri for performing ELISA for PEDF protein determinations with mouse samples; and Drs. Debbie Carper and Connie Cox for interesting discussions and proofreading the manuscript.
This research was supported by the Intramural Research Program of the NIH-NEI.
Glossary
The abbreviations used are
- CNV
choroidal neovascularization
- RPE
retinal pigment epithelium
- MMP-2
matrix metalloproteinase type 2
- MMP-9
matrix metalloproteinase type 9
- PEDF
pigment epithelium-derived factor
Footnotes
Disclosure: S. Samtani, None; J. Amaral, None; M. Campos, None; R. N. Fariss, None; S. P. Becerra, None
REFERENCES
- 1.Pasquale TR, Tan JS. Update on antimicrobial agents: new indications of older agents. Expert opinion on pharmacotherapy. 2005;6:1681–1691. doi: 10.1517/14656566.6.10.1681. [DOI] [PubMed] [Google Scholar]
- 2.Ochsendorf F. Systemic antibiotic therapy of acne vulgaris. J Dtsch Dermatol Ges. 2006;4:828–841. doi: 10.1111/j.1610-0387.2006.06053.x. [DOI] [PubMed] [Google Scholar]
- 3.Cunha BA. New uses for older antibiotics: nitrofurantoin, amikacin, colistin, polymyxin B, doxycycline, and minocycline revisited. The Medical clinics of North America. 2006;90:1089–1107. doi: 10.1016/j.mcna.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 4.Cunha BA, Sibley CM, Ristuccia AM. Doxycycline. Therapeutic drug monitoring. 1982;4:115–135. doi: 10.1097/00007691-198206000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Kim HS, Luo L, Pflugfelder SC, Li DQ. Doxycycline inhibits TGF-beta1-induced MMP-9 via Smad and MAPK pathways in human corneal epithelial cells. Investigative ophthalmology & visual science. 2005;46:840–848. doi: 10.1167/iovs.04-0929. [DOI] [PubMed] [Google Scholar]
- 6.Hashimoto T, Matsumoto MM, Li JF, Lawton MT, Young WL. Suppression of MMP-9 by doxycycline in brain arteriovenous malformations. BMC neurology. 2005;5:1. doi: 10.1186/1471-2377-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Paiva CS, Corrales RM, Villarreal AL, et al. Corticosteroid and doxycycline suppress MMP-9 and inflammatory cytokine expression, MAPK activation in the corneal epithelium in experimental dry eye. Experimental eye research. 2006;83:526–535. doi: 10.1016/j.exer.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Uitto VJ, Firth JD, Nip L, Golub LM. Doxycycline and chemically modified tetracyclines inhibit gelatinase A (MMP-2) gene expression in human skin keratinocytes. Annals of the New York Academy of Sciences. 1994;732:140–151. doi: 10.1111/j.1749-6632.1994.tb24731.x. [DOI] [PubMed] [Google Scholar]
- 9.Burns FR, Stack MS, Gray RD, Paterson CA. Inhibition of purified collagenase from alkali-burned rabbit corneas. Investigative ophthalmology & visual science. 1989;30:1569–1575. [PubMed] [Google Scholar]
- 10.Golub LM, Sorsa T, Lee HM, et al. Doxycycline inhibits neutrophil (PMN)-type matrix metalloproteinases in human adult periodontitis gingiva. Journal of clinical periodontology. 1995;22:100–109. doi: 10.1111/j.1600-051x.1995.tb00120.x. [DOI] [PubMed] [Google Scholar]
- 11.Haas TL, Davis SJ, Madri JA. Three-dimensional type I collagen lattices induce coordinate expression of matrix metalloproteinases MT1-MMP and MMP-2 in microvascular endothelial cells. The Journal of biological chemistry. 1998;273:3604–3610. doi: 10.1074/jbc.273.6.3604. [DOI] [PubMed] [Google Scholar]
- 12.Golub LM, Lee HM, Ryan ME, Giannobile WV, Payne J, Sorsa T. Tetracyclines inhibit connective tissue breakdown by multiple non-antimicrobial mechanisms. Advances in dental research. 1998;12:12–26. doi: 10.1177/08959374980120010501. [DOI] [PubMed] [Google Scholar]
- 13.Sorsa T, Ding Y, Salo T, et al. Effects of tetracyclines on neutrophil, gingival, and salivary collagenases. A functional and western-blot assessment with special reference to their cellular sources in periodontal diseases. Annals of the New York Academy of Sciences. 1994;732:112–131. doi: 10.1111/j.1749-6632.1994.tb24729.x. [DOI] [PubMed] [Google Scholar]
- 14.Garcia RA, Pantazatos DP, Gessner CR, Go KV, Woods VL, Jr., Villarreal FJ. Molecular interactions between matrilysin and the matrix metalloproteinase inhibitor doxycycline investigated by deuterium exchange mass spectrometry. Molecular pharmacology. 2005;67:1128–1136. doi: 10.1124/mol.104.006346. [DOI] [PubMed] [Google Scholar]
- 15.Raffetto JD, Khalil RA. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochemical pharmacology. 2008;75:346–359. doi: 10.1016/j.bcp.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campochiaro PA, Soloway P, Ryan SJ, Miller JW. The pathogenesis of choroidal neovascularization in patients with age-related macular degeneration. Molecular vision. 1999;5:34. [PubMed] [Google Scholar]
- 17.Chappelow AV, Kaiser PK. Neovascular age-related macular degeneration: potential therapies. Drugs. 2008;68:1029–1036. doi: 10.2165/00003495-200868080-00002. [DOI] [PubMed] [Google Scholar]
- 18.Sickenberg M, Schmidt-Erfurth U, Miller JW, et al. A preliminary study of photodynamic therapy using verteporfin for choroidal neovascularization in pathologic myopia, ocular histoplasmosis syndrome, angioid streaks, and idiopathic causes. Archives of ophthalmology. 2000;118:327–336. doi: 10.1001/archopht.118.3.327. [DOI] [PubMed] [Google Scholar]
- 19.Steen B, Sejersen S, Berglin L, Seregard S, Kvanta A. Matrix metalloproteinases and metalloproteinase inhibitors in choroidal neovascular membranes. Investigative ophthalmology & visual science. 1998;39:2194–2200. [PubMed] [Google Scholar]
- 20.Kvanta A, Shen WY, Sarman S, Seregard S, Steen B, Rakoczy E. Matrix metalloproteinase (MMP) expression in experimental choroidal neovascularization. Current eye research. 2000;21:684–690. [PubMed] [Google Scholar]
- 21.Kvanta A, Sarman S, Fagerholm P, Seregard S, Steen B. Expression of matrix metalloproteinase-2 (MMP-2) and vascular endothelial growth factor (VEGF) in inflammation-associated corneal neovascularization. Experimental eye research. 2000;70:419–428. doi: 10.1006/exer.1999.0790. [DOI] [PubMed] [Google Scholar]
- 22.Lambert V, Munaut C, Jost M, et al. Matrix metalloproteinase-9 contributes to choroidal neovascularization. The American journal of pathology. 2002;161:1247–1253. doi: 10.1016/S0002-9440(10)64401-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lambert V, Wielockx B, Munaut C, et al. MMP-2 and MMP-9 synergize in promoting choroidal neovascularization. Faseb J. 2003;17:2290–2292. doi: 10.1096/fj.03-0113fje. [DOI] [PubMed] [Google Scholar]
- 24.Bergers G, Brekken R, McMahon G, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nature cell biology. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chau KY, Sivaprasad S, Patel N, Donaldson TA, Luthert PJ, Chong NV. Plasma levels of matrix metalloproteinase-2 and -9 (MMP-2 and MMP-9) in age-related macular degeneration. Eye (London, England) 2008;22:855–859. [PubMed] [Google Scholar]
- 26.Notari L, Miller A, Martinez A, et al. Pigment epithelium-derived factor is a substrate for matrix metalloproteinase type 2 and type 9: implications for downregulation in hypoxia. Investigative ophthalmology & visual science. 2005;46:2736–2747. doi: 10.1167/iovs.04-1489. [DOI] [PubMed] [Google Scholar]
- 27.Amaral J, Becerra S. Pigment epithelium-derived factor and angiogenesis: Therapeutic implications. Springer; Dordrecht, The Nederlands: 2008. pp. 311–337. [Google Scholar]
- 28.Petersen SV, Valnickova Z, Enghild JJ. Pigment-epithelium-derived factor (PEDF) occurs at a physiologically relevant concentration in human blood: purification and characterization. The Biochemical journal. 2003;374:199–206. doi: 10.1042/BJ20030313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamagishi S, Adachi H, Abe A, et al. Elevated serum levels of pigment epithelium-derived factor in the metabolic syndrome. The Journal of clinical endocrinology and metabolism. 2006;91:2447–2450. doi: 10.1210/jc.2005-2654. [DOI] [PubMed] [Google Scholar]
- 30.Bouck N. PEDF: anti-angiogenic guardian of ocular function. Trends in molecular medicine. 2002;8:330–334. doi: 10.1016/s1471-4914(02)02362-6. [DOI] [PubMed] [Google Scholar]
- 31.Becerra SP. Focus on Molecules: Pigment epithelium-derived factor (PEDF) Experimental eye research. 2006;82:739–740. doi: 10.1016/j.exer.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 32.Gehlbach P, Demetriades AM, Yamamoto S, et al. Periocular injection of an adenoviral vector encoding pigment epithelium-derived factor inhibits choroidal neovascularization. Gene therapy. 2003;10:637–646. doi: 10.1038/sj.gt.3301931. [DOI] [PubMed] [Google Scholar]
- 33.Clark M, Fowler J, Penn J. Animal models of choroidal neovascularization. Springer; Dordretch, The Nederlands: 2008. pp. 41–56. [Google Scholar]
- 34.Dobi ET, Puliafito CA, Destro M. A new model of experimental choroidal neovascularization in the rat. Archives of ophthalmology. 1989;107:264–269. doi: 10.1001/archopht.1989.01070010270035. [DOI] [PubMed] [Google Scholar]
- 35.Frank RN, Das A, Weber ML. A model of subretinal neovascularization in the pigmented rat. Current eye research. 1989;8:239–247. doi: 10.3109/02713688908997565. [DOI] [PubMed] [Google Scholar]
- 36.Campos M, Amaral J, Becerra SP, Fariss RN. A novel imaging technique for experimental choroidal neovascularization. Investigative ophthalmology & visual science. 2006;47:5163–5170. doi: 10.1167/iovs.06-0156. [DOI] [PubMed] [Google Scholar]
- 37.Berglin L, Sarman S, van der Ploeg I, et al. Reduced choroidal neovascular membrane formation in matrix metalloproteinase-2-deficient mice. Investigative ophthalmology & visual science. 2003;44:403–408. doi: 10.1167/iovs.02-0180. [DOI] [PubMed] [Google Scholar]
- 38.Kohri T, Moriwaki M, Nakajima M, Tabuchi H, Shiraki K. Reduction of experimental laser-induced choroidal neovascularization by orally administered BPHA, a selective metalloproteinase inhibitor. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2003;241:943–952. doi: 10.1007/s00417-003-0761-2. [DOI] [PubMed] [Google Scholar]
- 39.Stellmach V, Crawford SE, Zhou W, Bouck N. Prevention of ischemia-induced retinopathy by the natural ocular antiangiogenic agent pigment epithelium-derived factor. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:2593–2597. doi: 10.1073/pnas.031252398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amaral J, Fariss RN, Campos MM, et al. Transscleral-RPE permeability of PEDF and ovalbumin proteins: implications for subconjunctival protein delivery. Investigative ophthalmology & visual science. 2005;46:4383–4392. doi: 10.1167/iovs.05-0492. [DOI] [PubMed] [Google Scholar]
- 41.Andreoli CM, Miller JW. Anti-vascular endothelial growth factor therapy for ocular neovascular disease. Current opinion in ophthalmology. 2007;18:502–508. doi: 10.1097/ICU.0b013e3282f0ca54. [DOI] [PubMed] [Google Scholar]
- 42.Yi X, Ogata N, Komada M, et al. Vascular endothelial growth factor expression in choroidal neovascularization in rats. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 1997;235:313–319. doi: 10.1007/BF01739641. [DOI] [PubMed] [Google Scholar]
- 43.Baffi J, Byrnes G, Chan CC, Csaky KG. Choroidal neovascularization in the rat induced by adenovirus mediated expression of vascular endothelial growth factor. Investigative ophthalmology & visual science. 2000;41:3582–3589. [PubMed] [Google Scholar]
- 44.Spilsbury K, Garrett KL, Shen WY, Constable IJ, Rakoczy PE. Overexpression of vascular endothelial growth factor (VEGF) in the retinal pigment epithelium leads to the development of choroidal neovascularization. The American journal of pathology. 2000;157:135–144. doi: 10.1016/S0002-9440(10)64525-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qiu G, Stewart JM, Sadda S, et al. A new model of experimental subretinal neovascularization in the rabbit. Experimental eye research. 2006;83:141–152. doi: 10.1016/j.exer.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 46.Oshima Y, Oshima S, Nambu H, et al. Increased expression of VEGF in retinal pigmented epithelial cells is not sufficient to cause choroidal neovascularization. Journal of cellular physiology. 2004;201:393–400. doi: 10.1002/jcp.20110. [DOI] [PubMed] [Google Scholar]
- 47.Hoffmann S, He S, Ehren M, Ryan SJ, Wiedemann P, Hinton DR. MMP-2 and MMP-9 secretion by rpe is stimulated by angiogenic molecules found in choroidal neovascular membranes. Retina (Philadelphia, Pa. 2006;26:454–461. doi: 10.1097/01.iae.0000238549.74626.33. [DOI] [PubMed] [Google Scholar]
- 48.Hollborn M, Stathopoulos C, Steffen A, Wiedemann P, Kohen L, Bringmann A. Positive feedback regulation between MMP-9 and VEGF in human RPE cells. Investigative ophthalmology & visual science. 2007;48:4360–4367. doi: 10.1167/iovs.06-1234. [DOI] [PubMed] [Google Scholar]
- 49.Lee S, Jilani SM, Nikolova GV, Carpizo D, Iruela-Arispe ML. Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors. The Journal of cell biology. 2005;169:681–691. doi: 10.1083/jcb.200409115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dejneka NS, Kuroki AM, Fosnot J, Tang W, Tolentino MJ, Bennett J. Systemic rapamycin inhibits retinal and choroidal neovascularization in mice. Molecular vision. 2004;10:964–972. [PubMed] [Google Scholar]
- 51.Ciulla TA, Criswell MH, Danis RP, Williams JI, McLane MP, Holroyd KJ. Squalamine lactate reduces choroidal neovascularization in a laser-injury model in the rat. Retina (Philadelphia, Pa. 2003;23:808–814. doi: 10.1097/00006982-200312000-00011. [DOI] [PubMed] [Google Scholar]
- 52.Takahashi K, Saishin Y, Saishin Y, et al. Topical nepafenac inhibits ocular neovascularization. Investigative ophthalmology & visual science. 2003;44:409–415. doi: 10.1167/iovs.02-0346. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.