Abstract
Background
Prescription of resistance training (RT) exercises is an essential aspect of management for knee osteoarthritis (OA). However, whether patients with knee OA who are randomly assigned to receive RT simply substitute RT for other modes of physical activity remains unclear.
Objective
The aim of this study was to determine the effect of a structured RT intervention on overall levels of moderate- and vigorous-intensity physical activity (MVPA) in patients with early-onset knee OA. The study compared patients with early-onset OA who participated in an RT program, those who participated in a self-management (SM) program, and those who participated in both RT and SM. Because participants randomly assigned to receive the RT intervention may simply switch activity modes, resulting in little net effect, we assessed total MVPA in addition to tracking changes in strength (force-generating capacity).
Design and Intervention
This study was a randomized controlled trial comparing the effectiveness of SM alone, RT alone, and combined RT+SM on MVPA in patients with early OA of the knee.
Setting
The study was conducted on a university campus, with patient recruitment from the local community.
Participants
The participants in this study were 171 patients (74% women, 26% men) with knee OA. They had a mean age of 55.1 (SD=7.1) years, a mean body mass index of 27.6 (SD=4.2) kg/m2, and radiographic status of grade II OA (and no higher) in at least one knee, as defined by the Kellgren and Lawrence classification. They wore an accelerometer while awake (X̄=14.2 [SD=2.2] hours) for 5 to 7 contiguous days (X̄=6.8 [SD=0.5] days) at baseline and at 3 and 9 months of intervention.
Results
The participants engaged in MVPA a mean of 26.2 (SD=19.3) minutes per day at baseline. Both groups significantly increased their MVPA from baseline to 3 months (RT group by 18% [effect size (d)=0.26]; SM group by 22% [effect size (d)=0.25]), but only the RT group sustained those changes at 9 months (RT group maintained a 10% increase [effect size (d)=0.15]; SM group maintained a 2% increase [effect size (d)=0.03]). A significant group × time interaction for MVPA indicated that the RT group maintained higher MVPA levels than the SM group.
Limitations.
Lack of direct measures of energy expenditure and physical function was a limitation of the study.
Conclusions
Patients with early-onset OA of the knee can engage in an RT program without sacrificing their overall MVPA levels. These results support the value of RT for management of knee OA.
The primary goals of knee osteoarthritis (OA) treatment are to reduce pain and improve function and quality of life. Declining enthusiasm for cyclo-oxygenase 2 inhibitors for knee OA pain relief and unsuccessful clinical trials of disease-modifying OA drugs have contributed to increased interest in nonpharmacologic treatments for OA.1 Resistance training (RT) exercise programs and educational self-management (SM) programs are 2 mainstays of nonpharmacologic treatment.
Physical activity (PA) refers to any bodily movement that results in energy expenditure. Physical activity is an essential recommendation included in all guidelines for management of knee OA.2–4 Moreover, PA is recommended by the US Centers for Disease Control and Prevention (CDC) and the American College of Sports Medicine (ACSM) for general health to reduce risks of obesity-linked health problems, including diabetes and cardiovascular disease,5,6 which often coexist with knee OA. Work group recommendations from the 2002 Exercise and Physical Activity Conference (EPAC)7 advise patients with knee OA to accumulate 30 minutes of at least moderate-intensity (≥3 metabolic equivalents [METs]*) PA on at least 3 days of the week. The expert EPAC panel concluded that promotion of PA in adults with arthritis should emphasize aerobic moderate- and vigorous-intensity physical activity (MVPA, ≥3 METs) and muscle strengthening resistance exercise. In a more recent statement, an expert consensus panel provided evidence-based recommendations for practical delivery of exercise therapy for patients with knee OA, stating that “both general (aerobic fitness training) and local (strengthening) exercises are essential, core aspects of management for every patient with knee OA.”8(p69)
In recent years, it has become clear that RT can have a positive effect on resting energy expenditure (REE), total free-living energy expenditure (TEE), and activity-related energy expenditure (AEE). Withers et al9 compared REE, TEE, and AEE of chronically active women who engaged in RT and chronically inactive women, aged 49 to 70 years. They reported that the chronically active women had increased REE, TEE, and AEE compared with the chronically inactive women. Hunter et al10 addressed this concern in elderly men and women who were healthy, aged 61 to 77 years. They found increases in REE, TEE, and AEE in response to 26 weeks of RT and showed that the TEE increase remained significant even after adjustment for the energy expenditure of the RT. These findings suggest that RT has value in increasing energy expenditure and lipid oxidation rates in older adults.
A potential concern when structured RT programs are prescribed is that participants may simply switch activity modes, resulting in a decrease in aerobic MVPA. For example, Goran and Poehlman11 and Meijer et al12 both observed a compensatory decrease in free-living PA levels of older adults after engaging in RT programs. However, we found no studies that have addressed this concern in a patient population such as patients with early OA of the knee. Although controlling mode is desirable for study purposes, in clinical and public health settings, replacement of one mode with another may defeat efforts to increase overall MVPA. In contexts such as the present study, participants randomly assigned to receive RT might engage in less overall MVPA, substituting RT for other modes of MVPA. Alternatively, if participants randomly assigned to receive RT increased or at least maintained their MVPA levels, they would benefit from both RT and aerobic MVPA. However, if RT inhibited participants from achieving recommended MVPA levels, the net result could interfere with exercise interventions aimed at improving cardiovascular function, insulin action, energy metabolism, and psychological health in patients with OA of the knee.13,14 Therefore, in the present analysis, we aimed to determine the effect of a structured RT intervention on overall daily levels of activity by using accelerometry to measure MVPA in individuals with early-onset knee OA who participated in an RT program and in those who participated in an SM program. We hypothesized that in addition to improving muscle strength (force-generating capacity), the RT groups would maintain similar levels of MVPA compared with the SM group.
Method
Design Overview
The data used for this analysis were obtained from the Multidimensional Intervention for Early Osteoarthritis of the Knee Study (the Knee Study), a randomized clinical trial comparing the effectiveness of SM alone, RT alone, and combined RT+SM on relevant knee OA outcomes over 24 months. After preliminary analyses of the 3 groups, the RT groups (RT alone and RT+SM) were collapsed into a single group and compared with the SM only group to test the question of whether participants randomly assigned to the RT group would substitute RT for MVPA. The analysis presented here was based on data from the first phase (baseline to 9 months) of the interventions described. Outcome measurements were obtained at baseline and at 3 and 9 months of intervention.
Participants
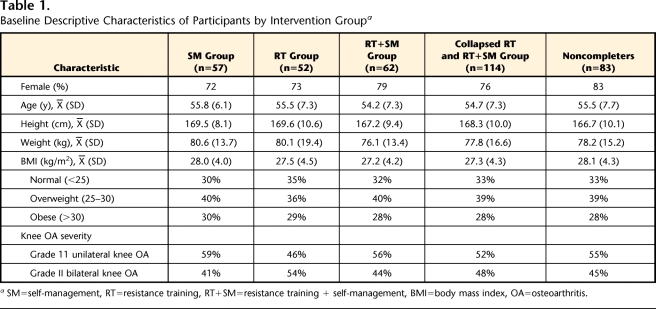
Participants were recruited from the Tucson, Arizona, general community and surrounding areas using mass mailings, media advertisements, periodic media coverage, and requests to local physicians for patient referrals. A total of 1,726 people were assessed for eligibility. Eligibility criteria were: age between 35 and 68 years to ensure an early-onset knee OA sample; pain on 4 or more days of the week in one or both knees for at least 4 months during the previous year; less than 5 years' symptom duration15,16; radiographic status of grade II OA (and no higher) in at least one knee, as defined by the Kellgren and Lawrence classification17; and disability due to knee OA, as assessed with the Western Ontario and McMaster Universities Osteoarthritis (WOMAC) Index.18 Participants gave written informed consent and self-reported demographic characteristics (Tab. 1). All participants enrolled in the study met American College of Rheumatology classification criteria for early OA of the knee.19
Table 1.
Baseline Descriptive Characteristics of Participants by Intervention Groupa
SM=self-management, RT=resistance training, RT+SM=resistance training + self-management, BMI=body mass index, OA=osteoarthritis.
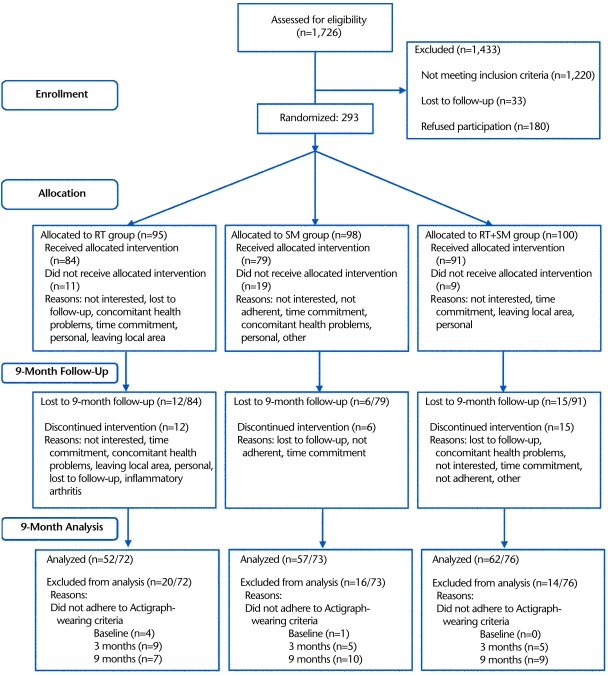
A CONSORT flowchart describing the progress of participants through the 9-month intervention is presented in Figure 1. Of the 1,726 people who were assessed for eligibility, 293 eligible participants were stratified by sex and randomly assigned via a random number table to 1 of the 3 treatment groups (SM, RT, or RT+SM). Concealed allocation was accomplished using envelopes to conceal computer-generated values. Manifest transparency of the treatments rendered blinding unfeasible. Essentially, given our outcome measures, interrater agreement bias was deemed a smaller threat to internal validity than ensuring treatment fidelity, which precluded effective blinding. Of the 293 eligible participants, 39 did not receive any of the allocated intervention and 33 discontinued the intervention prior to 9 months (Fig. 1).
Figure 1.
Flowchart describing the progress of participants through the Knee Study trial. RT=resistance training, SM=self-management, RT+SM=resistance training + self-management.
Interventions
Resistance training.
The overall goal of the RT intervention was to encourage participants to maintain a long-term exercise program to increase muscle strength, decrease impairment, maintain and restore function, and protect joint structures from further damage. The RT intervention paralleled programs developed by the ACSM5 and the National Strength Training and Conditioning Association20 and was designed to test expert panel recommendations.8 Sessions targeted improvement in each of 4 core areas: (1) stretching and balance, (2) range of motion (ROM) and flexibility, (3) isotonic muscle strengthening, and (4) aerobics. Participants met with certified physical trainers 3 times per week for 9 months, with a minimum of 1 day of rest between training sessions, to complete a 1-hour exercise regimen that emphasized RT. Supervised, small-group sessions were held to improve adherence. Each session consisted of: (1) 10-minute warm-up on either a bicycle ergometer or treadmill at 50% maximum heart rate, (2) 5 to 10 minutes of stretching and balance exercises, (3) 10 minutes of ROM exercises, (4) 30 minutes of RT exercises, and (5) 5 minutes of cool-down. Specific RT exercises included leg press, leg curl, hip abduction and adduction, straight leg lift, incline dumbbell press, seated row, and calf raise. The exercises were chosen primarily to directly strengthen the muscles supporting the knee, but also to improve the strength of muscles most involved in activities of daily living.
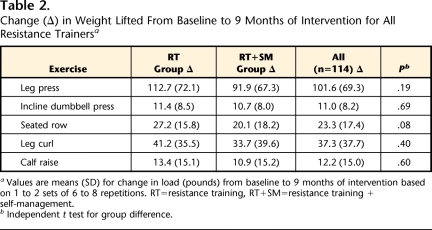
The strength protocol progressed through 2 phases: (1) resistance from body weight and Thera-Band exercise bands† and (2) free weights and machine weights. Participants started with one set per exercise, 6 to 8 repetitions per set, at an intensity of 50% of each individual's 3-repetition maximum (3-RM). During an orientation session, participants were familiarized with the equipment and instructed by certified trainers on proper lifting techniques for all exercises. All participants began training at a comfortable weight with proper form for each exercise based on standardized protocols developed by the ACSM.5 The program progressed from 1 to 2 sets, along with increases in load when participants were able to complete all repetitions with proper body position and joint alignment for 3 consecutive sessions.21 They then progressed to loads between 60% and 75% of their 3-RM and continued to increase loads to maintain vigorous intensity. The ROM exercises were increased for each participant when the exercises could be completed with a Borg scale score of difficulty of ≤6.22 Participants completed training logs during all sessions and reported sets, repetitions, and loads for each exercise. Certified physical trainers supervised all RT sessions, monitored progression, and tested participants following standard protocols.5 Throughout the intervention, trainers emphasized good form and encouraged participants to report soreness or pain during and after RT sessions. Changes in load from baseline to 9 months for the RT groups (RT and RT+SM) are shown in Table 2.
Table 2.
Change (Δ) in Weight Lifted From Baseline to 9 Months of Intervention for All Resistance Trainersa
Values are means (SD) for change in load (pounds) from baseline to 9 months of intervention based on 1 to 2 sets of 6 to 8 repetitions. RT=resistance training, RT+SM=resistance training + self-management.
b Independent t test for group difference.
Self-management.
The SM intervention was designed to target coping skills, promoting the use of more adaptive strategies and fewer avoidance or passive strategies based on existing self-help programs.23 The intervention also targeted self-efficacy through a variety of educational and behavioral techniques. Self-efficacy skills focused on increasing perceptions of control for physical functioning, pain management, and other ancillary arthritis symptoms. The 9-month program began with 12 weekly, 90-minute classroom sessions in which participants completed SM education modules addressing an overview of OA, general exercise principles and PA recommendations, stress management, foot care, pain management, analgesic and anti-inflammatory medications, nutrition for health, coping mechanisms, communication with health care providers, and healthy lifestyle practices. As part the of the exercise module, participants were introduced to the benefits of MVPA and RT for patients with OA of the knee and were given instructions for establishing a regular PA program. They also were provided with PA recommendations implemented by the CDC6 and the ACSM5 and work group PA recommendations from the 2002 EPAC for people with arthritis,7 but no further exercise instruction was given. Classroom sessions were followed by 24 weeks of a structured telephone intervention program that reinforced SM skills.
Combined treatment.
The combined treatment group (RT+SM) engaged in both the RT and SM interventions, with slight alterations to ensure equivalence of contact time across treatment groups. Specifically, participants in the RT+SM group were contacted by staff less during the 24 weeks of the telephone intervention program that followed classroom sessions.
Anthropometry.
Anthropometric measurements were obtained at baseline following standard protocols outlined in the Anthropometric Standardization Reference Manual.24 Total body mass was measured to the nearest 0.1 kg using a calibrated scale (Seca model 770),‡ and height was measured to the nearest 0.1 cm using a portable stadiometer (Shorr Height Measuring Board)§ after full inspiration.
Pain.
Knee pain was assessed using the WOMAC Index, which has been validated in patients with OA of the knee.18 The WOMAC pain subscale comprises 5 items eliciting patient ratings on visual analog scales (0–100) of pain severity during walking, stair use, lying in bed at night, sitting, and standing. The pain subscale has a maximum summed score of 500, with higher scores reflecting more pain.
Physical activity.
Baseline PA levels of the Knee Study participants have been published previously.25 Physical activity was measured using the MTI Actigraph accelerometer (model 7164).‖ The uniaxial Actigraph accelerometer measures vertical-plane accelerations and decelerations and records them as “counts” over a specific time interval (epoch), which provides information regarding the intensity of PA associated with movement.26 The accelerometer assesses accelerations ranging from 0.05 to 2.0g, with a frequency ranging from 0.25 to 2.5 Hz.27 These specifications allow for detection of normal body motion, while filtering out high-frequency vibration movements. Actigraph reliability and validity have been reviewed in detail.28 Counts have been shown to be highly correlated (r=.77–.88) with steady-state oxygen consumption during ambulatory activities26,29 and have been shown to be dependent upon movement frequency in a mechanical setup.30
For each assessment, the accelerometer was initialized and downloaded according to the manufacturer's specifications30 and set to record data in 60-second epochs. Participants were instructed to wear the accelerometer for 7 contiguous days during all waking hours, except while in water. A previous study31 has shown that when the Actigraph accelerometer is worn for 7 consecutive days, PA can be assessed with 90% reliability. The accelerometer was firmly secured to a belt worn around the waist and positioned on the right hip because this site permits measurement of whole-body movement, does not interfere with daily activities, and is the most frequently used site in epidemiological studies.25 The following measurements were obtained: days worn; registered wear time in hours per day; and average minutes per day spent in moderate-intensity physical activity (MPA, 3–6 METs), vigorous-intensity physical activity (VPA, >6 METs), and MVPA (≥3 METs). A number of studies26,29,32–34 have used criterion methods such as indirect calorimetry and heart rate monitoring to demonstrate the reliability and validity of the Actigraph accelerometer. As described in detail previously,25 we minimized sampling error by averaging the cutoff points reported by calibration studies using the MTI Actigraph model 716426,29,32–34 and applied the resulting cutoff points to differentiate among PA intensities. The applied cutoff points for MPA and VPA intensities were accelerometer recordings of 2,225 to 5,950 and >5,950 counts per minute, respectively. Moderate- and vigorous-intensity physical activity was defined as ≥2,225 counts per minute.
Leisure time PA and exercise habits were assessed at baseline and at 3 and 9 months of intervention using the Aerobics Center Longitudinal Study Physical Activity Questionnaire (ACLS).35 The ACLS elicits self-reports of frequency (sessions per week) and duration (minutes per session) of activities such as walking, running, treadmill, cycling, swimming, aerobics, yoga, weight lifting, and other sports (eg, golf, tennis, soccer).
Data Analysis
For the analyses presented here, a valid day of PA was defined as having 10 or more hours of accelerometer wearing, based on previous recommendations from analyses of the National Health and Nutrition Examination Survey (NHANES) accelerometer database.36 Furthermore, 5 to 7 days of valid accelerometer wearing was required for inclusion in the present analysis. We chose a minimum of 5 days of accelerometer wearing because PA levels vary greatly throughout the week and 1 to 4 days of PA may not be representative of habitual PA.28
In order to address the question of whether participants randomly assigned to receive RT would substitute RT for MVPA, the RT groups (RT and RT+SM, n=114) were collapsed into a single group and compared with the SM group (Tab. 1). Descriptions of the 171 participants randomly assigned to each of the 3 intervention groups (SM, RT, RT+SM) and the 114 participants who received the RT intervention (collapsed RT and RT+SM group) are shown in Table 1. Means, standard deviations, and 95% confidence intervals were calculated for continuous variables, and frequencies were calculated for categorical variables. Data were checked for missing values and normality prior to analyses. Moderate- and vigorous-intensity physical activity (minutes per day), which was skewed, was natural log transformed for analysis, resulting in a normal distribution.
Preliminary tests for baseline differences in descriptive characteristics among the 3 intervention groups were performed using an analysis of variance or the chi-square test for proportions as appropriate. For all subsequent analyses, the RT groups (RT and RT+SM) were collapsed into a single group and compared with the SM group to address the question of whether RT affects MVPA. The RT groups were combined because both groups of participants engaged in the RT intervention and no significant (P>.05) group differences using independent t tests were observed for any variables at any time point. Effect sizes representing the magnitude of difference between baseline and 3 and 9 months were calculated using Cohen's method37 based on adjusted means of MVPA for the 2 groups (SM and collapsed RT).
A repeated-measures analysis of covariance was performed with group (SM and collapsed RT) as the between-subjects factor and time (3 and 9 months) as the within-subjects factor. Baseline MPVA, age, BMI, knee OA pain, and sex were entered as covariates to account for baseline between-group differences in MVPA and the putative influence of these variables on MVPA. The specific effect of interest in this analysis was the group × time interaction. A significant group × time interaction would indicate that the degree of change in MVPA over time was different for the 2 groups (SM and collapsed RT). Statistical significance was set at P<.05 for all tests. Analyses were conducted using the Statistical Package for the Social Sciences, version 17.0.#
Results
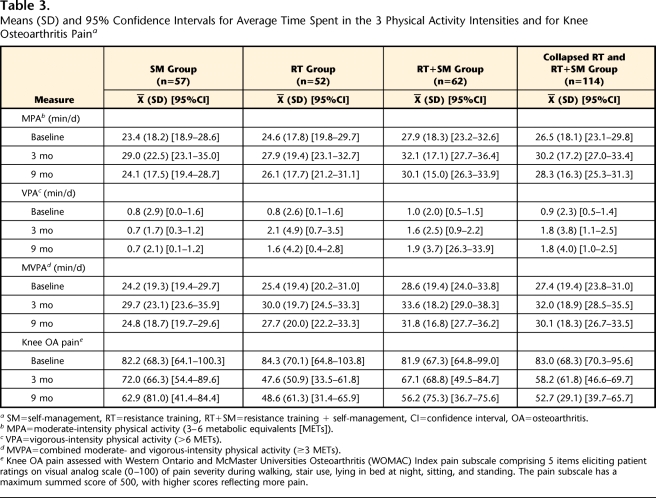
At baseline, 3 months, and 9 months, 5, 17, and 26 participants, respectively, either did not wear an accelerometer or did not meet the inclusion criteria. Comparisons of baseline descriptive characteristics for noncompleters (n=83) versus completers (n=171) showed no significant (P>.05) differences (Tab. 1). After 9 months of intervention, the numbers of participants who successfully completed all measures at baseline, 3 months, and 9 months were as follows: SM group=57, RT group=52, RT+SM group=62 (Fig. 1). The final sample of participants with early onset OA of the knee who successfully adhered to the accelerometer protocol comprised 171 participants (74% women, 26% men; mean age=55.1 (SD=7.1) years, and mean BMI=27.6 (SD=4.2) kg/m2). Preliminary baseline analyses of the 3 intervention groups showed no significant (P>.05) difference among groups or between completers (n=171) and noncompleters (n=83) at baseline for any variables (Tabs. 1 and 3). Participants wore the accelerometer, on average, 6.8 (SD=0.5) days and 14.2 (SD=2.2) hours per day over all 3 PA assessments. There were no significant group differences (P>.05) in number of days the accelerometer was worn or in accelerometer wearing time at any time interval. Significant differences were not observed for any of the measured variables (P>.05) when participants with 5 to 6 days of accelerometer data were compared with participants with 7 days of data. Unadjusted means, standard deviations, and 95% confidence intervals for MPA, VPA, and MVPA for the collapsed RT group and the SM group at baseline, 3 months, and 9 months are presented in Table 3.
Table 3.
Means (SD) and 95% Confidence Intervals for Average Time Spent in the 3 Physical Activity Intensities and for Knee Osteoarthritis Paina
SM=self-management, RT=resistance training, RT+SM=resistance training + self-management, CI=confidence interval, OA=osteoarthritis.
b MPA=moderate-intensity physical activity (3–6 metabolic equivalents [METs]).
c VPA=vigorous-intensity physical activity (>6 METs).
d MVPA=combined moderate- and vigorous-intensity physical activity (≥3 METs).
e Knee OA pain assessed with Western Ontario and McMaster Universities Osteoarthritis (WOMAC) Index pain subscale comprising 5 items eliciting patient ratings on visual analog scale (0–100) of pain severity during walking, stair use, lying in bed at night, sitting, and standing. The pain subscale has a maximum summed score of 500, with higher scores reflecting more pain.
Exercise session attendance was 75.9% (SD=17.9%) for the collapsed RT group, and SM class attendance was 85.9% (SD=14.2%) for the SM group. However, attendance did not significantly (P=.48) differ between the collapsed RT group and the SM group. The RT groups significantly (P<.001) increased their leg press, leg curl, incline dumbbell press, seated row, and calf raise loads from baseline to 9 months (Tab. 2). Data from the ACLS questionnaire showed that few SM group participants (n=11, 19%) reported engaging in any form of resistance exercise throughout the intervention.
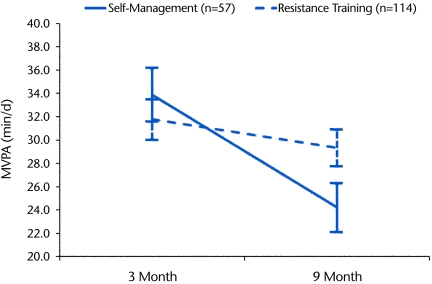
Despite high exercise session attendance and significant improvements in muscle strength, very little time (minutes per day) was spent in VPA as measured by the accelerometer, and there were no significant (P>.05) differences in VPA among the intervention groups at any time interval (Tab. 3). Consequently, average daily MVPA (≥3 METs) was representative of total time spent in health-enhancing PA intensities. The collapsed RT group participants increased their MVPA by 18% at 3 months (P=.001, effect size [d]=0.26) and by 10% at 9 months (P=.047, effect size [d]=0.15) compared with baseline levels. The SM group participants increased their MVPA by 22% at 3 months (P=.023, effect size [d]=0.25) and by 2% at 9 months (P=.80, effect size [d]=0.03) compared with baseline levels. After adjusting for baseline MVPA, age, BMI, sex, and knee OA pain, there was a significant (P=.034) group × time interaction for MVPA, which indicated that longitudinal MVPA decreased at a greater rate in the SM group than in the collapsed RT group (Fig. 2).
Figure 2.
Average daily moderate- and vigorous-intensity physical activity (MVPA, ≥3 metabolic equivalents) at 3 and 9 months of intervention for the self-management group (SM) and collapsed resistance training (RT) groups (RT and RT+SM). Values were adjusted for baseline MVPA, age, body mass index, sex, and knee osteoarthritis pain. There was a significant group × time interaction (P=.034) for MVPA.
Discussion
The overall increase in MVPA by the RT groups suggests that patients with early-onset OA of the knee can engage in a structured resistance exercise program without a compensatory decrease in MVPA levels. Compared with baseline, MVPA increased in the collapsed RT group by 18% at 3 months and 10% at 9 months, and the SM group showed a 22% increase in MVPA at 3 months but only a 2% increase at 9 months. These findings indicate that both SM and RT programs are effective for increasing short-term MVPA in patients with early OA of the knee, which is consistent with the findings of previous studies.38–40 The greater 3-month increase in MVPA in the SM group compared with the RT group may have resulted from differences in adherence (SM group=86%, RT group=76%) or because both programs encouraged PA. Although both treatments were effective in increasing short-term MVPA, RT was better than SM for maintaining long-term MVPA levels. Indeed, a significant group × time interaction indicated that the degree of change in MVPA was different between groups (ie, MVPA in the RT groups regressed between 3 and 9 months at a slower rate than in the SM group). Thus, rather than simply substituting RT for MVPA, the RT groups were able to maintain MVPA levels in addition to attaining strength benefits from RT. It is possible that participation in the RT sessions may have contributed to the long-term maintenance of MVPA levels. On the other hand, adherence and MVPA in the SM group may have dropped off because of the intervention content. For example, early sessions focused on developing SM skills, whereas later sessions focused on reinforcing those skills. Thus, those participants who already mastered the skills may have seen less utility in the continued support.
Previous studies have shown that when older adults participate in structured RT programs, there is a tendency for a compensatory decrease in aerobic MVPA.11,12 For example, Goran and Poehlman11 observed a decrease of >544 kJ per day in free-living PA in elderly adults who were healthy after an 8-week high-intensity (85% of maximal oxygen consumption) training program. It is possible that the high intensity of the exercise program used in that study was too vigorous, thereby fatiguing the participants to the extent that they were no longer able to engage their regular PA throughout the remainder of the day. Meijer et al12 reported that a 12-week, moderate-intensity combined aerobic and RT program resulted in improved physical fitness but had no effect on total daily PA (ie, after subtracting the PA of the exercise training sessions, this study showed that training PA was compensated for by a decrease in nontraining PA, consistent with the findings of Goran and Poehlman11). In contrast, a 26-week RT program in a study by Hunter et al10 was not associated with a compensatory drop in free-living PA. The findings of Hunter et al are consistent with our findings because participants were able to engage in RT without substituting RT for MVPA.
Although accelerometers allow for accurate measurements of daily time spent in various health-enhancing PA intensities (ie, MPA, VPA, and MVPA),26 hip placement may underestimate energy expenditure during certain activities (ie, biking, climbing stairs, and weight lifting) and provide no estimate of PA during water activities (eg, swimming) because accelerometers cannot be worn.28 Given the time spent standing, sitting, and lying during RT, this mode of activity is not measured well by accelerometers.28 This finding was evident in the present study because despite rather high exercise session attendance (RT group=76%) and significant (P<.001) increases in load from baseline to 9 months for a number of RT exercises (eg, leg press, leg curl, seated row, incline dumbbell press, calf raise), average daily VPA in the RT group remained extremely low at 3 months (1.8 [SD=3.8] minutes per day) and 9 months (1.8 [SD=4.0] minutes per day). Thus, based on the observed improvements in strength, the RT groups likely received additional benefits of RT compared with the SM group, such as improved REE, TEE, AEE, lipid oxidation rates, musculoskeletal function, and body composition, which have been observed in response to RT in older adults.9,10,41 Furthermore, the ACLS questionnaire indicated that few SM group participants (n=11, 19%) engaged in any form of RT, and thus the majority of participants in this group did not receive additional benefits of RT.
In addition to increasing TEE,10 RT has been shown to improve a number of functional limitations that lead to disability in patients with OA of the knee, such as quadriceps muscle weakness,42 neurological deficits,43 and decreased knee ROM.44 Furthermore, RT programs have been shown to improve psychological factors such as mood, self-efficacy, anxiety, and depression.45,46 Aerobic MVPA also can improve some of the same functional limitations in patients with OA of the knee and can reduce risks of obesity-linked health problems, including diabetes and cardiovascular disease,5,6 which often coexist with knee OA. Therefore, in clinical settings, the ability to engage in RT without sacrificing MVPA is important.
We acknowledge several limitations of our study. For example, 5 to 7 days of contiguous accelerometer recordings may not be representative of habitual PA, and adipose tissue around the waist might affect the validity of the accelerometer outputs.25 Furthermore, accelerometers may underestimate PA during activities such as biking, climbing stairs, and weight lifting and cannot be worn during water activities (eg, swimming).28 Thus, PA may be underestimated in individuals who engage in these activities on a regular basis. More detailed physiological studies are needed in patients with OA of the knee to measure directly different energy expenditures (ie, REE, TEE, and AEE), which have been shown to be elevated in older adults in response to RT.9,10 Lastly, we cannot establish a causal relationship between increased levels of MVPA and improved physical function because we did not measure functional changes. However, a number of randomized controlled trials8,39,40,47,48 in patients with OA of the knee have shown a strong association between increased levels of MVPA and RT and improved physical function, which provides support for improved knee function of individuals who undertook the RT intervention.
Conclusion
Patients with early-onset knee OA were able to engage in an RT program without a compensatory decrease in their overall MVPA levels. Because RT has been shown to increase energy expenditure in adults9,10,41 and has been shown to improve muscle strength and physical function and to reduce pain in patients with OA of the knee,8 it is a vital component of knee OA therapy. Given the relevant health benefits of RT and aerobic MVPA for management of knee OA, future studies are necessary to improve adherence to both modes of exercise.
Footnotes
Dr Going and Dr McKnight provided concept/idea/research design. Mr Farr, Dr McKnight, and Dr Kasle provided writing. Mr Farr, Dr McKnight, Dr Kasle, and Dr Cornett provided data collection. Dr Kaske provided data management. Mr Farr, Dr McKnight, and Ms Cussler provided data analysis. Dr Going, Dr McKnight, and Dr Cornett provided project management. Dr McKnight provided fund procurement. Dr Going and Ms Cussler provided consultation (including review of manuscript before submission).
The authors thank the men and women with knee OA who generously volunteered their time, the project coordinators for their oversight of all aspects of the study, and the other members of the Knee Study investigative team. Dr Isidro Villanueva is gratefully acknowledged for his contributions to the Knee Study.
This study was approved by the University of Arizona Institutional Review Board and conducted in accordance with the Helsinki Declaration.
This project was supported by National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases grant R01-AR-047595. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Arthritis and Musculoskeletal and Skin Diseases or the National Institutes of Health.
NIH Clinical Trials Registry: NCT00586300.
1 MET=3.5 mL O2·kg−1·min−1.
The Hygenic Corp, 1245 Home Ave, Akron, OH 44310-2575.
Seca GMBH and Co KG, Hammer Steindamm 9 25, 20089 Hamburg, Germany.
Shorr Productions, 17802 Shotley Bridge Place, Olney, MD 20832.
Manufacturing Technologies Inc, 70 Ready Ave NW, Fort Walton Beach, FL 32548.
SPSS Inc, 233 S Wacker Dr, Chicago, IL 60606.
References
- 1.Mazzuca SA. Is behavioral graded activity effective for the treatment of hip and knee osteoarthritis? Nat Clin Pract Rheumatol 2007;3:322–323 [DOI] [PubMed] [Google Scholar]
- 2.American College of Rheumatology Subcommittee on Osteoarthritis Guidelines Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. Arthritis Rheum 2000;43:1905–1915 [DOI] [PubMed] [Google Scholar]
- 3.Jordan KM, Arden NK, Doherty M, et al. EULAR Recommendations 2003: an evidence-based approach to the management of knee osteoarthritis: Report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis 2003;62:1145–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scott DL, Shipley M, Dawson A, et al. The clinical management of rheumatoid arthritis and osteoarthritis: strategies for improving clinical effectiveness. Br J Rheumatol 1998;37:546–554 [DOI] [PubMed] [Google Scholar]
- 5.American College of Sports Medicine Position Stand : The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med Sci Sports Exerc 1998;30:975–991 [DOI] [PubMed] [Google Scholar]
- 6.US Department of Health and Human Services Physical Activity and Health: A Report of the Surgeon General Atlanta, GA: Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion; 1996 [Google Scholar]
- 7.Minor MA. 2002 Exercise and Physical Activity Conference, St Louis, Missouri: exercise and arthritis “we know a little bit about a lot of things em leader.” Arthritis Rheum 2003;49:1–2 [DOI] [PubMed] [Google Scholar]
- 8.Roddy E, Zhang W, Doherty M, et al. Evidence-based recommendations for the role of exercise in the management of osteoarthritis of the hip or knee: the MOVE consensus. Rheumatology (Oxford) 2005;44:67–73 [DOI] [PubMed] [Google Scholar]
- 9.Withers RT, Smith DA, Tucker RC, et al. Energy metabolism in sedentary and active 49- to 70-year-old women. J Appl Physiol 1998;84:1333–1340 [DOI] [PubMed] [Google Scholar]
- 10.Hunter GR, Wetzstein CJ, Fields DA, et al. Resistance training increases total energy expenditure and free-living physical activity in older adults. J Appl Physiol 2000;89:977–984 [DOI] [PubMed] [Google Scholar]
- 11.Goran MI, Poehlman ET. Endurance training does not enhance total energy expenditure in healthy elderly persons. Am J Physiol 1992;263(5 pt 1):E950–E957 [DOI] [PubMed] [Google Scholar]
- 12.Meijer EP, Westerterp KR, Verstappen FT. Effect of exercise training on total daily physical activity in elderly humans. Eur J Appl Physiol Occup Physiol 1999;80:16–21 [DOI] [PubMed] [Google Scholar]
- 13.Booth FW, Gordon SE, Carlson CJ, Hamilton MT. Waging war on modern chronic diseases: primary prevention through exercise biology. J Appl Physiol 2000;88:774–787 [DOI] [PubMed] [Google Scholar]
- 14.Pate RR, Pratt M, Blair SN, et al. Physical activity and public health: a recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA 1995;273:402–407 [DOI] [PubMed] [Google Scholar]
- 15.Hart DJ, Spector TD, Brown P, et al. Clinical signs of early osteoarthritis: reproducibility and relation to x-ray changes in 541 women in the general population. Ann Rheum Dis 1991;50:467–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sullivan T, Allegrante JP, Peterson MG, et al. One-year followup of patients with osteoarthritis of the knee who participated in a program of supervised fitness walking and supportive patient education. Arthritis Care Res 1998;11:228–233 [DOI] [PubMed] [Google Scholar]
- 17.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis 1957;16:494–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bellamy N, Buchanan WW, Goldsmith CH, et al. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 1988;15:1833–1840 [PubMed] [Google Scholar]
- 19.Altman R, Asch E, Bloch D, et al. ; for the Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association Development of criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of the knee. Arthritis Rheum 1986;29:1039–1049 [DOI] [PubMed] [Google Scholar]
- 20.Pearson D, Faigenbaum A, Conley M, Kraemer WJ. The National Strength and Conditioning Association's basic guidelines for the resistance training of athletes. Strength and Conditioning Journal 2000;22:14–27 [Google Scholar]
- 21.Baker KR, Nelson ME, Felson DT, et al. The efficacy of home based progressive strength training in older adults with knee osteoarthritis: a randomized controlled trial. J Rheumatol 2001;28:1655–1665 [PubMed] [Google Scholar]
- 22.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982;14:377–381 [PubMed] [Google Scholar]
- 23.Lorig KR, Holman H. Self-management education: history, definition, outcomes, and mechanisms. Ann Behav Med 2003;26:1–7 [DOI] [PubMed] [Google Scholar]
- 24.Lohman TG, Roche AF, Martorell R. Anthropometric Standardization Reference Manual Champaign, IL: Human Kinetics Publishers; 1988 [Google Scholar]
- 25.Farr JN, Going SB, Lohman TG, et al. Physical activity levels in patients with early knee osteoarthritis measured by accelerometry. Arthritis Rheum 2008;59:1229–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications Inc accelerometer. Med Sci Sports Exerc 1998;30:777–781 [DOI] [PubMed] [Google Scholar]
- 27.Tryon W, Williams R. Fully proportional actigraphy: a new instrument. Behav Res Meth Instrum Comput 1996;28:392–403 [Google Scholar]
- 28.Matthew CE. Calibration of accelerometer output for adults. Med Sci Sports Exerc 2005;37(11 suppl):S512–S522 [DOI] [PubMed] [Google Scholar]
- 29.Hendelman D, Miller K, Baggett C, et al. Validity of accelerometry for the assessment of moderate intensity physical activity in the field. Med Sci Sports Exerc 2000;32(9 suppl): S442–S449 [DOI] [PubMed] [Google Scholar]
- 30.Computer Science and Applications Inc Activity Monitor Operator's Manual, Model 7164, Release 1.04 Shalimar, FL: Computer Science and Applications Inc; 1995 [Google Scholar]
- 31.Matthews CE, Ainsworth BE, Thompson RW, Bassett DR., Jr Sources of variance in daily physical activity levels as measured by an accelerometer. Med Sci Sports Exerc 2002;34:1376–1381 [DOI] [PubMed] [Google Scholar]
- 32.Brage S, Wedderkopp N, Franks PW, et al. Reexamination of validity and reliability of the CSA monitor in walking and running. Med Sci Sports Exerc 2003;35:1447–1454 [DOI] [PubMed] [Google Scholar]
- 33.Nichols JF, Morgan CG, Chabot LE, et al. Assessment of physical activity with the Computer Science and Applications Inc accelerometer: laboratory versus field validation. Res Q Exerc Sport 2000;71:36–43 [DOI] [PubMed] [Google Scholar]
- 34.Yngve A, Nilsson A, Sjostrom M, Ekelund U. Effect of monitor placement and of activity setting on the MTI accelerometer output. Med Sci Sports Exerc 2003;35:320–326 [DOI] [PubMed] [Google Scholar]
- 35.Kohl HW, Blair SN, Paffembarger RS, et al. The Aerobics Center Longitudinal Study Physical Activity Questionnaire: a collection of physical activity questionnaires for health-related research. Med Sci Sports Exerc 1997;29(suppl):S10–S14 [PubMed] [Google Scholar]
- 36.Troiano RP, Berrigan D, Dodd KW, et al. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc 2008;40:181–188 [DOI] [PubMed] [Google Scholar]
- 37.Cohen J. A power primer. Psychol Bull 1992;112:155–159 [DOI] [PubMed] [Google Scholar]
- 38.Evcik D, Sonel B. Effectiveness of a home-based exercise therapy and walking program on osteoarthritis of the knee. Rheumatol Int 2002;22:103–106 [DOI] [PubMed] [Google Scholar]
- 39.Kovar PA, Allegrante JP, MacKenzie CR, et al. Supervised fitness walking in patients with osteoarthritis of the knee: a randomized, controlled trial. Ann Intern Med 1992;116:529–534 [DOI] [PubMed] [Google Scholar]
- 40.Talbot LA, Gaines JM, Huynh TN, Metter EJ. A home-based pedometer-driven walking program to increase physical activity in older adults with osteoarthritis of the knee: a preliminary study. J Am Geriatr Soc 2003;51:387–392 [DOI] [PubMed] [Google Scholar]
- 41.Van Etten LM, Westerterp KR, Verstappen FT, et al. Effect of an 18-week weight-training program on energy expenditure and physical activity. J Appl Physiol 1997;82:298–304 [DOI] [PubMed] [Google Scholar]
- 42.Schilke JM, Johnson GO, Housh TJ, O'Dell JR. Effects of muscle-strength training on the functional status of patients with osteoarthritis of the knee joint. Nurs Res 1996;45:68–72 [DOI] [PubMed] [Google Scholar]
- 43.Hurley MV, Scott DL. Improvements in quadriceps sensorimotor function and disability of patients with knee osteoarthritis following a clinically practicable exercise regime. Br J Rheumatol 1998;37:1181–1187 [DOI] [PubMed] [Google Scholar]
- 44.Badley EM, Wagstaff S, Wood PH. Measures of functional ability (disability) in arthritis in relation to impairment of range of joint movement. Ann Rheum Dis 1984;43:563–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Byrne A, Byrne DG. The effect of exercise on depression, anxiety and other mood states: a review. J Psychosom Res 1993;37:565–574 [DOI] [PubMed] [Google Scholar]
- 46.McAuley E, Shaffer S, Rudolph D. Physical activity, aging, and psychological well-being. JAPA 1995;3:67–96 [Google Scholar]
- 47.Ettinger WH, Jr, Burns R, Messier SP, et al. A randomized trial comparing aerobic exercise and resistance exercise with a health education program in older adults with knee osteoarthritis: the Fitness Arthritis and Seniors Trial (FAST). JAMA 1997;277:25–31 [PubMed] [Google Scholar]
- 48.Bautch JC, Malone DG, Vailas AC. Effects of exercise on knee joints with osteoarthritis: a pilot study of biologic markers. Arthritis Care Res 1997;10:48–55 [DOI] [PubMed] [Google Scholar]