Abstract
Two subtypes of β-adrenoceptors, β1 and β2, mediate cardiac catecholamine effects. These two types differ qualitatively, e.g., regarding G protein coupling and calcium channel stimulation. Transgenic mice overexpressing human β2-adrenoceptors survive high-expression levels, unlike mice overexpressing β1-adrenoceptors. We examined the role of inhibitory Gi proteins, known to be activated by β2- but not β1-adrenoceptors, on the chronic effects of human β2-adrenoreceptor overexpression in transgenic mice. These mice were crossbred with mice where Gαi2, a functionally important cardiac Gi α-subunit, was inactivated by targeted gene deletion. Survival of β2-adrenoreceptor transgenic mice was reduced by heterozygous inactivation of Gαi2. Homozygous knockout/β2-adrenoreceptor transgenic mice died within 4 days after birth. Heterozygous knockout/β2-adrenoreceptor transgenic mice developed more pronounced cardiac hypertrophy and earlier heart failure compared with β2-adrenoreceptor transgenic mice. Single calcium-channel activity was strongly suppressed in heterozygous knockout/β2-adrenoreceptor transgenic mice. In cardiomyocytes from these mice, pertussis toxin treatment in vitro fully restored channel activity and enhanced channel activity in cells from homozygous Gαi2 knockout animals. Cardiac Gαi3 protein was increased in all Gαi2 knockout mouse strains. Our results demonstrate that Gαi2 takes an essential protective part in chronic signaling of overexpressed β2-adrenoceptors, leading to prolonged survival and delayed cardiac pathology. However, reduction of calcium-channel activity by β2-adrenoreceptor overexpression is due to a different pertussis-toxin-sensitive pathway, most likely by Gαi3. This result indicates that subtype-specific signaling of β2-adrenoreceptor functionally bifurcates at the level of Gi, leading to different effects depending on the Gα isoform.
Keywords: L-type calcium channel, single channel recording, mouse genetics, survival curve, pertussis toxin
Cardiac stimulatory catecholamine effects are mediated by both β1- and β2-adrenergic receptors (β-adrenoceptors), mainly through cAMP-dependent protein kinase A-catalyzed phosphorylation of cardiac proteins involved in calcium homeostasis, such as phospholamban and the L-type calcium channel. Evidently, this pathway is compromised in heart failure. Because of the clear-cut evidence that β2-adrenoceptors mediate acute functional effects in human cardiomyocyte in vitro (1, 2) and in vivo (3), a rationale for β2-adrenoreceptor gene therapy exists (4–6). Whether and where β2-adrenoreceptor stimulation or inhibition, by pharmacological or genetic means, will have its place in heart failure therapy is an open question (7–9).
Inherent in the clinical discussion is recent molecular insight into differences between cardiac β1- and β2-adrenoreceptor stimulation at the signal-transduction level (10, 11). In rat cardiomyocytes, a β2-agonist, zinterol, was shown to increase calcium current in a manner qualitatively distinct from β1-adrenoreceptor stimulation (12), consistent with single-channel effects reported later (13). Xiao et al. (14) also showed that β2- but not β1-effects were potentiated by inactivation of Gi/o protein by using pertussis toxin (PTX), and β2-adrenoreceptor coupling to Gαi2 and Gαi3 could be directly demonstrated in mice (15). Gi coupling then may lead to activation of protein phosphatases (16), or activate, by means of c-Src and Ras, the mitogen-activated protein kinase pathway (17, 18). In addition, β2- but not β1-adrenoreceptor activation modulates sodium/proton exchanger in a G protein-independent manner (19). The distinct effect of β1-adrenoreceptor vs. β2-adrenoreceptor stimulation on cardiac apoptosis (20) also likely reflects (antiapoptotic) Gi signaling through β2-adrenoreceptor activation (21, 22). Which of the abundant cardiac Gi proteins, i.e., Gαi2 or Gαi3, figures more prominently in these effects has hitherto not been addressed.
The most striking difference between β1-adrenoreceptor and β2-adrenoreceptor signaling in a pathophysiological sense comes from studies using a transgenic approach: mice overexpressing human β2-adrenoceptors in the heart from early life (by α-MHC promotor) display increased contractility (23, 24) and calcium currents (25) at a young age. These effects vanish during adulthood (26, 27). β2-Adrenoreceptor transgenic mice appear to have normal life expectancy (24) if expression levels are moderately high, i.e., <4,000–5,000 fmol of receptors per mg of membrane protein (28). In sharp contrast, mice containing a β1-adrenoreceptor construct (at ≈600 fmol/mg) develop severe hypertrophy and die of heart failure (29).
It is tempting to hypothesize that β2-adrenoreceptor stimulation, besides its known cardiac stimulatory effects, exerts some sort of protection against hypertrophy and failure, possibly by parallel chronic Gi signaling. Testing this idea could shed some light on the question whether the well known up-regulation of Gi found in human heart failure (e.g., ref. 30) is maladaptive or beneficial (31, 32).
PTX, an inhibitor of Gi/o signaling, has been a valuable tool to dissect Gi-dependent components of β2-adrenoreceptor signaling. This compound, because of its systemic toxicity, is ill-suited to study the phenomena associated with chronic β2-adrenoreceptor overexpression and signaling in vivo. Therefore, we used a genetic approach to determine the role of Gi in chronic effects of β2-adrenoreceptor signaling. To this end, we crossed mice with targeted deletion of Gαi2 (33, 34) with mice overexpressing the human β2-adrenoreceptor (23). We report here that the three most relevant endpoints, mortality, cardiac hypertrophy, and calcium-channel activity, are affected in heterozygous and homozygous Gαi2 knockout/β2-adrenoreceptor transgenic mice. We conclude that Gαi2 figures crucially in the prevention of hypertrophy and in survival, but not in the calcium-channel-suppressing function of chronic β2-adrenoreceptor signaling.
Materials and Methods
Animals. Mice with cardiac-specific heterozygous overexpression of human β2-adrenoceptors (TG β2, ref. 23) were fully back-crossed (>5 generations) into the C57/Bl6 strain and bred with their respective nontransgenic littermates. Littermates served as the WT (or Gαi2 +/+) controls. Gαi2 knockout animals (Gαi2 -/-, ref. 33) were fully back-crossed (>5 generations) into the C57/Bl6 strain. Offspring were generated by breeding heterozygous and homozygous knockout animals. Hybrid mice (Gαi2 +/TG β2 and Gαi2 -/TG β2) were generated by using three breeding schemes: mating of Gαi2 -/× Gαi2 +/+ TG β2 yielded offspring at approximately Mendelian distribution (Gαi2 +/TG β2, n = 21; Gαi2 +/-, n = 26). Mating of Giα2 -/× Giα2 +/TG β2 yielded less than expected Gαi2 -/TG β2 (n = 2), whereas the other genotypes occurred at the expected frequencies (Gαi2 +/TG β2, n = 8; Gαi2 +/-, n = 9; Gαi2 -/-, n = 6). In 23 breeding protocols using Gαi2 +/TG β2 × Gαi2+/-, the following genotypes were obtained at approximate Mendelian frequency: Gαi2 +/+, n = 26; Gαi2+/-, n = 28; Gαi2 -/-, n = 16; Gαi2 +/+ TG β2, n = 17; Gαi2 +/TG β2, n = 30; again except for Gαi2 -/TG β2, (n = 6), which fell below expectation by half (4.9% instead of 12.5%).
Genotyping. A tail-clip analysis was performed at 3–4 weeks of age. After preparation of genomic DNA, a PCR was run. To genotype Gαi2 (GenBank accession no. NM_008138), we used the following primer pairs: wild type (+), forward, 5′-GAT CAT CCA TGA AGA TGG CTA CTC AGA AG-3′; reverse, 5′-CCC CTC TCA CTC TTG ATT TCC TAC TGA CAC-3′. Knockout (-), forward, 5′-CAG GAT CAT CCA TGA AGA TGG CTA C-3′; reverse, 5′-GCA CTC AAA CCG AGG ACT TAC AGA AC-3′.
Both reactions were run over 35 cycles (saturation). Amplified sequences were 805 bp for the WT allele and 509 bp for the targeting construct.
A similar strategy was used to identify the presence of the human β2-adrenoreceptor transgene (accession no.Y00106) in mice (23). The following primers, human β2-adrenoreceptor, forward, 5′-ACA TTG TGC ATG TGA TCC-3′; reverse, 5′-ATT CCT CCC TTG TGA ATC-3′, when used over 35 PCR cycles, yielded a strong signal of the expected size (337 bp) in native human myocardial tissue and in transgenic animals. However, a weak signal could also be seen with endogenous murine β2-adrenoceptors, which are 82% homologous at the cDNA level. To eliminate these wrong positive results, PCR products were extracted, precipitated, washed, and subjected to digestion with EcoRV, which recognizes a restriction site unique to the human gene, leading to specific fragments (219 and 118 bp in length).
Isolation of Cardiac Myocytes. Single ventricular myocytes were isolated from the hearts of 3- to 9-month-old mice by enzymatic dissociation by using the method described (27). In brief, hearts were perfused with a collagenase solution (Worthington type I and II, 75 units·liter-1) in a Langendorff setup and subsequently cut into small chunks. Myocytes were harvested by pouring the suspension through cheesecloth.
Single-Channel Recording. Single-channel recordings were performed by using the cell-attached configuration of the patchclamp method as described (35). Cells were placed in disposable Petri dishes containing 3 ml of a high-potassium depolarizing solution [25 mM KCl/120 mM potassium glutamate/2 mM MgCl2·6H2O/10 mM Hepes/2 mM EGTA/10 mM dextrose/1 mM CaCl2/1 mM Na2-ATP (pH 7.3) with KOH]. The patch pipettes (borosilicate glass, 6–8 MΩ) were filled with the pipette solution (70 mM BaCl2·2H2O/110 mM sucrose/10 mM Hepes, with pH adjusted to 7.4 with tetraethylammonium hydroxide). Ba2+ currents were elicited by voltage steps (150 ms at 1.66 Hz) from -100 mV to +20 mV (≥180 sweeps per experiment). Data were sampled at 10 kHz and filtered at 2 kHz (-3 dB, four-pole Bessel) by using an Axopatch 200 A amplifier (Axon Instruments, Foster City, CA). PCLAMP software (CLAMPEX 5.5.1, FETCHAN, and PSTAT 6) was used for data acquisition and analysis (Axon Instruments).
Data Analysis and Statistics. Mostly, experiments with one single channel (i.e., no stacked openings above unitary amplitude level) were analyzed in this study. Linear leak and capacity currents (averaged nonactive sweeps) were digitally subtracted. Openings and closures were identified by the half-height criterion. The fraction of active sweeps within a channel-containing patch (availability), the open probability within active sweeps (open probability), and the peak value of single-channel ensemble average currents (Ipeak) were determined as described (35). In double-channel patches, these parameters were corrected for the number of channels as described (36). Time constants of open- and closed-time histograms were estimated by the maximum-likelihood method (37). For multiple comparisons among groups, ANOVA followed by Bonferroni-corrected posttest was done. For simple comparisons (e.g., PTX effects), an unpaired Student's two-tailed t test was used. Kaplan–Meier survival curves were statistically compared by log-rank test. Throughout, a level of P < 0.05 was considered significant. Values are given as mean ± SEM.
SDS/PAGE and Western Blot Analysis. For isolation of cardiac membrane protein fractions, mouse hearts where frozen in liquid nitrogen and homogenized in a buffer containing 50 mM Tris·HCl and 1 mM phenylmethylsulfonyl fluoride, pH 7.4. The homogenized tissue was centrifuged (15 min, 3,400 × g), and the resulting supernatant was recentrifuged (40 min, 100,000 × g). The resulting pellets were solubilized in sample buffer [50 mM Tris·HCl (pH 6.8)/2% (wt/vol) SDS/20% (vol/vol) glycerol/5 mM DTT], shaken for 30 min, and subjected to SDS/PAGE on a 12.5% acrylamide running gel and a 4% stacking gel. For protein analysis an equal amount of 15 μg per homogenate was loaded. SDS/PAGE was blotted to poly(vinylidene difluoride) membranes (Bio-Rad) by using a semidry Western blot system. Gαs, Gαi2, and Gαi3 proteins were detected by specific antisera (Sigma-Aldrich product nos. 65090, 64090, and 64915, respectively; dilution, 1:2,000). Protein bands were analyzed densitometrically.
Results and Discussion
Generation and Survival of Transgenic Mice. In all breeding schemes used to generate knockout (Gαi2 +/-, Gαi2 -/-), transgenic (TG β2), or hybrid, double-mutant (Gαi2 +/TG β2) mice, viable offspring were obtained at roughly Mendelian rates (see Materials and Methods). Notably, Gαi2 -/TG β2 were observed at less than half of the expected Mendelian distribution. At birth, these animals appeared smaller and weaker than their littermates.
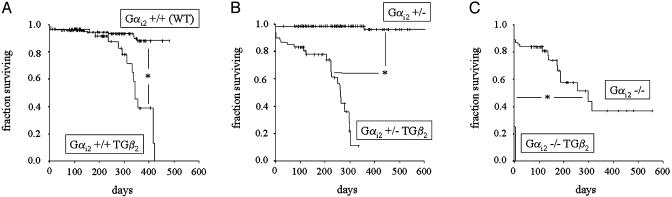
Survival was monitored for all genotypes for at least 1 year (Fig. 1). WT and Gαi2 +/did not reveal mortality or any clinical signs of disease during this period. (Fig. 1 A and B). Mean survival was 445 ± 8 days (WT, n = 227) and 642 ± 9 days (Gαi2 +/-, n = 191), respectively. In contrast, Gαi2 -/developed gastrointestinal symptoms (diarrhea, rectal prolapse, anal bleeding), loss of body weight (significant vs. Gαi2+/- and Gαi2 +/+ in male animals) and fatigue after a few months. Survival time (307 ± 40 days, n = 59) was significantly shorter than with heterozygous Gαi2 +/-. These findings qualitatively corroborate the original description of this mouse line (33).
Fig. 1.
Kaplan–Meier survival curves of knockout and transgenic mice. Vertical bars indicate a censoring event (usually representing killing of an animal for experiment). (A)WT(Gαi2 +/+) and β2-adrenoreceptor transgenic (TG β2) mice. (B) Heterozygous Gαi2 knockout mice without (Gαi2 +/-) or with (Gαi2 +/TG β2) cardiac overexpression of β2-adrenoceptors. (C) Homozygous Gαi2 knockout mice without (Gαi2 -/-) or with (Gαi2 -/TG β2) cardiac overexpression of β2-adrenoceptors. *, Significant difference (log-rank test) among the two curves depicted, respectively.
TG β2 mice, in agreement with Liggett et al. (28), developed fatigue, dyspnea, and cyanosis of mucous membranes when approaching 1 year of age. The survival (mean, 340 ± 17 days, n = 61) curve (Fig. 1 A) fell steeply in this age range, and most animals had died by 1 year of age (significant vs. WT). Heterozygous hybrid mice (Gαi2 +/TG β2) showed a similar clinical phenotype, but at earlier ages (Fig. 1B). Survival was significantly shortened (mean, 223 ± 15 days, n = 59), compared with both Gαi2 +/and nonhybrid TG β2 mice. At necropsy, these animals (Gαi2 +/TG β2) revealed lung and liver congestion, ascites, and an enlarged and dilated heart, often with thrombotic material in the atria. Only in a few cases (3 of 24), death occurred without prior clinical signs, and, in all these cases, massive thrombi were found in the atrial cavities. Heart weight of heterozygous hybrid mice was enhanced at the time of death, compared with the other genotypes (killed at a similar age range of 7–10 months). Together with a slightly reduced body weight, this gave rise to a highly significant increase of heart/body weight ratio (Table 1) in Gαi2 +/TG β2, indicating cardiac hypertrophy. At the same age, heart/body weight ratio was slightly but not significantly enhanced in simple transgenic TG β2 mice, in agreement with their clinical phenotype, developing heart failure and death at a higher age (Fig. 1).
Table 1. Body weight and heart weight of transgenic animals.
| Animals | Body weight, g | Heart weight, g | Heart/body weight ratio, % | Animals, n |
|---|---|---|---|---|
| WT | 29.7 ± 0.8 | 0.164 ± 0.007 | 0.55 ± 0.02 | 10 |
| Gαi2 +/– | 32.9 ± 1.3 | 0.154 ± 0.005 | 0.47 ± 0.02 | 10 |
| Gαi2 +/+ TGβ2 | 34.1 ± 4.2 | 0.221 ± 0.039 | 0.65 ± 0.12 | 6 |
| Gαi2 +/– TGβ2 | 25.3 ± 0.9† | 0.291 ± 0.012* | 1.15 ± 0.07* | 10 |
Body and heart weights were determined after killing or spontaneous death of 7- to 10-month-old animals of different genotypes. Mean ± SEM of n animals per group. Gαi2 +/– TGβ2 mice revealed. *, Significant (P < 0.05) differences versus all other genotypes; †, significant (P < 0.05) differences versus Gαi2 +/– and Gαi2 +/+ TGβ2 (ANOVA and posttests with Bonferroni correction, P < 0.05).
All homozygous hybrid mice (Gαi2 -/TG β2, n = 8) died between day 1 and 4 after birth (Fig. 1C). Necropsy revealed a dilated heart in the two cases examined macroscopically. Unfortunately, autolysis at the time of necropsy prevented meaningful histological examination. All these findings are in line with our hypothesis that protective effects (prolonged survival, suppression of cardiac hypertrophy and failure) are mediated by Gαi2 in mice overexpressing the β2-adrenoreceptor.
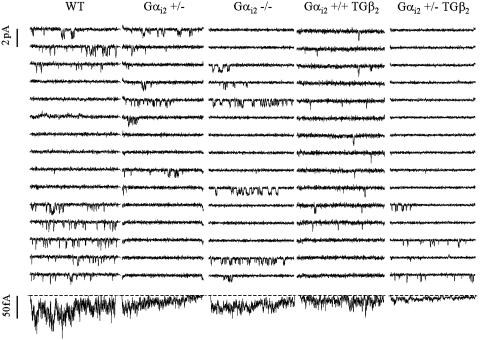
Calcium-Channel Gating Under Basal Conditions. Single channels were examined by using the cell-attached configuration, with the same protocol (holding potential, -100 mV; test potential, +20 mV; 70 mM BaCl2) as described (27). All genotypes except for the homozygous hybrid mice (Gαi2 -/TG β2) could be tested at adult age. The apparent channel density (i.e., the probability to find at least one channel in a technically stable patch) amounted to 18–21% in all genotypes, suggesting that our single-channel data are predictive for whole-cell current levels, which were not measured in this study. The majority of patches analyzed contained only one active channel (see Tables 2 and 3). Channel activity (Fig. 2 and Table 2) was similar to our report (27) regarding WT and TG β2 mice, although the reduction in single-channel activity in TG β2 turned out to be nonsignificant in this study. This outcome may be due to comparably large scatter (we used a wider age range, but age-dependent changes were not obvious), or perhaps because we now used monoallelic transgene carriers throughout (see Materials and Methods). Channel activity was not significantly different between WT and Gαi2 -/mice, confirming earlier studies at the level of whole-cell currents (38) or cardiac contractility (39). In striking contrast, a clear-cut reduction in single-channel activity and ensemble average currents (Fig. 2 and Table 2) was observed with heterozygous hybrid Gαi2 +/TG β2 mice. Both a reduced availability (fraction of active sweeps within a channel-containing patch) and a lower open probability due to altered closed time distribution contributed to this effect. The phenomenon is qualitatively similar to, but more pronounced than our observations with TG β2 mice (27). Therefore, loss of one allele of Gαi2 did not ameliorate but rather aggravated the suppression of calcium-channel activity induced by β2-adrenoreceptor overexpression, in contrast to our initial hypothesis. An explanation would be that other G proteins, such as Gαi3, mediate the suppressive effects of β2-adrenoceptors on the L-type calcium channel. To test this idea, myocytes of all available genotypes were pretreated with PTX (1.5 μg/ml, 3 h, 37°C), and single-channel activity was examined afterward.
Table 2. Calcium-channel gating under basal conditions.
| Conditions | WT | Gαi2 +/– | Gαi2 –/– | Gαi2 +/+ TGβ2 | Gαi2 +/– TGβ2 |
|---|---|---|---|---|---|
| Ipeak, fA | 49.2 ± 13.1 | 25.2 ± 6.3 | 26.4 ± 5.2 | 32.3 ± 8.8 | 20.8 ± 10.4 |
| Availability, % | 41.5 ± 5.3 | 34.7 ± 3.4 | 42.4 ± 5.5 | 47.7 ± 7.7 | 21.1 ± 4.1† |
| Open probability, % | 8.94 ± 1.99 | 7.00 ± 2.43 | 5.88 ± 1.11 | 4.75 ± 1.19 | 4.57 ± 2.25 |
| Mean open time, ms | 0.45 ± 0.03 | 0.44 ± 0.05 | 0.37 ± 0.05 | 0.46 ± 0.07 | 0.35 ± 0.06 |
| τopen, ms | 0.42 ± 0.04 | 0.43 ± 0.06 | 0.32 ± 0.05 | 0.46 ± 0.07 | 0.29 ± 0.06 |
| Mean closed time, ms | 4.27 ± 0.99 | 3.81 ± 0.92 | 3.99 ± 0.6 | 7.53 ± 1.89 | 8.57 ± 2.96 |
| τclosed1, ms | 0.49 ± 0.07 | 0.54 ± 0.07 | 0.64 ± 0.09 | 1.04 ± 0.29 | 0.45 ± 0.08† |
| Proportion | 0.66 ± 0.04 | 0.74 ± 0.04 | 0.75 ± 0.03 | 0.55 ± 0.07* | 0.72 ± 0.04 |
| τclosed2, ms | 12.0 ± 2.5 | 13.5 ± 2.6 | 14.0 ± 1.3 | 16.5 ± 2.6 | 23.3 ± 9.6 |
| Proportion | 0.34 ± 0.04 | 0.26 ± 0.04 | 0.25 ± 0.03 | 0.45 ± 0.07* | 0.28 ± 0.04 |
| n | 19 | 16 | 15 | 12 | 13 |
| no | 13 | 11 | 11 | 9 | 12 |
Measurements were carried out at a test potential of +20 mV (holding potential, –100 mV) by using 70 mM Ba2+ as charge carrier. Mean ± SEM of n experiments are given. Peak ensemble average current (Ipeak), availability (fraction of active sweeps within a channel containing patch), and open probability were corrected for the number of channels in multichannel patches. Closed times were analyzed for one-channel patches only (no).
,Significant versus Gαi2 +/– and Gαi2 –/– (ANOVA followed by posttests with Bonferroni correction, P<0.05);
,Significant versus Gαi2 +/+ TGβ2 (ANOVA followed by posttests with Bonferroni correction, P<0.05)
Table 3. Effect of PTX on calcium-channel gating.
| Conditions | WT | Gαi2 +/– | Gαi2 –/– | Gαi2 +/+ TGβ2 | Gαi2 +/– TGβ2 |
|---|---|---|---|---|---|
| Ipeak, fA | 60.3 ± 26.0 | 56.7 ± 12.2* | 68.8 ± 15.6* | 44.2 ± 11.0 | 93.3 ± 31.8* |
| Availability, % | 58.9 ± 10.0 | 47.8 ± 8.4 | 53.4 ± 6.2 | 53.2 ± 5.3 | 68.7 ± 7.7* |
| Open probability, % | 10.5 ± 2.56 | 16.0 ± 4.0 | 13.3 ± 4.2 | 7.2 ± 1.4 | 17.3 ± 9.0 |
| Mean open time, ms | 0.32 ± 0.06 | 0.49 ± 0.08 | 0.42 ± 0.07 | 0.65 ± 0.06 | 0.48 ± 0.11 |
| τopen, ms | 0.32 ± 0.08 | 0.50 ± 0.09 | 0.43 ± 0.14 | 0.61 ± 0.08 | 0.44 ± 0.08 |
| Mean closed time, ms | 3.67 ± 1.04 | 2.16 ± 0.74 | 2.05 ± 0.77 | 3.38 ± 0.83 | 2.00 ± 0.65 |
| τclosed1, ms | 0.47 ± 0.03 | 0.31 ± 0.07 | 0.34 ± 0.08 | 0.44 ± 0.07 | 0.24 ± 0.05 |
| Closed1 fraction, % | 70 ± 3 | 76 ± 4 | 78 ± 4 | 52 ± 6 | 74 ± 6 |
| τclosed2, ms | 11.2 ± 2.4 | 6.94 ± 2.72 | 7.04 ± 2.57* | 9.51 ± 2.64 | 5.69 ± 3.21 |
| n | 6† | 6 | 4 | 6† | 3 |
| no | 6 | 5 | 4 | 4 | 3 |
Measurements were carried out after a 3-h incubation with PTX (1.5 μg/ml, 37°C) at a test potential of +20mV (holding potential, –100 mV), using 70 mM Ba2+ as charge carrier. Mean ± SEM of n experiments are given. The peak value of ensemble average currents (Ipeak), availability (fraction of active sweeps within a channel containing patch), and open probability were corrected for the number of channels in multichannel patches. Closed times were analyzed for one-channel patches only (no). *,Significant versus baseline values (Table 2) of the respective genotype (t test, P < 0.05).
Includes data from unpublished experiments (n = 3 for WT and n = 4 for Gαi2 +/+ TGβ2) performed during our previous study (27)
Fig. 2.
Comparison of gating properties (70 mM Ba2+) observed for the different genotypes (for abbreviations, see Fig. 1). Fifteen consecutive single-channel sweeps (150-ms pulses to +20 mV; holding potential, -100 mV) are shown together with the ensemble average current of the whole experiment (bottom traces, from left to right, 180, 180, 240, 180, and 360 sweeps). Note that the single-channel activity is markedly lower in the Gαi2 +/TGβ2 cell (compare with Table 2).
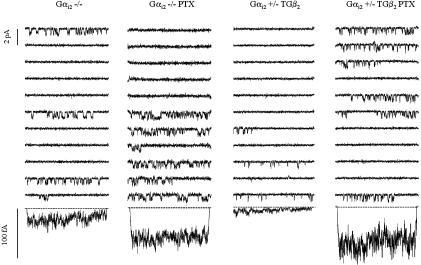
Effects of PTX on Single-Channel Gating. PTX pretreatment had no effect on WT (Gαi2 +/+) channels (Table 3), as reported (27). However, in channels from homozygous mice lacking Gαi2 (Gαi2 -/-), PTX significantly elevated single-channel activity (Fig. 3 and Table 3), and normal channel activity was fully restored in channels from heterozygous hybrid mice (Gαi2 +/TG β2), as depicted in Fig. 3 and statistically verified in Table 3. Even in heterozygous knockout mice (Gαi2 +/-), a significant elevation (compared with baseline, Table 2) of channel activity was observed after PTX pretreatment. The changes in ensemble average current were mostly due to an elevation of open probability and a shortening of closed times, exactly opposing the phenomena induced by β2-adrenoreceptor overexpression. This finding argues in favor of a role for a PTX-sensitive G protein other than Gαi2 to regulate calcium-channel activity. Gαi3 is a likely candidate, given its abundance in heart and stimulation by β2-adrenoreceptor activation (15). Therefore, levels of G proteins were measured by Western blot analysis.
Fig. 3.
PTX (3-h incubation with 1.5 μg/ml, 37°C) elevates single-channel activity in Gαi2 -/cells (Left) and restores unitary events and average currents toward WT levels in Gαi2 +/TGβ2 (Right). Note that control and PTX data are from different cells, respectively. Eleven consecutive single-channel sweeps (compare with Fig. 2) are shown together with the average current of the whole ensemble (bottom traces, from left to right, 240, 360, 360, and 240 sweeps).
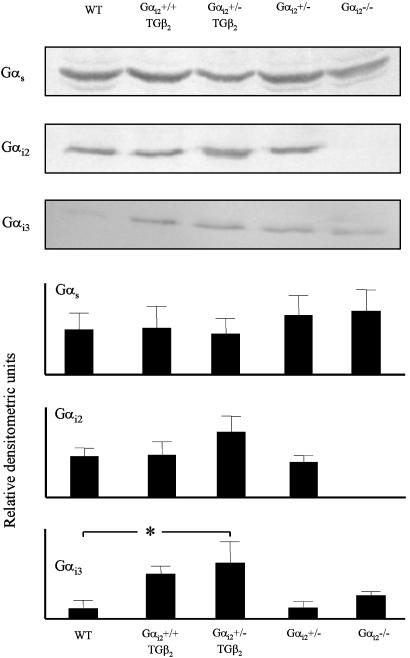
Expression Levels of G Proteins. Protein levels of Gαs, Gαi2, and Gαi3 were determined as depicted in Fig. 4. Gs levels were not significantly different among genotypes. Gαi2 was undetectable in homozygous mice (Gαi2 -/-) as expected. Levels seemed slightly enhanced in heterozygous hybrid mice (Gαi2 +/TG β2), confirming earlier findings in adult TG β2 mice (40). Gαi3 levels appeared to be increased in homozygous knockouts (Gαi2 -/-), in line with observations of Rudolph et al. (34) in other tissues and cells. More strikingly, a severalfold increase was observed in β2-adrenoreceptor transgenic hearts and in hybrid mutant mice (Gαi2 +/TG β2, significant vs. WT). Taken together, those genotypes reveal an increased expression of Gαi3, where reduction in single-channel activity was described in this (Table 2) and the previous study (27), and where PTX raises basal channel activity (Table 3). Up-regulation of Gαi3 probably represents compensation for lack of Gαi2 (function) (34, 41). Gαi3 is the most likely candidate to exert suppression of calcium-channel activity, although a role of other PTX substrates (Gαi1 or Gαo) cannot be safely excluded at present. Distinct roles of Gαi2 and Gαi3 have already been described for muscarinic regulation of cardiac ventricular calcium currents, which fully depends on Gαi2 but not Gαi3 (38, 42).
Fig. 4.
G protein expression in murine cardiac membrane homogenates depending on genotype (for abbreviations, see Fig. 1). Gs levels are unaltered, whereas Gαi2 is not detectable in Gαi2 -/and tends to be enhanced in Gαi2 +/TGβ2.Gαi3 appears somewhat up-regulated in Gαi2 -/and is markedly increased in TGβ2 and Gαi2 +/TGβ2 mouse heart. Statistics (mean ± SEM) are derived from n = 5–7 samples per genotype for Gs,Gαi2, and Gαi3. *, Significant difference (ANOVA and posttests with Bonferroni correction, P < 0.05).
In conclusion, our data strongly suggest that cardiac hypertrophy and failure due to β2-adrenoreceptor overexpression are compensated by Gαi2 activation, but single-channel regulation of L-type calcium channels follows a different pathway. We propose that calcium-channel regulation is rather mediated by Gαi3, and this G protein is indeed up-regulated. Therefore, the chronic β2-adrenoreceptor stimulation drives Gi protein isoforms to several distinct, subtype-specific signaling cascades, the details of which merit further investigation.
Acknowledgments
We thank Mrs. Sylvia Goitzsch and Mrs. Ramona Paura for excellent technical assistance and Mr. Jens Reifenrath for help with animal breeding. This study was supported by German Research Foundation Grant DFG He 1578/12-1 (to S.H.), intramural funding of the Medical Faculty Cologne (Köln Fortune 25/2001 to S.H.), and National Institutes of Health Research Grant DK-19318 (to L.B.).
Abbreviation: PTX, pertussis toxin.
References
- 1.Altschuld, R. A., Starling, R. C., Hamlin, R. L., Billman, G. E., Hensley, J., Castillo, L., Fertel, R. H., Hohl, M., Robitaille, P. M., Jones, L. R., et al. (1995) Circulation 92, 1612-1618. [DOI] [PubMed] [Google Scholar]
- 2.Kaumann, A., Bartel, S., Molenaar, P., Sanders, L., Burrell, K., Vetter, D., Hempel, P., Karczewski, P. & Krause, E. G. (1999) Circulation 99, 65-72. [DOI] [PubMed] [Google Scholar]
- 3.Newton, G. E., Azevedo, E. R. & Parker, J. D. (1999) Circulation 99, 2402-2407. [DOI] [PubMed] [Google Scholar]
- 4.Maurice, J. P., Hata, J. A., Shah, A. S., White, D. C., McDonald, P. H., Dolber, P. C., Wilson, K. H., Lefkowitz, R. J., Glower, D. D. & Koch, W. J. (1999) J. Clin. Invest. 104, 21-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawahira, Y., Sawa, Y., Nishimura, M., Sakakida, S., Ueda, H., Kaneda, Y. & Matsuda, H. (1998) Circulation 98, Suppl., II262-II267. [PubMed] [Google Scholar]
- 6.Shah, A. S., Lilly, R. E., Kypson, A. P., Tai, O., Hata, J. A., Pippen, A., Silvestry, S. C., Lefkowitz, R. J., Glower, D. D. & Koch, W. J. (2000) Circulation 101, 408-414. [DOI] [PubMed] [Google Scholar]
- 7.Limbird, L. E. & Vaughan, D. E. (1999) Proc. Natl. Acad. Sci. USA 96, 7125-7127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Houser, S. R. & Lakatta, E. G. (1999) Circulation 99, 600-604. [DOI] [PubMed] [Google Scholar]
- 9.Vatner, S. F., Vatner, D. E. & Homcy, C. J. (2000) Circ. Res. 86, 502-506. [DOI] [PubMed] [Google Scholar]
- 10.Steinberg, S. F. (1999) Circ. Res. 85, 1101-1111. [DOI] [PubMed] [Google Scholar]
- 11.Xiao, R. P., Cheng, H., Zhou, Y. Y., Kuschel, M. & Lakatta, E. G. (1999) Circ. Res. 85, 1092-1100. [DOI] [PubMed] [Google Scholar]
- 12.Xiao, R. P. & Lakatta, E. G. (1993) Circ. Res. 73, 286-300. [DOI] [PubMed] [Google Scholar]
- 13.Schröder, F. & Herzig, S. (1999) Am. J. Physiol. 276, H834-H843. [DOI] [PubMed] [Google Scholar]
- 14.Xiao, R. P., Ji, X. & Lakatta, E. G. (1995) Mol. Pharmacol. 47, 322-329. [PubMed] [Google Scholar]
- 15.Xiao, R. P., Avdonin, P., Zhou, Y. Y., Cheng, H., Akhter, S. A., Eschenhagen, T., Lefkowitz, R. J., Koch, W. J. & Lakatta, E. G. (1999) Circ. Res. 84, 43-52. [DOI] [PubMed] [Google Scholar]
- 16.Kuschel, M., Zhou, Y. Y., Cheng, H., Zhang, S. J., Chen, Y., Lakatta, E. G. & Xiao, R. P. (1999) J. Biol. Chem. 274, 22048-22052. [DOI] [PubMed] [Google Scholar]
- 17.Daaka, Y., Luttrell, L. M. & Lefkowitz, R. J. (1997) Nature 380, 88-91. [DOI] [PubMed] [Google Scholar]
- 18.Luttrell, L. M., Ferguson, S. S., Daaka, Y., Miller, W. E., Maudsley, S., Della Rocca, G. J., Lin, F., Kawakatsu, H., Owada, K., Luttrell, D. K., et al. (1999) Science 283, 655-661. [DOI] [PubMed] [Google Scholar]
- 19.Hall, R. A., Premont, R. T., Chow, C. W., Blitzer, J. T., Pitcher, J. A., Claing, A., Stoffel, R. H., Barak, L. S., Shenolikar, S., Weinman, E. J., et al. (1998) Nature 392, 626-630. [DOI] [PubMed] [Google Scholar]
- 20.Zaugg, M., Xu, W., Lucchinetti, E., Shafiq, S. A., Jamali, N. Z. & Siddiqui, M. A. (2000) Circulation 102, 344-350. [DOI] [PubMed] [Google Scholar]
- 21.Communal, C., Singh, K., Sawyer, D. B. & Colucci, W. S. (1999) Circulation 100, 2210-2212. [DOI] [PubMed] [Google Scholar]
- 22.Zhu, W. Z., Zheng, M., Koch, W. J., Lefkowitz, R. J., Kobilka, B. K. & Xiao, R. P. (2001) Proc. Natl. Acad. Sci. USA 98, 1607-1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Milano, C. A., Allen, L. F., Rockman, H. A., Dolber, P. C., McMinn, T. R., Chien, K. R., Johnson, T. D., Bond, R. A. & Lefkowitz, R. J. (1994) Science 264, 582-586. [DOI] [PubMed] [Google Scholar]
- 24.Rockman, H. A., Hamilton, R. A., Jones, L. R., Milano, C. A., Mao, L. & Lefkowitz, R. J. (1996) J. Clin. Invest. 97, 1618-1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.An, R., Heath, B. M., Higgins, J. P., Koch, W. J., Lefkowitz, R. J. & Kass, R. S. (1999) J. Physiol. (London) 516, 19-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heubach, J. F., Trebess, I., Wettwer, E., Himmel, H. M., Michel, M. C., Kaumann, A. J., Koch, W. J., Harding, S. E. & Ravens, U. (1999) Cardiovasc. Res. 42, 173-182. [DOI] [PubMed] [Google Scholar]
- 27.Heubach, J. F., Graf, E. M., Molenaar, P., Jäger, A., Schröder, F., Herzig, S., Harding, S. E. & Ravens, U. (2001) Br. J. Pharmacol. 133, 73-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liggett, S. B., Tepe, N. M., Lorenz, J. N., Canning, A. M., Jantz, T. D., Mitarai, S., Yatani, A. & Dorn, G. W., II (2000) Circulation 101, 1707-1714. [DOI] [PubMed] [Google Scholar]
- 29.Engelhardt, S., Hein, L., Wiesmann, F. & Lohse, M. J. (1999) Proc. Natl. Acad. Sci. USA 96, 7059-7064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neumann, J., Schmitz, W., Scholz, H., von Meyerinck, L., Döring, V. & Kalmar, P. (1988) Lancet ii, 936-947. [DOI] [PubMed] [Google Scholar]
- 31.Eschenhagen, T., Mende, U., Diederich, M., Hertle, B., Memmesheimer, C., Pohl, A., Schmitz, W., Scholz, H., Steinfath, M., Böhm, M., et al. (1996) Circulation 93, 763-771. [DOI] [PubMed] [Google Scholar]
- 32.Grimm, M., Gsell, S., Mittmann, C., Nose, M., Scholz, H., Weil, J. & Eschenhagen, T. (1998) J. Mol. Cell. Cardiol. 30, 1917-1928. [DOI] [PubMed] [Google Scholar]
- 33.Rudolph, U., Finegold, M. J., Rich, S. S., Harriman, G. R., Srinivasan, Y., Brabet, P., Boulay, G., Bradley, A. & Birnbaumer, L. (1995) Nat. Genet. 10, 143-150. [DOI] [PubMed] [Google Scholar]
- 34.Rudolph, U., Spicher, K. & Birnbaumer, L. (1996) Proc. Natl. Acad. Sci. USA 93, 3209-3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schröder, F., Handrock, R., Beuckelmann, D. J., Hirt, S., Hullin, R., Priebe, L., Schwinger, R. H. G., Weil, J. & Herzig, S. (1998) Circulation 98, 969-976. [DOI] [PubMed] [Google Scholar]
- 36.Hullin, R., Khan, I. F., Wirtz, S., Mohacsi, P., Varadi, G., Schwartz, A. & Herzig, S. (2003) J. Biol. Chem. 278, 21623-21630. [DOI] [PubMed] [Google Scholar]
- 37.Michels, M., Matthes, J., Handrock, R., Kuchinke, U., Groner, F., Cribbs, L. L., Pereverzev, A., Schneider, T., Perez-Reyes, E. & Herzig, S. (2002) Mol. Pharmacol. 61, 682-694. [DOI] [PubMed] [Google Scholar]
- 38.Chen, F., Spicher, K., Jiang, M., Birnbaumer, L. & Wetzel, G. T. (2001) Am. J. Physiol. 280, H1989-H1995. [DOI] [PubMed] [Google Scholar]
- 39.Jain, M., Lim, C. C., Nagata, K., Davis, V. M., Milstone, D. S., Liao, R. & Mortensen, R. M. (2001) Am. J. Physiol. 280, H569-H575. [DOI] [PubMed] [Google Scholar]
- 40.Gong, H., Adamson, D. L., Ranu, H. K., Koch, W. J., Heubach, J. F., Ravens, U., Zolk, O. & Harding, S. E. (2000) Br. J. Pharmacol. 131, 594-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Offermanns, S. (1999) Naunyn-Schmiedeberg's Arch. Pharmacol. 360, 5-13. [DOI] [PubMed] [Google Scholar]
- 42.Nagata, K., Ye, C., Jain, M., Milstone, D. S., Liao, R. & Mortensen, R. M. (2000) Circ. Res. 87, 903-909. [DOI] [PubMed] [Google Scholar]