Abstract
We and others have shown a persistently high induction of Fra-1 transcription factor (a dimeric partner of AP-1) levels by respiratory carcinogens in pulmonary epithelial cells. Fra-1 is frequently overexpressed in various human tumors and cancer cells. We have recently shown that Fra-1 significantly promotes growth, motility, and invasion of human pulmonary epithelial cells, the precise molecular mechanisms by which this enhancement occurs are unclear. Because matrix metalloproteinases (MMPs) play key roles in wound healing and lung tumor metastasis, we tested the hypothesis that Fra-1 promotes lung epithelial cell motility and invasion via MMP activation. We show here that MMP-9 and MMP-2 activated signaling plays a critical role in regulating Fra-1-induced lung epithelial cell growth and invasion. Ectopic Fra-1 markedly stimulates MMP-2 and MMP-9 mRNA expression. Inhibition of MMP-2 and MMP-9 activity significantly attenuated Fra-1-driven cell motility and invasion. Furthermore, Fra-1 induced EGFR phosphorylation in an MMP-dependent manner, and an EGFR-specific inhibitor was able to block Fra-1-enhanced cell motility and invasion. Taken together, our data suggest that Fra-1 enhances lung cancer epithelial cell motility and invasion by inducing the activity of MMPs, in particular MMP-2 and MMP-9, and EGFR-activated signaling.
Keywords: Lung, AP-1, wound healing, cell motility and invasion, EGFR
INTRODUCTION
Pulmonary epithelial cells are the primary targets of lung carcinogens such as tobacco smoke and asbestos (Wistuba et al., 2002). Dysregulation of proto-oncogene expression and/or activation; and an upregulation of genes that promote cell growth and transformation collectively contribute to tumor development (Sekido et al., 2003). The AP-1 transcription factor constitutes a group of Jun (c-Jun, Jun-B, Jun-D) and Fos (c-Fos, Fos-B, Fra-1 and Fra-2) protooncogenes, which regulate the expression of genes involved in various biological processes, including cell proliferation, differentiation, transformation, inflammation and pulmonary defense (Eferl and Wagner, 2003). However, abnormal induction and/or activation of AP-1 proteins by various toxicants and mitogens contribute to the development of various diseases, including cancer (Eferl and Wagner, 2003). However, the precise role of AP-1 family proteins in the development and severity of lung pathogenesis is unclear.
The expression of Fra-1 is induced in human bronchial epithelial cells by lung carcinogens such as tobacco smoke (Zhang et al., 2005), tumor-promoting phorbol esters and mitogens (Adiseshaiah et al., 2003; Adiseshaiah et al., 2005). Other studies have demonstrated a protracted induction of Fra-1 expression by asbestos, a potent lung carcinogen that causes mesothelioma. Fra-1 induces malignant phenotype of mesothelioma, while silencing of Fra-1 expression reverses such effect (Ramos-Nino et al., 2003; Ramos-Nino et al., 2002). Expression of Fra-1 was significantly elevated in emphysema lungs of mice exposed to cigarette smoke (Hirama et al., 2007). Recently, we have shown that the ectopic expression of Fra-1 in the human pulmonary malignant epithelial cell line, A549, is sufficient to enhance cell motility, invasion and anchorage-independent growth as well as tumor growth and lung metastases (Adiseshaiah et al., 2007). Although Fra-1 is known to regulate epithelial cell motility and invasion in various malignant epithelial cell types, including mammary (Belguise et al., 2005; Kustikova et al., 1998) and colon carcinomas (Pollock et al., 2005; Vial et al., 2003), the precise molecular mechanisms by which it regulates these malignant and invasive phenotypes are not completely understood. Delineating the mechanisms by which Fra-1 promotes epithelial cell motility and invasion may provide a further insight into injury repair as well as tumor growth and metastasis. In this report, we demonstrate that the Fra-1-enhanced motility and invasion of human lung adenocarcinoma cells requires the activity of matrix metalloproteinase (MMPs), in particular MMP-9 and MMP-2, and that their stimulatory effect is exerted through an activation of EGFR signaling.
MATERIALS AND METHODS
Reagents and plasmids
The pharmacological inhibitors GM6001, AG1478, MMP-2 inhibitor I (Cat # 444244) and MMP-9 inhibitor I (cat# 444278) were obtained from Calbiochem (La Jolla, CA). Native and phosphospecific antibodies or EGFR, and pEGFR (Tyr1068) were obtained from Cell Signaling Technology (Beverly, MA) and from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). The β-actin antibody was obtained from Cell Signaling Technology, Inc. (Danvers, MA). MMP-9 luciferase promoter construct, containing −634 to +32 bp 5′-flanking region of the human MMP-9 gene, was obtained from Douglas Boyd (M. D. Anderson Cancer Center, Houston, TX).
Cell culture and transfections
A549, a human non-small cell lung carcinoma cell line that exhibits type II-like alveolar epithelial characteristics (Lieber et al., 1976) was obtained from ATCC and cultured in RPMI medium supplemented with 5% FBS. Cells were transfected with a mouse Fra-1 wild-type cDNA cloned into the pCMV-neo mammalian expression vector (Kustikova et al., 1998). BLAST alignment analysis showed ~90% homology between human and mouse Fra1 protein sequences, with highly conserved leucine zipper domain and DNA binding domain. Stable cell lines expressing Fra-1 (hereafter referred to as A549-F1 cells) or bearing an empty vector (A549-C) were selected in the presence of G418 (600 μg/ml) and Fra-1 mRNA expression was analyzed by RT-PCR analysis using gene-specific primers, whereas protein expression was confirmed by Western analysis using an antibody specific for human and mouse Fra-1 as detailed elsewhere (Adiseshaiah et al., 2007).
RT-PCR analysis
Total RNA was isolated from A549-C or A549-F1 stable transfectants, and PCR was performed using forward (F) and reverse (R) primer sequences for MMP-2 (F, 5′-CACTTTCCTGGGCAACAAAT-3′ and R, 5′-TGATGTCATCCTGGGACAGA-3′), MMP-9 (F, 5′-TTCATCTTCCAAGGCCAATC-3′ and R, 5′-CAGAAGCCCCACTTCTTGTC-3′), and β-actin (F, 5′-GAGAAAATCTGGCACCACAC-3′ and R, 5′-TACCCCTCGTAGATGGGCAC-3′) amplification was used as an internal control. The RT-PCR assays were carried out in duplicate and confirmed in two independent experiments.
Western Analysis
For Western analysis, a comparable amount of whole cell lysate (40 μg) was separated by 10% SDS-PAGE and transferred to a PDVF membrane (Bio-Rad, CA) and probed with specific primary antibodies followed by horseradish peroxidase-conjugated secondary antibodies and visualized using ECL-Plus reagent (Amersham Biosci., NJ).
Reporter assays
Stable A549-C and A549-F1 cells at 80% confluence were transiently transfected with 100 ng of promoter reporter construct. To normalize the transfection efficiency, 1 ng of pRL-TK (Promega Corp, WI) was used as a reference plasmid. After 18–24 h of transfection, cells were lysed and luciferase activities were measured and normalized to that of Renilla luciferase.
Wound-healing assays
Cells were plated at equal density in 6-well plates and grown to confluence. Wounds were then generated with a sterile pipette tip, cells were rinsed two times with PBS and fresh culture medium was added (Adiseshaiah et al., 2007). Areas of wound were marked and photographed at various time points with a phase-contrast microscope (Nikon Eclipse TE 2000-S).
Cell invasion assays
For invasion assays, modified Boyden chamber transwell polycarbonate filters (6.5-mm in diameter, 8-μm pore size, Costar) were coated with 100 μl of Matrigel (Sigma) at a 1:20 dilution in serum-free RPMI 1640 medium and air-dried for 24 h. Cells were placed in serum-free medium for 24 h before experiments. Cells (1 ×105) were layered in the upper compartment of a Transwell inserts. Medium containing 10% FBS was used in the bottom wells. Cells were allowed to incubate for 48 h and invaded cells adherent to the undersurface of the insert were fixed and stained, and readings were taken at OD595 using appropriate controls as described previously (Adiseshaiah et al., 2007).
Detection of MMP activity
An equal density of stable cells (A549-C or A549-F1) was seeded to 80% confluence and incubated for 24 h in serum-free medium. The culture medium was collected and concentrated using Microcon centrifuge tubes (Millipore, MA), and protein was quantified using a standard protein assay (Bio-Rad, CA). MMP activity was assessed fluorimetrically using Enzolyte 520 Generic MMP assay kit (Anaspec, CA) according to the manufacturer’s protocol. This kit uses a 5-FAM/QXL™520 FRET peptide as a substrate with its fluorescence monitored at Ex/Em=490 nm/520 nm upon proteolytic cleavage by MMPs.
Small interfering RNA (siRNA) analysis
Functionally tested siRNAs for EGFR (cat # SI02660140), MMP-2 (cat # SI02780666), MMP-9 (cat # SI03648883), and a scrambled siRNA (cat # 1027280) were purchased from Qiagen (Valencia, CA). A549-C and A549-F1 cells at 30–40% confluence were transfected with siRNAs at 100 nM concentrations using HiPerFect transfection reagent (Cat # 301704) according to manufacturer recommendations. Cells transfected with HiPerFect reagent alone was used as control. Cells were harvested at 48 h and plated on Boyden chambers (25,000 cells/well) for cell migration analysis as detailed above. Remaining cells were used to determine the effect of siRNA on the expression of endogenous MMP-2 and MMP-9 expression by RT-PCR analysis as detailed above.
Statistical analysis
All assays were performed using two or three cell samples, and some experiments were repeated to assure reproducibility. The fold-difference between the treated cells to the untreated cells was calculated (i.e., normalization to the mean of untreated A549-C cells. Therefore the mean of untreated A549-C cells is pre-defined to be unit in each experiment). Data are presented in the form of means ± S.D. Statistical significance between the untreated A549-C and A549-F1 cells and the respective control and treated experimental groups was determined using Student t-tests.
RESULTS
MMPs regulate the Fra-1-induced aggressive phenotype of A549 cells
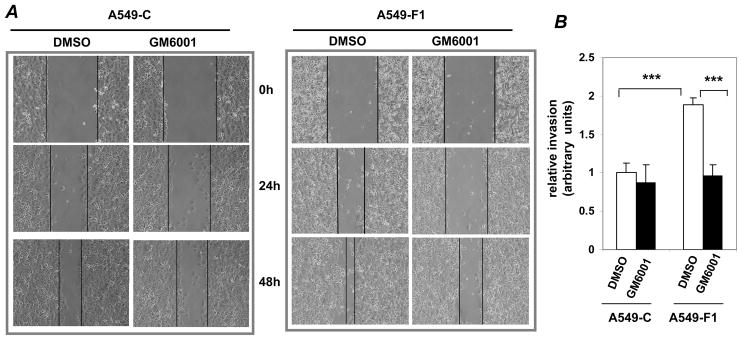
MMPs play key roles in the motility and the invasion of various cancer cells (Coussens et al., 2002). Since the activation of MMPs is known to be regulated by AP-1, we asked whether MMPs mediate the Fra-1-induced cell motility in vitro using A549-F1 and A549-C cells, which express exogenous Fra1 and empty vector, respectively. In contrast to the untreated condition, treatment with GM6001, a pan-MMP inhibitor, significantly delayed the migration of A549-F1 and A549-C cells into the wounded region (Fig. 1A). In the presence of GM6001, the invasive potential of A549-F1 (Fig. 1B, bar 4) was comparable to that of A549-C cells (Fig. 1B, bar 1). Thus, MMPs are critical for Fra-1-induced lung cancer cell motility and invasion.
Figure 1. Effect of an MMP inhibitor on Fra-1-enhanced cell motility and invasion.
In wound-healing assays, a uniform scratch was made in each confluent monolayer culture grown in the presence or presence of GM6001 (10 μM). DMSO was used as a vehicle control. The extent of closure was monitored under phase contrast microscopy at the indicated time points and photographed at different time points as indicated. (B) The effect of GM6001 on cell invasion was assessed using modified Boyden chambers. After 48 h of incubation, cells that migrated through the Matrigel-coated filters were stained with crystal violet, and the absorbance was read at OD595. The values of A549-C cell invasion were set as one arbitrary unit. Each assay was performed using three cell samples, the assay was repeated once (n = 6). Data are represented by mean ± S.D. *** = P<0.005.
Fra-1 activates the expression of select members of the MMP family
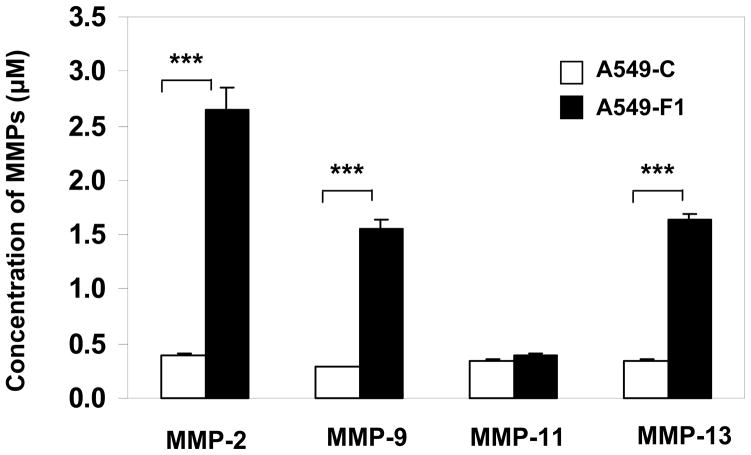
To determine if enhanced invasion of Fra-1-overexpressing cells was associated with an induction of specific members of MMP family, we analyzed the conditioned medium obtained from A549-F1 and A549-C cell cultures using ELISA (Fig. 2). We found a marked increase in the expression of MMPs -2, -9, and -13 in the conditioned medium of A549-F1 cells (filled bars) compared to A549-C cells (open bars). There was no difference between A549-F1 and A549-C cells with respect to MMP-11 levels. These results suggest that Fra-1 distinctly induces specific MMPs in human lung cancer epithelial cells.
Figure 2. Fra-1 strongly activates MMP family members.
Conditioned medium from the stable transfectants was used to analyze the activation of MMPs by fluorometrically using Enzolyte 520 Generic MMP assay kit (Anaspec, CA). The levels of MMP expression (μM) was calculated using standard graphs. Each assay was performed using three cell samples (n = 3). Data are represented by mean ± S.D. *** = P< 0.005.
Inhibition of MMP-2 and MMP-9 abrogates the Fra-1-induced invasive potential of A549 cells
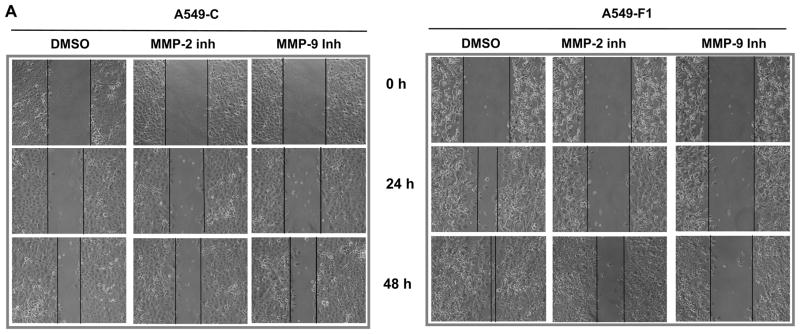
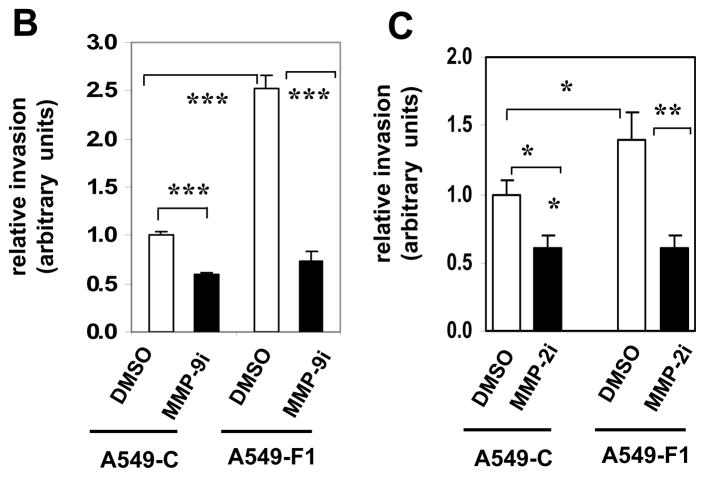
Since pharmacological inhibition of MMP activity blocked Fra-1-driven migration and invasion of A549 cells (Fig. 1), and Fra-1 induces the expression of various forms of MMPs, we next examined the role of MMP-2 and MMP-9 in the present study using small-molecule inhibitors. We have primarily focused on these MMPs because they are known to regulate lung tumorigenesis in vivo (Chetty et al., 2006; Hiratsuka et al., 2002; Itoh et al., 1999; Rao et al., 2005). As shown in Fig. 3, treatment of cells with an MMP-2 or MMP-9 inhibitor blocked the ability of A549-F1 cells to migrate effectively into the wound (panel A). However, the MMP-9 inhibitor had a greater effect than did the MMP-2 inhibitor, which had a more modest but significant effect. Treatment of cells with the MMP-2 or the MMP-9 inhibitor markedly suppressed the motility of A549-F1 cells, as analyzed in Boyden chamber assays (panel B). No such effect was noted in cells treated with vehicle (DMSO) alone. These data suggest that MMP-2 and MMP-9 act as major downstream effectors that regulate Fra-1-induced cell motility and invasiveness.
Figure 3. Effect of an MMP-2 and MMP-9 inhibitor on Fra-1 enhanced cell motility and invasion.
(A) A scratch was made in the presence or absence of 5 μM concentration of MMP-2 or MMP-9 inhibitors and photographed at different time points. DMSO was used as the vehicle control. Images are representatives of three independent experiments done in two cell samples. (B) Cells were seeded on the apical surface of the modified Boyden chambers coated with Matrigel in the absence or presence of MMP-9 inhibitor ((MMP-9i, 5 μM) for 48 h. The cells that migrated through the Matrigel-coated inserts were stained with crystal violet and quantified. The values of A549-C cell invasion were set as one arbitrary unit. Each assay was performed using three cell samples, the assay was repeated once (n = 6). Data are represented by mean ± S.D. *** = P< 0.005. (C) The effect of MMP-2 inhibitor (MMP-2i, 5 μM) on Fra-1 stimulated cell invasion. The values of A549-C cell invasion were set as one arbitrary unit. Each assay was performed using three cell samples, the assay was repeated once (n = 4). Data are means ± S.D. * = P< 0.05. **P< 0.01, and *** = P< 0.005.
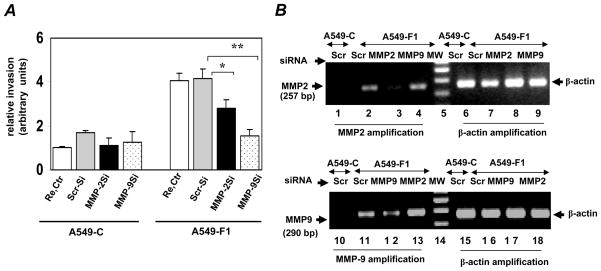
To confirm this result and to address the possibility that the pharmacologic inhibition we observed was non-specific, we have utilized an siRNA-mediated knockdown approach. Consistent with the results of our pharmacologic studies, we found that knockdown of MMP-2 or MMP-9 expression using specific siRNA sequences significantly attenuated the Fra1-enhanced migration of A549-F1 cells, but these sequences had no significant effect on the migration of A549-C cells (Fig. 4A). Knockdown of MMP-9 had a greater (70%) inhibitory effect on A549-F1 cell invasion than did MMP-2 knockdown (30%). To further assess the efficiency of functionally validated siRNA sequences in our experimental system, we analyzed the mRNA expression levels of MMP-2 and MMP-9 after transfection (Fig. 4B). For these experiments, total RNA was isolated from siRNA-transfected cell cultures. Because of the low or undetectable levels of MMP-2 and MMP-9 expression in vector-transfected A549-C cells, we performed RT-PCR analysis only in A549-F1 cells. Indeed, as shown in Fig. 4, panel B, MMP-2 and MMP-9 levels were very low in A549-C cells transfected with scrambled (control) siRNA (lanes 1 and 7), as compared to their high level of expression in A549-F1 cells transfected with the same control siRNA (lanes 2 and 8). The MMP-2- (lane 3) and MMP-9- (lane 9) specific siRNA sequences specifically knocked down the expression levels of their respective targets, as compared to cells that were transfected with the scrambled siRNA sequence. MMP-2 siRNA had no effect on MMP-9 mRNA expression and vice versa, further demonstrating the specificity of the MMP-2- and MMP-9-directed siRNA sequences used in our experimental system.
Figure 4.
MMP-2 and MMP-2 required for Fra-1 stimulated cell invasion. (A) A549-C and A549-F1 cells were transfected with the scrambled (Scr-si), MMP-2, or MMP-9 siRNA (100 nM) sequences as described in Methods. Transfection reagent was also used as control (Re. Ctr). After 48 h of transfection, cells were trypsinized and equal number of cells then plated on Boyden chambers to assess the cell invasion. After 48 h incubation, cells that migrated through the filters were stained with crystal violet, and the absorbance was read at OD595. The absorbance value for reagent transfected A549-C cells set to one unit. Each assay was performed using three cell samples (n = 3). Values are mean ± S. D. * = P≤ 0.05 and ** = P≤ 0.001. (B) Validation of MMP-2 and MMP-9 knockdown in siRNA transfected cells. Aliquot of the cell cultures transfected with siRNAs (as in panel A) were lysed in Trizol for RNA isolation. To confirm the knockdown of endogenous MMP-2 and MMP-9 expression, a semi-quantitative RT-PCR was performed using gene specific primers for 35 and 40 cycles, respectively. The quantity of cDNA used MMP-9 amplification in PCR amplification was three times greater than that used for MMP-2.
Fra-1 stimulates MMP-9 expression at the transcriptional level
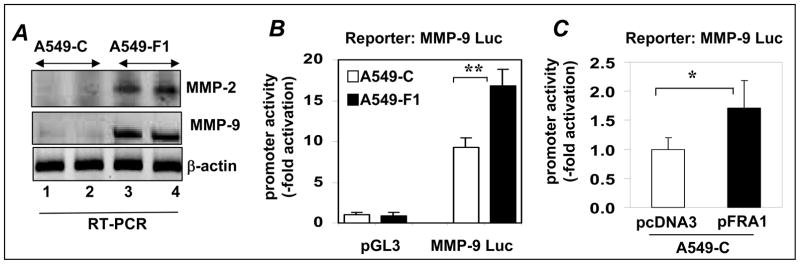
The levels of endogenous MMP-2 and MMP-9 mRNA were markedly higher in A549-F1 cells than in A549-C cells (Fig. 5A). To determine if Fra-1 stimulates the expression of MMP-9 at the transcription level, MMP-9 promoter activity in A549-C and A549-F1 cells was determined by transient transfection analysis. As shown in Fig. 5B, a two-fold higher induction of MMP-9 promoter was noted in A549-F1 cells than in A549-C cells suggesting that Fra-1 stimulates MMP-9 gene transcription. To test if this is a direct effect, we transiently transfected parental A549-C cells with an MMP-9 promoter reporter construct along with an empty (pcDNA) or a Fra-1 expression vector; and luciferase activity was quantified. As shown in Fig. 5C, Fra-1 significantly stimulated MMP-9 promoter activity compared to empty vector control. FRA-1- Fra-1 enhanced MMP promoter activity do not correlate with that of inducible MMP9 mRNA expression levels, suggesting that additional Fra-1-regulated mechanisms may contribute to MMP-9 gene regulation.
Figure 5. Fra-1 stimulates MMP-9 transcription.
(A) MMP-2 and MMP-9 mRNA expression in A549-C and A549-F1 cells was quantified by RT-PCR using β-actin as a reference. (B) A549-C and A549-F1 cells were cotransfected with 100 ng of MMP-9 promoter reporter construct and 1 ng of pRL-TK plasmid. The luciferase activity was measured and normalized to that of Renilla activity. The values obtained from the empty luciferase vector (pGL3) were set as 1.0. Data are means ± S.D of nine independent samples (n=9) from at least three independent experiments. The values obtained from the PGFL-3 transfected A549-C sample were set as 1.0. Each assay was performed using three cell samples (n = 3). Data are means ± S. D. ** = P≤ 0.01. (C) A549-C cells were co-transfected with 100 ng of MMP-9 Luc along with 100 ng of Fra-1 expression vector (pFra-1) or empty vector (pcDNA3). pRL-TK plasmid (1 ng) was used to normalize transfection variations between samples. The values obtained from the pcDNA3 transfected sample (pcDNA3) were set as 1.0. Each assay was performed using three cell samples (n = 3). Data are mean ± S.D. * = P< 0.05.
The EGFR acts as an effector of MMP-9 and promotes Fra-1-induced lung cancer cell phenotypes
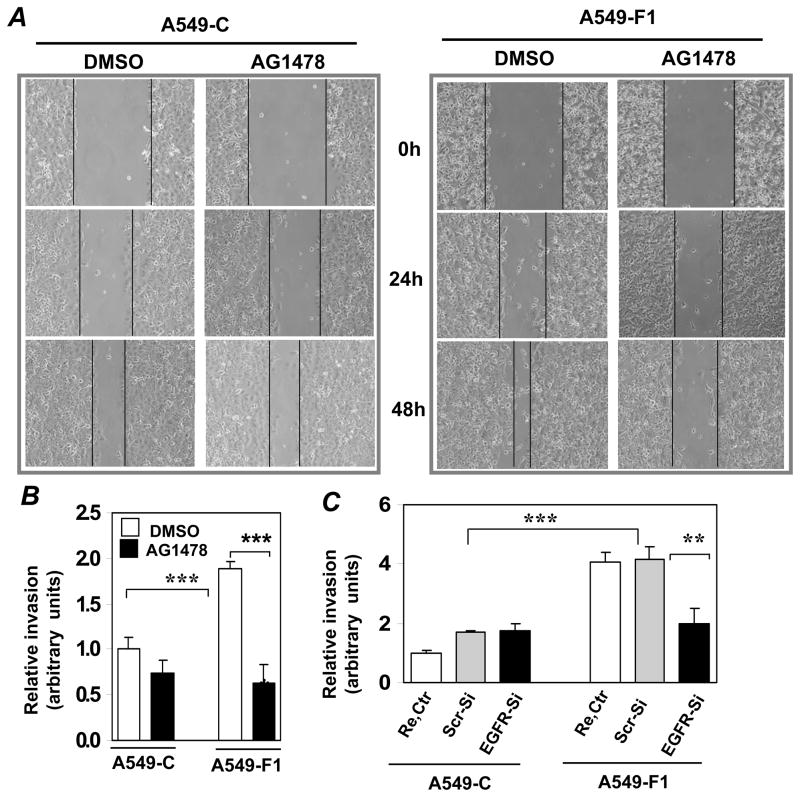
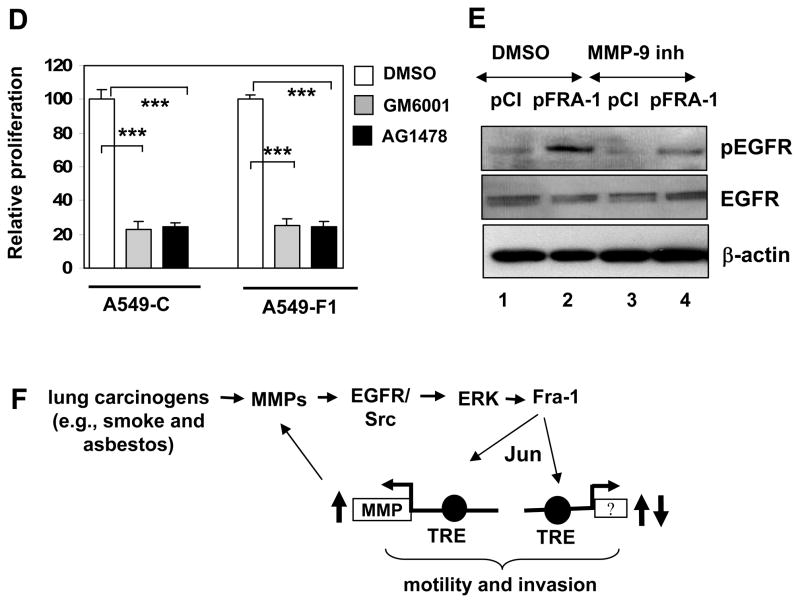
MMP activation has been implicated in receptor-dependent intracellular signaling that leads to transcription factor activation and gene expression (Coussens et al., 2002). One of the well-known targets of MMP is the EGFR (Fischer et al., 2003). An aberrant activation and/or expression of EGFRs have been detected in lung tumors and cancer cells (Franklin et al., 2002). To determine the role of EGFR-activated signaling, we treated the cells with AG1478 and analyzed its effect on Fra-1-enhanced cell motility (Fig. 6A) and invasion (Fig. 6B). Treatment of the A549-F1 cells with the EGFR inhibitor blocked the Fra-1-induced cell motility and invasion. Consistent with this result, we found that knockdown of EGFR expression using functionally validated specific siRNA sequence significantly attenuated Fra1-enhanced cell migration (Fig. 6C, bar 6), as compared to scrambled siRNA transfected A549-F1 cells (Fig. 6C, bar 5). But EGFR knockdown had no significant effect on A549-C cell migration (compare bar 2 and bar 3, Fig. 6C). To determine whether EGFR inhibition affects cell proliferation, an equal number of viable A549-C and A549-F1 cells were cultured in serum-containing medium in the presence or absence of GM6001 or AG1478 and the cell proliferation rate was measured after 48 h-incubation (Fig. 6D). Both inhibitors (filled bars) strikingly blocked the cell proliferation rate of both A549-C and A549-F1 cells, as compared to vehicle (DMSO)-treated respective controls (open bar). Consistent with previous result, no significant difference in proliferation rate was observed when cultured in serum medium (data not shown) (Adiseshaiah et al., 2007). Collectively these results demonstrate that MMP-EGFR activated signaling regulates both lung cancer cell proliferation and invasion. We next examined the status of EGFR activation in A549-F1 and A549-C cells using a Western analysis with phospho-EGFR-specific antibodies. An elevated level of EGFR phosphorylation was found in A549-F1 cells (Fig. 6E, lane 2), as compared to A549-C cells (lane 1). Treatment of cells with the MMP-9 inhibitor significantly attenuated the EGFR phosphorylation in A549-F1 cells (lane 4).
Figure 6. Effect of EGFR inhibitor on Fra-1-enhanced cell motility and invasion.
(A) Wound healing assays were performed as detailed above in the presence of AG1478 (2 μM). Images are representative of three independent experiments done in duplicate. (B) Cell invasion was assessed using modified Boyden chamber assay as detailed above. Each assay was performed using three cell samples, the assay was repeated once (n = 6). Data are means ± S.D. *** = P< 0.005. (C) A549-C and A549-F1 cells were transfected with transfection reagent (Re. Ctr), or scrambled (Scr-si) or EGFR-specific (EGFR-si,) siRNA (100 nM) sequences. After 48 h of transfection, cells were trypsinized and equal number of cells then plated on Boyden chambers, and cell invasion was quantified as in Fig. 4A. The absorbance value for reagent transfected A549-C control cells (Re.Ctr) set to one unit. Each assay was performed using three cell samples (n = 3). Data are mean ± S.D. **P< 0.01 and *** = P< 0.005. (D) A549-C and A549-F1 cells were seeded in a 48-well plate and maintained on 5% serum in the presence or absence of GM6001 or AG1478. After 48-h incubation, cell numbers were quantified using Cell Titer Glo reagent. Each assay was performed using six cell samples (n = 6). Data are means ± S.D. *** = P< 0.005. (E) Whole-cell lysates (40 μg) prepared from A549-C and A549-F1 cells treated with GM6001 or DMSO were subjected to Western analysis using anti-EGFR and anti-β-actin antibodies as indicated. (F) Working model of Fra-1-stimulated MMP activation and subsequent EGFR-activated MEK-ERK signaling that enhances carcinogen-induced lung cancer cell progression. Question mark indicates other yet unidentified target genes of Fra-1 involved in lung cancer cell progression.
DISCUSSION
Considerable progress has been made in our understanding of the components that regulate the motility and invasive phenotypes of various tumorigenic cells (Guo and Giancotti, 2004). Among the various effectors identified, a prominent role for members of the MMP family of proteins in the regulation of tumor cell progression and invasion has been established in animal models (Coussens, 2002 #1466}. Moreover, members of the MMP family are frequently overexpressed in various human cancer cells/tumors. Thus, delineating the upstream mechanisms activating MMPs will be of great importance in providing further insights into lung cancer development. The present study, for the first time demonstrates a prominent role for MMP-2 and MMP-9 in promoting Fra-1 proto-oncogene enhanced human lung epithelial adenocarcinoma cell growth and invasion. Various mitogens and carcinogens that promote growth, progression and metastasis potently stimulate both MMPs and Fra-1 induction in non-malignant and malignant lung epithelial cells. These results suggest that the activation of Fra-1 transcription factor and MMPs may play a critical role in enhancing lung tumorigenicity. Fra-1 strongly stimulates MMP-2 and MMP-9 expression (Fig. 4 and 5), and a rise in their expression was correlated with cell motility and invasion (Fig. 1). Conversely, blocking MMP-2 or MMP-9 activity with a chemical inhibitor (Fig. 4) or specifically knockdown of their expression by SiRNA-mediated approach significantly inhibited this process (Fig. 4). Thus, MMP-2 and MMP-9 act as major effectors of Fra-1-initiated lung cancer cell motility and invasion. Our findings are consistent with a recent report that MMP-9 is frequently overexpressed in human lung tumors (Gouyer et al., 2005) and expression of antisense MMP-9 transcripts inhibited lung cancer cell invasive phenotype (Rao et al., 2005). Importantly, tumor progression and invasion are suppressed in mice lacking the MMP-9 gene in an experimental model of lung metastasis (Hiratsuka et al., 2002; Itoh et al., 1999). MMP-2 overexpression is also frequently associated with the malignant phenotype (Hrabec et al., 2002; Lin et al., 2004; Yamamura et al., 2002) and SiRNA-mediated knockdown of MMP-2 expression in A549 cells suppresses tumor growth and lung metastasis in mice (Chetty et al., 2006). However, MMP-2 knockdown showed only a modest but significant effect on Fra-1-enhanced cell motility and invasion as compared to MMP-9 knockdown suggesting that MMP-9 plays a prominent role in promoting Fra-1 mediated effects in our model.
MMP family member gene expression is known to be regulated by AP-1 family members (Chakraborti et al., 2003). Treatment of cells with tumor promoting agents such as phorbol esters, which potential activates Jun and Fos proteins, strongly induces MMP family member transcription. Promoter-reporter analysis and DNA binding assays revealed the presence of at least a functional AP-1 site in the 5′-flanking region of several MMP family members (Benbow and Brinckerhoff, 1997). Among them, MMP-1, MMP-2, and MMP-9 transcription has been shown to be regulated AP-1 complex composed of Fra-1 in different cell types (Belguise et al., 2005; Bergman et al., 2003; Hong et al., 2005; Tower et al., 2003; Zhang et al., 2004). Our results demonstrate that Fra-1 stimulates MMP-9 promoter activity, but the level of induction does not mimic that of high level mRNA expression suggesting that other regulatory mechanisms also contributes to Fra-1 inducible MMP-9 expression. Elevated levels of MMP-2 mRNA expression observed in A549-F1 cells; whether MMP-2 is a direct transcriptional target of Fra-1 remains to be established.
EGFR plays a central role in various cellular processes, and its overexpression and/or activation have been implicated in various diseases, including lung cancer (Fischer et al., 2003). Our previous findings indicate that EGFR-mediated MAP kinase activation is an obligatory step in regulating cigarette smoke-stimulated Fra-1 induction in human bronchial epithelial cells (Zhang et al., 2005). Likewise, asbestos acts via EGFR-activated signaling to induce the expression of Fra-1 in murine alveolar epithelial cells (Scapoli et al., 2004). In the present study, we have shown that the EGFR inhibitor AG1478 can completely block Fra-1-enhanced lung cancer cell motility and invasion (Fig. 5A and 5B), suggesting an important role for this receptor-activated signaling in the process. Our results are in agreement with previous reports that EGFR-activated signaling regulates both motility and invasion of cancer cells in various tissues, including the lung (Festuccia et al., 2005; Lu et al., 2001; Xue et al., 2006; Zhang et al., 1996). Importantly, Fra-1 overexpression resulted in an enhanced EGFR phosphorylation without affecting its expression levels (Fig. 5C). The inhibition of MMP-9 activity with a specific inhibitor significantly, but not completely, attenuated Fra-1-enhanced EGFR phosphorylation indicating that Fra-1 stimulates EGFR activation through MMP-9-dependent and -independent manner. We found that Fra-1 stimulates Src activation and Src inhibitor PP2 blocks A549-F1 enhanced cell motility (data not shown). These observations suggest Src may play role in regulating Fra-1 driven A549 cell motility. In agreement with this notion, several studies have demonstrated a prominent role for Src in regulating cancer cell invasion (Park et al., 2007; Summy and Gallick, 2003). A cross talk between EGFR and Src kinases has been well established by several studies (Fischer et al., 2003). Although Fra-1 stimulated both EGFR and Src activation, it is unclear whether such a cross talk exists between these two kinases. In addition, the precise mechanisms (MMP-dependent and -independent) controlling the activation of EGFR and Src kinases and downstream targets that regulate cell motility and invasion in our model warrants a further investigation.
We found that the inhibition of EGFR or MMP activity markedly blocked proliferation of both empty vector (A549-C)- and Fra1-vector-transfected (A549-F1) cells (Fig. 6D). However, although inhibition of MMP activity had no significant effect on A549-C cell migration, it markedly blocked the invasive potential of A549-F1 cells (Fig. 1B). Intriguingly, neither EGFR inhibition nor its knockdown had any significant effect on A549-C cell migration (Fig. 6B and 6C), but both markedly blocked the migration of A549-F1 cells. Based on these results, we propose that elevated levels of MMP activity induced by FRA-1 are required for EGFR-mediated A549-F1 cell invasion, while the basal-level EGFR activity modulated by constitutive or low-level MMP activity regulates cell proliferation. In other words, EGFR acts as one of the major nodal points to regulate both proliferation and cell invasion modulated by MMPs.
In summary, our study indicates that MMP- and EGFR-activated signaling is a necessary component of Fra-1-enhanced lung cancer cell motility and invasion. We have recently shown that exposure to cigarette smoke induces Fra-1 expression in an MMP-dependent manner in non-malignant human lung epithelial cells (Zhang et al., 2005) and that EGFR-activated MEK/ERK signaling plays a crucial role in this process (Zhang et al., 2006). Our current findings demonstrate that ectopic Fra-1 expression alone can stimulate MMP expression and EGFR activation. Inhibition of either MMP or EGFR activity suppressed Fra-1-enhanced lung cancer cell motility and invasion. Inhibition of MMP activity blocked the induction of increased EGFR phosphorylation in A549-F1 cells, suggesting that Fra-1 regulates EGFR activity by acting via MMPs. We further observed that the inhibition of EGFR or MMP activity significantly attenuated the expression of ectopic Fra-1 in A549-F1 cells (data not shown). Based on these observations and previous results, we propose the existence of a positive feedback loop (see Fig. 6F) between Fra-1-stimulated MMP activation and subsequent EGFR-activated MEK-ERK signaling (and potentially other pathways) further enhances the activation of Fra-1, as well as other transcription factors. Dimerization of Fra-1, particularly with the Jun family of proteins, enhances the transcription of various gene network genes, including the MMPs. The feedback loop that we have postulated here may play a role in enhancing the lung tumorogenicity that is promoted by lung carcinogens such as cigarette smoke. Further studies are warranted in mouse model systems in which Fra-1 is conditionally deleted in the lung epithelium, as a means of firmly validating this notion and establishing other functional roles of this transcription factor in lung cancer development and progression in vivo.
Acknowledgments
Contract grant sponsor: NIH and contract grant number ES011863 and HL66109 (to SPR)
Contract grant sponsor: NCI and contract grant number CA78282 and CA105005 (to DVK).
The authors would like to thank Eugene Tulchinsky (University of Leicester, UK) and Douglas Boyd (M. D. Anderson Cancer Center, Houston, TX) for providing the Fra-1 expression vector and MMP-9 promoter construct used in this study. We thank Sining Chen (The Johns Hopkins Bloomberg School of Public Health, Baltimore, MD) for assistance in statistical analysis. This work was supported by NIH grant ES011863 and HL 66109 (to SPR) and DVK is supported by CA78282 and CA105005.
References
- Adiseshaiah P, Lindner DJ, Kalvakolanu DV, Reddy SP. FRA-1 proto-oncogene induces lung epithelial cell invasion and anchorage-independent growth in vitro, but is insufficient to promote tumor growth in vivo. Cancer Res. 2007;67(13):6204–6211. doi: 10.1158/0008-5472.CAN-06-4687. [DOI] [PubMed] [Google Scholar]
- Adiseshaiah P, Papaiahgari SR, Vuong H, Kalvakolanu DV, Reddy SP. Multiple cis-Elements Mediate the Transcriptional Activation of Human fra-1 by 12-O-Tetradecanoylphorbol-13-acetate in Bronchial Epithelial Cells. J Biol Chem. 2003;278(48):47423–47433. doi: 10.1074/jbc.M303505200. [DOI] [PubMed] [Google Scholar]
- Adiseshaiah P, Peddakama S, Zhang Q, Kalvakolanu DV, Reddy SP. Mitogen regulated induction of FRA-1 proto-oncogene is controlled by the transcription factors binding to both serum and TPA response elements. Oncogene. 2005;24(25):4193–4120. doi: 10.1038/sj.onc.1208583. [DOI] [PubMed] [Google Scholar]
- Belguise K, Kersual N, Galtier F, Chalbos D. FRA-1 expression level regulates proliferation and invasiveness of breast cancer cells. Oncogene. 2005;24(8):1434–1444. doi: 10.1038/sj.onc.1208312. [DOI] [PubMed] [Google Scholar]
- Benbow U, Brinckerhoff CE. The AP-1 site and MMP gene regulation: what is all the fuss about? Matrix Biol. 1997;15(8–9):519–526. doi: 10.1016/s0945-053x(97)90026-3. [DOI] [PubMed] [Google Scholar]
- Bergman MR, Cheng S, Honbo N, Piacentini L, Karliner JS, Lovett DH. A functional activating protein 1 (AP-1) site regulates matrix metalloproteinase 2 (MMP-2) transcription by cardiac cells through interactions with JunB-Fra1 and JunB-FosB heterodimers. Biochem J. 2003;369(Pt 3):485–496. doi: 10.1042/BJ20020707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborti S, Mandal M, Das S, Mandal A, Chakraborti T. Regulation of matrix metalloproteinases: an overview. Mol Cell Biochem. 2003;253(1–2):269–285. doi: 10.1023/a:1026028303196. [DOI] [PubMed] [Google Scholar]
- Chetty C, Bhoopathi P, Joseph P, Chittivelu S, Rao JS, Lakka S. Adenovirus-mediated small interfering RNA against matrix metalloproteinase-2 suppresses tumor growth and lung metastasis in mice. Mol Cancer Ther. 2006;5(9):2289–2299. doi: 10.1158/1535-7163.MCT-06-0169. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295(5564):2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- Eferl R, Wagner EF. AP-1: a double-edged sword in tumorigenesis. Nat Rev Cancer. 2003;3(11):859–868. doi: 10.1038/nrc1209. [DOI] [PubMed] [Google Scholar]
- Festuccia C, Angelucci A, Gravina GL, Biordi L, Millimaggi D, Muzi P, Vicentini C, Bologna M. Epidermal growth factor modulates prostate cancer cell invasiveness regulating urokinase-type plasminogen activator activity. EGF-receptor inhibition may prevent tumor cell dissemination. Thromb Haemost. 2005;93(5):964–975. doi: 10.1160/TH04-09-0637. [DOI] [PubMed] [Google Scholar]
- Fischer OM, Hart S, Gschwind A, Ullrich A. EGFR signal transactivation in cancer cells. Biochem Soc Trans. 2003;31(Pt 6):1203–1208. doi: 10.1042/bst0311203. [DOI] [PubMed] [Google Scholar]
- Franklin WA, Veve R, Hirsch FR, Helfrich BA, Bunn PA., Jr Epidermal growth factor receptor family in lung cancer and premalignancy. Semin Oncol. 2002;29(1 Suppl 4):3–14. doi: 10.1053/sonc.2002.31520. [DOI] [PubMed] [Google Scholar]
- Gouyer V, Conti M, Devos P, Zerimech F, Copin MC, Creme E, Wurtz A, Porte H, Huet G. Tissue inhibitor of metalloproteinase 1 is an independent predictor of prognosis in patients with nonsmall cell lung carcinoma who undergo resection with curative intent. Cancer. 2005;103(8):1676–1684. doi: 10.1002/cncr.20965. [DOI] [PubMed] [Google Scholar]
- Guo W, Giancotti FG. Integrin signalling during tumour progression. Nat Rev Mol Cell Biol. 2004;5(10):816–826. doi: 10.1038/nrm1490. [DOI] [PubMed] [Google Scholar]
- Hirama N, Shibata Y, Otake K, Machiya J, Wada T, Inoue S, Abe S, Takabatake N, Sata M, Kubota I. Increased surfactant protein-D and foamy macrophages in smoking-induced mouse emphysema. Respirology. 2007;12(2):191–201. doi: 10.1111/j.1440-1843.2006.01009.x. [DOI] [PubMed] [Google Scholar]
- Hiratsuka S, Nakamura K, Iwai S, Murakami M, Itoh T, Kijima H, Shipley JM, Senior RM, Shibuya M. MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer Cell. 2002;2(4):289–300. doi: 10.1016/s1535-6108(02)00153-8. [DOI] [PubMed] [Google Scholar]
- Hong S, Park KK, Magae J, Ando K, Lee TS, Kwon TK, Kwak JY, Kim CH, Chang YC. Ascochlorin inhibits matrix metalloproteinase-9 expression by suppressing activator protein-1-mediated gene expression through the ERK1/2 signaling pathway: inhibitory effects of ascochlorin on the invasion of renal carcinoma cells. J Biol Chem. 2005;280(26):25202–25209. doi: 10.1074/jbc.M413985200. [DOI] [PubMed] [Google Scholar]
- Hrabec E, Strek M, Nowak D, Greger J, Suwalski M, Hrabec Z. Activity of type IV collagenases (MMP-2 and MMP-9) in primary pulmonary carcinomas: a quantitative analysis. J Cancer Res Clin Oncol. 2002;128(4):197–204. doi: 10.1007/s00432-001-0320-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T, Tanioka M, Matsuda H, Nishimoto H, Yoshioka T, Suzuki R, Uehira M. Experimental metastasis is suppressed in MMP-9-deficient mice. Clin Exp Metastasis. 1999;17(2):177–181. doi: 10.1023/a:1006603723759. [DOI] [PubMed] [Google Scholar]
- Kustikova O, Kramerov D, Grigorian M, Berezin V, Bock E, Lukanidin E, Tulchinsky E. Fra-1 induces morphological transformation and increases in vitro invasiveness and motility of epithelioid adenocarcinoma cells. Mol Cell Biol. 1998;18(12):7095–7105. doi: 10.1128/mcb.18.12.7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber M, Smith B, Szakal A, Nelson-Rees W, Todaro G. A continuous tumor-cell line from a human lung carcinoma with properties of type II alveolar epithelial cells. Int J Cancer. 1976;17(1):62–70. doi: 10.1002/ijc.2910170110. [DOI] [PubMed] [Google Scholar]
- Lin TS, Chiou SH, Wang LS, Huang HH, Chiang SF, Shih AY, Chen YL, Chen CY, Hsu CP, Hsu NY, Chou MC, Kuo SJ, Chow KC. Expression spectra of matrix metalloproteinases in metastatic non-small cell lung cancer. Oncol Rep. 2004;12(4):717–723. [PubMed] [Google Scholar]
- Lu Z, Jiang G, Blume-Jensen P, Hunter T. Epidermal growth factor-induced tumor cell invasion and metastasis initiated by dephosphorylation and downregulation of focal adhesion kinase. Mol Cell Biol. 2001;21(12):4016–4031. doi: 10.1128/MCB.21.12.4016-4031.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SI, Shah AN, Zhang J, Gallick GE. Regulation of angiogenesis and vascular permeability by Src family kinases: opportunities for therapeutic treatment of solid tumors. Expert Opin Ther Targets. 2007;11(9):1207–1217. doi: 10.1517/14728222.11.9.1207. [DOI] [PubMed] [Google Scholar]
- Pollock CB, Shirasawa S, Sasazuki T, Kolch W, Dhillon AS. Oncogenic K-RAS is required to maintain changes in cytoskeletal organization, adhesion, and motility in colon cancer cells. Cancer Res. 2005;65(4):1244–1250. doi: 10.1158/0008-5472.CAN-04-1911. [DOI] [PubMed] [Google Scholar]
- Ramos-Nino ME, Scapoli L, Martinelli M, Land S, Mossman BT. Microarray Analysis and RNA Silencing Link fra-1 to cd44 and c-met Expression in Mesothelioma. Cancer Res. 2003;63(13):3539–3545. [PubMed] [Google Scholar]
- Ramos-Nino ME, Timblin CR, Mossman BT. Mesothelial cell transformation requires increased AP-1 binding activity and ERK-dependent Fra-1 expression. Cancer Res. 2002;62(21):6065–6069. [PubMed] [Google Scholar]
- Rao JS, Gondi C, Chetty C, Chittivelu S, Joseph PA, Lakka SS. Inhibition of invasion, angiogenesis, tumor growth, and metastasis by adenovirus-mediated transfer of antisense uPAR and MMP-9 in non-small cell lung cancer cells. Mol Cancer Ther. 2005;4(9):1399–1408. doi: 10.1158/1535-7163.MCT-05-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scapoli L, Ramos-Nino ME, Martinelli M, Mossman BT. Src-dependent ERK5 and Src/EGFR-dependent ERK1/2 activation is required for cell proliferation by asbestos. Oncogene. 2004;23(3):805–813. doi: 10.1038/sj.onc.1207163. [DOI] [PubMed] [Google Scholar]
- Sekido Y, Fong KM, Minna JD. Molecular genetics of lung cancer. Annu Rev Med. 2003;54:73–87. doi: 10.1146/annurev.med.54.101601.152202. [DOI] [PubMed] [Google Scholar]
- Summy JM, Gallick GE. Src family kinases in tumor progression and metastasis. Cancer Metastasis Rev. 2003;22(4):337–358. doi: 10.1023/a:1023772912750. [DOI] [PubMed] [Google Scholar]
- Tower GB, Coon CI, Belguise K, Chalbos D, Brinckerhoff CE. Fra-1 targets the AP-1 site/2G single nucleotide polymorphism (ETS site) in the MMP-1 promoter. Eur J Biochem. 2003;270(20):4216–4225. doi: 10.1046/j.1432-1033.2003.03821.x. [DOI] [PubMed] [Google Scholar]
- Vial E, Sahai E, Marshall CJ. ERK-MAPK signaling coordinately regulates activity of Rac1 and RhoA for tumor cell motility. Cancer Cell. 2003;4(1):67–79. doi: 10.1016/s1535-6108(03)00162-4. [DOI] [PubMed] [Google Scholar]
- Wistuba, Mao L, Gazdar AF. Smoking molecular damage in bronchial epithelium. Oncogene. 2002;21(48):7298–7306. doi: 10.1038/sj.onc.1205806. [DOI] [PubMed] [Google Scholar]
- Xue C, Wyckoff J, Liang F, Sidani M, Violini S, Tsai KL, Zhang ZY, Sahai E, Condeelis J, Segall JE. Epidermal growth factor receptor overexpression results in increased tumor cell motility in vivo coordinately with enhanced intravasation and metastasis. Cancer Res. 2006;66(1):192–197. doi: 10.1158/0008-5472.CAN-05-1242. [DOI] [PubMed] [Google Scholar]
- Yamamura T, Nakanishi K, Hiroi S, Kumaki F, Sato H, Aida S, Kawai T. Expression of membrane-type-1-matrix metalloproteinase and metalloproteinase-2 in nonsmall cell lung carcinomas. Lung Cancer. 2002;35(3):249–255. doi: 10.1016/s0169-5002(01)00431-7. [DOI] [PubMed] [Google Scholar]
- Zhang M, Singh RK, Wang MH, Wells A, Siegal GP. Epidermal growth factor modulates cell attachment to hyaluronic acid by the cell surface glycoprotein CD44. Clin Exp Metastasis. 1996;14(3):268–276. doi: 10.1007/BF00053900. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Adiseshaiah P, Kalvakolanu DV, Reddy SP. A phosphatidylinositol 3-kinase regulated Akt independent signaling promotes cigarette smoke induced FRA-1 expression. J Biol Chem. 2006 doi: 10.1074/jbc.M513008200. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Adiseshaiah P, Reddy SP. Matrix metalloproteinase/epidermal growth factor receptor/mitogen-activated protein kinase signaling regulate fra-1 induction by cigarette smoke in lung epithelial cells. Am J Respir Cell Mol Biol. 2005;32(1):72–81. doi: 10.1165/rcmb.2004-0198OC. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Kleeberger SR, Reddy SP. DEP-induced fra-1 expression correlates with a distinct activation of AP-1-dependent gene transcription in the lung. Am J Physiol Lung Cell Mol Physiol. 2004;286(2):L427–436. doi: 10.1152/ajplung.00221.2003. [DOI] [PubMed] [Google Scholar]