Abstract
Traumatic brain injury and stroke are major causes of mortality and morbidity worldwide. Unfortunately, almost all phase-III neuroprotective clinical trials for stroke and traumatic brain injury have shown no benefits; this has raised concerns regarding neuroprotective strategy alone as a therapy for acute brain injuries. There is therefore a compelling need to develop treatments that promote the repair and regeneration of injured brain tissue and functional recovery. Recent findings suggest that strategies to enhance angiogenesis and neurogenesis for brain injuries may provide promising opportunities to improve clinical outcomes during brain functional recovery. This article reviews current data on angiogenesis and neurogenesis in the adult brain after stroke and traumatic brain injury. Select cell-based and pharmacological therapies that promote angiogenesis and neurogenesis designed to restore neurological function after brain injuries are described. These findings highlight the need for a better understanding of injury- and therapy-induced angiogenesis and neurogenesis in the adult and suggest that the manipulation of endogenous neural precursors and endothelial cells is a potential therapy for brain injury.
Keywords: Angiogenesis, functional recovery, neurogenesis, neuroprotection, neurorestoration, neurovascular unit, stroke, traumatic brain injury
Introduction
Brain injuries caused by stroke and trauma remain major health problems worldwide, and are the leading causes of serious long-term disability. Focal ischemic stroke begins with a thrombus or embolus that occludes a cerebral artery. Ischemia also plays an important role in pathogenesis of traumatic brain injury (TBI). Pathophysiological responses in brain after stroke and TBI are highly complex and involve multiple mechanisms including excitotoxicity, free radical damage, and inflammation, leading to neuronal injury and cell death [1]. Currently, neuroprotection is a main strategy for the treatment of acute stroke and TBI. There are select excellent reviews on neuroprotection for stroke [2,3] and TBI [4–6]. Thus far, a monotherapy for saving neurons has not revealed any clinically effective neuroprotectants. Only one Food and Drug Administration (FDA)-approved drug, recombinant tissue plasminogen activator (tPA), for the treatment of clinical ischemic stroke shows therapeutic effects and this treatment is limited by its narrow therapeutic time window and related risks of brain hemorrhage [7]. Recent preclinical studies have revealed that brain injury induces neurogenesis (the generation of new neurons) and angiogenesis (the growth of new blood vessels). To the best of our knowledge, clinical trials in TBI and stroke have targeted neuroprotection and none of them have been aimed specifically at angiogenesis and neurogenesis. Agents and manipulations that boost angiogenesis and/or neurogenesis promote functional recovery after brain injuries [8]. This suggests that the manipulation of endogenous neural precursors and endothelial cells is a potential therapy for brain injury.
Neurogenesis and angiogenesis
Mammalian adult neurogenesis occurs in the subgranular zone (SGZ) of the hippocampus, subventricular zone (SVZ), and olfactory bulb (OB) [9,10]. Newly generated neuronal cells originate from neural stem cells (NSCs) in the adult brain. NSCs are the self-renewing, multipotent cells that generate the neuronal and glial cells of the nervous system [11]. Granule neurons in the dentate gyrus (DG) of the hippocampus continuously die and the progenitors may proliferate to maintain a constant cell number in the DG [12]. Similarly, the newly proliferated cells from SVZ replenish the dead OB neurons. In addition, the resident neural progenitors could be induced to replace neurons lost due to acute insults [13–15]. Newly generated neurons in the DG of the hippocampus are capable of projecting axons to the CA3 region in normal [10,16] and injured adult rats [17].
The adult brain vascular system is stable under normal conditions and is activated in response to pathological conditions including injuries [18]. Adult vascular remodeling includes angiogenesis by mature endothelial cells (growth of capillaries from pre-existing vessels) and by endothelial progenitor cells (EPCs). EPCs are present in the bone marrow and peripheral blood and are mobilized to peripheral blood after TBI. The number of CD34+ EPCs in peripheral blood increases at 24 h, peaks at 48 h and returns to normal at 168 h after TBI. These CD34+ cells are detected as early as 24 h after TBI in the area surrounding injured brain and the vessel-lumen structure with CD34+ endothelial-like cell lining being observed at 72 h after TBI [19]. Bone marrow-derived endothelial progenitor cells (EPCs) also promote endothelial repair and contribute to ischemia-induced neovascularization [20]. Pharmacological agents such as statins, angiotensin-converting enzyme inhibitors, angiotensin II type 1 receptor blockers, PPAR-γ agonists and erythropoietin increase the number, mobilization and functional activity of EPCs [21]. However, some papers show that EPCs hardly incorporate into newly developed blood vessels and suggest that the cells may support pre-existing endothelium-derived angiogenesis by secreting growth factors including bFGF and VEGF [22]. The induction of angiogenesis can be achieved by delivering angiogenic factors including vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), and angiopoietins (Ang) [23]. Mobilization or transplantation of EPCs into ischemic tissues may emerge as a promising approach in the therapy of diseases associated with blood vessel disorders including stroke and TBI.
Central nervous system (CNS) neurovascular units (NVUs) are multi-cellular complexes consisting of endothelial cells, pericytes, neurons, and glial cells as well as growth factors and extracellular matrix proteins that are in physical proximity to the endothelium [24,25]. NVUs are niches for neural stem/progenitor cells (NSPCs) in the adult brain. Within the NVUs, newly born, immature neurons closely associate with the remodeling vasculature. The generation of new vasculature facilitates highly coupled neurorestorative processes including neurogenesis and synaptogenesis, which in turn lead to improved functional recovery [26–28]. Several compounds with angiogenic activities currently tested in clinical trials include sildenafil, atorvastatin, erythropoietin (EPO) and carbamylated EPO (CEPO) [26]. Neurogenesis and angiogenesis are causally linked through vascular production of stromal-derived factor 1 (SDF1) and Ang1. Furthermore, SDF1 and Ang1 promote post-stroke neuroblast migration and behavioral recovery [29]. The disruption of the neurovascular coordination was observed in a variety of brain diseases such as infection, stroke, and trauma [30]. We and others demonstrate that injured brain can be stimulated to promote angiogenesis and neurogenesis [31–39], which are coupled restorative processes that contribute to functional recovery from stroke and TBI. Magnetic resonance imaging (MRI) indices of these neurorestorative events are highly correlative with neurologic function and may be used in real-time monitoring of recovery from stroke [40,41]. Some of the agents including EPO offer both neuroprotective and neurorestorative benefits [39,42–44].
Neurogenesis and angiogenesis after TBI and stroke
In the normal adult brain, SVZ cells migrate along the rostral migratory stream (RMS) to the OB where they differentiate into interneurons. There are several excellent reviews on neurogenesis after TBI [14] and stroke [13,15,45,46]. After stroke, neuroblasts generated in the SVZ migrate to the ischemic boundary zone (IBZ) where angiogenesis occurs, and during migration neuroblasts are closely associated with cerebral vessels [29,47]. Neuroblasts actively interact with the microenvironment to reach the ischemic striatum individually or in chains [48]. After migration, SVZ-derived neuroblasts differentiate into mature neurons in the IBZ [49–52]. Following injury activated endothelial cells of cerebral vessels secrete SDF-1α to attract neuroblasts expressing CXCR4, a receptor for SDF-1α [53–55], and blockage of CXCR4 abrogates migration of neuroblasts to the IBZ [29,53,55]. Experimental results show that stroke also induces neurogenesis in aged animals, although basal neurogenesis is attenuated in these animals [56–58]. Pharmaceutical agents such as statins and sildenafil substantially enhance angiogenesis and neurogenesis and improve functional outcome during stroke recovery in aged animals [58,59].
Cortical injury changes the migration routes of cells born in the SVZ of the adult brain from the RMS to injured areas. Cellular proliferation is found in the SVZ, corpus callosum, around the cortex, and subcortical areas anatomically connected to, but not directly injured by the impact in a rat TBI model [60]. Following TBI, cells from the SVZ can differentiate into neurons and glia in injured areas [61–63]. Brain injuries also stimulate neurogenesis in the SGZ of the hippocampus [34,39,63–66]. TBI induces hippocampal cell proliferation and the majority of the injury-induced cell population that survives for an extended period of time differentiates into mature granule neurons. Some mature granule neurons extend axonal projections into the CA3 region by 2 weeks post TBI [17]. The functional integration of the injury-induced population into the existing hippocampal circuitry appears as early as 2 weeks when cognitive recovery is observed in TBI rats [34]. In addition, there is a persistent proliferation of neurons and glia in the SVZ following brain trauma that does not diminish during aging (4 months up to 1 year) [67].
Endothelial cells in the IBZ proliferate as early as 12–24 h following stroke, leading to peri-infarcted angiogenesis 3 days following the ischemic injury [26]. Active angiogenesis is observed 3–4 days following the ischemic insult in humans [26]. Formation of neovessels in the adult brain after stroke and TBI is not restricted to angiogenesis [39,68,69] but also involves vasculogenesis contributed by circulating EPCs from bone marrow [19,20].
Angiogenesis and neurogenesis may play an important role in mediating functional recovery after experimental stroke and TBI [8,28,34,36,39,40,70,71]. Nearly all the neurorestorative agents that improve functional outcome after stroke and TBI increase angiogenesis and neurogenesis [8,40,44,71]. Specific ablation of adult-born hippocampal neurons impairs spatial and object recognition memory in adult rats [72]. These data imply a direct and causal relationship between neurogenesis and recovery of neurologic function after brain injury. Brain angiogenesis may provide the critical neurovascular niches for neuronal remodeling. Understanding how neurovascular signals and substrates make the transition from initial injury to angiogenic and neurogenic recovery will be important for developing new therapies for brain injuries [73]. Coupling of angiogenesis and neurogenesis has also been observed in the adult human [74]. Human neurosphere-forming SVZ cells can self-renew and are multipotential [75,76]. Human adult neural stem/progenitor cells derived from the SVZ during routine surgery are capable of generating both neurons and astrocytes in vitro and may provide a source for application in cell-based human transplantation paradigms [77].
Erythropoietin
Erythropoietin (EPO) stimulates the maturation, differentiation and survival of hematopoietic progenitor cells [78,79]. While EPO and its receptor (EPORs) are only weakly expressed in normal adult brain, expression of EPO and the EPORs is greatly increased in neurons, neuronal progenitor cells, glia and cerebrovascular endothelial cells in response to many different types of cell injury [80,81]. Intraperitoneal administration of EPO (5000 U/kg) crosses the blood–brain barrier (BBB) and protects against brain injury in rats [37,42]. EPO treatment reduces ischemic infarct and hemorrhage volume, decreases neuronal death, and improves survival rates in animal models of stroke [82,83]. EPO may direct cell fate away from gliogenesis toward neurogenesis in neonatal stroke [84]. EPO administration at a dose of 5000 U/kg starting day 1 for 14 days after TBI significantly increases DG neurogenesis, and promotes restoration of spatial memory after TBI [34]. Post-TBI treatment (6 h or 24 h) with EPO (5000 U/kg) or CEPO (50 μg/kg) significantly increases BDNF expression and improves spatial learning at 5 weeks after injury in rats [85]. Efficacy of EPO is independent of increased hematocrit [44] and a multiple-dose EPO treatment (5000 U/kg daily for 3 days starting at day 1 post injury) increases functional recovery more than single-dose EPO therapy (5000 U/kg at day 1 post injury) in TBI rats [86]. EPO treatment initiated as late as 6 h post-TBI provides neuroprotection (i.e., decrease lesion volume and cell loss) as well as enhances neurogenesis, and subsequently improves sensorimotor and spatial learning functions [39,42–44]. Thus, EPO provides neuroprotection [39,42,43,87,88] and neurorestoration via promotion of neurogenesis and angiogenesis [34,37,44].
Treatment with EPO also contributes to neurovascular remodeling, leading to improved neurobehavioral outcomes after ischemic brain injury [37,89]. EPO enhances VEGF secretion from neural progenitor cells and neural progenitor cells treated with EPO upregulate VEGFR2 expression in cerebral endothelial cells, thus promoting angiogenesis [90]. While the therapeutic benefits of the novel EPO derivatives continue to be characterized in preclinical studies, the experimental findings in support for the use of EPO in human brain diseases have already been translated to clinical studies in acute ischemic stroke, chronic schizophrenia, and chronic progressive multiple sclerosis [83]. In stroke patients, EPO treatment may reduce infarct volume and improve functional outcomes [91]. It should be noted that the high EPO doses used for treatment of stroke and TBI significantly increase hematocrit [39,86]. The concern is that increased hematocrit may pose potential adverse vascular effects seen in the critically ill patients treated with EPO [92]. Although EPO administration has proven safe in animal studies and adult human patients, safety and efficacy data in neonates and infants are incomplete and long-term multi-center patient evaluations are necessary [82]. Nonhematopoietic EPO analogues such as CEPO are as effective as hematopoietic EPO without potential side effects [85,93–96]. CEPO at a dose of 50 μg/kg has a therapeutic window of at least 3 h and effectively improves clinical rating scores and motor function in a small clot embolic stroke rabbit model [95,96]. Stroke- and TBI-related EPO or CEPO clinical trials can be found on www.clinicaltrials.gov [97]. A Phase-I safety study of CEPO to treat patients with acute ischemic stroke (AIS) has been completed (NCT00756249). Further safety and pharmacokinetic studies of CEPO are ongoing (NCT00870844). A Phase II/III multicenter efficacy study of EPO in AIS has been completed (NCT00604630) [98]. This clinical trial enrolled 522 patients with acute ischemic stroke with 460 patients treated within 6 h of symptom onset, at 24 and 48 h, with EPO infused intravenously (40,000 IU each). Systemic thrombolysis with recombinant tissue plasminogen activator (rtPA) was used in 63% of the stroke patients [98]. This is a negative trial that raises safety concerns, particularly in patients receiving systemic thrombolysis, but restorative effects of EPO should be pursued. However, we note, that to date, the interaction between EPO and rtPA has not been investigated. Also, this clinical trial points to the importance of fully evaluating CEPO and other EPO analogs for both acute protection and chronic restoration of function. A Phase II EPO combined with Beta-human chorionic gonadotropin in AIS trial has been completed (NCT00362414). A Phase III trial studying the effects of EPO on cerebral vascular dysfunction and anemia in TBI is underway (NCT00313716). A Phase II safety trial of Darbepoetin Alfa (a long-acting form of EPO) treatment in patients with severe TBI is ongoing (NCT00375869), as well as a Phase II/III study on the early administration of EPO to patients sustaining TBI (NCT00260052). A Phase III trial of EPO in ICU patients with TBI is being planned (NCT00987454). We are waiting for the reports from these clinical trials.
Cell therapy
Neuronal tissue has limited repair capability after injury. Cell therapies using NSPCs are promising approaches for the treatment of brain injury [99,100]. There are several excellent reviews on NSPC cell therapy for TBI [8,99–104]. NSPC therapy may replace lost brain cells, promote endogenous neurogenesis and improve functional recovery [100]. NSPCs also stabilize vasculature during ischemia, suggesting therapeutic application of NSPCs to promote revascularization and repair after brain injury [105]. There is little evidence to assess the applicability of NSPCs to brain injury patients, and well designed clinical trials are necessary to evaluate safety, toxicity and efficacy as well as optimal cell type, route and time of delivery for NSPCs [103,106].
Although embryonic stem cells or fetal tissues are suitable sources for cell therapy, their clinical application is limited by ethical considerations and other specific problems including tumorigenicity, viability, and antigenic compatibility [107]. Recent landmark experiments have shown that transient overexpression of a small number of transcription factors can reprogram differentiated cells into induced pluripotent stem (iPS) cells that resemble embryonic stem cells [108]. These iPS cells avoid the ethical issue inherent in embryonic tissues or oocytes and have the potential to generate patient-specific cell types for cell replacement therapy. iPS cells may offer promising opportunities for the treatment of brain injury. Mesenchymal stem cells (MSCs), mesoderm-derived cells, have been isolated from bone marrow, adipose tissue, umbilical cord blood, placenta and pancreas. MSCs exert powerful immunomodulatory effects, which include inhibition of proliferation and function of T cells, B cells, and natural killer cells, thus reducing immune reactions and increasing tolerance of MSC recipients [109]. This immunosuppressive property makes them an important alternative source for allogeneic cell therapy since MSCs can be isolated from donors, expanded and cryopreserved [28,110,111]. In this review, we focus on adult bone marrow-derived MSCs, which are able to give rise to neuronal cells and many tissue-specific cell phenotypes [28,112,113]. When grafted into the lateral ventricles of neonatal mouse brain, MSCs migrate and differentiate into OB granule cells and periventricular astrocytes [114]. Our studies indicate that systematically infused rat MSCs migrate into injured rat brain and survive [115–118]. Some implanted MSCs express the markers for neurons and astrocytes. MSC treatment significantly improves neurological functional recovery after TBI [115,119,120].
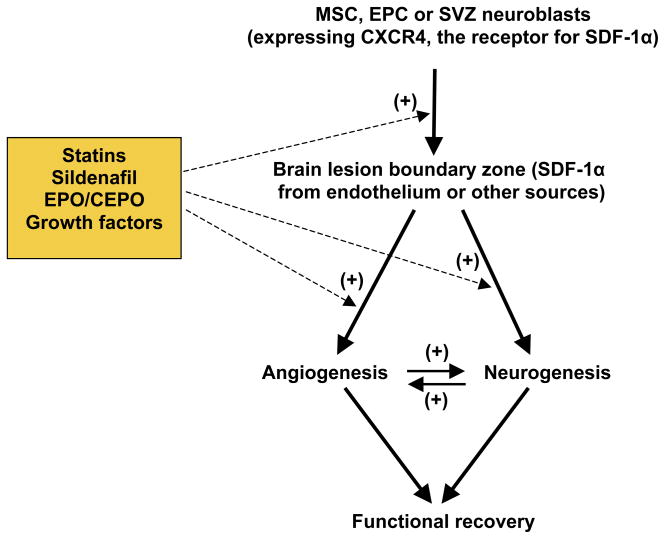
MSCs may provide neuroprotection when intrathecally given early (1 million cells, 6 h) in rats after TBI [121]. Delayed (24 h or 1 week after injury) administration of MSCs in the range of 2–8 million cells also significantly improves functional outcome after TBI and stroke [27,31,35,116,122–126]. MSC therapy at 1 month after stroke (3 million cells, i.v.) shows therapeutic benefits [127]. CXC-chemokine receptor-4 (CXCR4) is expressed in MSCs and the interaction of SDF-1/CXCR4 may contribute to the trafficking of transplanted MSCs into injured brain [127]. MSCs secrete various growth factors including BDNF, VEGF and FGF, thus amplifying their endogenous brain levels [122,128–130]. MSCs also induce intrinsic parenchymal cells to produce these growth factors [129]. These trophic factors enhance angiogenesis and vascular stabilization in the lesion boundary zone, where the majority of MSCs that survive in the brain are located [119,124]. MSCs increase VEGF expression and promote angiogenesis after stroke [122,131–134]. These growth factors also promote neurogenesis [130,135,136]. MSCs not only increase vascular density in the lesion boundary zone and hippocampus [126] but also enhance neurogenesis in the SGZ and SVZ [35] in rodents after TBI. In addition, MSCs induce expression of bone morphogenetic proteins BMP2 and BMP4 and connexin 43 in astrocytes in injured brain, promoting synaptogenesis [137]. In concert with enhancing angiogenesis, neurogenesis, and synaptogenesis, MSCs significantly decrease glial scar formation and promote glial–axonal remodeling in rats after stroke [138]. Thus, MSC therapeutic benefits are probably not attributable to the very few MSCs that differentiate into brain cells [116]. However, MSCs appear to work as neurotrophic generators to promote brain functional recovery via angiogenesis, neurogenesis, synaptogenesis, and axonal remodeling (Figure 1). With delayed treatment, MSCs alone does not reduce the lesion volume after TBI [117], while collagen scaffolds populated with MSCs do reduce the lesion volume, foster the migration of MSCs into the lesion boundary zone, and improve spatial learning and sensorimotor function compared with MSCs alone after TBI in rats [139].
Figure 1. A simplified schematic diagram summarizing injury- and therapy-induced angiogenesis and neurogenesis after TBI and stroke.
Following brain injury, CXC-chemokine receptor-4 (CXCR4)-expressing subventricular zone (SVZ) neuroblasts are attracted by increased stromal-derived factor 1 (SDF-1)α to migrate into the lesion boundary zone, where they differentiate into neural cells. Similarly, mesenchymal stem cells (MSCs) and endothelial precursor cells (EPCs) are directed to injured brain regions, where they secret growth factors to promote angiogenesis and neurogenesis. Erythropoietin (EPO) and statins promote the migration of SVZ neuroblasts into injured brain regions. Treatment with EPO/carbamylated erythropoietin (CEPO), statins, PDE5 inhibitors, MSCs, VEGF or basic FGF increases angiogenesis and neurogenesis after brain injury. In addition, angiogenesis and neurogenesis are coupled through VEGF, angiopoietins (Ang)1 and SDF1α. Brain remodeling, including angiogenesis and neurogenesis, may contribute to spontaneous and therapy-promoted functional recovery after brain injury. The symbol (+) in the figure indicates positive effects.
MSCs transferred with ex vivo hepatocyte growth factor gene are more therapeutically efficient for treating stroke rats than MSCs alone [140]. Cellular delivery of placental growth factor gene-modified MSCs provides better neuroprotection and angiogenesis, and better improvement in functional recovery in cerebral ischemia than MSCs alone [141]. Intravenous administration of human MSCs transfected with the Ang1 and VEGF genes shows the greatest structural-functional recovery as compared to monotherapy groups after cerebral ischemia [142]. Strategies to maximizing angiogenesis may prove valuable for brain injury therapies. Thus, genetically engineered MSCs and NSPCs with overexpression of growth factors may be an improved source for cell therapy for stroke and TBI. An excellent review of genetically modified stem cells used in experimental models of stroke can be found in reference [143].
Although the first clinical trial of autologous MSC therapy in stroke showed promising results [144], the optimal approach (types of MSCs, cell dose, timing of treatment, route of cell delivery) has yet to be determined. The safety and feasibility of autologous MSC treatment of TBI patients have been recently assessed [145]. No toxicity related to the cell therapy was observed within the 6-month follow-up period. Neurologic function was significantly improved by 6 months after the MSC therapy [146]. Clinical trials of cell therapy by intravenous injection of MSCs after ischemic stroke are ongoing (NCT00535197, NCT00761982, NCT00473057) and more are being planned (NCT00875654, NCT00908856) [97]. The safety of autologous stem cell treatment in children with TBI (NCT00254722) [97] is being examined. The potential short-term and long-term toxicities of MSCs still need to be determined before use in the clinic [146].
Statins
Statins, cholesterol biosynthesis inhibitors used for lowering cholesterol, show neuroprotective and neurorestorative benefits in animal models of TBI and stroke [71,72,147–151]. Many of the pleiotropic effects of statins are cholesterol independent, such as improvement of endothelial function, increased nitric oxide (NO) bioavailability, antioxidant properties, inhibition of inflammatory responses, immunomodulatory actions, upregulation of endothelial NO synthase (eNOS), and decrease of platelet activation [37,59,152]. Statins induce angiogenesis, neurogenesis, and synaptogenesis and enhance functional recovery after stroke in rats [71]. VEGF, VEGFR2 and BDNF likely contribute to these restorative processes [148]. Oral administration of atorvastatin at a dose of 1 mg/kg daily for 14 days starting at 1 day after TBI significantly reduces the neurological functional deficits, increases neuronal survival [33,149,154] and synaptogenesis in the boundary zone of the lesion and in the CA3 regions of the hippocampus, and induces angiogenesis in these regions [148] and increases neurogenesis in the DG [33,70]. When administered in combination with MSCs, atorvastatin increases MSC access and/or survival within the injured brain and enhances functional recovery compared with monotherapy [155]. Statins induce neuroglial differentiation of human MSCs [156]. These cholesterol-lowering agents might be used in conjunction with MSC transplantation for treating neurological disorders and injuries.
Simvastatin therapy elevates the expression of BDNF and VEGF; increases cell proliferation and differentiation in the DG; and enhances the recovery of spatial learning after TBI [70]. Protective mechanisms for lovastatin may be partly attributed to dampening inflammatory response after TBI [147,154]. Simvastatin treatment provides long-lasting (3-month) functional improvement after TBI in rats. Lovastatin is currently approved by the FDA for the treatment of acute ischemic stroke patients 3 days after ictus and the maximum tolerated dose is estimated to be 8 mg/kg/day [157]. Another clinical trial showed that simvastatin-treated stroke patients improve significantly by the third day when simvastatin is given at 3–12 h after symptom onset (with an initial dose of 40 mg/day for the 1 week followed by a dose of 20 mg/kg until day 90 day) to 60 patients with cortical strokes. However, a non-significant increase in mortality and greater proportion of infections in the simvastatin group are the main safety concerns [158]. Therefore, a larger clinical trial is needed to confirm the net benefit of the statin therapeutic approach. It has been demonstrated that rosuvastatin (hydrophilic), but not simvastatin (lipophilic), provides end-organ protection in stroke-prone rats [159]. The property of hydrophilicity/hydrophobicity may contribute to the different statin pharmacology, for lipophilic statins are more susceptible to metabolism [160]. Consideration of the differences among the statins provides a rational basis for their preclinical research and clinical practice. Given the wide use of statins, their favorable safety profile and positive clinical data in patients, rare serious adverse events, and the extensive preclinical data showing neuroprotection and neurorestoration, further clinical studies are warranted to determine the neuroprotective and neurorestorative properties of statins after stroke and TBI. The effect of rosuvastatin on TBI-induced cytokine change is being studied in a clinical trial (NCT00990028) [97], as are the neuroprotective effects of lovastatin therapy on ischemic stroke recovery (NCT00243880) [97].
Phosphodiesterase type 5 inhibitors
Guanosine 3′, 5′-cyclic monophosphate (cGMP) acts as a relaxant second messenger in the blood vessels. cGMP-specific phosphodiesterase type 5 (PDE5) inhibitors elevate intracellular cGMP levels, increase cerebral blood flow and improve functional recovery after stroke [161,162]. Administration of a PDE5 inhibitor sildenafil (at a dose of 10 mg/kg administered subcutaneously 24 h after stroke and daily for an additional 6 days) to rats with embolic stroke enhances angiogenesis and selectively increases the cerebral blood flow level in the ischemic boundary, and improves neurological functional recovery compared to saline-treated rats [163]. MRI measurements, confirmed by histology, show that sildenafil treatment simultaneously enhances angiogenesis and axonal remodeling [164]. Sildenafil evokes neurogenesis and reduces neurological deficits when given to rats 2 or 24 h after stroke [165]. Treatment with sildenafil at a dose of 3 mg/kg daily for 7 consecutive days starting 7 days after focal ischemia significantly enhances neurogenesis and improves functional recovery in aged rats after focal cerebral ischemia [58]. Treatment of ischemic stroke with a long-acting PDE5 tadalafil improves functional recovery, which is associated with increases of brain cGMP levels and enhancement of angiogenesis and neurogenesis [166]. Our recent clinical safety study of sildenafil shortly after ischemic stroke onset shows that sildenafil (25 mg daily for 2 weeks) appears to be safe in patients with mild to moderately severe stroke [167].
Conclusions
Brain injury induces angiogenesis and neurogenesis. Strategies to enhance angiogenesis and neurogenesis improve brain functional recovery in experimental stroke and TBI. A better understanding of cross talk between neurogenesis and angiogenesis will further lead to novel therapeutic avenues for TBI and stroke. Cell therapeutic intervention involves the stimulation of endogenous NSPCs or the transplantation of adult-derived NSPCs. NSPCs have been isolated from human post-mortem tissues, providing an alternative source of tissues for allogeneic cellular therapy which needs donor-recipient matching. Autologous transplantation does not require a matching donor and immune-suppressive drugs; NSPCs would be isolated from an undamaged area of the CNS, expanded, and grafted back to restore brain function. However, harvesting NSPCs from patients is time-consuming, may delay the therapy, and involves invasive surgery that destroys healthy brain tissue, limiting its clinical application. The select cell-based and pharmacological therapies (ie, MSCs, EPO, CEPO, statins) in the review induce endogenous neurorestorative remodeling by increasing angiogenesis, neurogenesis, and synaptogenesis, subsequently improving neurological functional recovery after stroke and TBI (Figure 1). However, several aspects should be considered during the preclinical studies and clinical trials in stroke and TBI. Prior to translation of an agent or cell into clinical trial, preclinical evidence should be sufficiently strong, based on multiple experiments, preferably in several models, and including optimal administration routes, single doses versus multiple doses, bolus dose versus continuous infusion, and therapeutic windows. Extensive pharmacokinetic data on agents for treating injured brain should also be obtained, ensuring adequate brain tissue penetration through the BBB. Timing for manipulation of these factors is also critical for achieving effective outcome after injury. In addition, effective translation of agents into clinical trials may require multiple functional agents including EPO, CEPO, statins or combination therapy. These potential combinations include agents (eg, pharmaceuticals or cytokines) with cells (eg, MSCs, neural stem cells, iPS cells, and genetically modified cells) or with other approaches (physical or electric stimulation). Combination of neuroprotective and neurorestorative therapy may facilitate recovery of the injured brain.
Acknowledgments
This work was supported by NINDS grants PO1 NS23393, PO1 NS42345, RO1 NS42259 and RO1 NS62002.
References
•• of outstanding interest
• of special interest
- 1.Greve MW, Zink BJ. Pathophysiology of traumatic brain injury. Mt Sinai J Med. 2009;76(2):97–104. doi: 10.1002/msj.20104. [DOI] [PubMed] [Google Scholar]
- 2.Ginsberg MD. Current status of neuroprotection for cerebral ischemia: Synoptic overview. Stroke. 2009;40(Suppl 3):S111–114. doi: 10.1161/STROKEAHA.108.528877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barone FC. Ischemic stroke intervention requires mixed cellular protection of the penumbra. Curr Opin Investig Drugs. 2009;10(3):220–223. [PubMed] [Google Scholar]
- 4•.Narayan RK, Michel ME, Ansell B, Baethmann A, Biegon A, Bracken MB, Bullock MR, Choi SC, Clifton GL, Contant CF, Coplin WM, et al. Clinical trials in head injury. J Neurotrauma. 2002;19(5):503–557. doi: 10.1089/089771502753754037. An excellent early review of failed clinical trials in head injury from clinical, research, and pharmaceutical views. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tolias CM, Bullock MR. Critical appraisal of neuroprotection trials in head injury: What have we learned? NeuroRx. 2004;1(1):71–79. doi: 10.1602/neurorx.1.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jain KK. Neuroprotection in traumatic brain injury. Drug Discov Today. 2008;13(23–24):1082–1089. doi: 10.1016/j.drudis.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Weintraub MI. Thrombolysis (tissue plasminogen activator) in stroke: A medicolegal quagmire. Stroke. 2006;37(7):1917–1922. doi: 10.1161/01.STR.0000226651.04862.da. [DOI] [PubMed] [Google Scholar]
- 8•.Zhang ZG, Chopp M. Neurorestorative therapies for stroke: underlying mechanisms and translation to the clinic. Lancet Neurol. 2009;8(5):491–500. doi: 10.1016/S1474-4422(09)70061-4. A comprehensive review of neurorestorative therapies for stroke. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hagg T. From neurotransmitters to neurotrophic factors to neurogenesis. Neuroscientist. 2009;15(1):20–27. doi: 10.1177/1073858408324789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10••.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132(4):645–660. doi: 10.1016/j.cell.2008.01.033. An important review of regulation of adult neurogenesis and functional integration. [DOI] [PubMed] [Google Scholar]
- 11.Taupin P. The therapeutic potential of adult neural stem cells. Curr Opin Mol Ther. 2006;8(3):225–231. [PubMed] [Google Scholar]
- 12.Gage FH. Mammalian neural stem cells. Science. 2000;287(5457):1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 13.Greenberg DA. Neurogenesis and stroke. CNS Neurol Disord Drug Targets. 2007;6(5):321–325. doi: 10.2174/187152707783220901. [DOI] [PubMed] [Google Scholar]
- 14•.Richardson RM, Sun D, Bullock MR. Neurogenesis after traumatic brain injury. Neurosurg Clin N Am. 2007;18(1):169–181. xi. doi: 10.1016/j.nec.2006.10.007. An excellent review of brain injury-induced neurogenesis. [DOI] [PubMed] [Google Scholar]
- 15.Zhang RL, Zhang ZG, Chopp M. Neurogenesis in the adult ischemic brain: generation, migration, survival, and restorative therapy. Neuroscientist. 2005;11(5):408–416. doi: 10.1177/1073858405278865. [DOI] [PubMed] [Google Scholar]
- 16.Hastings NB, Gould E. Rapid extension of axons into the CA3 region by adult-generated granule cells. J Comp Neurol. 1999;413(1):146–154. doi: 10.1002/(sici)1096-9861(19991011)413:1<146::aid-cne10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 17.Emery DL, Fulp CT, Saatman KE, Schutz C, Neugebauer E, McIntosh TK. Newly born granule cells in the dentate gyrus rapidly extend axons into the hippocampal CA3 region following experimental brain injury. J Neurotrauma. 2005;22(9):978–988. doi: 10.1089/neu.2005.22.978. [DOI] [PubMed] [Google Scholar]
- 18.Greenberg DA, Jin K. From angiogenesis to neuropathology. Nature. 2005;438(7070):954–959. doi: 10.1038/nature04481. [DOI] [PubMed] [Google Scholar]
- 19.Guo X, Liu L, Zhang M, Angela B, Zhang J. Correlation of CD34+ cells with tissue angiogenesis after traumatic brain injury in a rat model. J Neurotrauma. 2009 Feb 18; doi: 10.1089/neu.2008.0733. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang ZG, Zhang L, Jiang Q, Chopp M. Bone marrow-derived endothelial progenitor cells participate in cerebral neovascularization after focal cerebral ischemia in the adult mouse. Circ Res. 2002;90(3):284–288. doi: 10.1161/hh0302.104460. [DOI] [PubMed] [Google Scholar]
- 21.Besler C, Doerries C, Giannotti G, Lüscher TF, Landmesser U. Pharmacological approaches to improve endothelial repair mechanisms. Expert Rev Cardiovasc Ther. 2008;6(8):1071–1082. doi: 10.1586/14779072.6.8.1071. [DOI] [PubMed] [Google Scholar]
- 22.Ziegelhoeffer T, Fernandez B, Kostin S, Heil M, Voswinckel R, Helisch A, Schaper W. Bone marrow-derived cells do not incorporate into the adult growing vasculature. Circ Res. 2004;94(2):230–238. doi: 10.1161/01.RES.0000110419.50982.1C. [DOI] [PubMed] [Google Scholar]
- 23.Madeddu P. Therapeutic angiogenesis and vasculogenesis for tissue regeneration. Exp Physiol. 2005;90(3):315–326. doi: 10.1113/expphysiol.2004.028571. [DOI] [PubMed] [Google Scholar]
- 24.Lok J, Gupta P, Guo S, Kim WJ, Whalen MJ, van Leyen K, Lo EH. Cell-cell signaling in the neurovascular unit. Neurochem Res. 2007;32(12):2032–2045. doi: 10.1007/s11064-007-9342-9. [DOI] [PubMed] [Google Scholar]
- 25.Guo S, Lo EH. Dysfunctional cell-cell signaling in the neurovascular unit as a paradigm for central nervous system disease. Stroke. 2009;40(Suppl 3):S4–7. doi: 10.1161/STROKEAHA.108.534388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beck H, Plate KH. Angiogenesis after cerebral ischemia. Acta Neuropathol. 2009;117(5):481–496. doi: 10.1007/s00401-009-0483-6. [DOI] [PubMed] [Google Scholar]
- 27.Chopp M, Li Y. Treatment of stroke and intracerebral hemorrhage with cellular and pharmacological restorative therapies. Acta Neurochir Suppl (Wien) 2008;105:79–83. doi: 10.1007/978-3-211-09469-3_16. [DOI] [PubMed] [Google Scholar]
- 28.Li Y, Chopp M. Marrow stromal cell transplantation in stroke and traumatic brain injury. Neurosci Lett. 2009;456(3):120–123. doi: 10.1016/j.neulet.2008.03.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26(50):13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han HS, Suk K. The function and integrity of the neurovascular unit rests upon the integration of the vascular and inflammatory cell systems. Curr Neurovasc Res. 2005;2(5):409–423. doi: 10.2174/156720205774962647. [DOI] [PubMed] [Google Scholar]
- 31.Chopp M, Li Y, Zhang ZG. Mechanisms underlying improved recovery of neurological function after stroke in the rodent after treatment with neurorestorative cell-based therapies. Stroke. 2009;40(Suppl 3):S143–145. doi: 10.1161/STROKEAHA.108.533141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu D, Mahmood A, Zhang R, Copp M. Upregulation of neurogenesis and reduction in functional deficits following administration of DEtA/NONOate, a nitric oxide donor, after traumatic brain injury in rats. J Neurosurg. 2003;99(2):351–361. doi: 10.3171/jns.2003.99.2.0351. [DOI] [PubMed] [Google Scholar]
- 33.Lu D, Qu C, Goussev A, Jiang H, Lu C, Schallert T, Mahmood A, Chen J, Li Y, Chopp M. Statins increase neurogenesis in the dentate gyrus, reduce delayed neuronal death in the hippocampal CA3 region, and improve spatial learning in rat after traumatic brain injury. J Neurotrauma. 2007;24(7):1132–1146. doi: 10.1089/neu.2007.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu D, Mahmood A, Qu C, Goussev A, Schallert T, Chopp M. Erythropoietin enhances neurogenesis and restores spatial memory in rats after traumatic brain injury. J Neurotrauma. 2005;22(9):1011–1017. doi: 10.1089/neu.2005.22.1011. [DOI] [PubMed] [Google Scholar]
- 35.Mahmood A, Lu D, Chopp M. Marrow stromal cell transplantation after traumatic brain injury promotes cellular proliferation within the brain. Neurosurgery. 2004;55:1185–1193. doi: 10.1227/01.neu.0000141042.14476.3c. [DOI] [PubMed] [Google Scholar]
- 36.Chopp M, Li Y, Zhang J. Plasticity and remodeling of brain. J Neurol Sci. 2008;265(1–2):97–101. doi: 10.1016/j.jns.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 37•.Wang L, Zhang Z, Wang Y, Zhang R, Chopp M. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke. 2004;35(7):1732–1737. doi: 10.1161/01.STR.0000132196.49028.a4. An important paper demonstrating beneficial effects of delayed erythropoietin treatment in stroke rats. [DOI] [PubMed] [Google Scholar]
- 38.Xiong Y, Mahmood A, Lu D, Qu C, Kazmi H, Goussev A, Zhang ZG, Noguchi CT, Schallert T, Chopp M. Histological and functional outcomes after traumatic brain injury in mice null for the erythropoietin receptor in the central nervous system. Brain Res. 2008;1230:247–257. doi: 10.1016/j.brainres.2008.06.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiong Y, Lu D, Qu C, Goussev A, Schallert T, Mahmood A, Chopp M. Effects of erythropoietin on reducing brain damage and improving functional outcome after traumatic brain injury in mice. J Neurosurg. 2008;109(3):510–521. doi: 10.3171/JNS/2008/109/9/0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chopp M, Zhang ZG, Jiang Q. Neurogenesis, angiogenesis, and MRI indices of functional recovery from stroke. Stroke. 2007;38(Suppl 2):827–831. doi: 10.1161/01.STR.0000250235.80253.e9. [DOI] [PubMed] [Google Scholar]
- 41.Ding G, Jiang Q, Li L, Zhang L, Zhang ZG, Panda S, Ewing JR, Chopp M. MRI of combination treatment of embolic stroke in rat with rtPA and atorvastatin. J Neurol Sci. 2006;246(1–2):139–147. doi: 10.1016/j.jns.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 42••.Brines ML, Ghezzi P, Keenan S, Agnello D, de Lanerolle NC, Cerami C, Itri LM, Cerami A. Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc Natl Acad Sci U S A. 2000;97(19):10526–10531. doi: 10.1073/pnas.97.19.10526. A landmark paper demonstrating erythropoietin is neuroprotective in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cherian L, Goodman JC, Robertson C. Neuroprotection with erythropoietin administration following controlled cortical impact injury in rats. J Pharmacol Exp Ther. 2007;322(2):789–794. doi: 10.1124/jpet.107.119628. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y, Xiong Y, Mahmood A, Meng Y, Qu C, Schallert T, Chopp M. Therapeutic effects of erythropoietin on histological and functional outcomes following traumatic brain injury in rats are independent of hematocrit. Brain Res. 2009;1294:153–164. doi: 10.1016/j.brainres.2009.07.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ohab JJ, Carmichael ST. Poststroke neurogenesis: emerging principles of migration and localization of immature neurons. Neuroscientist. 2008;14(4):369–380. doi: 10.1177/1073858407309545. [DOI] [PubMed] [Google Scholar]
- 46.Parent JM. Injury-induced neurogenesis in the adult mammalian brain. Neuroscientist. 2003;9(4):261–272. doi: 10.1177/1073858403252680. [DOI] [PubMed] [Google Scholar]
- 47.Thored P, Wood J, Arvidsson A, Cammenga J, Kokaia Z, Lindvall O. Long-term neuroblast migration along blood vessels in an area with transient angiogenesis and increased vascularization after stroke. Stroke. 2007;38(11):3032–3039. doi: 10.1161/STROKEAHA.107.488445. [DOI] [PubMed] [Google Scholar]
- 48.Zhang RL, Chopp M, Gregg SR, Toh Y, Roberts C, Letourneau Y, Buller B, Jia L, SPND, Zhang ZG. Patterns and dynamics of subventricular zone neuroblast migration in the ischemic striatum of the adult mouse. J Cereb Blood Flow Metab. 2009;29(7):1240–1250. doi: 10.1038/jcbfm.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Katakowski M, Chen J, Zhang ZG, Santra M, Wang Y, Chopp M. Stroke-induced subventricular zone proliferation is promoted by tumor necrosis factor-alpha-converting enzyme protease activity. J Cereb Blood Flow Metab. 2007;27(4):669–678. doi: 10.1038/sj.jcbfm.9600390. [DOI] [PubMed] [Google Scholar]
- 50.Yamashita T, Ninomiya M, Hernandez Acosta P, Garcia-Verdugo JM, Sunabori T, Sakaguchi M, Adachi K, Kojima T, Hirota Y, Kawase T, Araki N, et al. Subventricular zone-derived neuroblasts migrate and differentiate into mature neurons in the post-stroke adult striatum. J Neurosci. 2006;26(24):6627–6636. doi: 10.1523/JNEUROSCI.0149-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang R, Zhang Z, Zhang C, Zhang L, Robin A, Wang Y, Lu M, Chopp M. Stroke transiently increases subventricular zone cell division from asymmetric to symmetric and increases neuronal differentiation in the adult rat. J Neurosci. 2004;24(25):5810–5815. doi: 10.1523/JNEUROSCI.1109-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang RL, LeTourneau Y, Gregg SR, Wang Y, Toh Y, Robin AM, Zhang ZG, Chopp M. Neuroblast division during migration toward the ischemic striatum: a study of dynamic migratory and proliferative characteristics of neuroblasts from the subventricular zone. J Neurosci. 2007;27(12):3157–3162. doi: 10.1523/JNEUROSCI.4969-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thored P, Arvidsson A, Cacci E, Ahlenius H, Kallur T, Darsalia V, Ekdahl CT, Kokaia Z, Lindvall O. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells. 2006;24(3):739–747. doi: 10.1634/stemcells.2005-0281. [DOI] [PubMed] [Google Scholar]
- 54.Hill WD, Hess DC, Martin-Studdard A, Carothers JJ, Zheng J, Hale D, Maeda M, Fagan SC, Carroll JE, Conway SJ. SDF-1 (CXCL12) is upregulated in the ischemic penumbra following stroke: association with bone marrow cell homing to injury. J Neuropathol Exp Neurol. 2004;63(1):84–96. doi: 10.1093/jnen/63.1.84. [DOI] [PubMed] [Google Scholar]
- 55.Imitola J, Raddassi K, Park KI, Mueller FJ, Nieto M, Teng YD, Frenkel D, Li J, Sidman RL, Walsh CA, Snyder EY, et al. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci U S A. 2004;101(52):18117–18122. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jin K, Minami M, Xie L, Sun Y, Mao XO, Wang Y, Simon RP, Greenberg DA. Ischemia-induced neurogenesis is preserved but reduced in the aged rodent brain. Aging Cell. 2004;3(6):373–377. doi: 10.1111/j.1474-9728.2004.00131.x. [DOI] [PubMed] [Google Scholar]
- 57.Maslov AY, Barone TA, Plunkett RJ, Pruitt SC. Neural stem cell detection, characterization, and age-related changes in the subventricular zone of mice. J Neurosci. 2004;24(7):1726–1733. doi: 10.1523/JNEUROSCI.4608-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang RL, Zhang Z, Zhang L, Wang Y, Zhang C, Chopp M. Delayed treatment with sildenafil enhances neurogenesis and improves functional recovery in aged rats after focal cerebral ischemia. J Neurosci Res. 2006;83(7):1213–1219. doi: 10.1002/jnr.20813. [DOI] [PubMed] [Google Scholar]
- 59•.Chen J, Chopp M. Neurorestorative treatment of stroke: cell and pharmacological approaches. NeuroRx. 2006;3(4):466–473. doi: 10.1016/j.nurx.2006.07.007. A focused review of bone marrow mesenchymal cells, statins, sildenafil for treatment of stroke. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ramaswamy S, Goings GE, Soderstrom KE, Szele FG, Kozlowski DA. Cellular proliferation and migration following a controlled cortical impact in the mouse. Brain Res. 2005;1053(1–2):38–53. doi: 10.1016/j.brainres.2005.06.042. [DOI] [PubMed] [Google Scholar]
- 61.Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8(9):963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 62.Nakatomi H, Kuriu T, Okabe S, Yamamoto S, Hatano O, Kawahara N, Tamura A, Kirino T, Nakafuku M. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell. 2002;110(4):429–441. doi: 10.1016/s0092-8674(02)00862-0. [DOI] [PubMed] [Google Scholar]
- 63.Kernie SG, Erwin TM, Parada LF. Brain remodeling due to neuronal and astrocytic proliferation after controlled cortical injury in mice. J Neurosci Res. 2001;66(3):317–326. doi: 10.1002/jnr.10013. [DOI] [PubMed] [Google Scholar]
- 64.Urrea C, Castellanos DA, Sagen J, Tsoulfas P, Bramlett HM, Dietrich WD. Widespread cellular proliferation and focal neurogenesis after traumatic brain injury in the rat. Restor Neurol Neurosci. 2007;25(1):65–76. [PubMed] [Google Scholar]
- 65.Zhang R, Zhang L, Zhang Z, Wang Y, Lu M, Lapointe M, Chopp M. A nitric oxide donor induces neurogenesis and reduces functional deficits after stroke in rats. Ann Neurol. 2001;50(5):602–611. doi: 10.1002/ana.1249. [DOI] [PubMed] [Google Scholar]
- 66.Kadam SD, Mulholland JD, McDonald JW, Comi AM. Neurogenesis and neuronal commitment following ischemia in a new mouse model for neonatal stroke. Brain Res. 2008;1208:35–45. doi: 10.1016/j.brainres.2008.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen XH, Iwata A, Nonaka M, Browne KD, Smith DH. Neurogenesis and glial proliferation persist for at least one year in the subventricular zone following brain trauma in rats. J Neurotrauma. 2003;20(7):623–631. doi: 10.1089/089771503322144545. [DOI] [PubMed] [Google Scholar]
- 68.Zhang R, Wang L, Zhang L, Chen J, Zhu Z, Zhang Z, Chopp M. Nitric oxide enhances angiogenesis via the synthesis of vascular endothelial growth factor and cGMP after stroke in the rat. Circ Res. 2003;92(3):308–313. doi: 10.1161/01.res.0000056757.93432.8c. [DOI] [PubMed] [Google Scholar]
- 69.Morgan R, Kreipke CW, Roberts G, Bagchi M, Rafols JA. Neovascularization following traumatic brain injury: Possible evidence for both angiogenesis and vasculogenesis. Neurol Res. 2007;29(4):375–381. doi: 10.1179/016164107X204693. [DOI] [PubMed] [Google Scholar]
- 70.Wu H, Lu D, Jiang H, Xiong Y, Qu C, Li B, Mahmood A, Zhou D, Chopp M. Simvastatin-mediated upregulation of VEGF and BDNF, activation of the PI3K/Akt pathway, and increase of neurogenesis are associated with therapeutic improvement after traumatic brain injury. J Neurotrauma. 2008;25(2):130–139. doi: 10.1089/neu.2007.0369. [DOI] [PubMed] [Google Scholar]
- 71.Chen J, Zhang ZG, Li Y, Wang Y, Wang L, Jiang H, Zhang C, Lu M, Katakowski M, Feldkamp CS, Chopp M. Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann Neurol. 2003;53(6):743–751. doi: 10.1002/ana.10555. [DOI] [PubMed] [Google Scholar]
- 72.Jessberger S, Clark RE, Broadbent NJ, Clemenson GD, Jr, Consiglio A, Lie DC, Squire LR, Gage FH. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn Mem. 2009;16(2):147–154. doi: 10.1101/lm.1172609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arai K, Jin G, Navaratna D, Lo EH. Brain angiogenesis in developmental and pathological processes: neurovascular injury and angiogenic recovery after stroke. FEBS J. 2009;276(17):4644–4652. doi: 10.1111/j.1742-4658.2009.07176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Sloan R, Gage FH, Brown TR, Small SA. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci U S A. 2007;104(13):5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Palmer TD, Schwartz PH, Taupin P, Kaspar B, Stein SA, Gage FH. Cell culture. Progenitor cells from human brain after death. Nature. 2001;411(6833):42–43. doi: 10.1038/35075141. [DOI] [PubMed] [Google Scholar]
- 76.Sanai N, Tramontin AD, Quinones-Hinojosa A, Barbaro NM, Gupta N, Kunwar S, Lawton MT, McDermott MW, Parsa AT, Manuel-Garcia Verdugo J, Berger MS, et al. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427(6976):740–744. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- 77.Ayuso-Sacido A, Roy NS, Schwartz TH, Greenfield JP, Boockvar JA. Long-term expansion of adult human brain subventricular zone precursors. Neurosurgery. 2008;62(1):223–229. doi: 10.1227/01.NEU.0000311081.50648.4C. [DOI] [PubMed] [Google Scholar]
- 78.Wojchowski DM, Gregory RC, Miller CP, Pandit AK, Pircher TJ. Signal transduction in the erythropoietin receptor system. Exp Cell Res. 1999;253(1):143–156. doi: 10.1006/excr.1999.4673. [DOI] [PubMed] [Google Scholar]
- 79.Naranda T, Kaufman RI, Li J, Wong K, Boge A, Hallen D, Fung KY, Duncan MW, Andersen N, Goldstein A, Olsson L. Activation of erythropoietin receptor through a novel extracellular binding site. Endocrinology. 2002;143(6):2293–2302. doi: 10.1210/endo.143.6.8860. [DOI] [PubMed] [Google Scholar]
- 80.Marti HH. Erythropoietin and the hypoxic brain. J Exp Biol. 2004;207(Pt 18):3233–3242. doi: 10.1242/jeb.01049. [DOI] [PubMed] [Google Scholar]
- 81.Grasso G, Sfacteria A, Cerami A, Brines M. Erythropoietin as a tissue- protective cytokine in brain injury: what do we know and where do we go? Neuroscientist. 2004;10(2):93–98. doi: 10.1177/1073858403259187. [DOI] [PubMed] [Google Scholar]
- 82.Liu XB, Wang JA, Yu SP, Keogh CL, Wei L. Therapeutic strategy of erythropoietin in neurological disorders. CNS Neurol Disord Drug Targets. 2008;7(3):227–234. doi: 10.2174/187152708784936617. [DOI] [PubMed] [Google Scholar]
- 83••.Siren AL, Fasshauer T, Bartels C, Ehrenreich H. Therapeutic potential of erythropoietin and its structural or functional variants in the nervous system. Neurotherapeutics. 2009;6(1):108–127. doi: 10.1016/j.nurt.2008.10.041. A focused review of erythropoietin and its derivatives for treatment of the nervous system disorders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gonzalez FF, McQuillen P, Mu D, Chang Y, Wendland M, Vexler Z, Ferriero DM. Erythropoietin enhances long-term neuroprotection and neurogenesis in neonatal stroke. Dev Neurosci. 2007;29(4–5):321–330. doi: 10.1159/000105473. [DOI] [PubMed] [Google Scholar]
- 85.Mahmood A, Lu D, Qu C, Goussev A, Zhang ZG, Lu C, Chopp M. Treatment of traumatic brain injury in rats with erythropoietin and carbamylated erythropoietin. J Neurosurg. 2007;107(2):392–397. doi: 10.3171/JNS-07/08/0392. [DOI] [PubMed] [Google Scholar]
- 86.Xiong Y, Mahmood A, Meng Y, Zhang Y, Qu C, Schallert T, Chopp M. Delayed administration of erythropoietin reducing hippocampal cell loss, enhancing angiogenesis and neurogenesis, and improving functional outcome following traumatic brain injury in rats: comparison of treatment with single and triple dose. J Neurosurg. 2009 Oct 9; doi: 10.3171/2009.9.JNS09844. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Grasso G, Sfacteria A, Meli F, Fodale V, Buemi M, Iacopino DG. Neuroprotection by erythropoietin administration after experimental traumatic brain injury. Brain Res. 2007;1182:99–105. doi: 10.1016/j.brainres.2007.08.078. [DOI] [PubMed] [Google Scholar]
- 88.Mammis A, McIntosh TK, Maniker AH. Erythropoietin as a neuroprotective agent in traumatic brain injury Review. Surg Neurol. 2009;71(5):527–531. doi: 10.1016/j.surneu.2008.02.040. discussion 531. [DOI] [PubMed] [Google Scholar]
- 89.Iwai M, Cao G, Yin W, Stetler RA, Liu J, Chen J. Erythropoietin promotes neuronal replacement through revascularization and neurogenesis after neonatal hypoxia/ischemia in rats. Stroke. 2007;38(10):2795–2803. doi: 10.1161/STROKEAHA.107.483008. [DOI] [PubMed] [Google Scholar]
- 90.Wang L, Chopp M, Gregg SR, Zhang RL, Teng H, Jiang A, Feng Y, Zhang ZG. Neural progenitor cells treated with EPO induce angiogenesis through the production of VEGF. J Cereb Blood Flow Metab. 2008;28(7):1361–1368. doi: 10.1038/jcbfm.2008.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91••.Ehrenreich H, Hasselblatt M, Dembowski C, Cepek L, Lewczuk P, Stiefel M, Rustenbeck HH, Breiter N, Jacob S, Knerlich F, Bohn M, et al. Erythropoietin therapy for acute stroke is both safe and beneficial. Mol Med. 2002;8(8):495–505. A landmark clinical trial of erythropoietin in stroke. [PMC free article] [PubMed] [Google Scholar]
- 92.Corwin HL, Gettinger A, Fabian TC, May A, Pearl RG, Heard S, An R, Bowers PJ, Burton P, Klausner MA, Corwin MJ. Efficacy and safety of epoetin alfa in critically ill patients. N Engl J Med. 2007;357(10):965–976. doi: 10.1056/NEJMoa071533. [DOI] [PubMed] [Google Scholar]
- 93.Wang Y, Zhang ZG, Rhodes K, Renzi M, Zhang RL, Kapke A, Lu M, Pool C, Heavner G, Chopp M. Post-ischemic treatment with erythropoietin or carbamylated erythropoietin reduces infarction and improves neurological outcome in a rat model of focal cerebral ischemia. Br J Pharmacol. 2007;151(8):1377–1384. doi: 10.1038/sj.bjp.0707285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Adembri C, Massagrande A, Tani A, Miranda M, Margheri M, De Gaudio R, Pellegrini-Giampietro DE. Carbamylated erythropoietin is neuroprotective in an experimental model of traumatic brain injury. Crit Care Med. 2008;36(3):975–978. doi: 10.1097/CCM.0B013E3181644343. [DOI] [PubMed] [Google Scholar]
- 95.Lapchak PA. Carbamylated erythropoietin to treat neuronal injury: new development strategies. Expert Opin Investig Drugs. 2008;17(8):1175–1186. doi: 10.1517/13543784.17.8.1175. [DOI] [PubMed] [Google Scholar]
- 96.Lapchak PA, Kirkeby A, Zivin JA, Sager TN. Therapeutic window for nonerythropoietic carbamylated-erythropoietin to improve motor function following multiple infarct ischemic strokes in New Zealand white rabbits. Brain Res. 2008;1238:208–214. doi: 10.1016/j.brainres.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 97.ClinicalTrials.gov. Available at: http://www.clinicaltrials.gov/. 2009 cited.
- 98.Ehrenreich H, Weissenborn K, Prange H, Schneider D, Weimar C, Wartenberg K, Schellinger PD, Bohn M, Becker H, Wegrzyn M, Jähnig P, Herrmann M, Knauth M, Bähr M, Heide W, Wagner A, Schwab S, Reichmann H, Schwendemann G, Dengler R, Kastrup A, Bartels C EPO Stroke Trial Group. Recombinant human erythropoietin in the treatment of acute ischemic stroke. Stroke. 2009;40(12):e647–e656. doi: 10.1161/STROKEAHA.109.564872. [DOI] [PubMed] [Google Scholar]
- 99.Miljan EA, Sinden JD. Stem cell treatment of ischemic brain injury. Curr Opin Mol Ther. 2009;11(4):394–403. [PubMed] [Google Scholar]
- 100•.Richardson RM, Singh A, Sun D, Fillmore HL, Dietrich DW, Bullock MR. Stem cell biology in traumatic brain injury: effects of injury and strategies for repair. J Neurosurg. 2009 June 5; doi: 10.3171/2009.4.JNS081087. [Epub ahead of print]. An excellent review of stem cell treatment of traumatic brain injury. [DOI] [PubMed] [Google Scholar]
- 101.Jain KK. Cell therapy for CNS trauma. Mol Biotechnol. 2009;42(3):367–376. doi: 10.1007/s12033-009-9166-8. [DOI] [PubMed] [Google Scholar]
- 102.Harting MT, Baumgartner JE, Worth LL, Ewing-Cobbs L, Gee AP, Day MC, Cox CS., Jr Cell therapies for traumatic brain injury. Neurosurg Focus. 2008;24(3–4):E18. doi: 10.3171/FOC/2008/24/3-4/E17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Locatelli F, Bersano A, Ballabio E, Lanfranconi S, Papadimitriou D, Strazzer S, Bresolin N, Comi GP, Corti S. Stem cell therapy in stroke. Cell Mol Life Sci. 2009;66(5):757–772. doi: 10.1007/s00018-008-8346-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kondziolka D, Wechsler L. Stroke repair with cell transplantation: neuronal cells, neuroprogenitor cells, and stem cells. Neurosurg Focus. 2008;24(3–4):E13. doi: 10.3171/FOC/2008/24/3-4/E12. [DOI] [PubMed] [Google Scholar]
- 105.Roitbak T, Li L, Cunningham LA. Neural stem/progenitor cells promote endothelial cell morphogenesis and protect endothelial cells against ischemia via HIF-1alpha-regulated VEGF signaling. J Cereb Blood Flow Metab. 2008;28(9):1530–1542. doi: 10.1038/jcbfm.2008.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bersano A, Ballabio E, Lanfranconi S, Boncoraglio GB, Corti S, Locatelli F, Baron P, Bresolin N, Parati E, Candelise L. Clinical studies in stem cells transplantation for stroke: A review. Curr Vasc Pharmacol. 2010 doi: 10.2174/157016110790226570. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 107.Vescovi AL, Gritti A, Galli R, Parati EA. Isolation and intracerebral grafting of nontransformed multipotential embryonic human CNS stem cells. J Neurotrauma. 1999;16(8):689–693. doi: 10.1089/neu.1999.16.689. [DOI] [PubMed] [Google Scholar]
- 108.Yamanaka S. Strategies and new developments in the generation of patient-specific pluripotent stem cells. Cell Stem Cell. 2007;1(1):39–49. doi: 10.1016/j.stem.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 109.Siegel G, Schäfer R, Dazzi F. The immunosuppressive properties of mesenchymal stem cells. Transplantation. 2009;87(Suppl 9):S45–S49. doi: 10.1097/TP.0b013e3181a285b0. [DOI] [PubMed] [Google Scholar]
- 110.Torrente Y, Polli E. Mesenchymal stem cell transplantation for neurodegenerative diseases. Cell Transplant. 2008;17(10–11):1103–1113. doi: 10.3727/096368908787236576. [DOI] [PubMed] [Google Scholar]
- 111.Kode JA, Mukherjee S, Joglekar MV, Hardikar AA. Mesenchymal stem cells: immunobiology and role in immunomodulation and tissue regeneration. Cytotherapy. 2009;11(4):377–391. doi: 10.1080/14653240903080367. [DOI] [PubMed] [Google Scholar]
- 112.Kassem M, Abdallah BM. Human bone-marrow-derived mesenchymal stem cells: biological characteristics and potential role in therapy of degenerative diseases. Cell Tissue Res. 2008;331(1):157–163. doi: 10.1007/s00441-007-0509-0. [DOI] [PubMed] [Google Scholar]
- 113.Greco SJ, Rameshwar P. Enhancing effect of IL-1alpha on neurogenesis from adult human mesenchymal stem cells: implication for inflammatory mediators in regenerative medicine. J Immunol. 2007;179(5):3342–3350. doi: 10.4049/jimmunol.179.5.3342. [DOI] [PubMed] [Google Scholar]
- 114.Deng J, Petersen BE, Steindler DA, Jorgensen ML, Laywell ED. Mesenchymal stem cells spontaneously express neural proteins in culture and are neurogenic after transplantation. Stem Cells. 2006;24(4):1054–1064. doi: 10.1634/stemcells.2005-0370. [DOI] [PubMed] [Google Scholar]
- 115.Lu D, Li Y, Wang L, Chen J, Mahmood A, Chopp M. Intraarterial administration of marrow stromal cells in a rat model of traumatic brain injury. J Neurotrauma. 2001;18(8):813–819. doi: 10.1089/089771501316919175. [DOI] [PubMed] [Google Scholar]
- 116.Lu D, Mahmood A, Wang L, Li Y, Lu M, Chopp M. Adult bone marrow stromal cells administered intravenously to rats after traumatic brain injury migrate into brain and improve neurological outcome. Neuroreport. 2001;12(3):559–563. doi: 10.1097/00001756-200103050-00025. [DOI] [PubMed] [Google Scholar]
- 117.Chen J, Li Y, Wang L, Lu M, Zhang X, Chopp M. Therapeutic benefit of intracerebral transplantation of bone marrow stromal cells after cerebral ischemia in rats. J Neurol Sci. 2001;189(1–2):49–57. doi: 10.1016/s0022-510x(01)00557-3. [DOI] [PubMed] [Google Scholar]
- 118.Seyfried DM, Han Y, Yang D, Ding J, Savant-Bhonsale S, Shukairy MS, Chopp M. Mannitol enhances delivery of marrow stromal cells to the brain after experimental intracerebral hemorrhage. Brain Res. 2008;1224:12–19. doi: 10.1016/j.brainres.2008.05.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mahmood A, Lu D, Yi L, Chen JL, Chopp M. Intracranial bone marrow transplantation after traumatic brain injury improving functional outcome in adult rats. J Neurosurg. 2001;94(4):589–595. doi: 10.3171/jns.2001.94.4.0589. [DOI] [PubMed] [Google Scholar]
- 120.Mahmood A, Lu D, Wang L, Chopp M. Intracerebral transplantation of marrow stromal cells cultured with neurotrophic factors promotes functional recovery in adult rats subjected to traumatic brain injury. J Neurotrauma. 2002;19(12):1609–1617. doi: 10.1089/089771502762300265. [DOI] [PubMed] [Google Scholar]
- 121.Walker PA, Harting MT, Jimenez F, Shah SK, Pati S, Dash PK, Cox CS. Direct intrathecal implantation of mesenchymal stromal cells leads to enhanced neuroprotection via an NFkappaB mediated increase in Interleukin 6 (IL-6) production. Stem Cells Dev. 2009 Sept 23; doi: 10.1089/scd.2009.0188. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chopp M, Li Y. Treatment of neural injury with marrow stromal cells. Lancet Neurol. 2002;1(2):92–100. doi: 10.1016/s1474-4422(02)00040-6. [DOI] [PubMed] [Google Scholar]
- 123.Mahmood A, Lu D, Qu C, Goussev A, Chopp M. Human marrow stromal cell treatment provides long-lasting benefit after traumatic brain injury in rats. Neurosurgery. 2005;57(5):1026–1031. doi: 10.1227/01.neu.0000181369.76323.50. discussion 1026–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mahmood A, Lu D, Qu C, Goussev A, Chopp M. Long-term recovery after bone marrow stromal cell treatment of traumatic brain injury in rats. J Neurosurg. 2006;104(2):272–277. doi: 10.3171/jns.2006.104.2.272. [DOI] [PubMed] [Google Scholar]
- 125.Qu C, Mahmood A, Lu D, Goussev A, Xiong Y, Chopp M. Treatment of traumatic brain injury in mice with marrow stromal cells. Brain Res. 2008;1208:234–239. doi: 10.1016/j.brainres.2008.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chen J, Li Y, Katakowski M, Chen X, Wang L, Lu D, Lu M, Gautam SC, Chopp M. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J Neurosci Res. 2003;73(6):778–786. doi: 10.1002/jnr.10691. [DOI] [PubMed] [Google Scholar]
- 127.Shen LH, Li Y, Chen J, Zacharek A, Gao Q, Kapke A, Lu M, Raginski K, Vanguri P, Smith A, Chopp M. Therapeutic benefit of bone marrow stromal cells administered 1 month after stroke. J Cereb Blood Flow Metab. 2007;27(1):6–13. doi: 10.1038/sj.jcbfm.9600311. [DOI] [PubMed] [Google Scholar]
- 128.Chen X, Katakowski M, Li Y, Lu D, Wang L, Zhang L, Chen J, Xu Y, Gautam S, Mahmood A, Chopp M. Human bone marrow stromal cell cultures conditioned by traumatic brain tissue extracts: growth factor production. J Neurosci Res. 2002;69(5):687–691. doi: 10.1002/jnr.10334. [DOI] [PubMed] [Google Scholar]
- 129.Mahmood A, Lu D, Chopp M. Intravenous administration of marrow stromal cells (MSCs) increases the expression of growth factors in rat brain after traumatic brain injury. J Neurotrauma. 2004;21(1):33–39. doi: 10.1089/089771504772695922. [DOI] [PubMed] [Google Scholar]
- 130.Yoshimura S, Teramoto T, Whalen MJ, Irizarry MC, Takagi Y, Qiu J, Harada J, Waeber C, Breakefield XO, Moskowitz MA. FGF-2 regulates neurogenesis and degeneration in the dentate gyrus after traumatic brain injury in mice. J Clin Invest. 2003;112(8):1202–1210. doi: 10.1172/JCI16618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chen J, Zhang ZG, Li Y, Wang L, Xu YX, Gautam SC, Lu M, Zhu Z, Chopp M. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ Res. 2003;92(6):692–699. doi: 10.1161/01.RES.0000063425.51108.8D. [DOI] [PubMed] [Google Scholar]
- 132.Kinnaird T, Stabile E, Burnett MS, Lee CW, Barr S, Fuchs S, Epstein SE. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94(5):678–685. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- 133.Zacharek A, Chen J, Cui X, Li A, Li Y, Roberts C, Feng Y, Gao Q, Chopp M. Angiopoietin1/Tie2 and VEGF/Flk1 induced by MSC treatment amplifies angiogenesis and vascular stabilization after stroke. J Cereb Blood Flow Metab. 2007;27(10):1684–1691. doi: 10.1038/sj.jcbfm.9600475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pavlichenko N, Sokolova I, Vijde S, Shvedova E, Alexandrov G, Krouglyakov P, Fedotova O, Gilerovich EG, Polyntsev DG, Otellin VA. Mesenchymal stem cells transplantation could be beneficial for treatment of experimental ischemic stroke in rats. Brain Res. 2008;1233:203–213. doi: 10.1016/j.brainres.2008.06.123. [DOI] [PubMed] [Google Scholar]
- 135.Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A. 2002;99(18):11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lee J, Duan W, Mattson MP. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem. 2002;82(6):1367–1375. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- 137.Zhang C, Li Y, Chen J, Gao Q, Zacharek A, Kapke A, Chopp M. Bone marrow stromal cells upregulate expression of bone morphogenetic proteins 2 and 4, gap junction protein connexin-43 and synaptophysin after stroke in rats. Neuroscience. 2006;141(2):687–695. doi: 10.1016/j.neuroscience.2006.04.054. [DOI] [PubMed] [Google Scholar]
- 138.Li Y, Chen J, Zhang CL, Wang L, Lu D, Katakowski M, Gao Q, Shen LH, Zhang J, Lu M, Chopp M. Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia. 2005;49(3):407–417. doi: 10.1002/glia.20126. [DOI] [PubMed] [Google Scholar]
- 139.Lu D, Mahmood A, Qu C, Hong X, Kaplan D, Chopp M. Collagen scaffolds populated with human marrow stromal cells reduce lesion volume and improve functional outcome after traumatic brain injury. Neurosurgery. 2007;61(3):596–602. doi: 10.1227/01.NEU.0000290908.38438.B2. discussion 602–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhao MZ, Nonoguchi N, Ikeda N, Watanabe T, Furutama D, Miyazawa D, Funakoshi H, Kajimoto Y, Nakamura T, Dezawa M, Shibata MA, et al. Novel therapeutic strategy for stroke in rats by bone marrow stromal cells and ex vivo HGF gene transfer with HSV-1 vector. J Cereb Blood Flow Metab. 2006;26(9):1176–1188. doi: 10.1038/sj.jcbfm.9600273. [DOI] [PubMed] [Google Scholar]
- 141.Liu H, Honmou O, Harada K, Nakamura K, Houkin K, Hamada H, Kocsis JD. Neuroprotection by PlGF gene-modified human mesenchymal stem cells after cerebral ischaemia. Brain. 2006;129(Pt 10):2734–2745. doi: 10.1093/brain/awl207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Toyama K, Honmou O, Harada K, Suzuki J, Houkin K, Hamada H, Kocsis JD. Therapeutic benefits of angiogenetic gene-modified human mesenchymal stem cells after cerebral ischemia. Exp Neurol. 2009;216(1):47–55. doi: 10.1016/j.expneurol.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 143.van Velthoven CT, Kavelaars A, van Bel F, Heijnen CJ. Regeneration of the ischemic brain by engineered stem cells: fuelling endogenous repair processes. Brain Res Rev. 2009;61(1):1–13. doi: 10.1016/j.brainresrev.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 144.Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57(6):874–882. doi: 10.1002/ana.20501. [DOI] [PubMed] [Google Scholar]
- 145•.Zhang ZX, Guan LX, Zhang K, Zhang Q, Dai LJ. A combined procedure to deliver autologous mesenchymal stromal cells to patients with traumatic brain injury. Cytotherapy. 2008;10(2):134–139. doi: 10.1080/14653240701883061. An important paper demonstrating safety and benefits of autologous mesenchymal stromal cells for treatment of patients with traumatic brain injury. [DOI] [PubMed] [Google Scholar]
- 146.Dharmasaroja P. Bone marrow-derived mesenchymal stem cells for the treatment of ischemic stroke. J Clin Neurosci. 2009;16(1):12–20. doi: 10.1016/j.jocn.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 147.Chen SF, Hung TH, Chen CC, Lin KH, Huang YN, Tsai HC, Wang JY. Lovastatin improves histological and functional outcomes and reduces inflammation after experimental traumatic brain injury. Life Sci. 2007;81(4):288–298. doi: 10.1016/j.lfs.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 148.Chen J, Zhang C, Jiang H, Li Y, Zhang L, Robin A, Katakowski M, Lu M, Chopp M. Atorvastatin induction of VEGF and BDNF promotes brain plasticity after stroke in mice. J Cereb Blood Flow Metab. 2005;25(2):281–290. doi: 10.1038/sj.jcbfm.9600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lu D, Goussev A, Chen J, Pannu P, Li Y, Mahmood A, Chopp M. Atorvastatin reduces neurological deficit and increases synaptogenesis, angiogenesis, and neuronal survival in rats subjected to traumatic brain injury. J Neurotrauma. 2004;21(1):21–32. doi: 10.1089/089771504772695913. [DOI] [PubMed] [Google Scholar]
- 150.Lu D, Mahmood A, Goussev A, Schallert T, Qu C, Zhang ZG, Li Y, Lu M, Chopp M. Atorvastatin reduction of intravascular thrombosis, increase in cerebral microvascular patency and integrity, and enhancement of spatial learning in rats subjected to traumatic brain injury. J Neurosurg. 2004;101(5):813–821. doi: 10.3171/jns.2004.101.5.0813. [DOI] [PubMed] [Google Scholar]
- 151.Wu H, Lu D, Jiang H, Xiong Y, Qu C, Li B, Mahmood A, Zhou D, Chopp M. Increase in phosphorylation of Akt and its downstream signaling targets and suppression of apoptosis by simvastatin after traumatic brain injury. J Neurosurg. 2008;109(4):691–698. doi: 10.3171/JNS/2008/109/10/0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zacharek A, Chen J, Cui X, Yang Y, Chopp M. Simvastatin increases notch signaling activity and promotes arteriogenesis after stroke. Stroke. 2009;40(1):254–260. doi: 10.1161/STROKEAHA.108.524116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rodriguez-Yanez M, Agulla J, Rodriguez-Gonzalez R, Sobrino T, Castillo J. Statins and stroke. Ther Adv Cardiovasc Dis. 2008;2(3):157–166. doi: 10.1177/1753944708091776. [DOI] [PubMed] [Google Scholar]
- 154.Wang H, Lynch JR, Song P, Yang HJ, Yates RB, Mace B, Warner DS, Guyton JR, Laskowitz DT. Simvastatin and atorvastatin improve behavioral outcome, reduce hippocampal degeneration, and improve cerebral blood flow after experimental traumatic brain injury. Exp Neurol. 2007;206(1):59–69. doi: 10.1016/j.expneurol.2007.03.031. [DOI] [PubMed] [Google Scholar]
- 155.Mahmood A, Lu D, Qu C, Goussev A, Chopp M. Treatment of traumatic brain injury with a combination therapy of marrow stromal cells and atorvastatin in rats. Neurosurgery. 2007;60(3):546–553. doi: 10.1227/01.NEU.0000255346.25959.99. discussion 553–544. [DOI] [PubMed] [Google Scholar]
- 156.Lee OK, Ko YC, Kuo TK, Chou SH, Li HJ, Chen WM, Chen TH, Su Y. Fluvastatin and lovastatin but not pravastatin induce neuroglial differentiation in human mesenchymal stem cells. J Cell Biochem. 2004;93(5):917–928. doi: 10.1002/jcb.20241. [DOI] [PubMed] [Google Scholar]
- 157.Elkind MS, Sacco RL, MacArthur RB, Fink DJ, Peerschke E, Andrews H, Neils G, Stillman J, Corporan T, Leifer D, Cheung K. The Neuroprotection with Statin Therapy for Acute Recovery Trial (NeuSTART): an adaptive design phase I dose-escalation study of high-dose lovastatin in acute ischemic stroke. Int J Stroke. 2008;3(3):210–218. doi: 10.1111/j.1747-4949.2008.00200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Montaner J, Chacon P, Krupinski J, Rubio F, Millan M, Molina CA, Hereu P, Quintana M, Alvarez-Sabin J. Simvastatin in the acute phase of ischemic stroke: a safety and efficacy pilot trial. Eur J Neurol. 2008;15(1):82–90. doi: 10.1111/j.1468-1331.2007.02015.x. [DOI] [PubMed] [Google Scholar]
- 159.Sironi L, Gianazza E, Gelosa P, Guerrini U, Nobili E, Gianella A, Cremonesi B, Paoletti R, Tremoli E. Rosuvastatin, but not simvastatin, provides end-organ protection in stroke-prone rats by antiinflammatory effects. Arterioscler Thromb Vasc Biol. 2005;25(3):598–603. doi: 10.1161/01.ATV.0000157145.98200.55. [DOI] [PubMed] [Google Scholar]
- 160.Schachter M. Chemical, pharmacokinetic and pharmacodynamic properties of statins: an update. Fundam Clin Pharmacol. 2005;19(1):117–125. doi: 10.1111/j.1472-8206.2004.00299.x. [DOI] [PubMed] [Google Scholar]
- 161.Gao F, Sugita M, Nukui H. Phosphodiesterase 5 inhibitor, zaprinast, selectively increases cerebral blood flow in the ischemic penumbra in the rat brain. Neurol Res. 2005;27:638–643. doi: 10.1179/016164105X25135. [DOI] [PubMed] [Google Scholar]
- 162.Bednar MM. The role of sildenafil in the treatment of stroke. Curr Opin Investig Drugs. 2008;9(7):754–759. [PubMed] [Google Scholar]
- 163.Li L, Jiang Q, Zhang L, Ding G, Gang Zhang Z, Li Q, Ewing JR, Lu M, Panda S, Ledbetter KA, Whitton PA, et al. Angiogenesis and improved cerebral blood flow in the ischemic boundary area detected by MRI after administration of sildenafil to rats with embolic stroke. Brain Res. 2007;1132(1):185–192. doi: 10.1016/j.brainres.2006.10.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ding G, Jiang Q, Li L, Zhang L, Zhang ZG, Ledbetter KA, Panda S, Davarani SP, Athiraman H, Li Q, Ewing JR, et al. Magnetic resonance imaging investigation of axonal remodeling and angiogenesis after embolic stroke in sildenafil-treated rats. J Cereb Blood Flow Metab. 2008;28(8):1440–1448. doi: 10.1038/jcbfm.2008.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhang R, Wang Y, Zhang L, Zhang Z, Tsang W, Lu M, Chopp M. Sildenafil (Viagra) induces neurogenesis and promotes functional recovery after stroke in rats. Stroke. 2002;33(11):2675–2680. doi: 10.1161/01.str.0000034399.95249.59. [DOI] [PubMed] [Google Scholar]
- 166.Zhang L, Zhang Z, Zhang RL, Cui Y, LaPointe MC, Silver B, Chopp M. Tadalafil, a long-acting type 5 phosphodiesterase isoenzyme inhibitor, improves neurological functional recovery in a rat model of embolic stroke. Brain Res. 2006;1118(1):192–198. doi: 10.1016/j.brainres.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 167.Silver B, McCarthy S, Lu M, Mitsias P, Russman AN, Katramados A, Morris DC, Lewandowski CA, Chopp M. Sildenafil treatment of subacute ischemic stroke: a safety study at 25-mg daily for 2 weeks. J Stroke Cerebrovasc Dis. 2009;18(5):381–383. doi: 10.1016/j.jstrokecerebrovasdis.2009.01.007. [DOI] [PubMed] [Google Scholar]