Abstract
Radix Astragali (RA), known as “Huangqi” in China, is one of the most popular herbal medicines known worldwide to reinforce “Qi”. RA is traditionally prepared from the dried roots of Astragalus membranaceus (MJHQ) and A. membranaceus var. mongholicus (MGHQ). Radix Hedysari is named “Hongqi” (HQ), which is similar to RA. We assessed and compared the chemical constituents and bioactivity of RA and HQ. Different constituents were extracted into five major parts and were analyzed using different methods. Comparison of the immunological effects of extracts was done by using two immunological models. Results showed that flavonoids and saponins present in RA and HQ were not only structurally significantly different but also different in their immunological effect. Amino acids extract (AE) in MGHQ shows immunological effect while AE in MJHQ and HQ did not. Polysaccharides comprised the major constituents in RA and HQ. All polysaccharides extract (PE) of the three herbs showed similar levels of immunological effect in both immunological assays.
1. Introduction
Radix Astragali (RA), known as Huangqi, which is the root either of Astragalus membranaceus (Fisch.) Bge. var. mongholicus (Bge.) Hsiao (MGHQ) or A. membranaceus (Fisch.) Bge. (MJHQ), has been widely used as an immunostimulant, cardiotonic, hepatoprotective, antidiabetic, and antiviral drug [1–3]. It is said to benefit the deficiency of “Qi” (the vital energy) of the spleen that symptomatically presents as fatigue, diarrhea, and lack of appetite. The constituents most often associated with the activity of RA are flavonoids, saponins, polysaccharides, amino acids, and various trace elements [4–6]. In recent years, RA is mostly obtained from cultivated plants, as wild ones are increasingly scarce. Most of the herbs sold commercially are MGHQ [7].
Radix Hedysari, known as Hongqi (HQ), is the dried root of Hedysarum polybotrys Hand.-Mazz., which belongs to the same family as RA. Although HQ has been considered an authentic and specifically different medicine since 1985, as recorded in the Chinese Pharmacopoeia, traditionally these two herbs were often used in the same way [8]. Today in the northwest of China, HQ is still widely used in clinical practice instead of RA. The main chemical constituents of HQ are flavonoids, saponins, benzofurans, organic acids, lignans, and polysaccharides [8–10]. Comparison of the quality of these two herbs has a long history. In “shen nong ben cao jing”, HQ is recorded as one of the species used as RA, and as being of better quality. HQ is mainly cultivated in Gansu province and especially used in the Southeast Asia market.
As far as the known analysis methods, scanning thin-layer chromatography (TLC), high-performance liquid chromatography (HPLC), high-speed counter-current chromatography (HSCCC), capillary HPLC, and a colorimetric method were used to determine the identify, and levels of the main constituents in RA and HQ [11–15]. In order to separate, identify and quantify multicomponents, reverse-phase HPLC/photodiode array detector, evaporative light scattering detector, and MS detection were used.
It has been reported, that the main pharmacological bioactivities of RA included cardioprotective, hepatoprotective, hypotensive, immunostimulant, antiageing, antioxidant, antidiabetic, and antiinflammatory activities [16–20]. HQ has also showed activity in cardiovascular and circulatory functions, immunological benefits, antiaging effect, antiviral activity, hepatoprotective, antiinflammatory and analgesic activities [8, 21].
Compared to RA, HQ differs in several ways. HQ seems to have weaker activities in hypotension, circulatory improvement, and protection from cerebral ischemia, as demonstrated by animal model testing [22]. Therefore, in the context of clinical application, comparative evaluation of the pharmacological effects of RA and HQ is urgently needed. The present study just represents such a comparative study on the chemical constituents and immunological effect between RA and HQ for clinical application.
2. Materials and Methods
2.1. Experimental Animals
NIH mice (Grade II, 5 weeks old) weighing 18–22 g were purchased from Guangdong Experimental Animal Center (Certificate no. SCXK2008A021, Guangdong, China). Half of them were males and half were females. All procedures were in strict accordance with the PR China legislation on the use and care of laboratory animals and with the guidelines established by Guangdong Pharmacological University.
2.2. Plant Material
MGHQ and MJHQ were obtained from Chifeng, Neimenggu; HQ was obtained from Wudu, Gansu. The plant materials were identified by Dr. CHEN Hu-Biao, School of Chinese Medicine, Hong Kong Baptist University. The voucher specimens are deposited at the Herbarium, School of Chinese Medicine, Hong Kong Baptist University.
2.3. Extraction of Chemical Constituents
Reagent grade methanol, ethanol, acetic ether, n-butanol, ammonia water, hydrochloric acid, and sodium hydroxide were purchased from Lab-scan (Bangkok, Thailand). IR-120H ion-exchange resin was purchased from Aldrich (USA). Water was purified using a Milli-Q water system (Millipore; Bedford, MA, USA).
We extracted the different constituents into five major parts. There were total extract (TE), flavonoids extract (FE), saponins extract (SE), polysaccharides extract (PE), and amino acids extract (AE).
The method of TE extraction: 0.5 kg of powdered dried roots were refluxed 3 times with 3 L of MeOH (MeOH/ H2O, 50/50) for 1 hour, then filtered; the residues were refluxed 3 times with 3 L of water for 1 hour in a glass flask, then filtered. The final extracts were concentrated to 500 mL in vacuum.
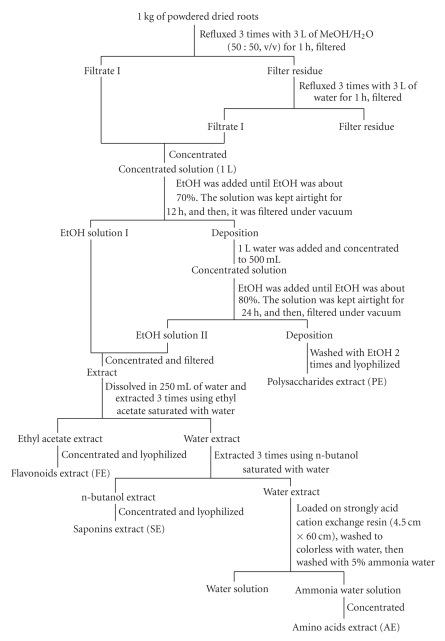
The procedure for the other extractions was shown in Figure 1. Flavonoids extract (FE), saponins extract (SE), polysaccharides extract (PE), and amino acids extract (AE) were dried in a vacuum oven (Cornelius, OR, USA) to constant weight.
Figure 1.
Procedures for FE, SE, PE, AE.
2.4. Analysis of Flavonoids and Saponins
The flavonoids and saponins in MGHQ, MJHQ, and HQ were analyzed by ultra-performance liquid chromatography/mass spectrometry (UPLC/MS). 2 mL of TE of MGHQ, MJHQ, and HQ and 1 mg of FE, SE of MGHQ, MJHQ, and HQ were weighed for determination. The samples were dissolved in a 10-mL volumetric flask with MeOH/H2O (50 : 50, v/v) and then filtered through a syringe filter (0.2 μm, Alltech, Beerfield, IL, USA). An aliquot of 4 μL sample solution was injected for UPLC/TOF-MS analysis. UPLC was performed on a Waters ACQUITY UPLC system (Waters, Milford, MA), which was equipped with a binary solvent delivery manager, column manager, sample manager, and diode array detector (DAD); it was used for quantitative analysis and UV acquisition. For mass spectrometric determination, the UPLC-DAD system was hyphenated to a Bruker MicrOTOFQ system by an electrospray ionization (ESI) interface (Bruker Daltonics, Bremen, Germany). For chromatographic separation, an Acquity UPLC BEH C18 column, 1.7 μm, 2.1×150 mm (Waters, Milford, MA) was used. The mobile phase consisted of 0.1% of formic acid in water (A) and 0.1% of formic acid in acetonitrile (B) using a gradient program of 10–40% B in 0–12 minutes, 40–70% B in 12–18 minutes, and 70–100% B in 18–22 minutes. The solvent flow rate was 0.3 mL/min and the column temperature was set at 30°C. The conditions of MS analysis in the negative ion mode were as follows: capillary voltage, 2.0 kV; cone voltage, 0.035 kV; the desolvation gas flow, 500 L/h; the desolvation temperature, 350°C; and source temperature, 100°C. The acquired m/z was from 100 to 1500. Acetonitrile of LC grade and methanol of analytical grade were purchased from Lab-scan (Bangkok, Thailand). Formic acid of LC grade was purchased from Merck (Darmstadt, Germany). Water was purified using a Milli-Q water system (Millipore, Bedford, MA, USA).
2.5. Quantitative Analysis of Polysaccharides
Polysaccharides extracts (PE) were redissolved in water and were hydrolyzed, dehydrated, and polymerized to form a colored complex in the presence of sulfuric acid and anthrone. The colored complex was measured quantitatively with an ultraviolet/visible spectrophotometer (Jasco V-530 UV/Vis spectrophotometer, Japan) at 625 nm [3]. 10 mg of PE of MGHQ, MJHQ, and HQ was dissolved in 100 mL of water for polysaccharide determination. Prepared samples were adjusted to a volume of 2.0 mL, and then 6.0 mL of 0.1% anthrone-sulfuric acid (prepared just before use) was added to each tube. The tubes were placed in a water bath (99°C) for 15 minutes with occasional shaking and then cooled in an ice water bath for 15 minutes. The samples were then used for UV/Visible analysis.
2.6. Quantitative Analysis of Amino Acids
500 mg of AE of MGHQ, MJHQ, and HQ was dissolved in 600 μL of water for amino acid determination. A Hitachi 855-350 auto amino acid detector (Hitachi, Japan), 150 mm × 2.6 mm ion-exchange column at 57°C and 10297 kPa, flow rate 0.225 mL/h, running time 59 minutes, and 20 μL sample, was used. The detection wavelengths were set at 570 nm and 440 nm [11].
2.7. Assay of Immune-Organ Index and Index of Carbon Grain Elimination
Cyclophosphamide and levamisole hydrochloride were purchased from Shanghai Hualian Pharmaceutical Ltd. (Shanghai, China). Drawing ink was purchased from Shanghai Haiwen Ltd. (Shanghai, China).
A total of 180 NIH mice were randomly divided into 18 groups, namely, normal control (NC), model (M), positive control (PC), and 15 experimental groups designed as: MGHQ-TE, MGHQ-FE, MGHQ-SE, MGHQ-PE, MGHQ-AE, MJHQ-TE, MJHQ-FE, MJHQ-SE, MJHQ-PE, MJHQ-AE, HQ-TE, HQ-FE, HQ-SE, HQ-PE, and HQ-AE.
Herbal extracts was administered to the animals in experimental groups intragastrically at a dose of 560 mg/kg of body weight per day for ten consecutive days. Distilled water was administered to the animals in the normal control and model groups at a dose of 560 mg/kg of body weight per day for ten consecutive days. The solution of levamisole hydrochloride was administered to the animals in the positive control group at a dose of 12 mg/kg of body weight per day for ten consecutive days. The solution of cyclophosphamide were administered to the animals in the groups (except the normal control group) intragastrically at a dose of 100 mg/kg of body weight after 1 hour following the drug administration in the second day for two consecutive days. After 1 hour following the last drug administration, mice were injected via the tail vein with colloidal carbon suspension, consisting of drawing ink. At 30 s and 6 minutes after the injection of the carbon suspension, 20 μL aliquot of the blood were taken from the orbital plexus with a heparinised haematocrit tube, then immediately mixed with 2 ml of 0.1% (w/v) Na2CO3. The concentration of the colloidal carbon was estimated by absorbance using a 721-spectrophotometer at 675 nm.
The clearance rate of carbon was expressed as the index of phagocytosis in the blood (K), calculated by means of the following equation: K = (log A1 − log A2)/(t1 − t2), where A1 and A2 are the optical densities at times t1 and t2, respectively. After blood sample collection, all the mice were sacrificed by dislocation of the cervical vertebrae and the immune organs including spleens and thymus glands were isolated and weighed. The indexes of the immune organs were calculated.
2.8. Assay of Serum Hemolysin to SRBC
Cyclophosphamide and levamisole hydrochloride were purchased from Shanghai Hualian Pharmaceutical Ltd. (Shanghai, China). Sheep erythrocytes were purchased from Jingmei Ltd. (Guangzhou, China).
A total of 180 NIH mice were randomly divided into 18 groups, namely, normal control (NC), model (M), positive control (PC), and 15 experimental groups designed as: MGHQ-TE, MGHQ-FE, MGHQ-SE, MGHQ-PE, MGHQ-AE, MJHQ-TE, MJHQ-FE, MJHQ-SE, MJHQ-PE, MJHQ-AE, HQ-TE, HQ-FE, HQ-SE, HQ-PE, and HQ-AE.
Herbal extracts was administered to the animals in experimental groups intragastrically at a dose of 560 mg/kg of body weight per day for ten consecutive days. Distilled water was administered to the animals in the normal control and model groups at a dose of 560 mg/kg of body weight per day for ten consecutive days. The solution of levamisole hydrochloride was administered to the animals in the positive control group at a dose of 12 mg/kg of body weight per day for ten consecutive days. The solution of cyclophosphamide were administered to the animals in the groups except the normal control group by hypodermic injection at a dose of 40 mg/kg of body weight following the drug administration on the first day for three consecutive days. Except for the normal control group, 5% suspensions of sheep erythrocyte were administered to the animals by intraperitoneal injection at a dose of 0.2 mL per mouse following the drug administration in the second day.
On the seventh day, 1 mL blood was collected by retro-orbital venous plexus puncture. 0.5 mL of 20% sheep red blood cell (SBRC) was added to 1 mL diluted sera, followed by 1 mL guinea pig plasma in sequence. For the normal controls, the complement was substituted with saline. The sample and control were incubated for 10 minutes at 37°C, and then immersed in an ice-water bath to stop the reaction. Each sample was centrifuged 10 minutes at 2000 rpm, and the supernatant was analyzed by hemoglobinometry. Color comparisons were detected using a 721-spectrophotometer at 540 nm wavelength. The absorbance for each sample was recorded and hemolytic complement activity (HC50) was calculated by means of the following equation: HC50 = OD1/OD2 × dilution factors, where OD1 and OD2 are the optical densities of sample and SRBC, respectively.
2.9. Statistical Analysis
The data were analyzed using one-way ANOVA and expressed as mean ± standard deviation (S.D.), n = 10. Significant differences between groups were detected by Duncan's multiple range test using SPSS 12.0 software. Student's t-test was used for comparison between two groups.
3. Results and Discussion
3.1. Contents of Each Extract
Flavonoids extract (FE), saponins extract (SE), polysaccharides extract (PE), and amino acids extract (AE) were dried in the vacuum oven to constant weight. The results are listed in Table 1. We can find out that HQ had the greatest amounts of all five constituents. And the contents of PE (85.0800 g/kg) and SE (17.7941 g/kg) in HQ were much higher in both of them than in MGHQ and MJHQ. MGHQ had the smallest amounts of FE (3.3611 g/kg) and PE (49.5570 g/kg), while MJHQ had the smallest amounts of SE (9.9575 g/kg) and AE (12.8310 g/kg).
Table 1.
Weights of extracts of MGHQ, MJHQ and HQ (g/kg).
| MGHQ | MJHQ | HQ | |
|---|---|---|---|
| FE | 3.3611 | 4.2065 | 4.9359 |
| SE | 11.5036 | 9.9575 | 17.7941 |
| PE | 49.5570 | 61.2505 | 85.0800 |
| AE | 16.2292 | 12.8310 | 18.4242 |
3.2. UPLC/TOF-MS Results
The mass spectrometric conditions were optimized in both positive and negative ion modes; the negative ion mode was found to be more sensitive. Most constituents exhibited their quasimolecular ions [M − H]−and [M + HCOOH − H]− in the negative ion mode.
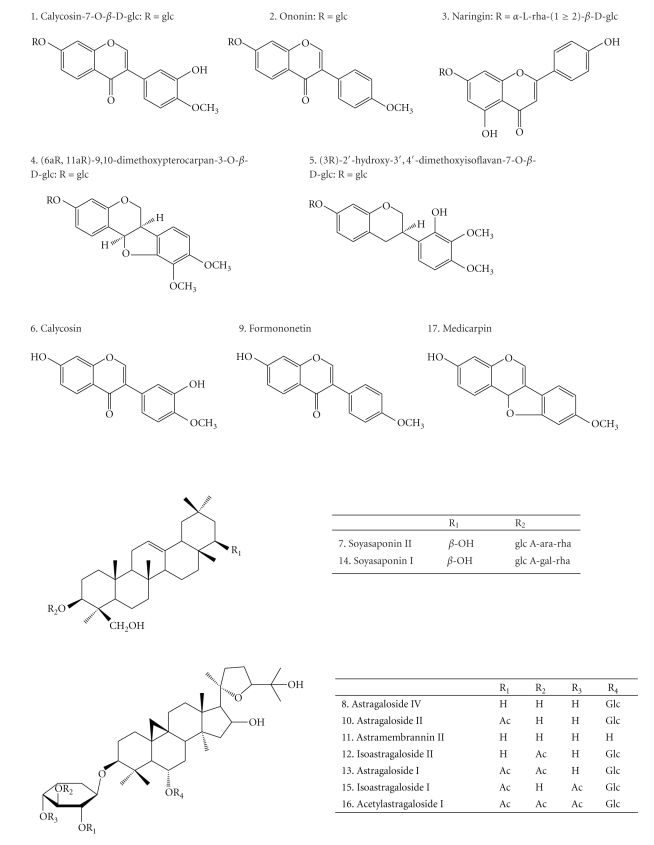
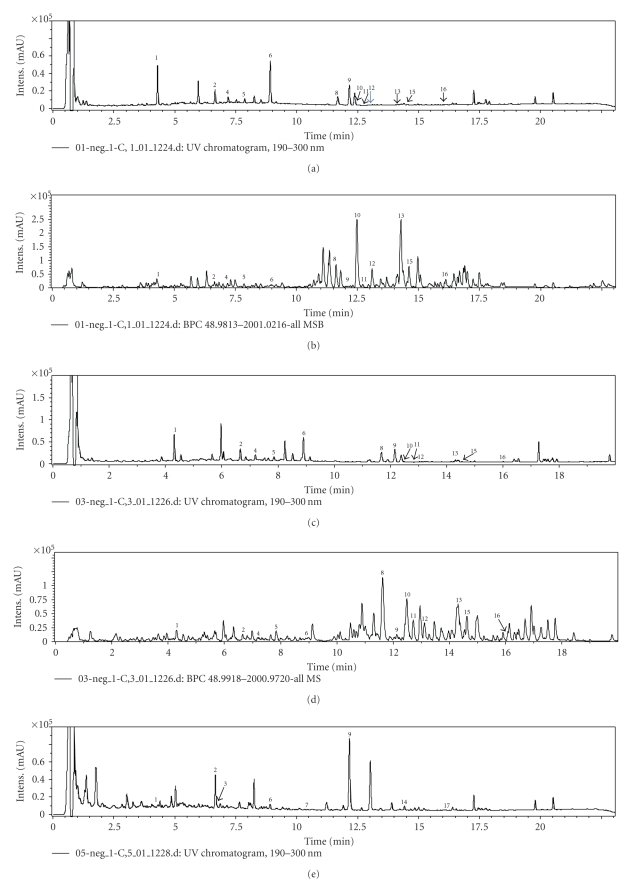
Based on comparison with chromatograms of standards compounds and comparison of their m/z values and UV spectra with the literature [23–25], 17 peaks were identified. They are: calycosin-7-O-β-D-glucoside (1), ononin (2), naringin (3), (6aR, 11aR)-9,10-dimethoxypterocarpan-3-O-β-D-glucopyranoside (4), (3R)- 2′-hydroxy-3′, 4′-dimethyl-isoflavan-7-O-β-D-glucopyranoside (5), calycosin (6), soyasaponin II (7), astragaloside IV (8), formononetin (9), astragaloside II (10), astramembrannin II (11), isoatragalosides II (12), astragaloside I (13), soyasaponin I (14), isoastragaloside I (15), acetylastragaloside I (16), medicarpin (17). The structures of the identified compounds were shown in Figure 2. The UPLC/TOF-MS analysis, giving accurate mass measurements of the compounds in TE of MGHQ, MJHQ, and HQ is shown in Table 2.
Figure 2.
Chemical structures of the compounds identified in the UPLC chromatograms.
Table 2.
Accurate mass measurements of the compounds in MGHQ, MJHQ, and HQ as revealed by UPLC/TOF-MS analysis in negative mode.
| Peak no. | tR (min) | Selected ion | m/z | Compound identification |
|---|---|---|---|---|
| 1 | 4.33 | [M + HCOOH − H]− | 491.1221 | calycosin-7-O-β-D-glucoside |
| 2 | 6.66 | [M + HCOOH − H]− | 475.1225 | ononin |
| 3 | 6.75 | [M + HCOOH − H]− | 625.2927 | naringin |
| 4 | 7.19 | [M + HCOOH − H]− | 507.1475 | (6aR, 11aR)-9,10-dimethoxypterocarpan-3-O-β-D-glucopyranoside |
| 5 | 7.86 | [M − H]− | 463.1603 | (3R)-2′-hydroxy-3′, 4′-dimethoxyisoflavan-7-O-β-D-glucopyranoside |
| 6 | 8.93 | [M − H]− | 283.061 | calycosin |
| 7 | 10.46 | [M + HCOOH − H]− | 957.5071 | soyasaponin II |
| 8 | 11.69 | [M + HCOOH − H]− | 829.462 | astragaloside IV |
| 9 | 12.16 | [M − H]− | 267.0686 | formononetin |
| 10 | 12.47 | [M + HCOOH − H]− | 871.4798 | astragaloside II |
| 11 | 12.70 | [M + HCOOH − H]− | 667.4065 | astramembrannin II |
| 12 | 13.09 | [M + HCOOH − H]− | 871.4722 | isoastragaloside II |
| 13 | 14.28 | [M + HCOOH − H]− | 913.4913 | astragaloside I |
| 14 | 14.37 | [M − H]− | 941.5078 | soyasaponin I |
| 15 | 14.60 | [M + HCOOH − H]− | 913.4836 | isoastragaloside I |
| 16 | 16.05 | [M + HCOOH − H]− | 955.4894 | acetylastragaloside I |
| 17 | 16.28 | [M + HCOOH − H]− | 315.2524 | medicarpin |
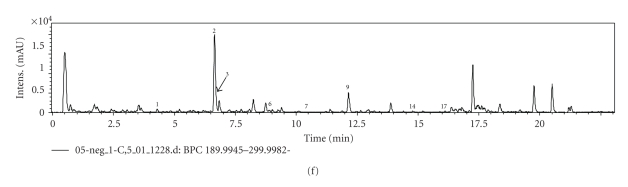
The UPLC-UV chromatogram (190–440 nm) and base peak chromatograms (BPC) of samples were analyzed, and results are shown in Figure 3. 13 compounds including 6 flavonoids and 7 saponins as calycosin-7-O-β-D-glucoside (1), ononin (2), (6aR, 11aR)-9,10-dimethoxypterocarpan-3-O-β-D-glucopyranoside (4), (3R)- 2′-hydroxy-3′, 4′-dimethyl-isoflavan-7-O-β-D-glucopyranoside (5), calycosin (6), astragaloside IV (8), formononetin (9), astragaloside II (10), astramembrannin II (11), isoatragaloside II (12), astragaloside I (13), isoastragaloside I (15), acetylastragaloside I (16) were detected in both MGHQ and MJHQ. 8 compounds comprising 6 flavonoids and 2 saponins namely, calycosin-7-O-β-D-glucoside (1), ononin (2), naringin (3), calycosin (6), soyasaponin II (7), formononetin (9), soyasaponin I (14), and medicarpin (17) were detected in HQ; Naringin (3), soyasaponin II (7), soyasaponin I (14), and medicarpin (17) were only detected in HQ. The results show that MGHQ and MJHQ have similar flavonoid and saponin constituents, while flavonoids and saponins constituents in HQ were significantly different. The saponins in HQ are oleanane pentacyclic triterpenoids, while the saponins in MGHQ and MJHQ are cycloartane tetracyclic triterpenoids.
Figure 3.
UPLC-UV chromatogram and BPC of the samples. (a): UV of MGHQ-TE, (b): BPC of MGHQ-TE, (c): UV of MJHQ-TE, (d): BPC of MJGHQ-TE, (e): UV of HQ-TE, (f): BPC of HQ-TE.
3.3. Determination of Polysaccharides
The polysaccharide contents of MGHQ, MJHQ, and HQ were calculated and are shown in Table 3. The results show that the contents of polysaccharides in HQ (21.238 g/kg) are much higher than, indeed, almost double, that in MGHQ (10.621 g/kg) and MJHQ (11.242 g/kg). MGHQ and MJHQ had similar polysaccharide contents, which agrees with earlier work [26, 27]. The references, however, show that the polysaccharide contents in MJHQ differ in specimens from different places [26], thus it is hard to draw conclusion about the difference in the polysaccharide contents of MGHQ and MJHQ in this study. Further work on specimens of MGHQ and MJHQ from different places is needed.
Table 3.
Polysaccharide content in MGHQ, MJHQ and HQ.
| Sample | Conc. | Absorbance | G Conc. | Amount in | PE Content | G Content |
|---|---|---|---|---|---|---|
| (mg/L) | (mg/L) | Sample (%) | (g) | (g/kg) | ||
| MGHQ-PE | 100.00 | 0.1873 | 21.43038 | 21.43 | 49.5570 | 10.621 |
| MJHQ-PE | 100.00 | 0.1630 | 18.35443 | 18.35 | 61.2505 | 11.242 |
| HQ-PE | 100.00 | 0.2152 | 24.96203 | 24.96 | 85.0800 | 21.238 |
3.4. Determination of Amino Acids
Seventeen amino acids were determined to be present in the extracts as follows: aspartic acid (Asp), serine (Ser), glutamic acid (Glu), glycine (Gly), alanine (Ala), cysteine (Cys), valine (Val), methionine (Met), isoleucine (Ile), leucine (Leu), tyrosine (Tyr), phenylalanine (Phe), lysine (Lys), asparagines (Asn), histidine (His), arginine (Arg), proline (Pro). Val, Met, Ile, Leu, Phe, and Lys are essential amino acids. His and Arg are semidispensable amino acids.
The amino acid contents of MGHQ, MJHQ and HQ were calculated and are shown in Table 4. The results show that the contents of total amino acids in MGHQ (3.7111 g/kg) and HQ (3.5333 g/kg) were higher than in MJHQ (2.3244 g/kg), and highest in MGHQ. The contents of Glu, Cys, Met, Tyr and Phe in MJHQ were higher thanthosein MGHQ and HQ. In addition, the content of Ser in MJHQ is too low to be quantified.
Table 4.
Amino acid contents of MGHQ, MJHQ, and HQ (g/kg).
| No. | Amino acid | MGHQ-AE | MJHQ-AE | HQ-AE |
|---|---|---|---|---|
| 1 | Asp | 0.7859 | 0.4103 | 0.8778 |
| 2 | Ser | 0.0327 | * | 0.0411 |
| 3 | Glu | 0.5772 | 0.6792 | 0.5534 |
| 4 | Gly | 0.0121 | 0.0096 | 0.0108 |
| 5 | Ala | 0.0965 | 0.0718 | 0.1895 |
| 6 | Cys | 0.0099 | 0.0241 | 0.0096 |
| 7 | Val | 0.0335 | 0.0297 | 0.0424 |
| 8 | Met | 0.0120 | 0.0477 | 0.0169 |
| 9 | Ile | 0.0077 | 0.0037 | 0.0064 |
| 10 | Leu | 0.0063 | 0.0052 | 0.0023 |
| 11 | Tyr | 0.0249 | 0.0274 | 0.0229 |
| 12 | Phe | 0.0157 | 0.0611 | 0.0324 |
| 13 | Lys | 0.0301 | 0.0203 | 0.0061 |
| 14 | Asn | 0.0926 | 0.0816 | 0.1148 |
| 15 | His | 0.0077 | 0.0049 | 0.0189 |
| 16 | Arg | 0.3093 | 0.2103 | 0.0346 |
| 17 | Pro | 1.6571 | 0.6375 | 1.5731 |
| 18 | Total | 3.7111 | 2.3244 | 3.5533 |
*Can be detected, but cannot be quantified.
3.5. Effect on the Immune-Organs and Macrophages
The nonspecific immunological effects of the extracts were tested by measuring the immune-organ weights and phagocytic activity in mice. Macrophages play a significant role in the host defense mechanism. Macrophages have the strong ability of phagocytosis and clearance variant particle diameter or some dissolvability xenoma. When mice are injected via the tail vein with carbon particles of a definite size, the carbon particles will be cleared up in blood by macrophages in the liver and spleen and by the total entire mononuclear phagocytic system [28].
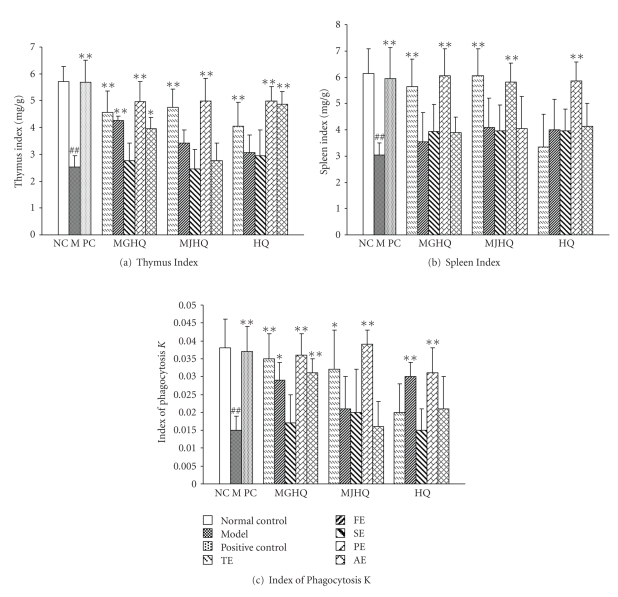
The effects on mice immune organs and phagocytosis from treatment with different extracts are shown in Table 5 and Figure 4. Figure 4(a) shows that compared with the model, the thymus indexes of mice treated with TE and PE of the three herbs groups significantly increased (P < .01), while PE groups wereobserved to have bettereffect. In addition, the thymus indexes of MGHQ-FE and HQ-AE groups significantly increased (P < .01), and MGHQ-AE increased the thymus index significantly (P < .05). Figure 4(b) shows that the spleen indexes in all experimental groups increased compared with the model, but this only became significant (P < .01) in MGHQ-TE, MJHQ-TE, MGHQ-PE, MJHQ-PE, and HQ-PE groups. Figure 4(c) shows that compared with the model, the indexes of phagocytosis K in mice treated with PE groups of all three herbs, MGHQ-TE, MGHQ-AE, and HQ-FE groups significant increased (P < .01). In addition, MGHQ-FE and MJHQ-TE increased the indexes of phagocytosis K. The results showed that MGHQ-TE, MGHQ-PE, MJHQ-TE, MJHQ-PE, and HQ-PE increased the indexes of thymus, spleen, and phagocytosis K. The polysaccharides extract (PE) may contain the bioactive compounds which stimulated the nonspecificity immunological activity in mice. PE in HQ showed similar activities with those in MGHQ and MJHQ.
Table 5.
Effects of different extracts on mice immune organs and phagocytosis.
| Group | Thymus index (mg/g) | Spleen index (mg/g) | Index of Phagocytosis K |
|---|---|---|---|
| Normal control | 5.72 ± 0.56 | 6.15±.0.94 | 0.038 ± 0.008 |
| Model | 2.53 ± 0.43## | 3.04 ± 0.46## | 0.015 ± 0.004## |
| Positive control | 5.69 ± 0.81** | 5.95 ± 1.18** | 0.037 ± 0.007** |
| MGHQ-TE | 4.56 ± 0.81** | 5.65 ± 1.04** | 0.035 ± 0.007** |
| MGHQ-FE | 4.26 ± 0.16** | 3.54 ± 1.12 | 0.029 ± 0.005* |
| MGHQ-SE | 2.76 ± 0.66 | 3.93 ± 1.04 | 0.017 ± 0.008 |
| MGHQ-PE | 4.96 ± 0.76** | 6.05 ± 1.04** | 0.036 ± 0.006** |
| MGHQ-AE | 3.96 ± 0.42* | 3.89 ± 0.58 | 0.031 ± 0.004** |
| MJHQ-TE | 4.76 ± 0.66** | 6.05 ± 1.04** | 0.032 ± 0.011* |
| MJHQ-FE | 3.42 ± 0.48 | 4.08 ± 1.12 | 0.021 ± 0.009 |
| MJHQ-SE | 2.46 ± 0.73 | 3.95 ± 0.98 | 0.020 ± 0.012 |
| MJHQ-PE | 4.98 ± 0.85** | 5.82 ± 0.72** | 0.039 ± 0.004** |
| MJHQ-AE | 2.76 ± 0.66 | 4.05 ± 1.21 | 0.016 ± 0.007 |
| HQ-TE | 4.04 ± 0.89** | 3.34 ± 1.24 | 0.020 ± 0.008 |
| HQ-FE | 3.06 ± 0.67 | 4.01 ± 1.14 | 0.030 ± 0.004** |
| HQ-SE | 2.96 ± 0.96 | 3.95 ± 0.84 | 0.015 ± 0.006 |
| HQ-PE | 4.99 ± 0.54** | 5.85 ± 0.74** | 0.031 ± 0.007** |
| HQ-AE | 4.87 ± 0.46** | 4.14 ± 0.87 | 0.021 ± 0.009 |
##P < .01 and #P < .05 versus the normal control group. **P < .01 and *P < .05 versus the model group.
Figure 4.
Effects of different extracts on mice immune organs and phagocytosis ##P < .01 and #P < .05 versus the normal control group. **P < .01 and *P < .05 versus the model group.
3.6. Effect on Humoral Immune Responses
The level of a specific antibody in the serum can be used as a measure of the functional status of all three development phases of the humoral immune response-antigen recognition, activation, and expression [29]. Hemolysin level is usually considered to be an indicator of humoral immunity. To determine an extract's effect on the regulation of hemolysin, mice were immunized with SRBC (T-dependent particulate antigen).
HC50, the absorbance at the time when hemolysin takes place in half of SRBC, shows decreasing hemolysin and a reduction in humoral immunity [30].
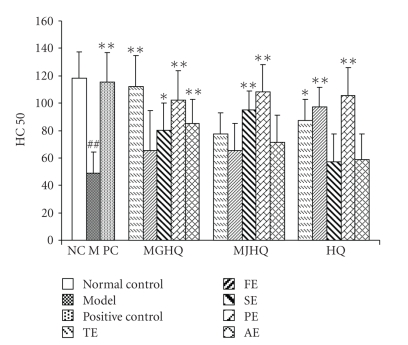
The results are shown in Table 6. Figure 5 showed that the HC50 in all experimental groups increased compared with the model, but this only became significantly (P < .01) in PE groups of all the three herbs, MGHQ-TE, MGHQ-AE, MJHQ-SE, and HQ-FE groups. In addition, the HC50 of mice treated with MGHQ-SE and HQ-TE significantly increased (P < .05). The results show that polysaccharides extract (PE) may contain the bioactive compounds that influence specific immunological activities, and PE in HQ showed similar activities with those in MGHQ and MJHQ. HQ-FE showed immunological activity in the assay of serum hemolysin to SRBC, while FE of MGHQ and MJHQ did not. SE in MGHQ and MJHQ showed immunological activity, while HQ-SE did not. These results were consistent with the significant differences in the flavonoid and saponin constituents in RA and HQ.
Table 6.
Effects of different extracts on mice serum hemolysin.
| Group | HC50 |
|---|---|
| Normal control | 118.3 ± 19.4 |
| Model | 48.7 ± 15.6## |
| Positive control | 115.3 ± 21.6* |
| MGHQ-TE | 112.3 ± 22.5** |
| MGHQ-FE | 65.3 ± 29.4 |
| MGHQ-SE | 80.1 ± 17.1* |
| MGHQ-PE | 102.3 ± 21.5** |
| MGHQ-AE | 85.3 ± 17.3** |
| MJHQ-TE | 77.3 ± 15.4 |
| MJHQ-FE | 65.7 ± 19.5 |
| MJHQ-SE | 95.3 ± 13.7** |
| MJHQ-PE | 108.3 ± 20.4** |
| MJHQ-AE | 71.3±20.3 |
| HQ-TE | 87.3±15.4* |
| HQ-FE | 97.1±14.7** |
| HQ-SE | 57.3±21.5 |
| HQ-PE | 105.7±22.8 ** |
| HQ-AE | 59.1±18.6 |
##P < .01 and #P < .05 versus the normal control group. **P < .01 and *P < .05 versus the model group.
Figure 5.
Effects of different extracts treatment on mice serum hemolysin ##P < .01 and #P < .05 versus the normal control group. **P < .01 and *P < .05 versus the model group.
4. Conclusions
In the present work, we have compared the chemical constituents and the immunological effects of MGHQ, MJHQ, and HQ. We extracted and analyzed the components of the three herbs, in five different forms: total extract (TE), flavonoids extract (FE), saponins extract (SE), polysaccharides extract (PE), and amino acids extract (AE), and compared the immunological effects of different extracts by using two immunological models.
The results showed that MGHQ and MJHQ had the similar flavonoid and saponin constituents, while HQ was significantly different. Naringin and medicarpin were only detected in HQ. The saponins in HQ are oleanane pentacyclic triterpenoids, while the saponins in MGHQ and MJHQ are cycloartane tetracyclic triterpenoids. In this comparative study of specific immunological activity, HQ-FE showed immunological activity in the assay of serum hemolysin to SRBC, while FE of MGHQ and MJHQ did not. SE in MGHQ and MJHQ showed immunological activity, while HQ-SE did not.
The contents of total amino acids were higher in MGHQ and HQ than those in MJHQ. In this comparative study of immunological activity, AE increased the indexes of thymus, phagocytosis K and HC50 in mice significantly, while AE in MJHQ and HQ did not. The results show that AE in MGHQ is very special. We could do more research on the relationship of the percents of different amino acids and the immunological effects in future.
Polysaccharides were the major constituents in MGHQ, MJHQ, and HQ. The contents of polysaccharides in HQ were almost double of those in MGHQ and MJHQ, but were similar in MGHQ and MJHQ. In this comparative study of nonspecific immunological activity, PE increased the indexes of thymus, spleen, and phagocytosis K in mice significantly. In this comparative study of specific immunological activity, PE increased HC50 in mice significantly. In a conclusion, PE showed regulatory effects on both specific and nonspecific immunological functions in mice. PE in HQ showed similar activities with those in MGHQ and MJHQ. In a conclusion, polysaccharides may be the main bioactive compounds that are responsible for immunoregulatory function of RA and HQ. Our studies provide the scientific data for that HQ can be used instead of RA in the immunoregulatory function aspect in the clinical application.
Acknowledgment
This project was supported by the Faculty Research Grant of Hong Kong Baptist University (FRG/06-07/II-24).
References
- 1.Toda S, Shirataki Y. Inhibitory effects of Astragali Radix, a crude drug in Oriental medicines, on lipid peroxidation and protein oxidative modification by copper. Journal of Ethnopharmacology. 1999;68(1–3):331–333. doi: 10.1016/s0378-8741(99)00104-x. [DOI] [PubMed] [Google Scholar]
- 2.Shon YH, Kim JH, Nam KS. Effect of Astragali radix extract on lipopolysaccharide-induced inflammation in human amnion. Biological & Pharmaceutical Bulletin. 2002;25(1):77–80. doi: 10.1248/bpb.25.77. [DOI] [PubMed] [Google Scholar]
- 3.Zheng XY. Pharmacopoeia of the People’s Republic of China. Beijing, China: Chemical Industry Press; 2005. [Google Scholar]
- 4.Gui S-Y, Wei W, Wang H, et al. Effects and mechanisms of crude astragalosides fraction on liver fibrosis in rats. Journal of Ethnopharmacology. 2006;103(2):154–159. doi: 10.1016/j.jep.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 5.Song ZH, Ji ZN, Lo CK, et al. Chemical and biological assessment of a traditional Chinese herbal decoction prepared from Radix Astragali and Radix Angelicae Sinensis: orthogonal array design to optimize the extraction of chemical constituents. Planta Medica. 2004;70(12):1222–1227. doi: 10.1055/s-2004-835855. [DOI] [PubMed] [Google Scholar]
- 6.Liu R, Xu S, Li J, Hu Y, Lin Z. Expression profile of a PAL gene from Astragalus membranaceus var. Mongholicus and its crucial role in flux into flavonoid biosynthesis. Plant Cell Reports. 2006;25(7):705–710. doi: 10.1007/s00299-005-0072-7. [DOI] [PubMed] [Google Scholar]
- 7.Liu J, Chen HB, Zhao ZZ, et al. Quality evaluation of Radix Astragali from different sources in China. Journal of Chinese Pharmaceutical Sciences. 2009;18(1):p. 6. [Google Scholar]
- 8.Li GM, Wang WN, Hu MS. Pharmacognosy of radix Hedysari. Zhong Yao Tong Bao. 1987;12(8):5–8. [PubMed] [Google Scholar]
- 9.Liu Y, Zhang Q-Y, Zhao Y-Y, et al. Saponins from the roots of Hedysarum polybotrys. Biochemical Systematics and Ecology. 2007;35(6):389–391. [Google Scholar]
- 10.Yang M, Chan GCF, Deng R, et al. An herbal decoction of Radix astragali and Radix angelicae sinensis promotes hematopoiesis and thrombopoiesis. Journal of Ethnopharmacology. 2009;124(1):87–97. doi: 10.1016/j.jep.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Ma XQ, Shi Q, Duan JA, Dong TTX, Tsim KWK. Chemical analysis of Radix Astragali (Huangqi) in China: a comparison with its adulterants and seasonal variations. Journal of Agricultural and Food Chemistry. 2002;50(17):4861–4866. doi: 10.1021/jf0202279. [DOI] [PubMed] [Google Scholar]
- 12.Xiao W, Han L, Shi B. Isolation and purification of flavonoid glucosides from Radix Astragali by high-speed counter-current chromatography. (AnalyticalTechnologies in the Biomedical and Life Sciences).Journal of Chromatography B. 2009;877(8-9):697–702. doi: 10.1016/j.jchromb.2009.01.034. [DOI] [PubMed] [Google Scholar]
- 13.Xiao HB, Krucker M, Albert K, Liang XM. Determination and identification of isoflavonoids in Radix astragali by matrix solid-phase dispersion extraction and high-performance liquid chromatography with photodiode array and mass spectrometric detection. Journal of Chromatography A. 2004;1032(1-2):117–124. doi: 10.1016/j.chroma.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 14.Qi L-W, Yu Q-T, Li P, et al. Quality evaluation of Radix Astragali through a simultaneous determination of six major active isoflavonoids and four main saponins by high-performance liquid chromatography coupled with diode array and evaporative light scattering detectors. Journal of Chromatography A. 2006;1134(1-2):162–169. doi: 10.1016/j.chroma.2006.08.085. [DOI] [PubMed] [Google Scholar]
- 15.Zhao J, Yu Q-T, Li P, Zhou P, Zhang Y-J, Wang W. Determination of nine active components in Radix Hedysari and Radix Astragali using capillary HPLC with diode array detection and MS detection. Journal of Separation Science. 2008;31(2):255–261. doi: 10.1002/jssc.200700379. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Li J, Fang C. Inhibitory effects of anti-SARS traditional Chinese medicines on the UV irradiation of λ-lysogen. The American Journal of Chinese Medicine. 2006;34(1):147–155. doi: 10.1142/S0192415X06003710. [DOI] [PubMed] [Google Scholar]
- 17.Wu X-L, Wang Y-Y, Cheng J, Zhao Y-Y. Calcium channel blocking activity of calycosin, a major active component of Astragali Radix, on rat aorta. Acta Pharmacologica Sinica. 2006;27(8):1007–1012. doi: 10.1111/j.1745-7254.2006.00349.x. [DOI] [PubMed] [Google Scholar]
- 18.Wu T, Annie Bligh SW, Gu L-H, et al. Simultaneous determination of six isoflavonoids in commercial Radix Astragali by HPLC-UV. Fitoterapia. 2005;76(2):157–165. doi: 10.1016/j.fitote.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 19.Zhang X, Feng J, Mu K, et al. Effects of single herbal drugs on adhesion and migration of melanocytes. Journal of Traditional Chinese Medicine. 2005;25(3):219–221. [PubMed] [Google Scholar]
- 20.Toda S, Shirataki Y. Inhibitory effects of isoflavones in roots of Astragalus membranaceus BUNGE (Astragali Radix) on lipid peroxidation by reactive oxygen species. Phytotherapy Research. 1998;12(1):59–61. doi: 10.1002/(SICI)1099-1573(199903)13:2<163::AID-PTR405>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 21.Lan Z, Zhang Z, Cheng G, Wang G, Xi S. Effects of radix hedysari polysaccharides on immunological function and transplanted tumors in mice. Zhongguo Yaoli Xuebao. 1987;8(3):275–277. [PubMed] [Google Scholar]
- 22.Mao X, Wang J, Wang F. Effect of Hedysari and Astragli polysaccharides on humoral immune function in mice. Zhongguo Mianyixue Zazhi. 1998;4(3):158–161. [Google Scholar]
- 23.Qi L-W, Yu Q-T, Yi L, et al. Simultaneous determination of 15 marker constituents in various Radix Astragali preparations by solid-phase extraction and high-performance liquid chromatography. Journal of Separation Science. 2008;31(1):97–106. doi: 10.1002/jssc.200700286. [DOI] [PubMed] [Google Scholar]
- 24.Qi L-W, Li P, Ren M-T, Yu Q-T, Wen X-D, Wang Y-X. Application of high-performance liquid chromatography-electrospray ionization time-of-flight mass spectrometry for analysis and quality control of Radix Astragali and its preparations. Journal of Chromatography A. 2009;1216(11):2087–2097. doi: 10.1016/j.chroma.2008.02.095. [DOI] [PubMed] [Google Scholar]
- 25.Li X, Zhu Z-Y, Wang B, Lou Z-Y, Chai Y-F. Determination of three constituents in Radix stragali by HPLC-MS. Yao Xue Xue Bao. 2006;41(8):793–796. [PubMed] [Google Scholar]
- 26.Zhao CY, Qin H, Liu Y. Study on comparing Astragalan Polysaccharide from two kinds of astragalus. Journal of Systems Science and Complexity. 2006;19(6):441–446. [Google Scholar]
- 27.Yang L, Wang ZH, Tao JS, et al. Comparison of the methods for determination of Astraglus polysaccharides in radix astragali. Chinese Journal of Pharmaceuticals. 2005;36(9):562–563. [Google Scholar]
- 28.Gao LS, Zeng FP, Ning LX, et al. Researches on effects of magnetized Codonopsis pilosula (Franch.) nannf. medicinal solution on carbon expurgatory function of small white rats. Biomagnetism. 2004;4(4):1–4. [Google Scholar]
- 29.Silkworth JB, Loose LD. Assessment of environmental contaminant-induced lymphocyte dysfunction. Environmental Health Perspectives. 1981;39:105–128. doi: 10.1289/ehp.8139105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wen X-M, Zhang Y-l, Liu X-M, Guo S-X, Wang H. Immune responses in mice to arecoline mediated by lymphocyte muscarinic acetylcholine receptor. Cell Biology International. 2006;30(12):1048–1053. doi: 10.1016/j.cellbi.2006.09.015. [DOI] [PubMed] [Google Scholar]