Abstract
The selective γ-aminobutyric acid B-subtype receptor agonist baclofen activates both pre- and post-synaptic receptors in the brain. Microinjection of baclofen into the nucleus of the solitary tract increases arterial pressure, heart rate and sympathetic nerve discharge consistent with inhibition of the arterial baroreflex. The magnitude of these responses is enhanced in hypertension and is associated with increased post-synaptic GABAB receptor function. We tested whether a pre-synaptic mechanism contributes to the enhanced baclofen inhibition in hypertension. Whole-cell recordings of second-order baroreceptor neurons, identified by 4-(4-(dihexadecylamino)styryl)-N-methylpyridinium iodide labeling of aortic nerve, were obtained in brainstem slices from normotensive control and renal-wrap hypertensive rats. After 4 weeks, arterial blood pressure was 162±9 mmHg in hypertensive (n=6) and 107±3 mmHg in control rats (n=6/11, p<0.001). Baclofen reduced the amplitude of excitatory post-synaptic currents evoked by solitary tract stimulation and the EC50 of this inhibition was greater in control (1.5±0.5 µmol/L, n=6) than hypertensive cells (0.6±0.1 µmol/L, n=9, p<0.05). Baclofen (1 µmol/L) elicited greater inhibition on evoked response in hypertensive (58±6%, n=9) than control cells (40±6%, n=8, p<0.05). Another index of pre-synaptic inhibition, the paired-pulse ratio (ratio of second to first evoked response amplitudes at stimulus intervals of 40 ms), was greater in hypertensive (0.60±0.08, n=8) than control cells (0.48±0.06. n=5, p<0.05). The results suggest that in renal-wrap hypertensive rats, baclofen causes an enhanced pre-synaptic inhibition of glutamate release from baroreceptor afferent terminals to second-order neurons in the nucleus of the solitary tract. This enhanced pre-synaptic inhibition could contribute to altered baroreflex function in hypertension.
Keywords: cardiovascular regulation, baroreceptor, baroreflex, hypertension, blood pressure
INTRODUCTION
The nucleus of the solitary tract (NTS) is the first central integration site of arterial baroreceptor afferents1–2. Baroreceptor afferent terminals release glutamate to activate second-order neurons in the NTS. This excitatory transmission is under dynamic modulation from various inhibitory neurotransmitters or neuromodulators, including the γ-aminobutyric acid (GABA) via both ionotropic GABAA receptors and metabotropic GABAB receptors3–5.
GABAB receptor-mediated modulation of excitatory baroreceptor inputs in the NTS has been demonstrated and this inhibition has both pre- and post-synaptic components. Microinjection of selective GABAB receptor agonist baclofen into the NTS results in an increase in arterial blood pressure, heart rate, and renal sympathetic nerve discharge6–8. These baclofen-induced responses could be mediated by GABAB receptor-mediated inhibition of pre-synaptic glutamate release and/or post-synaptic neuronal responses to glutamate in NTS neurons integrating baroreceptor afferent inputs5, 9–11. NTS microinjection of baclofen-induced cardiovascular responses are enhanced in several animal models of chronic hypertension, including spontaneously hypertensive rats12–13, deoxycorticosterone (DOCA)-salt hypertensive rats14, and one-kidney, renal wrap rat model of hypertension4,9–11, 15. However, the cellular mechanisms underlying the enhanced effects of baclofen in hypertension are still not well understood.
Previous studies from this lab have demonstrated that one kidney renal-wrap hypertension is associated with increased GABAB receptor-mediated inhibition of baroreceptor evoked discharge in NTS neurons10, and increased expression of GABAB receptor mRNA in the NTS9. These data support the concept that enhanced GABAB receptor function may contribute to enhanced baclofen inhibition observed in chronic hypertension. We also demonstrated in a brain slice preparation from the same rat model of chronic arterial hypertension, that the post-synaptic effect of baclofen (increased potassium conductance) was enhanced4. However, it is not known whether pre-synaptic baclofen inhibition also contributes to the enhanced responses to baclofen observed in hypertension.
To further clarify GABAB receptor-mediated cellular mechanisms in chronic hypertension, the present study investigated pre-synaptic inhibition by baclofen on synaptic transmission of baroreceptor afferents to NTS neurons using the same one kidney renal-wrap hypertension rat model. An in vitro patch-clamp method was used to investigate the effect of baclofen on pre-synaptic release of glutamate to second-order baroreceptor neurons in the NTS. The results demonstrated that after chronic hypertension, there is enhanced baclofen-mediated pre-synaptic inhibition of glutamate release from baroreceptor afferents. This enhanced pre-synaptic baclofen inhibition could contribute to the enhanced baclofen-induced pressor response observed in chronic hypertension9–11, 15.
METHODS
All experimental protocols in this work were reviewed and approved by the Institutional Animal Care and Use Committee at the University of Texas Health Science Center at San Antonio.
Surgical preparation for labeling aortic nerve and renal-wrap chronic hypertension model
Male Sprague-Dawley rats (100–125 g, Charles River, Wilmington, MA) were anaesthetized with a combination of ketamine (75 mg/kg, i.p., Fort Dodge, Madison, NJ) and medetomidine (0.5 mg/kg, i.p., Pfizer, New York, NY). Under aseptic conditions, crystals of anterograde fluorescent dye 4-(4-(dihexadecylamino)styryl)-N-methylpyridinium iodide (DiA, D-3883, Molecular Probes, Carlsbad, CA) were gently applied unilaterally to the intact aortic nerve to visualize baroreceptor synaptic terminals and neurons receiving these synaptic contacts3–4, 16–17. The area was then embedded with silicone adhesive (Kwik-Sil, WPI, Sarasota, FL).
Hypertension was induced using a one-kidney renal-wrap procedure. Immediately following above labeling procedures, a figure-8 renal wrap and contralateral nephrectomy were performed on these animals18. Control animals were sham-operated rats that only received a unilateral nephrectomy but no wrap of the contralateral kidney. Anesthesia was terminated by atipamezole (1 mg/kg i.p., Pfizer, New York, NY) at the conclusion of the surgical procedures. Post-operative analgesics (Nubaine, i.m.) were available as needed.
Four weeks after renal wrap or sham surgery, an arterial catheter was placed in the femoral artery while the animal was under ketamine/medetomidine anesthesia as described. After a 2-day recovery period, the blood pressure of the conscious animal was measured by connecting the arterial catheter to a pressure transducer (Kobe) and displayed by means of Cambridge Electronics Design A/D converters (CED, Cambridge, UK). Blood pressure was measured for 3 hours in all hypertensive (HT) rats and in 6/11 normotensive (NT) rats, and measurements made during the last hour were used as an index of mean arterial pressure (MAP).
Brainstem slice preparation
The next day after blood pressure measurement, rats were anesthetized with isoflurane and the brainstem rapidly removed and placed in ice-cold, high-sucrose, artificial cerebrospinal fluid (aCSF) that contained (in mmol/l): 3 KCl, 1 MgCl2, 1 CaCl2, 2 MgSO4, 1.25 NaH2PO4, 26 NaHCO3, 10 glucose, and 206 sucrose, pH 7.4 when continuously bubbled with 95% O2/5% CO2. Brainstem horizontal slices (250 µm thick) were cut with a sapphire knife (Delaware Diamond Knives, Wilmington, DE) mounted in a vibrating microtome (VT 1000E, Leica Microsystems, Bannockburn, IL). Then slices were incubated for at least one hour in normal aCSF that contained (in mmol/l): 124 NaCl, 3 KCl, 2 MgSO4, 1.25 NaH2PO4, 26 NaHCO3, 10 glucose and 2 CaCl2, pH 7.4 when continuously bubbled with 95% O2/5% CO2.
Electrophysiological recording
For recordings, a single slice was transferred to the recording chamber on an upright epifluorescent microscope (Olympus BX50WI, Tokyo) equipped with infrared differential interference contrast (IR-DIC) and an optical filter set for visualization of DiA. The slice was held in place with a nylon mesh, submerged in normal aCSF equilibrated with 95% O2/5% CO2 and superfused at a rate of approximately 2 ml/min. All images were captured with a charge-coupled device (CCD) camera (IR-1000, CCD-100; Dage-MTI, Michigan City, IN) and displayed on a TV monitor. Patch pipettes were pulled from borosilicate glass capillaries with an inner filament (0.90 mm ID, 1.2 mm OD, WPI, Sarasota, FL) and were filled with a solution of the following composition (in mmol/l): 125 CsCl, l MgCl2, 10 HEPEs, 1.1 EGTA, 2 Mg2ATP, 0.3 Na3GTP, and 5 QX-314. The pH was adjusted to 7.3 with CsOH. The combination of CsCl and QX-314 in pipette solution reliably blocks the post-synaptic effect of baclofen which is primarily an increase in potassium conductance19–20. The pipette resistance ranged from 2 to 4 MΩ. A seal resistance of at least 1 GΩ or above, and an access resistance < 20 MΩ which changed <15% during recording were considered acceptable. Series resistance was optimally compensated. Cells were clamped at a membrane potential of −60 mV. Recordings of post-synaptic currents began 5 min later, after whole cell access was established and the current reached a steady state. Recordings were made with the AxoPatch 200B amplifier and pClamp software version 8 (Axon Instruments, Union City, CA). Whole-cell currents were filtered at 2 kHz, digitized at 10 kHz with the DigiData 1200 Interface (Axon Instruments) and stored in a PC computer for offline analysis. All experiments were performed at room temperature.
Whole-cell recording experiments were performed in DiA labeled second-order baroreceptor neurons in the NTS (Fig. 1A and B). Evoked EPSCs (eEPSCs) were elicited by electrical stimulation of the ipsilateral solitary tract (ST) using a concentric bipolar electrodes (FHC, Bowdoinham, ME) with a tip diameter of 0.2 mm. Square electric pulses of 0.1 ms duration with a frequency of 0.2 Hz were delivered through a stimulus isolator A360 (WPI, Sarasota, FL), in series with a programmable stimulator (Master8, AMPI, Jerusalem, Israel). Recordings of eEPSCs were performed in the presence of the GABAA receptor antagonist picrotoxin (100 µmol/L). Bath application of drugs typically lasted about 3–5 min to achieve steady state and begin drug effect tests. For establishing a dose-response curve of baclofen-induced inhibition of eEPSCs, sequential bath application of baclofen was performed at concentrations of 0.03, 0.1, 0.3, 1, 3, and 10 µmol/L. Each concentration was applied for at least three min before tract stimulation. For paired-pulse stimulation, two synaptic responses (A1 and A2) were evoked by a pair of stimuli given at an interval ranging from 20 to 200 ms. Paired-pulse ratio (PPR) was calculated as the amplitude ratio of the second synaptic response to the first synaptic response (A2/A1).
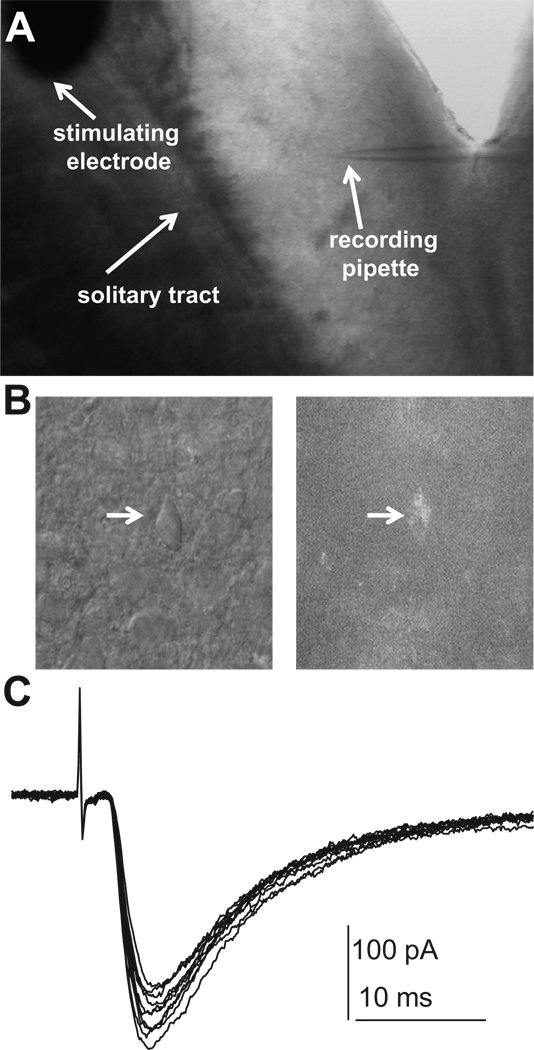
Figure 1. Whole-cell recordings in the nucleus of the solitary tract (NTS).
A: An in vitro whole-cell recording setup on a horizontal brainstem slice containing the NTS. B: A photograph shows one DiA labeled neuron viewed with fluorescence (right panel) and brightfield (left panel). C: A raw data of 10 tracings of EPSCs evoked by electrical stimulation of ipsilateral tract. Notice minimal variability of onset latency in all tracings, indicating mono-synaptic inputs. All experiments were performed in the presence of GABAA receptor antagonist picrotoxin (100 µmol/L).
Data analysis
All data were presented as means ± SEM. For baclofen-induced inhibition on eEPSCs, dose-response curves were fitted by the Hill equation: I=Imax{1/[1+(EC50/[Ligand])nH]}, where Imax is the maximum response, EC50 is the concentration of ligand producing a half-maximal response, and nH is the Hill coefficient. Differences in hypertensive effects were tested by un-paired t-test. For the comparison of hypertension or baclofen effect on PPR, a two-way ANOVA (factors: hypertension and baclofen) was performed. For the comparison of PPR at different pulse intervals, a one-way Repeated Measures ANOVA was performed. Statistics were performed using SigmaStat (v2.03, SPSS software, Chicago, IL), and graphs were made with SigmaPlot (v8.0, SPSS software, Chicago, IL). Differences were considered statistically significant for p values < 0.05.
RESULTS
Four weeks after renal wrap/sham-operated procedures, renal wrap HT rats had significantly higher MAP (162±9 mmHg, n=6) than control NT rats (107±3 mmHg, n=6/11, p<0.05), indicating the successful results of renal wrap surgery. This result is consistent with our previous studies with the same hypertension model3, 4, 15, 21–22.
Whole-cell recordings in second-order baroreceptor neurons in the NTS
All in vitro whole-cell recording experiments were performed in second-order baroreceptor neurons in the NTS, identified by the presence of fluorescent dye DiA labeled boutons (Fig. 1B). In our preparation of horizontal brainstem slices, fluorescent puncta were located medial to the solitary tract, usually in characteristic clusters. Clusters of dye puncta lay in close proximity to the soma membrane of individual neurons, usually forming a circle or near circle leaving the center of the soma void of labeling, and such positioned cells were considered to be anatomically identified second-order baroreceptive NTS neurons. Non-labeled neurons might be: second-order neurons contacted by non-baroreceptor afferents; higher order NTS neurons receiving baroreceptor afferent inputs or other afferent inputs; neurons that do not receive any tractus input. Analysis of these types of neurons was beyond the scope of this study. Electrical stimulation of ipsilateral tract evoked excitatory post-synaptic currents (eEPSCs) in voltage-clamp mode (Fig. 1C). There is little variability of onset latencies of eEPSCs (standard deviation of eEPSCs onset latency in each cell ranging from 44 µs to 187 µs with a mean variability of 121±12 µs), further indicating a monosynaptic input from afferent terminals of baroreceptors23.
Baclofen effect on evoked EPSCs in the NTS
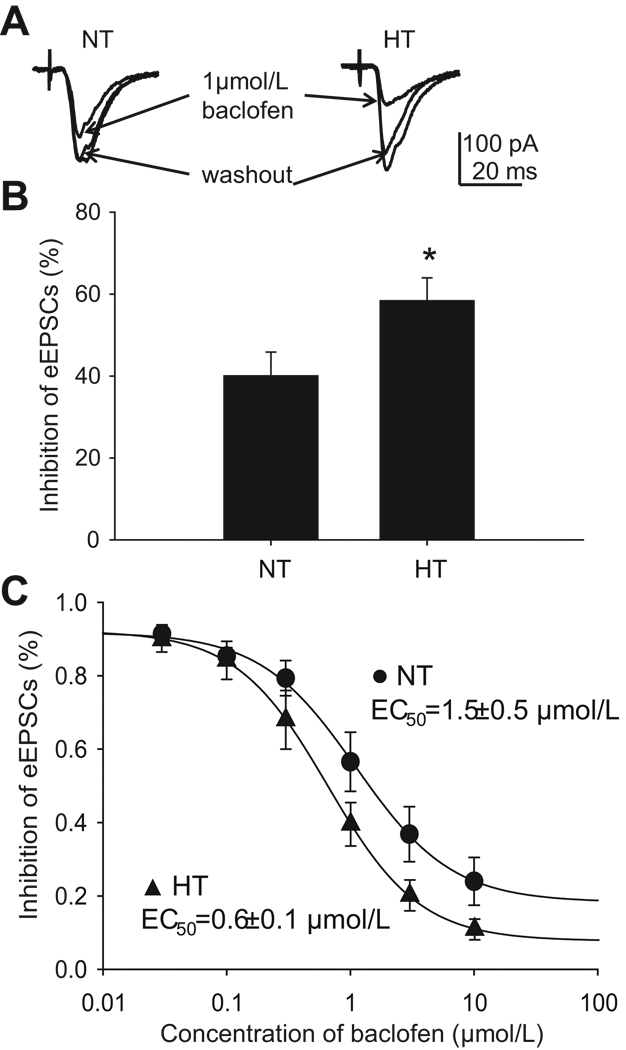
Application of baclofen did not elicit a discernable change in holding current, indicating that our pipette solution successfully blocked post-synaptic effects of baclofen. We did not observe a difference in the amplitudes of eEPSCs between NT and HT NTS neurons (211.0±30.5 pA, n=10 vs 172.5±20.7 pA, n=11, p>0.05), and onset latency of eEPSCs (4.9±0.6 ms vs 4.2±0.5 ms, p>0.05). Baclofen inhibited eEPSC amplitude in both HT and NT NTS neurons (Fig. 2A), but the inhibition was significantly greater in HT neurons (155.4±20.1 pA to 60.6±9.2 pA, 58±6% inhibition, n=9) than in NT neurons (226.9±29.1 pA to 143.4±25.1 pA, 40±6% inhibition, n=8) (p<0.05, Fig. 2B). No significant effect of baclofen on onset latency was observed in both NT (4.5±0.7 ms vs 4.4±0.7 ms, p>0.05) and HT cells (4.8±0.5 ms vs 5.1±0.4 ms, p>0.05). Baclofen effects were blocked by the selective GABAB receptor antagonist CGP 35348 (200 µmol/L, n=3, data not shown), confirming that baclofen was acting on GABAB receptors. Dose-response curves revealed that the EC50 of baclofen inhibition of eEPSC amplitude, calculated from the mean of the EC50 of each single neuron exposed to whole dose range of baclofen, was significantly smaller in HT neurons than in NT neurons (1.5±0.5 µmol/L, n=6 vs 0.6±0.1 µmol/L, n=9, p<0.05, Fig. 2C).
Figure 2. Baclofen effect on tract stimulation evoked responses.
A: Raw data showing that baclofen (1 µmol/L) inhibits eEPSCs in a second-order NTS neuron collect from a normotensive (NT) and a hypertensive (HT) rat. This is the average of 10 tracings, and electrical stimulation is delivered every 5 sec. Notice there is greater inhibition in HT cell and no discernable difference in onset latency in both neurons. B: At a concentration of 1 µmol/L, baclofen induces greater inhibition in HT NTS neurons (n=8) than in NT NTS neurons (n=9). C: Dose-response curve of baclofen-induced inhibition on eEPSCs in second-order baroreceptor neurons in the NTS. This curve is established from the mean value of baclofen inhibition at each concentration. HT (n=9) rats had significantly lower EC50 than NT rats (n=6). All experiments were performed in the presence of GABAA receptor antagonist picrotoxin (100 µmol/L). *: p<0.05.
Baclofen effect on paired-pulse tract stimulation evoked EPSCs
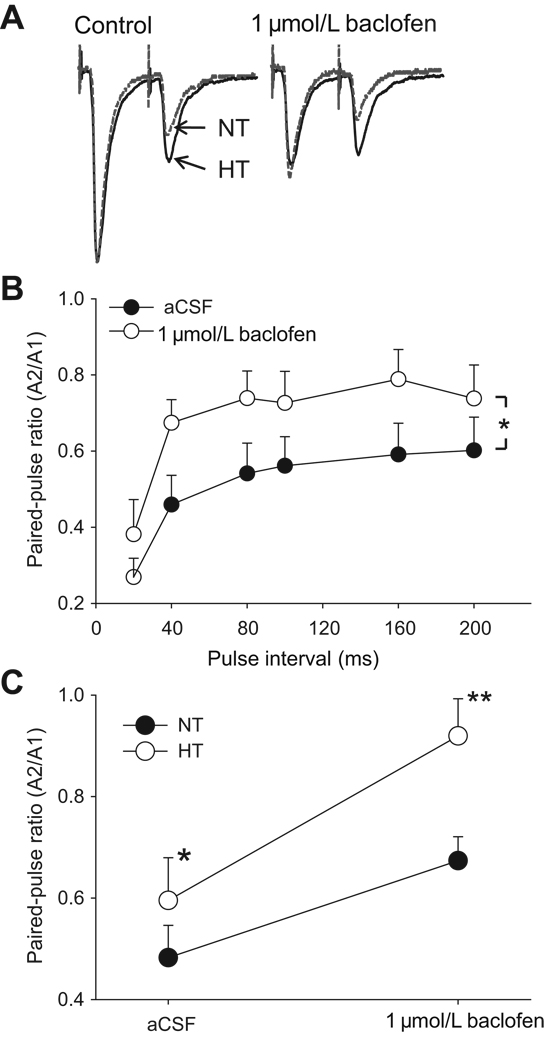
A paired-pulse stimulation protocol was used as one means to identify potential neural plasticity in synaptic transmission mediated by pre-synaptic mechanisms24. Differences in paired-pulse ratio (PPR) suggest alterations in the probability of release of available transmitter or the size of a release-ready pool of vesicles. At pulse intervals ranging from 20 to 200 ms the amplitude of second eEPSCs was consistently smaller than that of first one; therefore the PPR value was less than 1 (Fig. 3A & B). PPRs of less than one were observed in all neurons tested. The PPR was attenuated as the paired-pulse interval was increased. PPR at a paired-pulse interval of 20 ms was significantly smaller than PPRs at all other pulse intervals tested (p<0.001, in both control and baclofen group). When paired-pulse stimuli were applied in the presence of baclofen, the second eEPSC was minimally reduced by baclofen although the first eEPSC was greatly attenuated, leading to increased PPR (Fig. 3A). The baclofen effect on PPR was observed between pulse intervals from 20 to 200 ms (Fig. 3B). At a paired-pulse interval of 40 ms, the PPR was significantly greater in HT neurons (0.60±0.08, n=8) than in NT neurons (0.48±0.06. n=5, p<0.05). Baclofen increased the PPR in both types of neurons, however the baclofen effect was significantly greater in HT neurons (0.92±0.07, 172±22% increase, n=8) than in NT neurons (0.67±0.05, 148±16% increase, n=5) (p<0.05, Fig. 3C).
Figure 3. Baclofen effect on paired-pulse stimulation.
A. Raw data showing the effect of baclofen (1 µmol/L) on evoked EPSCs of paired-pulse stimulation in both NT (gray line) and HT (black line) neurons. This is the average of 10 tracings and tracings are matched in size on the first evoked response. Paired-pulse interval is 40 ms. Notice that baclofen greatly reduces the amplitude of first EPSCs, but has little effect on second EPSCs, leading to increased paired-pulse ratio (PPR: amplitude of second EPSCs/amplitude of first EPSCs). Also notice that the inhibition on first peak is greater in HT neuron than in NT neuron, and baclofen effect on PPR is greater in HT neuron (0.42 to 0.81) than in NT neuron (0.30 to 0.42). B. Baclofen increases PPR in paired-pulse interval ranging from 20 ms to 200 ms. C. At paired-pulse interval of 40 ms, PPR of eEPSCs are greater in HT NTS neurons (n=8) than NT neurons (n=5). Baclofen increases PPR in both NT and HT neurons. However, the slope of baclofen effect on PPR is significantly greater in HT neurons than in NT neurons. All experiments were performed in the presence of GABAA receptor antagonist picrotoxin (100 µmol/L). *: p<0.05; **: p<0.01.
DISCUSSION
These results demonstrate that activation of pre-synaptic GABAB receptors with baclofen inhibits glutamate release from baroreceptor afferent terminals to second-order baroreceptor neurons in the NTS and GABAB receptor-mediated pre-synaptic inhibition was enhanced in chronic hypertension. Considered along with our previous report of enhanced post-synaptic GABAB receptor function4, enhanced pre-synaptic GABAB receptor function could contribute to the enhanced blood pressure and sympathetic responses to NTS microinjections of baclofen observed in various animal models of chronic hypertension. Our present study provides strong evidence that chronic arterial hypertension induces profound changes in the function of both pre-synaptic and post-synaptic GABAB receptors.
The nucleus of the solitary tract (NTS) is the first site central integration site of arterial baroreceptor afferents1–2. Glutamate is the primary neurotransmitter relaying information from baroreceptor afferent terminals to second-order neurons within the NTS23, 25. This robust glutamatergic transmission is under constant modulation by various receptors and ion channels, including GABAB receptors. Baclofen activates GABAB receptors and inhibits NTS neurons receiving baroreceptor afferent inputs which will blunt the baroreflex and induce a pressor response6–10, 12–15, 26. GABAB inhibition is tonically active as injections of GABAB receptor antagonists into the NTS lower blood pressure and heart rate9. Activation of GABAB receptors can have dual effects to pre-synaptically inhibit excitatory neurotransmitter release and decrease post-synaptic neuronal excitability in the NTS5, thus reducing the transmission of the integrated afferent input to other sites in central baroreflex pathways2.
Enhanced arterial blood pressure, heart rate and sympathetic nerve discharge induced by NTS injections of baclofen have been described in several animal models of chronic hypertension4, 9–11, 15. Using in vitro whole-cell recordings, we previously demonstrated that chronic hypertension significantly enhanced baclofen-induced post-synaptic outward currents, as significantly lower EC50 for baclofen effect in HT than NT cells. This is consistent with previous studies showing that chronic hypertension increases mRNA expression of GABAB receptors9 and baclofen binding in the NTS27. The current study extends these previous findings and demonstrates that hypertension also enhances baclofen inhibition of glutamate release from baroreceptor afferent terminals. We examined pre-synaptic inhibition using two complementary analyses: the amplitudes of eEPSCs and PPR. Both approaches indicate that baclofen inhibition of glutamate release from baroreceptor afferent terminals in enhanced in hypertension. Insights into pre-synaptic transmitter release can also be obtained by analysis of spontaneous and miniature EPSCs. The frequency of m/sEPSCs represents the glutamate release probability from pre-synaptic terminals. However, our focus was on modulation of transmitter release from baroreceptor primary afferent fibers and analyses of m/sEPSCs cannot differentiate EPSCs that originate from peripheral as opposed to central sources.
The mechanisms underlying the development of hypertension-enhanced function of GABAB receptors in the NTS are unknown. Recent studies suggest that the availability of surface GABAB receptor could be modulated by glutamate in central neurons28–29, suggesting the alteration in GABAB receptor could be the direct result of high blood pressure-induced increase in afferent inputs. In addition, elevated circulating angiotensin during hypertension may cause enhanced function of GABAB receptor in the NTS30. Obviously these potential mechanisms will need further investigation.
The EC50 of pre-synaptic baclofen inhibition is noticeably smaller than the post-synaptic baclofen inhibition described in our previous study4, 1.5±0.5 µmol/L vs 9.1±3.2 µmol/L. This is consistent with an earlier in vitro current clamp study which found the EC50 of pre-synaptic inhibition was an order of magnitude lower than that of post-synaptic inhibition5. Although it is difficult to directly compare these two values, it suggests that depending upon GABA concentrations within the synaptic cleft and the precise location of GABAergic terminals, GABAB receptors could preferentially mediate pre-synaptic inhibition. Enhanced pre-synaptic inhibition of glutamate release in hypertension could reduce information flow within central pathways of the arterial baroreflex. However, it is possible that at least some of the second-order baroreceptor neurons reported in this study are GABAergic neurons31–32. Therefore, reduced excitation of GABAergic neurons could also determine the ultimate physiological significance of enhanced GABAB receptor inhibition in hypertension.
PERSPECTIVES
The baroreflex serves to stabilize arterial blood pressure fluctuations under physiological normotensive conditions and in chronic hypertension. However, in chronic hypertension, baroreflex function is reset to higher levels of blood pressures, representing a new balance between increased excitatory afferent inputs due to increased blood pressure and GABA-mediated inhibition2, 22. Enhanced GABAB receptor-mediated inhibition could be critical in baroreflex adaptation in chronic hypertension2, 13, 22. Along with our previous study4, we provided direct evidence of enhanced baclofen effect at both pre- and post-synaptic sites of second-order baroreceptor neurons in the NTS. Altered function of GABAB receptors could be crucial in determining baroreflex function in chronic hypertension.
ACKNOWLEDGEMENTS
Authors acknowledge expert technical assistance from Myrna Herrera-Rosales and Melissa Vitela.
SOURCE OF FUNDING
This work was supported by National Institutes of Health grant HL-56637.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
None.
References
- 1.Andresen MC, Kunze DL. Nucleus tractus solitarius--gateway to neural circulatory control. Annu Rev Physiol. 1994;56:93–116. doi: 10.1146/annurev.ph.56.030194.000521. [DOI] [PubMed] [Google Scholar]
- 2.Mifflin SW. What does the brain know about blood pressure? News in Physiol Sci. 2001;16:266–271. doi: 10.1152/physiologyonline.2001.16.6.266. [DOI] [PubMed] [Google Scholar]
- 3.Tolstykh G, Belugin S, Tolstykh O, Mifflin SW. Responses to GABA-A receptor activation are altered in NTS neurons isolated from renal-wrap hypertensive rats. Hypertension. 2003;42:732–736. doi: 10.1161/01.HYP.0000084371.17927.02. [DOI] [PubMed] [Google Scholar]
- 4.Zhang W, Herrera-Rosales M, Mifflin S. Chronic hypertension enhances the postsynaptic effect of baclofen in the nucleus tractus solitarius. Hypertension. 2007;49:659–663. doi: 10.1161/01.HYP.0000253091.82501.c0. [DOI] [PubMed] [Google Scholar]
- 5.Brooks PA, Glaum SR, Miller RJ, Spyer KM. The actions of baclofen on neurones and synaptic transmission in the nucleus tractus solitarius of the rat in vitro. J Physiol. 1992;457:115–129. doi: 10.1113/jphysiol.1992.sp019367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Callera JC, Bonagamba LG, Nosjean A, Laguzzi R, Machaalani R. Activation of GABA receptors in the NTS of awake rats reduces the gain of baroreflex bradycardia. Auton Neurosci. 2000;84:58–67. doi: 10.1016/S1566-0702(00)00184-3. [DOI] [PubMed] [Google Scholar]
- 7.Sved JC, Sved AF. Cardiovascular responses elicited by gamma-aminoibutyric acid in the nucleus tractus solitarius: evidence for action at the GABAB receptor. Neuropharmacology. 1989;28:515–520. doi: 10.1016/0028-3908(89)90088-9. [DOI] [PubMed] [Google Scholar]
- 8.Florentino A, Varga K, Kunos G. Mechanism of the cardiovascular effects of GABAB receptor activation in the nucleus tractus solitarii of the rat. Brain Res. 1990;535:264–270. doi: 10.1016/0006-8993(90)91609-k. [DOI] [PubMed] [Google Scholar]
- 9.Durgam VR, Vitela M, Mifflin SW. Enhanced GABA-B receptor agonist responses and mRNA within the nucleus of the solitary tract in hypertension. Hypertension. 1999;35:530–536. doi: 10.1161/01.hyp.33.1.530. [DOI] [PubMed] [Google Scholar]
- 10.Mei L, Zhang J, Mifflin SW. Hypertension alters GABA receptor mediated inhibition of neurons in the nucleus of the solitary tract. Am J Physiol Reg Int Comp Physiol. 2003;285:R1276–R1286. doi: 10.1152/ajpregu.00255.2003. [DOI] [PubMed] [Google Scholar]
- 11.Zhang J, Mifflin SW. Receptor subtype specific effects of GABA agonists on neurons receiving aortic depressor nerve inputs within the nucleus of the solitary tract. J Auton Nerv Syst. 1998;73:170–181. doi: 10.1016/s0165-1838(98)00140-4. [DOI] [PubMed] [Google Scholar]
- 12.Catelli JM, Sved AF. Enhanced pressor response to GABA in the nucleus tractus solitarii of the spontaneously hypertensive rat. Eur J Pharmacol. 1988;151:243–248. doi: 10.1016/0014-2999(88)90804-7. [DOI] [PubMed] [Google Scholar]
- 13.Yin M, Sved AF. Role of gamma-aminobutyric acid B receptors in baroreceptor reflexes in hypertensive rats. Hypertension. 1996;27:1291–1298. doi: 10.1161/01.hyp.27.6.1291. [DOI] [PubMed] [Google Scholar]
- 14.Tsukamoto K, Sved AF. Enhanced GABA-mediated responses in nucleus tractus solitarius of hypertensive rats. Hypertension. 1993;22:819–825. doi: 10.1161/01.hyp.22.6.819. [DOI] [PubMed] [Google Scholar]
- 15.Vitela M, Mifflin SW. Gamma-Aminobutyric acid (B) receptor-mediated responses in the nucleus tractus solitarius are altered in acute and chronic hypertension. Hypertension. 2001;37:619–622. doi: 10.1161/01.hyp.37.2.619. [DOI] [PubMed] [Google Scholar]
- 16.Mendelowitz D, Yang M, Andresen MC, Kunze DL. Localization and retention in vitro of fluorescently labeled aortic baroreceptor terminals on neurons from the nucleus tractus solitarius. Brain Res. 1992;581:339–343. doi: 10.1016/0006-8993(92)90729-s. [DOI] [PubMed] [Google Scholar]
- 17.Belugin S, Mifflin SW. Transient voltage-dependent potassium currents are reduced in NTS neurons isolated from renal wrap hypertensive rats. J Neurophysiol. 2005;94:3849–3859. doi: 10.1152/jn.00573.2005. [DOI] [PubMed] [Google Scholar]
- 18.Grollman A. A simplified procedure for inducing chronic renal hypertension in the mammal. Proc Soc Exp Biol Med. 1944;57:102–104. [Google Scholar]
- 19.Perkins KL, Wong RK. Ionic basis of the postsynaptic depolarizing GABA response in hippocampal pyramidal cells. J Neurophysiol. 1996;76:3886–3894. doi: 10.1152/jn.1996.76.6.3886. [DOI] [PubMed] [Google Scholar]
- 20.Nathan T, Jensen MS, Lambert JD. The slow inhibitory postsynaptic potential in rat hippocampal CA1 neurones is blocked by intracellular injection of QX-314. Neurosci Lett. 1990;110:309–313. doi: 10.1016/0304-3940(90)90865-7. [DOI] [PubMed] [Google Scholar]
- 21.Mei L, Zhang J, Mifflin S. Hypertension alters GABA receptor-mediated inhibition of neurons in the nucleus of the solitary tract. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1276–R1286. doi: 10.1152/ajpregu.00255.2003. [DOI] [PubMed] [Google Scholar]
- 22.Vitela M, Herrera-Rosales M, Haywood JR, Mifflin SW. Baroreflex regulation of renal sympathetic nerve activity and heart rate in renal wrap hypertensive rats. Am J Physiol Reg Int Comp Physiol. 2005;288:R856–R862. doi: 10.1152/ajpregu.00620.2004. [DOI] [PubMed] [Google Scholar]
- 23.Andresen MC, Peters JH. Comparison of baroreceptive to other afferent synaptic transmission to the medial solitary tract nucleus. Am J Physiol Heart Circ Physiol. 2008;295:H2032–H2042. doi: 10.1152/ajpheart.00568.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
- 25.Mifflin SW, Felder RB. Synaptic mechanisms regulating cardiovascular afferent inputs to solitary tract nucleus. Am J Physiol. 1990;259:H653–H661. doi: 10.1152/ajpheart.1990.259.3.H653. [DOI] [PubMed] [Google Scholar]
- 26.Sved AF, Ito S, Sved JC. Brainstem mechanisms of hypertension: role of the rostral ventrolateral medulla. Curr Hypertens Rep. 2003;5:262–268. doi: 10.1007/s11906-003-0030-0. [DOI] [PubMed] [Google Scholar]
- 27.Singh R, Ticku MK. Comparison of [3H]baclofen binding to GABA-B receptors in spontaneously hypertensive and normotensive rats. Brain Res. 1985;358:1–9. doi: 10.1016/0006-8993(85)90941-2. [DOI] [PubMed] [Google Scholar]
- 28.Vargas KJ, Terunuma M, Tello JA, Pangalos MN, Moss SJ, Couve A. The availability of surface GABA B receptors is independent of gamma-aminobutyric acid but controlled by glutamate in central neurons. J Biol Chem. 2008;283:24641–24648. doi: 10.1074/jbc.M802419200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilkins ME, Li X, Smart TG. Tracking cell surface GABAB receptors using an alpha-bungarotoxin tag. J Biol Chem. 2008;283:34745–34752. doi: 10.1074/jbc.M803197200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yao F, Sumners C, O'Rourke ST, Sun C. Angiotensin II increases GABAB receptor expression in nucleus tractus solitarii of rats. Am J Physiol Heart Circ Physiol. 2008;294:H2712–H2720. doi: 10.1152/ajpheart.00729.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawai Y, Senba E. Electrophysiological and morphological characterization of cytochemically-defined neurons in the caudal nucleus of tractus solitarius of the rat. Neuroscience. 1999;89:1347–1355. doi: 10.1016/s0306-4522(98)00393-5. [DOI] [PubMed] [Google Scholar]
- 32.Bailey TW, Appleyard SM, Jin YH, Andresen MC. Organization and properties of GABAergic neurons in solitary tract nucleus (NTS) J Neurophysiol. 2008;99:1712–1722. doi: 10.1152/jn.00038.2008. [DOI] [PubMed] [Google Scholar]