Abstract
The diverse actions of the incretin hormone glucagon-like peptide (GLP)-1 include insulinotropic, beta-cell preservative, cardioprotective and vasodilatory effects. This spectrum makes GLP-1 an appealing therapeutic option for patients with type 2 diabetes. However, its rapid metabolism by the enzyme dipeptidyl peptidase (DPP)-4 renders it impractical. Incretin-based analogues have been developed to extend endogenous GLP-1 action (GLP-1 receptor agonists) and to hamper its degradation (DPP-4 inhibitors). Evidence suggests that GLP-1 receptor agonists and DPP-4 inhibitors have different pharmacodynamic and pharmacokinetic effects. For example, GLP-1 receptor agonists deliver supraphysiologic levels of GLP-1 analogues designed to resist inactivation by DPP-4, whereas DPP-4 inhibition conserves native GLP-1 resulting in concentrations within the physiologic range. Furthermore, GLP-1 receptor agonists induce glucose-dependent insulin secretion, beta-cell protection, and other extraglycemic benefits such as weight loss and improvement in markers of cardiovascular risk. In contrast, DPP-4 inhibitors are weight neutral and have modest effects on glucose control. DPP-4 inhibition is dependent on the availability of endogenous GLP-1, which appears to be adversely affected by type 2 diabetes and its progression. Therefore, DPP-4 inhibitors may be better suited for patients with mild hyperglycemia without comorbidities. This review examines the present understanding of the pancreatic effects of endogenous GLP-1, and the extrapancreatic actions it exerts on human bodily systems. Also, it analyzes available preclinical and clinical data on incretin therapies with respect to glycemia, lipids, blood pressure, and weight.
Keywords: type 2 diabetes, beta-cell, DPP-4 inhibitors, extrapancreatic effects, incretin, weight loss, glycemia, insulinotropic polypeptide
Introduction
Glucagon-like peptide (GLP)-1 was first characterized as an incretin hormone. In the years after its discovery, diverse actions of GLP-1 were described. These include: 1. insulinotropic effects [1, 2], 2. neogenesis, differentiation, and preservation of pancreatic β-cells [3-7], and 3. cardioprotective and vasodilatory properties. The discrete mechanisms governing the latter two effects have not yet been fully clarified [8]. Despite early encouraging results with intravenous infusion [9-11], native GLP-1 was determined to be an impractical therapeutic tool, due to its rapid extensive metabolism by dipeptidyl peptidase 4 (DPP-4) [12, 13]. Consequently, attempts to adapt GLP-1 to therapeutic advantages in the treatment of type 2 diabetes resulted in the development of GLP-1 analogues that protract endogenous GLP-1 action. Also, DPP-4 inhibitors have been developed to impede the enzymatic inactivation of the incretin hormone.
Current evidence suggests that GLP-1 receptor agonists and DPP-4 inhibitors have differential pharmacodynamic and pharmacokinetic effects. GLP-1 receptor agonists deliver supraphysiologic levels of GLP-1 analogues designed to resist DPP-4 degradation. Whereas, DPP-4 inhibition conserves native GLP-1, resulting in concentrations within the physiologic range [12]. DPP-4 inhibitors are associated with weight neutrality and modest effects on glucose control (HbA1c reductions of 0.6% to 0.8%) [14]. DPP-4 inhibition is dependent on islet function, which is adversely affected by type 2 diabetes and its progression [15]. Therefore, DPP-4 inhibitors may be better suited for patients with early type 2 diabetes without comorbidities [16]. DPP-4 inhibition is nonspecific; thus, it may compromise the function of additional peptide substrates such as GLP-2, glucose-dependent insulinotropic polypeptide, peptide YY, neuropeptide Y, growth hormone-releasing hormone, as well as various paracrine chemokines and immune system substrates [17, 18].
GLP-1 receptor agonist therapy supplements native GLP-1 with pharmacologic doses of GLP-1 analogues. The analogues are fully capable of binding to the GLP-1 receptor and inducing glucose-dependent insulin secretion. They also provide β-cell protection and other extraglycemic benefits, such as weight loss and improvements in markers of cardiovascular (CV) risk [1, 4, 7, 12, 19-21]. GLP-1 receptor agonists are designed to retain the β-cell-potentiating and -preserving properties of the incretin hormones, while incorporating resistance to inactivation by DPP-4 [22].
DPP-4 inhibitors have less robust antiglycemic and extraglycemic effects than GLP-1 receptor agonists. This may be explained by different GLP-1 plasma concentrations. Furthermore, DPP-4 inhibitors may convey humoral and neuroendocrine effects of GLP-1 by inhibiting GLP-1 degradation in various tissues. Another explanation may involve the potential bioactivity of GLP-1 (9-36), a rapidly produced metabolite of GLP-1. In animal models, GLP-1 (9-36) appears to have cardioprotective benefits independent of the GLP-1 receptor [23]. It may be that agents of the incretin class are associated with substantial differences in circulating levels of GLP-1 (9-36) [23]. Whether inhibition of DPP-4 has beneficial consequences independent of its antidiabetic effects remains to be demonstrated [24].
GLP-1 receptor agonists improve glycemic control by increasing glucose-stimulated insulin secretion and suppressing glucagon secretion [7]. GLP-1 receptor agonists are associated with robust effects on glucose control (HbA1c reductions of 0.4% to 1.5%) as well as weight loss (~-3 kg) and other beneficial extraglycemic effects [25-33]. The insulinotropic effects of these agents have been amply demonstrated in large, placebo-controlled, clinical trials. GLP-1 receptor agonists promote weight loss by inhibiting gastric secretion and motility [34-36], which delays carbohydrate absorption and contributes to satiety by delaying gastric emptying [37, 38]. GLP-1 receptor agonists increase β-cell mass in rodents [39-43] and inhibit β-cell apoptosis in vitro and in vivo [44, 45]. While direct effects on β-cell mass cannot be quantified in humans, the results of these preclinical studies suggest potential protective effects on β-cell volume and morphology. Clinical evidence of effects on contractility, blood pressure, cardiac output, and cardioprotection in animals and humans, has also been reported [23, 46-50].
Ample preclinical and clinical evidence substantiates the need for a multifactorial risk-reduction strategy to address hyperglycemia and comorbidities in type 2 diabetes. Type 2 diabetes is highly correlated with dyslipidemia, hypertension, and a spectrum of cardiovascular and metabolic derangements. Adiposity increases the risk of type 2 diabetes [51, 52], and is often accompanied by a distinct pattern of plasma lipid abnormalities. Elevated triglyceride-rich lipoprotein levels, low high-density lipoprotein cholesterol (HDL-C) levels, and structural alterations of low-density lipoprotein cholesterol (LDL-C) cause a predominance of dense, highly proatherogenic particles [53]. The dramatic increase in mortality in type 2 diabetes associated with cardiovascular disease (CVD) and comorbid adiposity underscores an urgent need to address these risk factors in type 2 diabetes [54-56]. The benefits of weight reduction in type 2 diabetes are evident. They include improved insulin sensitivity, restored β-cell sensitivity, enhanced β-cell capacity [57-59], a less atherogenic lipid profile [60], and reduced systolic blood pressure (SBP, -5 mmHg to -20 mmHg) [61]. Weight reduction of as little as 5% to 7% from baseline has been shown to reduce the risk of developing diabetes mellitus by >50% in patients with impaired glucose tolerance [62].
The present review examines what is known about the pancreatic effects of endogenous GLP-1 and the extrapancreatic actions it exerts on the central nervous, gastrointestinal, and CV system (Figure 1) [63]. It concludes with an analysis of the available preclinical and clinical data on incretin therapeutics with respect to glycemia, lipids, blood pressure, and weight.
Figure 1. Glucagon-like peptide-1: pancreatic and extrapancreatic actions.
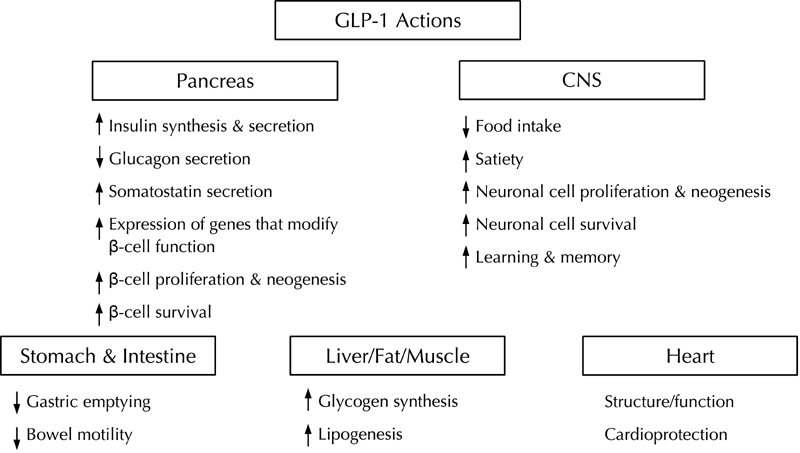
The various organs or organ systems affected by GLP-1 actions are depicted in the figure. In the pancreas, GLP-1 action causes short term effects that result in increased glucose-dependent insulin- and somatostatin secretion, increased insulin synthesis, and inhibition of glucagon secretion. Long-term effects of GLP-1 action on the pancreas include increased expression of genes that modify beta-cell function and survival in a beneficial way by inhibiting beta-cell apoptosis and stimulating beta-cell replication. In the stomach and intestine, GLP-1 slows motility resulting in delayed gastric emptying and a retardation of intestinal motility. In the CNS, GLP-1 is an important neurotransmitter for regulating appetite and eating behavior. GLP-1 promotes satiety and leads to reduced food intake. Additional long-term effects of GLP-1 on the CNS comprise an improvement of learning and memory, as well as a stimulation of neuronal cell survival and replication. In liver, adipose tissue and muscle, GLP-1 action causes increased glycogen synthesis and liogenesis. These effects are mainly mediated by the increase in insulin secretion and suppression of glucagon secretion mediated by GLP-1. In the heart GLP-1 improves left ventricular function and has preventive effects on ischemic damage of the heart muscle. Reproduced from Best Pract Res Clin Endocrinol Metab, Vol 18, Baggio LL, Drucker DJ, Clinical endocrinology. Glucagon-like peptide-1 and glucagon-like peptide-2, 531-554, 2004, with permission from Elsevier [63].
GLP-1 synthesis, secretion, and degradation
GLP-1 is a potent gut hormone that stimulates insulin secretion and inhibits glucagon release in a glucose-dependent manner. Therefore, it reduces postprandial glycemia without causing hypoglycemia. GLP-1 is present in two circulating molecular forms, glycine-extended GLP-1 (7-37) and GLP-1 (7-36) amide [17]. Following secretion by enteroendocrine L-cells located primarily in the distal ileum and colon, GLP-1 (7-36) amide is rapidly degraded and inactivated by DPP-4 to its metabolite GLP-1 (9-36) [64, 65]. The half-life of intact circulating GLP-1 is less than 2 minutes in vivo [65].
Unlike other insulin secretagogues, GLP-1 promotes insulin gene transcription and messenger-RNA (mRNA) biosynthesis. Therefore, it has the capacity to restore depleted β-cell insulin [66]. Studies in rodents and isolated human islets have shown that GLP-1 has insulinotropic effects on pancreatic islet β-cells by enhancing differentiation and proliferation and reducing apoptosis [3, 7, 67]. The peptide sequences of GLP-1 in rodents and humans, have been found to be identical, suggesting that those effects may occur in vivo in both species [68]. In clinical studies, exogenous administration of GLP-1 has normalized β-cell responsiveness to glucose and restored both first- and second-phase insulin responses in patients with type 2 diabetes, regardless of disease severity [11, 69].
The inhibitory effects of GLP-1 on the pancreatic islet α-cell may occur indirectly through GLP-1-mediated stimulation of insulin secretion [70] or via direct interaction with GLP-1 receptors on α-cells [71]. GLP-1 reduces α-cell insulin resistance in type 2 diabetes [72-74], thereby it helps inhibiting postprandial glucagon secretion [26, 75-77]. Additionally, GLP-1 is able to inhibit glucagon secretion from α-cells indirectly by stimulating somatostatin secretion from δ-cells. Somatostatin then binds to somatostatin receptor 2 on α-cells, leading to a decrease in glucagon secretion [78]. Thus, GLP-1 elicits multiple actions resulting in glucose homeostasis and appropriate islet cell activity. However, the precise mechanisms of GLP-1 action (e.g. insulinotropic effects on pancreatic islets) independent of the insulin to glucagon ratio is not yet fully known.
Extrapancreatic effects of GLP-1: central nervous, gastrointestinal, and skeletal systems
GLP-1 exerts its actions through the engagement of structurally distinct G-protein-coupled receptors. These receptors are present in islet α-cells and β-cells and in regions of the central nervous system (CNS) that regulate diverse homeostatic functions, including gastric motility and glucoregulation [79-81]. Consistent with the distribution of GLP-1-receptor expression, GLP-1 inhibits glucagon secretion and gastric empting, and may reduce caloric intake [82, 83]. In the presence of food, GLP-1 may mediate gut-brain signaling from the gastrointestinal tract to GLP-1 receptors in the hypothalamus and brainstem. This constitutes a feeding control via neural and endocrine mechanisms [84].
The coordinated actions of GLP-1 in feeding and glucose homeostasis are evident at multiple sites. GLP-1 is a key regulator of appetite, food intake, body weight, and gut motility. It has been shown to inhibit food intake and promote satiety in normal, obese, or diabetic individuals [36-38, 85, 86]. In vivo studies suggest that GLP-1 exerts effects on fuel sensing; it was able to reduce food intake in Göttingen minipigs [87], and craving in rats [88], for simple carbohydrates and/or fat. Manganese-enhanced magnetic resonance imaging in fasted mice following exogenous-administered GLP-1 supports the observation that the anorexigenic effects of GLP-1 may be mediated via "nausea" circuits within the hypothalamus and brainstem [89]. Collectively, the evidence indicates that the inhibitory effects of GLP-1 on gastric emptying and acid secretion involve vagus nerve stimulation and activation of GLP-1 receptors located in the CNS, and/or on the vagal afferent fibers that relay sensory information to the brainstem [7].
GLP-1 may also regulate bone metabolism, possibly through a calcitonin-dependent pathway [90]. A study evaluated bone resorption in GLP-1 receptor knockout mice compared with wild-type mice. The investigators found higher urine levels of the bone resorption marker deoxypyridinoline and reduced thyroid levels of calcitonin mRNA transcripts in the knockout mice, but no evidence of a direct effect of GLP-1 on osteoclasts or osteoblasts [90]. However, treatment with calcitonin effectively suppressed urinary concentrations of deoxypyridinoline in the knockout mice. The GLP-1 receptor agonist exendin-4 increased calcitonin gene expression in the thyroid of wild-type mice. These findings are preliminary and need confirmation and replication through in vivo studies.
Extrapancreatic effects of GLP-1: cardiovascular tissue, adipose tissue, liver, and kidney
GLP-1 receptors are widely expressed in CV, adipose, hepatic, and renal tissue [80, 81]. High-affinity GLP-1 receptors are present in autonomic nuclei that control CV functions [91, 92], and have been isolated in rodent and human cardiomyocytes, endothelial cells, and vascular smooth muscle cells [23, 93]. Although the specific localization and functional relevance of those receptors has not been completely defined, it is noteworthy that mice lacking functional GLP-1 receptors have structural and functional cardiac abnormalities. These include diastolic dysfunction, alterations in resting heart rate, heart wall thickness, and abnormalities in the ratio of heart weight to body weight [94]. Stimulation of central GLP-1 systems has been associated with activation of autonomic regulatory neurons and increased heart rate and blood pressure [91, 92, 95]. In a recent study, GLP-1-receptor activation improved survival after myocardial infarction (MI) in the normal and diabetic mouse heart. This finding suggests that GLP-1-receptor activation is accompanied by effects on the modulation of mediators important for cardiomyocyte survival, including peroxisome proliferator-activated receptors (PPAR)-β/δ, heme oxygenase (HO)-1, Akt, and glycogen synthase kinase (GSK)-3β [96].
GLP-1 receptor agonists appear to have CV effects independent of the autonomic nervous system, and even independent of known GLP-1 receptor-linked pathways [97]. Preclinical evidence suggests a novel two-pathway schema for the CV actions of GLP-1. One pathway depends on the GLP-1 receptor for glucose uptake, ischemic preconditioning, and mild vasodilatory actions. Another appears to involve GLP-1 receptor-independent effects on postischemic recovery of cardiac function and vasodilation [23, 48].
Recent studies suggest a role for GLP-1 as a cardioactive peptide, with demonstrable effects on contractility, cardiac output, arterial blood flow, and cardioprotection [23, 46, 48-50, 98]. At supraphysiologic concentrations, GLP-1 behaves as a molecular signal, linking CV and metabolic functions in vivo. Treatment with GLP-1 at supraphysiologic concentrations resulted in increased femoral arterial blood flow correlated with whole-body insulin-stimulated glucose utilization. In a murine model, a strong correlation was observed between glucose utilization and blood flow rates. This correlation was not observed in experimental conditions where brain GLP-1 signaling was abolished in GLP-1-receptor knockout mice, and even more selectively, in mice whose brains were directly infused with a GLP-1 receptor antagonist [99]. GLP-1 has demonstrated an inotropic effect in dogs with heart failure [49, 100], and exerts salutary cardioprotective effects in patients with acute MI when administered as a 72-hour infusion following angioplasty [50]. Clinical studies, involving patients with type 2 diabetes and comorbid coronary artery disease, have shown that GLP-1 infusion improved endothelial function [93], and blood pressure and cardiac function in the immediate postoperative state after bypass surgery [98]. Recent in vitro studies indicate that GLP-1 attenuates tumor necrosis factor alpha (TNF-α)-induced expression of plasminogen activator inhibitor 1 (PAI-1) in vascular endothelial cells, suggesting a possible mechanism for observed ameliorative effects on endothelial dysfunction [101]. Of salient interest, studies have shown cardioprotective and vasodilatory actions of GLP-1 independent of the proposed GLP-1-receptor pathway [23].
The beneficial effects of GLP-1 on CV parameters may also occur indirectly through GLP-1-mediated improvements in fatty free acid levels and glucose disposal. GLP-1 has been shown to stimulate lypolysis in rat [102] and human [103] adipocytes. In a small study of 20 patients with type 2 diabetes, a continuous 6-week infusion of GLP-1 produced reductions in fasting and 8-hour mean concentrations of free fatty acids [104]. There is preliminary evidence that GLP-1 suppresses endogenous glucose production under fasting conditions independently of its action on islet hormone secretion [105]. GLP-1 may also help to regulate glucose homeostasis via influencing islet cell hormone secretion and modulating gastric emptying. Also, it may have an impact on hepatic glucose production via stimulation of GLP-1 receptor in the arcuate [106]. D'Alessio et al. have postulated that GLP-1 facilitates enhanced glucose disposal in peripheral tissues independently of its effects on islet hormone secretions (insulin and glucagon) [107]. However, other studies have failed to demonstrate a GLP-1-mediated, insulin-independent effect on glucose disposal [107, 108].
Intravenous infusion of GLP-1 revealed renal-protective properties, including enhanced sodium excretion and reduction in hyperfiltration associated with kidney damage [109]. The antihypertensive effects of GLP-1 observed in salt-sensitive Dahl S rats, coupled with reductions in renal and cardiac end organ damage, has been attributed to the GLP-1-dependent increase in salt and water excretion [110].
Beyond glucose control: preclinical and clinical effects of GLP-1 receptor agonists on lipid and cardiovascular biomarkers
Recognition of the sustained insulinotropic and glucagon-lowering activity of GLP-1 has fostered interest in the use of GLP-1 receptor agonists for the treatment of patients with type 2 diabetes. GLP-1-based therapy could be especially valuable in patients with comorbid overweight/obesity and/or CVD. Observations elucidating a role for GLP-1 in the potentiation of glucose-dependent insulin secretion have been followed by clinical trials. They confirm the efficacy of GLP-1 receptor agonists in controlling the glycemic disorders associated with type 2 diabetes. Similarly, in vivo and small proof-of-concept studies confirming the extrapancreatic actions of endogenous GLP-1 have provided the rationale for investigations of the extrapancreatic effects of GLP-1-based therapeutics.
Exenatide
Exenatide (synthetic exendin-4) was originally identified during research for biologically active peptides in Gila monster venom [111]. The drug has a 53% homology to human GLP-1 [111] and a half-life of 2.4 hours. It must be injected within 60 minutes of morning and evening meals [112]. In addition to potentiating effects on glucose-dependent insulin secretion, exenatide appears to have beneficial effects on indices of β-cell function [113, 114]. However, these benefits have not been sustained after discontinuation of the therapy [115]. In three double-blind, placebo-controlled trials, patients with type 2 diabetes receiving exenatide added to conventional oral antidiabetics (metformin, sulfonylurea) experienced a mean weight loss ranging from 0.9 kg to 2.8 kg at 30 weeks coupled with reductions from baseline HbA1c of 0.40% (5 μg bid) to 0.86% (10 μg bid) [31-33]. In an open-label extension of a 30-week trial (n = 283), exenatide produced mean body weight reductions of 4.7 kg at week 104 (p < 0.001) [116]; HbA1c of ≤7% was achieved in approximately 50% of patients. In a subgroup of these patients, from whom homeostasis model assessment of β-cell function data were collected (n = 112), significant improvement (p < 0.01) was observed from baseline. Biomarkers of hepatic injury, alanine aminotransferase (ALT) and aspartate aminotransferase (AST), progressively and significantly declined (p < 0.05 for both ALT and AST) from baseline to week 104. β-cell function was also measured in a 1-year trial, in which exenatide was compared with insulin glargine, in type 2 diabetes patients inadequately controlled on metformin. Exenatide-treated patients demonstrated significantly increased first- and second-phase insulin secretion (as measured by C-peptide secretion response to arginine during hyperglycemic clamp) compared with insulin glargine (p < 0.0001) [115]. With respect to cardioprotective effects independent of weight loss, exenatide improved cardiac function and reduced infarct size in a porcine model of ischemia [117]. In vivo results showed infarct-limiting action against ischemia-reperfusion injury in rat heart [118].
In clinical studies, exenatide is associated with modest improvements in lipid parameters across a range of changes in HbA1c values, including increased HDL-C concentrations (+4.6 mg/dl, +0.12 mmol/l), and reductions in triglycerides (-38.6 mg/dl, -0.44 mmol/l) and LDL-C (-1.6 mg/dl, -0.04 mmol/l). Improvement in lipid profiles occurred even in the absence of clinically significant weight loss [119]. Blood pressure parameters were modestly improved, as indicated by reductions in SBP (-1.3 mmHg) and diastolic blood pressure (DBP; -2.7 mmHg). In the 82-week extension study, the largest reductions in weight relative to baseline were associated with the greatest baseline-to-end point improvements in SBP (-3.9 mmHg) and DBP (-4.4 mmHg) [119].
Reductions in CV risk factors were also observed in a post hoc analysis of patients who underwent exenatide therapy for at least 3 years in open-label extensions of 30-week, double-blind, randomized trials. These patients experienced a progressive weight loss from baseline (a mean of -5.3 kg at 3 years, p < 0.0001). Reductions in HbA1c observed at 12 weeks were sustained for up to 3 years (mean reduction, 1.0%). A subgroup of patients treated with exenatide for 3.5 years experienced reductions in triglycerides (12%, p = 0.0003), total cholesterol (5%, p = 0.0007), and LDL-C (6%, p < 0.0001), and an increase in HDL-C (24%, p < 0.0001) [120]. As reported at the 2009 Annual Meeting of the European Association for the Study of Diabetes, a study of 56 type 2 diabetes patients randomized to 3-month treatment with exenatide or insulin glargine, showed exenatide to be significantly better than insulin glargine regarding the improvement of central hemodynamic biomarkers of CV risk (e.g., central pulse pressure and augmentation pressure; p < 0.05 for both measures) [121]. Analysis of blood pressure reductions in the same patient group found that exenatide was associated with a significantly greater reduction in DBP, when tested versus insulin glargine (-4.85 ± 7.3 vs. -0.15 ± 9.7 mmHg for exenatide vs. insulin glargine, p = 0.038). A non-significant trend towards greater SBP reduction with exenatide versus insulin glargine was also reported [122]. A 1-year extension study of a 30-week trial of once-weekly exenatide in type 2 diabetes patients with elevated baseline cardiometabolic values, reported significant reductions from baseline in SBP and DBP as well as LDL-C and triglycerides (p < 0.05 for all cardiometabolic parameters) [123].
Liraglutide
Liraglutide is a once-daily GLP-1 receptor agonist with a 97% human homology to the native hormone [124]. The key modification (an acyl side-chain addition and an amino acid substitution at position 34) facilitates albumin binding and self-association. By these modifications, the half-life of liraglutide is prolonged to approximately 13 hours, enabling once-daily administration [125-127].
In the Liraglutide Effect and Action in Diabetes (LEAD) trials (6 randomized, controlled phase 3 trials involving ~4500 subjects), liraglutide was associated with significant reductions in HbA1c, weight, SBP, and plasma lipids. In LEAD-3, a 52-week, double-blind, active-control, parallel-group trial, liraglutide monotherapy reduced HbA1c by 1.6% in patients previously treated with diet and exercise only, representing the drug-naive population [26]. Significant weight reduction relative to insulin glargine (-3.43 kg; p < 0.0001) was seen in LEAD-5, accompanied by a significant reduction in waist circumference (-1.5 cm liraglutide vs. +0.89 cm glargine; p < 0.0001) [29]. Dose-dependent weight reductions of up to 3.2 kg were observed in liraglutide patients in the 24-week, head-to-head trial of liraglutide or exenatide added-on to metformin or sulfonylurea or both (LEAD-6) [25]. Weight loss was statistically similar in both treatment groups but numerically greater in the liraglutide group (-3.2 kg, liraglutide; -2.9 kg, exenatide; p = NS) [25]. Liraglutide also had significantly greater reductions in HbA1c than exenatide (-1.1% vs. -0.8%, p < 0.0001) [25]. Results from a 14-week extension of this trial demonstrated additional benefits in glycemic control (HbA1c -0.3%; p < 0.001), as well as a further reduction in SBP (-3.8 mmHg; p < 0.001), and additional weight loss (-0.9 kg; p < 0.001) in 186 patients who switched from exenatide to liraglutide [128].
Data from four clinical trials of liraglutide in combination with one or two oral therapies (metformin plus sulfonylurea or thiazolidinedione) achieved HbA1c reductions of 1.0% to 1.5% for liraglutide 1.2 mg (all reductions were significant vs. placebo), and 1.0% to 1.5% for 1.8 mg (all reductions were significant vs. placebo). Liraglutide 1.2 and 1.8 mg achieved HbA1c reductions of at least 1.0% regardless of whether it was used in conjunction with metformin, a sulfonylurea, or two oral therapies [27-30]. Additional results from the LEAD studies demonstrated that liraglutide combination therapy (metformin plus sulfonylurea or thiazolidinedione) produced SBP reductions from 4.5 mmHg (vs. insulin with metformin plus sulfonylurea) to 6.7 mmHg (vs. placebo with metformin plus rosiglitazone) [29, 30]. SBP reductions occurred within 2 weeks and after reductions in body weight, suggesting an ameliorative effect on blood pressure independent of weight loss [129]. A meta-analysis of results from all six LEAD trials found significant improvements for liraglutide versus baseline in plasma concentrations of total cholesterol (-5.03 mg/dl, -0.13 mmol/l, p < 0.01), LDL-C (-7.73 mg/dl, -0.20 mmol/l, p < 0.0001), free fatty acids (-0.09 mmol/l, p < 0.0001), and triglycerides (-17.72 mg/dl, -0.20 mmol/l, p < 0.01) [130].
Liraglutide is also associated with action on other markers of CV and metabolic risk. It conferred cardioprotection and survival advantages over metformin after MI in diabetic mice, including reduction of cardiac rupture and improvement of cardiac output. The results suggested that liraglutide treatment modulated cardioprotective genes in the mouse heart, including PPAR-β/δ, HO-1, NF-E2-related factor-2, Akt, and GSK-3β [96]. An in vitro investigation of liraglutide effects on markers of endothelial dysfunction found a significant inhibition of TNF-α or hyperglycemia-mediated induction of PAI-1, intercellular adhesion molecule-1, and vascular cell adhesion molecule-1 in human vascular endothelial cell lines [131]. An exploratory analysis of a 14-week study of 165 patients with type 2 diabetes treated with liraglutide reported PAI-1 reductions of 29%; levels of B-type natriuretic peptide, a marker of left ventricular dysfunction, were reduced by 38% [132]. These results support an earlier 14-week study that found a significant decrease in triglycerides (-22%) in patients with type 2 diabetes receiving liraglutide 1.9 mg; SBP was also reduced by 8 mmHg in these patients [125]. These reductions in CV risk markers were accompanied by reductions in HbA1c from baseline of 1.45% (liraglutide 1.9 mg daily) and 1.40% (liraglutide 1.25 mg daily) versus an increase of 0.29% for placebo (p < 0.0001). The percentage of patients reaching target HbA1c goal of <7% was 46% with 1.90 mg liraglutide, and 48% with liraglutide 1.25 mg versus 5% for placebo. The 1.9-mg dose of liraglutide was associated with a mean weight loss of 3.0 kg at week 14.
Recent clinical trial data suggest that liraglutide may significantly reduce deposits of metabolically active visceral fat when compared with glimepiride/metformin combination therapy. A trend towards visceral fat reduction was observed in patients who received combination therapy with liraglutide and metformin (a 13%-17% reduction from baseline) compared with the glimepiride/metformin treatment group [133].
Safety of GLP-1 analogues
Antiexenatide antibodies have been found in 27% to 49% of patients treated with exenatide [31-33, 134, 135]. Of the 6% who developed high-titer antiexenatide antibodies, approximately half showed an attenuated glycemic response [112]. Likely due to its closer homology to human GLP-1, liraglutide is associated with antiliraglutide antibodies in up to 13% of patients treated [27-30].
Nausea may be frequently observed with exenatide (incidence 3%-51%), although it typically subsides within 8 weeks of therapy initiation [31-33, 136]. Incidence of nausea is less frequent with liraglutide (11%-40%) and tends to abate within 4 weeks [25-30].
A number of cases of acute pancreatitis have been reported in patients with type 2 diabetes treated with exenatide. The exenatide product label cautions vigilance for signs and symptoms of acute pancreatitis [112]. A claims-based safety surveillance system report assessing the risk of acute pancreatitis with either exenatide or sitagliptin found no risk differential between the two therapies [137]. Currently available clinical trial data indicate that the incidence rate among subjects using liraglutide or a comparable product is in line with what one would expect in any type 2 diabetes population [25-30]. It is important to note that patients with type 2 diabetes have a three-times higher risk of developing pancreatitis than the general population [138]. To date, the number of pancreatitis cases is not sufficiently high to determine whether there is an association between the development of acute pancreatitis and liraglutide treatment [139, 140].
In preclinical rodent studies, liraglutide induced calcitonin-producing cell (c-cell) hyperplasia, c-cell adenoma, and, at the highest doses, c-cell carcinoma. Similar findings did not occur in nonhuman primates at an exposure of 60-fold that of the human dose of 1.8 mg. The cumulative data suggest that rodent c-cells are sensitive to activation by GLP-1 agonists, but human and nonhuman primate c-cells not [139, 140].
Conclusions
In recent years, research into type 2 diabetes has generated a wealth of discoveries concerning the pleiotropic effects of GLP-1. The research initiatives revealed an activity profile beyond the stimulation of insulin secretion. The profile includes actions potentiating the secretory activity, proliferation and preservation of the β-cell, as well as cardioprotective actions.
GLP-1 appears to have broader biological action on the pancreas and on extrapancreatic tissues than previously expected. Indeed, the results of recent preliminary investigations suggest that the cardioprotective effects of GLP-1 may manifest via two distinct pathways. One dependent on the GLP-1 receptor for glucose uptake, mild vasodilatory effects, and ischemic preconditioning. Another is accompanied by actions on postischemic recovery of vasodilation and cardiac function independent of the GLP-1 receptor [23].
Limited data exist on the question whether GLP-1 receptor agonists affect strong end points such as CVD morbidity and mortality. However, it is evident that GLP-1 receptor agonists may have other CV, CNS, and gastointestinal consequences than DPP-4 inhibitors. The latter prolongs the activity of native GLP-1, but secretion and bioactivity is progressively impaired in type 2 diabetes. The pleiotropic effects of GLP-1 receptor agonists may benefit patients with type 2 diabetes with hypertension, dyslipidemia, and other risk factors for CV disease, such as overweight/obesity. Further studies in GLP-1 receptor agonists assessing surrogate parameters, and strong end point studies, are warranted to support promising but preliminary emerging evidence to date.
Disclosures (conflict of interests statement): The author is member of advisory boards for AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Eli Lilly, Novartis, Novo Nordisk, Merck, Roche, and Takeda, and has also received honoraria from these companies for giving lectures.
Acknowledgments
The author thanks Rob McCarthy, PhD, of AdelphiEden Health Communications for providing medical writing and editorial support funded by Novo Nordisk.
References
- 1.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 2.Holst JJ, Gromada J. Role of incretin hormones in the regulation of insulin secretion in diabetic and nondiabetic humans. Am J Physiol Endocrinol Metab. 2004;287:E199–E206. doi: 10.1152/ajpendo.00545.2003. [DOI] [PubMed] [Google Scholar]
- 3.Cornu M, Yang JY, Jaccard E, Poussin C, Widmann C, Thorens B. Glucagon-like peptide-1 protects beta-cells against apoptosis by increasing the activity of an IGF-2/IGF-1 receptor autocrine loop. Diabetes. 2009;58:1816–1825. doi: 10.2337/db09-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buteau J. GLP-1 receptor signaling: effects on pancreatic beta-cell proliferation and survival. Diabetes Metab. 2008;34(Suppl 2):S73–S77. doi: 10.1016/S1262-3636(08)73398-6. [DOI] [PubMed] [Google Scholar]
- 5.Farilla L, Bulotta A, Hirshberg B, Li Calzi S, Khoury N, Noushmehr H, Bertolotto C, Di Mario U, Harlan DM, Perfetti R. Glucagon-like peptide 1 inhibits cell apoptosis and improves glucose responsiveness of freshly isolated human islets. Endocrinology. 2003;144:5149–5158. doi: 10.1210/en.2003-0323. [DOI] [PubMed] [Google Scholar]
- 6.Perfetti R, Zhou J, Doyle ME, Egan JM. Glucagon-like peptide-1 induces cell proliferation and pancreatic-duodenum homeobox-1 expression and increases endocrine cell mass in the pancreas of old, glucose-intolerant rats. Endocrinology. 2000;141:4600–4605. doi: 10.1210/endo.141.12.7806. [DOI] [PubMed] [Google Scholar]
- 7.Stoffers DA, Kieffer TJ, Hussain MA, Drucker DJ, Bonner-Weir S, Habener JF, Egan JM. Insulinotropic glucagon-like peptide 1 agonists stimulate expression of homeodomain protein IDX-1 and increase islet size in mouse pancreas. Diabetes. 2000;49:741–748. doi: 10.2337/diabetes.49.5.741. [DOI] [PubMed] [Google Scholar]
- 8.Nyström T, Gonon AT, Sjoholm A, Pernow J. Glucagon-like peptide-1 relaxes rat conduit arteries via an endothelium-independent mechanism. Regul Pept. 2005;125:173–177. doi: 10.1016/j.regpep.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 9.Nauck MA, Niedereichholz U, Ettler R, Holst JJ, Ørskov C, Ritzel R, Schmiegel WH. Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. Am J Physiol. 1997;273(5 Pt 1):E981–E988. doi: 10.1152/ajpendo.1997.273.5.E981. [DOI] [PubMed] [Google Scholar]
- 10.Nauck MA, Heimesaat MM, Ørskov C, Holst JJ, Ebert R, Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 (7-36 amide) but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest. 1993;91:301–307. doi: 10.1172/JCI116186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nauck MA, Kleine N, Ørskov C, Holst JJ, Willms B, Creutzfeldt W. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7-36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1993;36:741–744. doi: 10.1007/BF00401145. [DOI] [PubMed] [Google Scholar]
- 12.Nauck MA. Unraveling the science of incretin biology. Am J Med. 2009;122:S3–S10. doi: 10.1016/j.amjmed.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Deacon CF, Johnsen AH, Holst JJ. Degradation of glucagon-like peptide-1 by human plasma in vitro yields an N-terminally truncated peptide that is a major endogenous metabolite in vivo. J Clin Endocrinol Metab. 1995;80:952–957. doi: 10.1210/jcem.80.3.7883856. [DOI] [PubMed] [Google Scholar]
- 14.Aschner P, Kipnes MS, Lunceford JK, Sanchez M, Mickel C, Williams-Herman DE for the Sitagliptin Study 021 Group. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care. 2006;29:2632–2637. doi: 10.2337/dc06-0703. [DOI] [PubMed] [Google Scholar]
- 15.Barnett AH. New treatments in type 2 diabetes: a focus on the incretin-based therapies. Clin Endocrinol (Oxf) 2009;70:343–353. doi: 10.1111/j.1365-2265.2008.03396.x. [DOI] [PubMed] [Google Scholar]
- 16.Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA. 2007;298:194–206. doi: 10.1001/jama.298.2.194. [DOI] [PubMed] [Google Scholar]
- 17.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–1705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 18.Mentlein R. Dipeptidyl-peptidase IV (CD26) - role in the inactivation of regulatory peptides. Regul Pept. 1999;85:9–24. doi: 10.1016/s0167-0115(99)00089-0. [DOI] [PubMed] [Google Scholar]
- 19.Gilbert MP, Pratley RE. Efficacy and safety of incretin-based therapies in patients with type 2 diabetes mellitus. Am J Med. 2009;122(Suppl):S11–S24. doi: 10.1016/j.amjmed.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 20.DeFronzo RA, Okerson T, Viswanathan P, Guan X, Holcombe JH, MacConell L. Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Curr Med Res Opin. 2008;24:2943–2952. doi: 10.1185/03007990802418851. [DOI] [PubMed] [Google Scholar]
- 21.Stonehouse A, Okerson T, Kendall D, Maggs D. Emerging incretinbased therapies for type 2 diabetes: incretin mimetics and DPP-4 inhibitors. Curr Diabetes Rev. 2008;4:101–109. doi: 10.2174/157339908784220705. [DOI] [PubMed] [Google Scholar]
- 22.Knop FK, Holst JJ, Vilsbøll T. Replacing SUs with incretin-based therapies for type 2 diabetes mellitus: challenges and feasibility. IDrugs. 2008;11:497–501. [PubMed] [Google Scholar]
- 23.Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS, Drucker DJ, Husain M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation. 2008;117:2340–2350. doi: 10.1161/CIRCULATIONAHA.107.739938. [DOI] [PubMed] [Google Scholar]
- 24.Holst JJ, Deacon CF. Glucagon-like peptide 1 and inhibitors of dipeptidyl peptidase IV in the treatment of type 2 diabetes mellitus. Curr Opin Pharmacol. 2004;4:589–596. doi: 10.1016/j.coph.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 25.Buse JB, Rosenstock J, Sesti G, Schmidt WE, Montanya E, Brett JH, Zychma M, Blonde L LEAD-6 Study Group. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6) Lancet. 2009;374:39–47. doi: 10.1016/S0140-6736(09)60659-0. [DOI] [PubMed] [Google Scholar]
- 26.Garber A, Henry R, Ratner R, Garcia-Hernandez PA, Rodriguez-Pattzi H, Olvera-Alvarez I, Hale PM, Zdravkovic M, Bode B LEAD-3 (Mono) Study Group. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373:473–481. doi: 10.1016/S0140-6736(08)61246-5. [DOI] [PubMed] [Google Scholar]
- 27.Marre M, Shaw J, Brändle M, Bebakar WM, Kamaruddin NA, Strand J, Zdravkovic M, Le Thi TD, Colagiuri S LEAD-1 SU study group. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD-1 SU) Diabet Med. 2009;26:268–278. doi: 10.1111/j.1464-5491.2009.02666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nauck M, Frid A, Hermansen K, Shah NS, Tankova T, Mitha IH, Zdravkovic M, During M, Matthews DR LEAD-2 Study Group. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care. 2009;32:84–90. doi: 10.2337/dc08-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Russell-Jones D, Vaag A, Schmitz O, Sethi BK, Lalic N, Antic S, Zdravkovic M, Ravn GM, Simo R Liraglutide Effect and Action in Diabetes 5 (LEAD-5) met+SU Study Group. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trial. Diabetologia. 2009;52:2046–2055. doi: 10.1007/s00125-009-1472-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zinman B, Gerich J, Buse JB, Lewin A, Schwartz S, Raskin P, Hale PM, Zdravkovic M, Blonde L LEAD-4 Study Investigators. Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met+TZD) Diabetes Care. 2009;32:1224–1230. doi: 10.2337/dc08-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care. 2005;28:1092–1100. doi: 10.2337/diacare.28.5.1092. [DOI] [PubMed] [Google Scholar]
- 32.Kendall DM, Riddle MC, Rosenstock J, Zhuang D, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care. 2005;28:1083–1091. doi: 10.2337/diacare.28.5.1083. [DOI] [PubMed] [Google Scholar]
- 33.Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD for the Exenatide-113 Clinical Study Group. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care. 2004;27:2628–2635. doi: 10.2337/diacare.27.11.2628. [DOI] [PubMed] [Google Scholar]
- 34.Lugari R, Dei Cas A, Ugolotti D, Finardi L, Barilli AL, Ognibene C, Luciani A, Zandomeneghi R, Gnudi A. Evidence for early impairment of glucagon-like peptide 1-induced insulin secretion in human type 2 (non insulin-dependent) diabetes. Horm Metab Res. 2002;34:150–154. doi: 10.1055/s-2002-23199. [DOI] [PubMed] [Google Scholar]
- 35.Schirra J, Wank U, Arnold R, Göke B, Katschinski M. Effects of glucagon-like peptide-1(7-36)amide on motility and sensation of the proximal stomach in humans. Gut. 2002;50:341–348. doi: 10.1136/gut.50.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Näslund E, Bogefors J, Skogar S, Grybäck P, Jacobsson H, Holst JJ, Hellström PM. GLP-1 slows solid gastric emptying and inhibits insulin, glucagon, and PYY release in humans. Am J Physiol. 1999;277(3 Pt 2):R910–R916. doi: 10.1152/ajpregu.1999.277.3.R910. [DOI] [PubMed] [Google Scholar]
- 37.Gutzwiller JP, Drewe J, Göke B, Schmidt H, Rohrer B, Lareida J, Beglinger C. Glucagon-like peptide-1 promotes satiety and reduces food intake in patients with diabetes mellitus type 2. Am J Physiol. 1999;276(5 Pt 2):R1541–R1544. doi: 10.1152/ajpregu.1999.276.5.R1541. [DOI] [PubMed] [Google Scholar]
- 38.Flint A, Raben A, Astrup A, Holst JJ. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest. 1998;101:515–520. doi: 10.1172/JCI990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gedulin BR, Nikoulina SE, Smith PA, Gedulin G, Nielsen LL, Baron AD, Parkes DG, Young AA. Exenatide (exendin-4) improves insulin sensitivity and beta-cell mass in insulin-resistant obese fa/fa Zucker rats independent of glycemia and body weight. Endocrinology. 2005;146:2069–2076. doi: 10.1210/en.2004-1349. [DOI] [PubMed] [Google Scholar]
- 40.Bock T, Pakkenberg B, Buschard K. The endocrine pancreas in non-diabetic rats after short-term and long-term treatment with the long-acting GLP-1 derivative NN2211. APMIS. 2003;111(12):1117–1124. doi: 10.1111/j.1600-0463.2003.apm1111207.x. [DOI] [PubMed] [Google Scholar]
- 41.Sturis J, Gotfredsen CF, Rømer J, Rolin B, Ribel U, Brand CL, Wilken M, Wassermann K, Deacon CF, Carr RD. et al. GLP-1 derivative liraglutide in rats with beta-cell deficiencies: influence of metabolic state on beta-cell mass dynamics. Br J Pharmacol. 2003;140:123–132. doi: 10.1038/sj.bjp.0705397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rolin B, Larsen MO, Gotfredsen CF, Deacon CF, Carr RD, Wilken M, Knudsen LB. The long-acting GLP-1 derivative NN2211 ameliorates glycemia and increases beta-cell mass in diabetic mice. Am J Physiol Endocrinol Metab. 2002;283:E745–E752. doi: 10.1152/ajpendo.00030.2002. [DOI] [PubMed] [Google Scholar]
- 43.Xu G, Stoffers DA, Habener JF, Bonner-Weir S. Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes. 1999;48:2270–2276. doi: 10.2337/diabetes.48.12.2270. [DOI] [PubMed] [Google Scholar]
- 44.Chen J, Couto FM, Minn AH, Shalev A. Exenatide inhibits beta-cell apoptosis by decreasing thioredoxin-interacting protein. Biochem Biophys Res Commun. 2006;346:1067–1074. doi: 10.1016/j.bbrc.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 45.Bregenholt S, Møldrup A, Blume N, Karlsen AE, Nissen Friedrichsen B, Tornhave D, Knudsen LB, Petersen JS. The long-acting glucagon-like peptide-1 analogue, liraglutide, inhibits beta-cell apoptosis in vitro. Biochem Biophys Res Commun. 2005;330:577–584. doi: 10.1016/j.bbrc.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 46.Sokos GG, Bolukoglu H, German J, Hentosz T, Magovern GJ Jr, Maher TD, Dean DA, Bailey SH, Marrone G, Benckart DH. et al. Effect of glucagon-like peptide-1 (GLP-1) on glycemic control and left ventricular function in patients undergoing coronary artery bypass grafting. Am J Cardiol. 2007;100:824–829. doi: 10.1016/j.amjcard.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 47.Zhao T, Parikh P, Bhashyam S, Bolukoglu H, Poornima I, Shen YT, Shannon RP. Direct effects of glucagon-like peptide-1 on myocardial contractility and glucose uptake in normal and postischemic isolated rat hearts. J Pharmacol Exp Ther. 2006;317:1106–1113. doi: 10.1124/jpet.106.100982. [DOI] [PubMed] [Google Scholar]
- 48.Bose AK, Mocanu MM, Carr RD, Brand CL, Yellon DM. Glucagon-like peptide 1 can directly protect the heart against ischemia/reperfusion injury. Diabetes. 2005;54:146–151. doi: 10.2337/diabetes.54.1.146. [DOI] [PubMed] [Google Scholar]
- 49.Nikolaidis LA, Doverspike A, Hentosz T, Zourelias L, Shen YT, Elahi D, Shannon RP. Glucagon-like peptide-1 limits myocardial stunning following brief coronary occlusion and reperfusion in conscious canines. J Pharmacol Exp Ther. 2005;312:303–308. doi: 10.1124/jpet.104.073890. [DOI] [PubMed] [Google Scholar]
- 50.Nikolaidis LA, Mankad S, Sokos GG, Miske G, Shah A, Elahi D, Shannon RP. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation. 2004;109:962–965. doi: 10.1161/01.CIR.0000120505.91348.58. [DOI] [PubMed] [Google Scholar]
- 51.Balkau B, Picard P, Vol S, Fezeu L, Eschwege E for the DESIR Study Group. Consequences of change in waist circumference on cardiometabolic risk factors over 9 years: Data from an Epidemiological Study on the Insulin Resistance Syndrome (DESIR) Diabetes Care. 2007;30:1901–1903. doi: 10.2337/dc06-2542. [DOI] [PubMed] [Google Scholar]
- 52.Montague CT, O'Rahilly S. The perils of portliness: causes and consequences of visceral adiposity. Diabetes. 2000;49:883–888. doi: 10.2337/diabetes.49.6.883. [DOI] [PubMed] [Google Scholar]
- 53.Libby P. Fat fuels the flame: triglyceride-rich lipoproteins and arterial inflammation. Circ Res. 2007;100:299–301. doi: 10.1161/01.RES.0000259393.89870.58. [DOI] [PubMed] [Google Scholar]
- 54.Fox CS, Pencina MJ, Wilson PW, Paynter NP, Vasan RS, D'Agostino RB Sr. Lifetime risk of cardiovascular disease among individuals with and without diabetes stratified by obesity status in the Framingham heart study. Diabetes Care. 2008;31:1582–1584. doi: 10.2337/dc08-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fox CS, Coady S, Sorlie PD, D'Agostino RB Sr, Pencina MJ, Vasan RS, Meigs JB, Levy D, Savage PJ. Increasing cardiovascular disease burden due to diabetes mellitus: the Framingham Heart Study. Circulation. 2007;115:1544–1550. doi: 10.1161/CIRCULATIONAHA.106.658948. [DOI] [PubMed] [Google Scholar]
- 56.American Diabetes Association. Complications of diabetes in the United States. http://schoolwalk.diabetes.org/swfd/swfd_mshs_attach.pdf. Accessed December 3, 2009.
- 57.Utzschneider KM, Carr DB, Barsness SM, Kahn SE, Schwartz RS. Diet-induced weight loss is associated with an improvement in beta-cell function in older men. J Clin Endocrinol Metab. 2004;89:2704–2710. doi: 10.1210/jc.2003-031827. [DOI] [PubMed] [Google Scholar]
- 58.Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, Ilanne-Parikka P, Keinänen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M. et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 59.Goodpaster BH, Kelley DE, Wing RR, Meier A, Thaete FL. Effects of weight loss on regional fat distribution and insulin sensitivity in obesity. Diabetes. 1999;48:839–847. doi: 10.2337/diabetes.48.4.839. [DOI] [PubMed] [Google Scholar]
- 60.Markovic TP, Campbell LV, Balasubramanian S, Jenkins AB, Fleury AC, Simons LA, Chisholm DJ. Beneficial effect on average lipid levels from energy restriction and fat loss in obese individuals with or without type 2 diabetes. Diabetes Care. 1998;21:695–700. doi: 10.2337/diacare.21.5.695. [DOI] [PubMed] [Google Scholar]
- 61.Wexler R, Feldman D. Initiation of therapy for patients with essential hypertension or comorbid conditions. Prim Care. 2006;33:887–901. doi: 10.1016/j.pop.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 62.Pi-Sunyer FX. The effects of pharmacologic agents for type 2 diabetes mellitus on body weight. Postgrad Med. 2008;120:5–17. doi: 10.3810/pgm.2008.07.1785. [DOI] [PubMed] [Google Scholar]
- 63.Baggio LL, Drucker DJ. Clinical endocrinology and metabolism. Glucagon-like peptide-1 and glucagon-like peptide-2. Best Pract Res Clin Endocrinol Metab. 2004;18:531–554. doi: 10.1016/j.beem.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 64.Deacon CF, Nauck MA, Toft-Nielsen M, Pridal L, Willms B, Holst JJ. Both subcutaneously and intravenously administered glucagon-like peptide I are rapidly degraded from the NH2-terminus in type II diabetic patients and in healthy subjects. Diabetes. 1995;44:1126–1131. doi: 10.2337/diab.44.9.1126. [DOI] [PubMed] [Google Scholar]
- 65.Kieffer TJ, McIntosh CH, Pederson RA. Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology. 1995;136:3585–3596. doi: 10.1210/endo.136.8.7628397. [DOI] [PubMed] [Google Scholar]
- 66.Fehmann HC, Habener JF. Galanin inhibits proinsulin gene expression stimulated by the insulinotropic hormone glucagon-like peptide-I(7-37) in mouse insulinoma beta TC-1 cells. Endocrinology. 1992;130:2890–2896. doi: 10.1210/endo.130.5.1374016. [DOI] [PubMed] [Google Scholar]
- 67.Perfetti R, Merkel P. Glucagon-like peptide-1: a major regulator of pancreatic beta-cell function. Eur J Endocrinol. 2000;143:717–725. doi: 10.1530/eje.0.1430717. [DOI] [PubMed] [Google Scholar]
- 68.Irwin DM. Molecular evolution of proglucagon. Regul Pept. 2001;98:1–12. doi: 10.1016/s0167-0115(00)00232-9. [DOI] [PubMed] [Google Scholar]
- 69.Rachman J, Barrow BA, Levy JC, Turner RC. Near-normalisation of diurnal glucose concentrations by continuous administration of glucagon-like peptide-1 (GLP-1) in subjects with NIDDM. Diabetologia. 1997;40:205–211. doi: 10.1007/s001250050664. [DOI] [PubMed] [Google Scholar]
- 70.Samols E, Bonner-Weir S, Weir GC. Intra-islet insulin-glucagon-somatostatin relationships. Clin Endocrinol Metab. 1986;15:33–58. doi: 10.1016/s0300-595x(86)80041-x. [DOI] [PubMed] [Google Scholar]
- 71.Heller RS, Kieffer TJ, Habener JF. Insulinotropic glucagon-like peptide I receptor expression in glucagon-producing alpha-cells of the rat endocrine pancreas. Diabetes. 1997;46:785–791. doi: 10.2337/diab.46.5.785. [DOI] [PubMed] [Google Scholar]
- 72.Kawamori D, Kurpad AJ, Hu J, Liew CW, Shih JL, Ford EL, Herrera PL, Polonsky KS, McGuinness OP, Kulkarni RN. Insulin signaling in alpha cells modulates glucagon secretion in vivo. Cell Metab. 2009;9:350–361. doi: 10.1016/j.cmet.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Göke B. Islet cell function: alpha and beta cells - partners towards normoglycaemia. Int J Clin Pract Suppl. 2008;159:2–7. doi: 10.1111/j.1742-1241.2007.01686.x. [DOI] [PubMed] [Google Scholar]
- 74.Quesada I, Tuduri E, Ripoll C, Nadal A. Physiology of the pancreatic alpha-cell and glucagon secretion: role in glucose homeostasis and diabetes. J Endocrinol. 2008;199:5–19. doi: 10.1677/JOE-08-0290. [DOI] [PubMed] [Google Scholar]
- 75.Burcelin R, Knauf C, Cani PD. Pancreatic alpha-cell dysfunction in diabetes. Diabetes Metab. 2008;34(Suppl 2):S49–S55. doi: 10.1016/S1262-3636(08)73395-0. [DOI] [PubMed] [Google Scholar]
- 76.Degn KB, Juhl CB, Sturis J, Jakobsen G, Brock B, Chandramouli V, Rungby J, Landau BR, Schmitz O. One week's treatment with the long-acting glucagon-like peptide 1 derivative liraglutide (NN2211) markedly improves 24-h glycemia and alpha- and beta-cell function and reduces endogenous glucose release in patients with type 2 diabetes. Diabetes. 2004;53:1187–1194. doi: 10.2337/diabetes.53.5.1187. [DOI] [PubMed] [Google Scholar]
- 77.Juhl CB, Hollingdal M, Sturis J, Jakobsen G, Agersø H, Veldhuis J, Pørksen N, Schmitz O. Bedtime administration of NN2211, a long-acting GLP-1 derivative, substantially reduces fasting and postprandial glycemia in type 2 diabetes. Diabetes. 2002;51:424–429. doi: 10.2337/diabetes.51.2.424. [DOI] [PubMed] [Google Scholar]
- 78.de Heer J, Rasmussen C, Coy DH, Holst JJ. Glucagon-like peptide-1, but not glucose-dependent insulinotropic peptide, inhibits glucagon secretion via somatostatin (receptor subtype 2) in the perfused rat pancreas. Diabetologia. 2008;51:2263–2270. doi: 10.1007/s00125-008-1149-y. [DOI] [PubMed] [Google Scholar]
- 79.Schirra J, Nicolaus M, Woerle HJ, Struckmeier C, Katschinski M, Göke B. GLP-1 regulates gastroduodenal motility involving cholinergic pathways. Neurogastroenterol Motil. 2009;21:609–618. doi: 10.1111/j.1365-2982.2008.01246.x. [DOI] [PubMed] [Google Scholar]
- 80.Bullock BP, Heller RS, Habener JF. Tissue distribution of messenger ribonucleic acid encoding the rat glucagon-like peptide-1 receptor. Endocrinology. 1996;137:2968–2978. doi: 10.1210/endo.137.7.8770921. [DOI] [PubMed] [Google Scholar]
- 81.Wei Y, Mojsov S. Distribution of GLP-1 and PACAP receptors in human tissues. Acta Physiol Scand. 1996;157:355–357. doi: 10.1046/j.1365-201X.1996.42256000.x. [DOI] [PubMed] [Google Scholar]
- 82.Meier JJ, Gallwitz B, Salmen S, Goetze O, Holst JJ, Schmidt WE, Nauck MA. Normalization of glucose concentrations and deceleration of gastric emptying after solid meals during intravenous glucagon-like peptide 1 in patients with type 2 diabetes. J Clin Endocrinol Metab. 2003;88:2719–2725. doi: 10.1210/jc.2003-030049. [DOI] [PubMed] [Google Scholar]
- 83.Verdich C, Flint A, Gutzwiller JP, Näslund E, Beglinger C, Hellström PM, Long SJ, Morgan LM, Holst JJ, Astrup A. A meta-analysis of the effect of glucagon-like peptide-1 (7-36) amide on ad libitum energy intake in humans. J Clin Endocrinol Metab. 2001;86:4382–4389. doi: 10.1210/jcem.86.9.7877. [DOI] [PubMed] [Google Scholar]
- 84.Kim W, Egan JM. The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol Rev. 2008;60:470–512. doi: 10.1124/pr.108.000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hellstrom PM. GLP-1: Broadening the incretin concept to involve gut motility. Regul Pept. 2009;156:9–12. doi: 10.1016/j.regpep.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 86.Williams DL. Minireview: finding the sweet spot: peripheral versus central glucagon-like peptide 1 action in feeding and glucose homeostasis. Endocrinology. 2009;150:2997–3001. doi: 10.1210/en.2009-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Raun K, von Voss P, Knudsen LB. Liraglutide, a once-daily human glucagon-like peptide-1 analog, minimizes food intake in severely obese minipigs. Obesity (Silver Spring) 2007;15:1710–1716. doi: 10.1038/oby.2007.204. [DOI] [PubMed] [Google Scholar]
- 88.Raun K, von Voss P, Gotfredsen CF, Golozoubova V, Rolin B, Knudsen LB. Liraglutide, a long-acting glucagon-like peptide-1 analog, reduces body weight and food intake in obese candy-fed rats, whereas a dipeptidyl peptidase-IV inhibitor, vildagliptin, does not. Diabetes. 2007;56:8–15. doi: 10.2337/db06-0565. [DOI] [PubMed] [Google Scholar]
- 89.Parkinson JR, Chaudhri OB, Kuo YT, Field BC, Herlihy AH, Dhillo WS, Ghatei MA, Bloom SR, Bell JD. Differential patterns of neuronal activation in the brainstem and hypothalamus following peripheral injection of GLP-1, oxyntomodulin and lithium chloride in mice detected by manganese-enhanced magnetic resonance imaging (MEMRI) Neuroimage. 2009;44:1022–1031. doi: 10.1016/j.neuroimage.2008.09.047. [DOI] [PubMed] [Google Scholar]
- 90.Yamada C, Yamada Y, Tsukiyama K, Yamada K, Udagawa N, Takahashi N, Tanaka K, Drucker DJ, Seino Y, Inagaki N. The murine glucagon-like peptide-1 receptor is essential for control of bone resorption. Endocrinology. 2008;149:574–579. doi: 10.1210/en.2007-1292. [DOI] [PubMed] [Google Scholar]
- 91.Nakagawa A, Satake H, Nakabayashi H, Nishizawa M, Furuya K, Nakano S, Kigoshi T, Nakayama K, Uchida K. Receptor gene expression of glucagon-like peptide-1, but not glucose-dependent insulinotropic polypeptide, in rat nodose ganglion cells. Auton Neurosci. 2004;110:36–43. doi: 10.1016/j.autneu.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 92.Yamamoto H, Lee CE, Marcus JN, Williams TD, Overton JM, Lopez ME, Hollenberg AN, Baggio L, Saper CB, Drucker DJ. et al. Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J Clin Invest. 2002;110:43–52. doi: 10.1172/JCI15595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nyström T, Gutniak MK, Zhang Q, Zhang F, Holst JJ, Ahren B, Sjöholm A. Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am J Physiol Endocrinol Metab. 2004;287:E1209–E1215. doi: 10.1152/ajpendo.00237.2004. [DOI] [PubMed] [Google Scholar]
- 94.Gros R, You X, Baggio LL, Kabir MG, Sadi AM, Mungrue IN, Parker TG, Huang Q, Drucker DJ, Husain M. Cardiac function in mice lacking the glucagon-like peptide-1 receptor. Endocrinology. 2003;144:2242–2252. doi: 10.1210/en.2003-0007. [DOI] [PubMed] [Google Scholar]
- 95.Barragan JM, Eng J, Rodriguez R, Blazquez E. Neural contribution to the effect of glucagon-like peptide-1-(7-36) amide on arterial blood pressure in rats. Am J Physiol. 1999;277(5 Pt 1):E784–E791. doi: 10.1152/ajpendo.1999.277.5.E784. [DOI] [PubMed] [Google Scholar]
- 96.Noyan-Ashraf MH, Momen MA, Ban K, Sadi AM, Zhou YQ, Riazi AM, Baggio LL, Henkelman RM, Husain M, Drucker DJ. GLP-1R agonist liraglutide activates cytoprotective pathways and improves outcomes after experimental myocardial infarction in mice. Diabetes. 2009;58:975–983. doi: 10.2337/db08-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sulistio M, Carothers C, Mangat M, Lujan M, Oliveros R, Chilton R. GLP-1 agonist-based therapies: an emerging new class of antidiabetic drug with potential cardioprotective effects. Curr Atheroscler Rep. 2009;11:93–99. doi: 10.1007/s11883-009-0015-9. [DOI] [PubMed] [Google Scholar]
- 98.Müssig K, Oncü A, Lindauer P, Heininger A, Aebert H, Unertl K, Ziemer G, Häring HU, Holst JJ, Gallwitz B. Effects of intravenous glucagon-like peptide-1 on glucose control and hemodynamics after coronary artery bypass surgery in patients with type 2 diabetes. Am J Cardiol. 2008;102:646–647. doi: 10.1016/j.amjcard.2008.06.029. [DOI] [PubMed] [Google Scholar]
- 99.Cabou C, Campistron G, Marsollier N, Leloup C, Cruciani-Guglielmacci C, Pénicaud L, Drucker DJ, Magnan C, Burcelin R. Brain glucagon-like peptide-1 regulates arterial blood flow, heart rate, and insulin sensitivity. Diabetes. 2008;57:2577–2587. doi: 10.2337/db08-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nikolaidis LA, Elahi D, Hentosz T, Doverspike A, Huerbin R, Zourelias L, Stolarski C, Shen YT, Shannon RP. Recombinant glucagon-like peptide-1 increases myocardial glucose uptake and improves left ventricular performance in conscious dogs with pacing-induced dilated cardiomyopathy. Circulation. 2004;110:955–961. doi: 10.1161/01.CIR.0000139339.85840.DD. [DOI] [PubMed] [Google Scholar]
- 101.Liu H, Hu Y, Simpson RW, Dear AE. Glucagon-like peptide-1 attenuates tumour necrosis factor-alpha-mediated induction of plasmogen activator inhibitor-1 expression. J Endocrinol. 2008;196:57–65. doi: 10.1677/JOE-07-0387. [DOI] [PubMed] [Google Scholar]
- 102.Ruiz-Grande C, Alarcon C, Mérida E, Valverde I. Lipolytic action of glucagon-like peptides in isolated rat adipocytes. Peptides. 1992;13:13–16. doi: 10.1016/0196-9781(92)90134-o. [DOI] [PubMed] [Google Scholar]
- 103.Villanueva-Peñacarrillo ML, Márquez L, González N, Díaz-Miguel M, Valverde I. Effect of GLP-1 on lipid metabolism in human adipocytes. Horm Metab Res. 2001;33:73–77. doi: 10.1055/s-2001-12428. [DOI] [PubMed] [Google Scholar]
- 104.Zander M, Madsbad S, Madsen JL, Holst JJ. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet. 2002;359:824–830. doi: 10.1016/S0140-6736(02)07952-7. [DOI] [PubMed] [Google Scholar]
- 105.Prigeon RL, Quddusi S, Paty B, D’Alessio DA. Suppression of glucose production by GLP-1 independent of islet hormones: a novel extrapancreatic effect. Am J Physiol Endocrinol Metab. 2003;285:E701–E707. doi: 10.1152/ajpendo.00024.2003. [DOI] [PubMed] [Google Scholar]
- 106.Sandoval DA, Bagnol D, Woods SC, D’Alessio DA, Seeley RJ. Arcuate glucagon-like peptide 1 receptors regulate glucose homeostasis but not food intake. Diabetes. 2008;57:2046–2054. doi: 10.2337/db07-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.D’Alessio DA, Prigeon RL, Ensinck JW. Enteral enhancement of glucose disposition by both insulin-dependent and insulin-independent processes. A physiological role of glucagon-like peptide I. Diabetes. 1995;44:1433–1437. doi: 10.2337/diab.44.12.1433. [DOI] [PubMed] [Google Scholar]
- 108.Larsson H, Holst JJ, Ahren B. Glucagon-like peptide-1 reduces hepatic glucose production indirectly through insulin and glucagon in humans. Acta Physiol Scand. 1997;160:413–422. doi: 10.1046/j.1365-201X.1997.00161.x. [DOI] [PubMed] [Google Scholar]
- 109.Gutzwiller JP, Tschopp S, Bock A, Zehnder CE, Huber AR, Kreyenbuehl M, Gutmann H, Drewe J, Henzen C, Göke B. et al. Glucagon-like peptide 1 induces natriuresis in healthy subjects and in insulin-resistant obese men. J Clin Endocrinol Metab. 2004;89:3055–3061. doi: 10.1210/jc.2003-031403. [DOI] [PubMed] [Google Scholar]
- 110.Yu M, Moreno C, Hoagland KM, Dahly A, Ditter K, Mistry M, Roman RJ. Antihypertensive effect of glucagon-like peptide 1 in Dahl salt-sensitive rats. J Hypertens. 2003;21:1125–1135. doi: 10.1097/00004872-200306000-00012. [DOI] [PubMed] [Google Scholar]
- 111.Eng J, Kleinman WA, Singh L, Singh G, Raufman JP. Isolation and characterization of exendin-4, an exendin-3 analogue, from Heloderma suspectum venom. Further evidence for an exendin receptor on dispersed acini from guinea pig pancreas. J Biol Chem. 1992;267:7402–7405. [PubMed] [Google Scholar]
- 112.Byetta (exenatide injection) Prescribing Information. Amylin Pharmaceuticals. San Diego, California, 2008.
- 113.Ghofaili KA, Fung M, Ao Z, Meloche M, Shapiro RJ, Warnock GL, Elahi D, Meneilly GS, Thompson DM. Effect of exenatide on beta cell function after islet transplantation in type 1 diabetes. Transplantation. 2007;83:24–28. doi: 10.1097/01.tp.0000251379.46596.2d. [DOI] [PubMed] [Google Scholar]
- 114.Mari A, Nielsen LL, Nanayakkara N, DeFronzo RA, Ferrannini E, Halseth A. Mathematical modeling shows exenatide improved beta-cell function in patients with type 2 diabetes treated with metformin or metformin and a sulfonylurea. Horm Metab Res. 2006;38:838–844. doi: 10.1055/s-2006-956505. [DOI] [PubMed] [Google Scholar]
- 115.Bunck MC, Diamant M, Corner A, Eliasson B, Malloy JL, Shaginian RM, Deng W, Kendall DM, Taskinen MR, Smith U. et al. One-year treatment with exenatide improves beta-cell function, compared with insulin glargine, in metformin-treated type 2 diabetic patients: a randomized, controlled trial. Diabetes Care. 2009;32:762–768. doi: 10.2337/dc08-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Buse JB, Klonoff DC, Nielsen LL, Guan X, Bowlus CL, Holcombe JH, Maggs DG, Wintle ME. Metabolic effects of two years of exenatide treatment on diabetes, obesity, and hepatic biomarkers in patients with type 2 diabetes: an interim analysis of data from the open-label, uncontrolled extension of three double-blind, placebo-controlled trials. Clin Ther. 2007;29:139–153. doi: 10.1016/j.clinthera.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 117.Timmers L, Henriques JP, de Kleijn DP, Devries JH, Kemperman H, Steendijk P, Verlaan CW, Kerver M, Piek JJ, Doevendans PA. et al. Exenatide reduces infarct size and improves cardiac function in a porcine model of ischemia and reperfusion injury. J Am Coll Cardiol. 2009;53:501–510. doi: 10.1016/j.jacc.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 118.Sonne DP, Engstrøm T, Treiman M. Protective effects of GLP-1 analogues exendin-4 and GLP-1(9-36) amide against ischemia-reperfusion injury in rat heart. Regul Pept. 2008;146:243–249. doi: 10.1016/j.regpep.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 119.Blonde L, Klein EJ, Han J, Zhang B, Mac SM, Poon TH, Taylor KL, Trautmann ME, Kim DD, Kendall DM. Interim analysis of the effects of exenatide treatment on A1C, weight and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes. Diabetes Obes Metab. 2006;8:436–447. doi: 10.1111/j.1463-1326.2006.00602.x. [DOI] [PubMed] [Google Scholar]
- 120.Klonoff DC, Buse JB, Nielsen LL, Guan X, Bowlus CL, Holcombe JH, Wintle ME, Maggs DG. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin. 2008;24:275–286. doi: 10.1185/030079908x253870. [DOI] [PubMed] [Google Scholar]
- 121.Cohen A, Horton E, Gibson H, Lamparello B, Herzlinger Botein S, McFarland L. Effects of exenatide vs insulin glargine on central haemodynamics in subjects with type 2 diabetes. Vienna, Austria. September 29-October 2, 2009; 45th Annual Meeting of the European Association for the Study of Diabetes (EASD); Abstract 757. [Google Scholar]
- 122.Horton ES, Cohen A, Gibson H, Lamparello B, Herzlinger S, McFarland L. Effects of exenatide vs insulin glargine on cardiovascular risk factors in subjects with type 2 diabetes. Vienna, Austria. September 29-October 2, 2009; 45th Annual Meeting of the European Association for the Study of Diabetes (EASD); Abstract 760. [Google Scholar]
- 123.Bergenstal R. Exenatide once weekly improved cardiometabolic risk factors in subjects with type 2 diabetes during once year of treatment. Vienna, Austria. September 29-October 2, 2009; 45th Annual Meeting of the European Association for the Study of Diabetes (EASD); Abstract 758. [Google Scholar]
- 124.Victoza (Summary of Product Characteristics) Novo Nordisk A/S; Bagsværd, Denmark: [November 12, 2009]. http://www.emea.europa.eu/humandocs/PDFs/EPAR/victoza/H-1026 -PI-en.pdf. [Google Scholar]
- 125.Vilsbøll T, Zdravkovic M, Le-Thi T, Krarup T, Schmitz O, Courrèges JP, Verhoeven R, Bugánová I, Madsbad S. Liraglutide, a long-acting human glucagon-like peptide-1 analog, given as monotherapy significantly improves glycemic control and lowers body weight without risk of hypoglycemia in patients with type 2 diabetes. Diabetes Care. 2007;30:1608–1610. doi: 10.2337/dc06-2593. [DOI] [PubMed] [Google Scholar]
- 126.Agerso H, Jensen LB, Elbrond B, Rolan P, Zdravkovic M. The pharmacokinetics, pharmacodynamics, safety and tolerability of NN2211, a new long-acting GLP-1 derivative, in healthy men. Diabetologia. 2002;45:195–202. doi: 10.1007/s00125-001-0719-z. [DOI] [PubMed] [Google Scholar]
- 127.Knudsen LB, Nielsen PF, Huusfeldt PO, Johansen NL, Madsen K, Pedersen FZ, Thøgersen H, Wilken M, Agersø H. Potent derivatives of glucagon-like peptide-1 with pharmacokinetic properties suitable for once daily administration. J Med Chem. 2000;43:1664–1669. doi: 10.1021/jm9909645. [DOI] [PubMed] [Google Scholar]
- 128.Buse J, Sesti G, Schmidt W, Montanya E, Chang CT, Xu Y, Blonde L, Rosenstock J. Switching from twice-daily exenatide to once daily liraglutide improves glycemic control in type 2 diabetes on oral agents. New Orleans, Louisiana. June 5-9, 2009; American Diabetes Association (ADA) 69th Annual Scientific Sessions; Poster 591. [Google Scholar]
- 129.Colagiuri S, Frid A, Zdravkovic M, Thi T, Vaag A. The once-daily human glucagon-like peptide-1 analog liraglutide reduces systolic blood pressure in patients with type 2 diabetes. San Francisco, CA. June 6-10, 2008; American Diabetes Association (ADA) 68th Annual Scientific Sessions; Abstract 554. [Google Scholar]
- 130.Plutzky J, Garber A, Toft AD, Poulter NR. Meta-analysis demonstrates that liraglutide, a once-daily human GLP-1 analogue, significantly reduces lipids and other markers of cardiovascular risk in type 2 diabetes. Vienna, Austria. September 29-October 2, 2009; 45th Annual Meeting of the European Association for the Study of Diabetes (EASD); Abstract 762. [Google Scholar]
- 131.Liu H, Dear AE, Knudsen LB, Simpson RW. A long-acting glucagon-like peptide-1 analogue attenuates induction of plasminogen activator inhibitor type-1 and vascular adhesion molecules. J Endocrinol. 2009;201:59–66. doi: 10.1677/JOE-08-0468. [DOI] [PubMed] [Google Scholar]
- 132.Courreges JP, Vilsbøll T, Zdravkovic M, Le-Thi T, Krarup T, Schmitz O, Verhoeven R, Bugáñová I, Madsbad S. Beneficial effects of once-daily liraglutide, a human glucagon-like peptide-1 analogue, on cardiovascular risk biomarkers in patients with type 2 diabetes. Diabet Med. 2008;25:1129–1132. doi: 10.1111/j.1464-5491.2008.02484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jendle J, Nauck M, Matthews D, Frid A, Hermansen K, Düring M, Zdravkovic M, Strauss BJ for the LEAD-2 and LEAD-2 Study Groups. Weight loss with liraglutide, a once-daily human glucagon-like peptide-1 analogue for type 2 diabetes treatment as monotherapy or added to metformin, is primarily as a result of reduction in fat tissue. Diabetes Obes Metab. 2009;11:1162–1172. doi: 10.1111/j.1463-1326.2009.01158.x. [DOI] [PubMed] [Google Scholar]
- 134.Drucker DJ, Buse JB, Taylor K, Kendall DM, Trautmann M, Zhuang D, Porter L DURATION-1 Study Group. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet. 2008;372:1240–1250. doi: 10.1016/S0140-6736(08)61206-4. [DOI] [PubMed] [Google Scholar]
- 135.Moretto TJ, Milton DR, Ridge TD, Macconell LA, Okerson T, Wolka AM, Brodows RG. Efficacy and tolerability of exenatide monotherapy over 24 weeks in antidiabetic drug-naive patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther. 2008;30:1448–1460. doi: 10.1016/j.clinthera.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 136.Zinman B, Hoogwerf BJ, Durán García S, Milton DR, Giaconia JM, Kim DD, Trautmann ME, Brodows RG. The effect of adding exenatide to a thiazolidinedione in suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med. 2007;146:477–485. doi: 10.7326/0003-4819-146-7-200704030-00003. [DOI] [PubMed] [Google Scholar]
- 137.Dore DD, Seeger JD, Arnold Chan K. Use of a claims-based active drug safety surveillance system to assess the risk of acute pancreatitis with exenatide or sitagliptin compared to metformin or glyburide. Curr Med Res Opin. 2009;25:1019–1027. doi: 10.1185/03007990902820519. [DOI] [PubMed] [Google Scholar]
- 138.Noel RA, Braun DK, Patterson RE, Bloomgren G. Increased risk of acute pancreatitis observed in patients with type 2 diabetes. Copenhagen, Denmark. August 17-20, 2008; 24th International Conference on Pharmacoepidemiology and Therapeutic Risk Management; Abstract 409. [Google Scholar]
- 139.Food and Drug Administration. FDA Briefing Materials: Liraglutide. [December 3, 2009]. Available at: http://www.fda.gov/ohrms/dockets/ac/09/briefing/2009-4422b2-01-FDA.pdf.
- 140.Novo Nordisk. Liraglutide (injection) NDA22-34 - Endocrine and Metabolic Drug Advisory Committee. [December 3, 2009]. Available at: http://www.fda.gov/ohrms/dockets/ac/09/briefing/2009-4422b2-02-NovoNordisk.pdf.


