Abstract
Cellular microenvironment is known to play a critical role in the maintenance of human bone marrow-derived mesenchymal stem cells (BM-MSCs). It was uncertain whether BM-MSCs obtained from a 'diabetic milieu' (dBM-MSCs) offer the same regenerative potential as those obtained from healthy (non-diabetic) individuals (hBM-MSCs). To investigate the effect of diabetic microenvironment on human BM-MSCs, we isolated and characterized these cells from diabetic patients (dBM-MSCs). We found that dBM-MSCs expressed mesenchymal markers such as vimentin, smooth muscle actin, nestin, fibronectin, CD29, CD44, CD73, CD90, and CD105. These cells also exhibited multilineage differentiation potential, as evident from the generation of adipocytes, osteocytes, and chondrocytes when exposed to lineage specific differentiation media. Although the cells were similar to hBM-MSCs, 6% (3/54) of dBM-MSCs expressed proinsulin/C-peptide. Emanating from the diabetic microenvironmental milieu, we analyzed whether in vitro reprogramming could afford the maturation of the islet-like clusters (ICAs) derived from dBM-MSCs. Upon mimicking the diabetic hyperglycemic niche and the supplementation of fetal pancreatic extract, to differentiate dBM-MSCs into pancreatic lineage in vitro, we observed rapid differentiation and maturation of dBM-MSCs into islet-like cell aggregates. Thus, our study demonstrated that diabetic hyperglycemic microenvironmental milieu plays a major role in inducing the differentiation of human BM-MSCs in vivo and in vitro.
Keywords: diabetes, beta-cell, stem cell, differentiation, bone marrow, NGN3, NKX6.1, PAX6
Abbreviations: α-MEM - α-modified Eagle's medium (used for cell culture); AGE - advanced glycation end-product; ALL - acute lymphoblastic leukemia; ALS - amyotrophic lateral sclerosis; AML - acute myeloid leukemia; BM-MSC - bone marrow-derived mesenchymal stem cell; BRN4 - Brain 4 (transcription factor expressed in the brain and glucagon-expressing cells in the pancreas, also known as POU3F4); C-peptide - connecting peptide; Ct - cycle threshold; CXCR4 - alpha-chemokine receptor (also called fusin) specific for stromal-derived-factor-1 (SDF-1, also called CXCL12), a molecule endowed with potent chemotactic activity for lymphocytes; dBM-MSC - human diabetic BM-MSC; DME meduim - Dulbecco's modified Eagle’s medium; E-cadherin - epithelial cadherin (CDh1); EDTA - ethylenediaminetetraacetic acid (used as chelating agent that binds to calcium and prevents joining of cadher-ins between cells; it also prevents clumping of cells grown in liquid suspension, and is able to detach adherent cells for passaging); EGFP - enhanced green fluorescence protein; F(ab’)2 - antigen-binding fragment of an antibody; FACS - fluorescence-activated cell sorting; GATA6 - binding protein that binds (A/T/C)GAT(A/T)(A) of the binding sequence; Glut2 - glucose transporter 2 (also known as solute carrier family 2 member 2 SLC2A2); GCG - glucagons gene; hBM-MSC - normal human BM-MSC; HD - Hodgkin disease; ICA - islet-like cell aggregate; ICAM-5 - intercellular adhesion molecule 5 (also known as telencephalin, CD# not yet assigned); ISL1 - insulin gene enhancer protein gene 1; NCAM-1 - neural cell adhesion molecule 1 (CD56); NDS - normal donkey serum; NGN-3 - neurogenin-3 (controls islet cell fate specification in pancreatic progenitor cells); NHL - non-Hodgkin lymphoma; NKX6-1 - NK6 homeobox 1 (transcription factor required for the development of beta-cells); Oil-Red-O - Solvent Red 27 (fat-soluble dye used for stain-ing of triglycerides and lipids); PBS - phosphate-buffered saline; PECAM-1 - platelet endothelial cell adhesion molecule-1 (CD31); PE - phycoerythrin (fluorescent dye for labeling antibodies); Pdx1 - pancreatic and duodenal homeobox 1 (transcription factor necessary for pancreatic development and beta-cell maturation); PFA - paraformaldehyde (used to fix cells); POU - class of genes that produce transcription factors; POU3F4 - POU class 3 homeobox 4 gene or gene product (also known as BRN4); RNA - ribonucleic acid; RPE - rat pancreatic extract; RT-PCR - reverse transcriptase polymerase chain reaction; TPVG - trypsin phosphate versene glucose; UCBS - human umbilical cord blood serum
Introduction
Bone marrow-derived mesenchymal stem cells (BM-MSCs) are able to differentiate into many cell types, and to proliferate ex vivo. These attributes makes them a potential therapeutic tool for cell replacement therapy in diabetes and other diseases. Stem cell differentiation is controlled by extracellular cues, the environment, and intrinsic genetic programs within stem cells [1, 2]. The fate of stem cell differentiation is influenced by both soluble and insoluble factors from the surrounding microenvironment. Several signaling cascades mediate the balance response of the stem cell to the need of the organism. Pathological conditions induced by dysregulation result in aberrant functions of stem cells or other targets [3-6].
Previous studies have demonstrated the characteristics of BM-MSCs isolated from healthy donors [7, 8]. BM-MSCs typically lack hematopoietic antigens like CD45, CD34, and CD14, express mesenchymal antigens such as CD105, CD90, CD73, CD44, and CD29, exhibit self-renewal potential, and are able to differentiate into multiple lineages. Whereas, the characteristics and differentiation potential for BM-MSCs obtained from diabetic individuals are unknown. Optimization of their in vitro expansion and characterization of disease-specific BM-MSCs would provide crucial information to maximize the beneficial effects of autologous BM-MSC therapy in diabetes [9]. Studies have shown that BM-MSCs isolated from patients having Parkinson's disease [10], sporadic Amyotrophic lateral sclerosis (ALS) [11], acute lymphoblastic leukemia (ALL), Hodgkin disease (HD), and non-Hodgkin lymphoma (NHL) [12] were similar to normal adult BM-MSCs in morphology, surface epitopes, and differentiation ability in vitro. They also showed a normal karyotype and ultrastructure. However, BM-MSCs isolated from patients with acute myeloid leukemia (AML) [12], chronic renal failure [13], myelodysplastic syndrome [14], multiple myeloma [15], and rheumatoid arthritis [16] showed abnormal biological properties, and limited proliferation capacity.
We demonstrated earlier that BM-MSCs isolated from experimental diabetic mice maintained stem cell characteristics under sustained hyperglycemia, and that they were able to restore normoglycemia when transplanted in syngenic diabetic mice [17]. In an interesting study, Kojima et al. showed that extrapancreatic proinsulin-positive cells were present in liver, adipose tissue, and bone marrow of diabetic rats [18]. Transplantation of genetically marked bone marrow cells in diabetic rats showed hyperglycemia-induced proinsulin expression in bone marrow cells. Subsequently, these cells colonized other tissues of diabetic animals, including liver and adipose tissue. Oh et al. demonstrated that incubation of isolated rat bone marrow cells in high glucose medium induced insulin gene expression in about fifty percent of the cells within a week [19].
Some studies have also demonstrated that differentiation may be mediated through paracrine mechanisms. Chang et al. suggested that injured islets (pancreas pieces) could provide an environment under which bone marrow-derived stem cells could contribute to functional beta-cell phenotypes [20]. The interaction between MSCs and injured islet cells may initiate differentiation by direct cell-to-cell contact and by soluble paracrine factors. In another study, the same group reported that mesenchymal stromal cells (MStC) could improve hyperglycemia and insulin production in a diabetic microenvironment [21]. They transplanted male porcine bone marrow-derived EGFP-expressing MStCs directly into female diabetic porcine pancreas by multi-point injection, and observed that MStCs could adopt beta-cell fate [22] in the diabetic microenvironmental niche, and restore normoglycemia without obvious immune rejections.
In mammals, liver and pancreas exhibit a strong regenerative potential. In experimental studies, the process of regeneration followed an operative insult involving the removal of a part of the organ [23]. Pancreatectomy has already been established as a model of experimental diabetes [24], and substantial regeneration of both exocrine and endocrine pancreas after 90% pancreatectomy has been documented [25]. It is believed that paracrine or cytosolic factors released from the regenerating pancreas are responsible for inducing islet neogenesis that ultimately resulted in the regeneration of the pancreas [26, 27]. Rat pancreatic extract (RPE) from a regenerating pancreas is also known to simulate the microenvironment in vitro, inducing MSCs to differentiate into insulin-producing cells [28, 29].
To determine whether the diabetic microenvironmental niche has an impact on the mesenchymal stem cell status in vivo, we isolated and characterized human diabetic BM-MSCs (dBM-MSCs). We found that dBM-MSCs were similar to normal human BM-MSCs (hBM-MSCs) regarding phenotype and morphology. However, undifferentiated dBM-MSCs expressed insulin, C-peptide, and other pancreatic markers. This observation indicated that the diabetic microenvironment influenced the stem cells in early stages. We then exposed dBM-MSCs to high glucose-containing media supplemented by fetal pancreatic extract to differentiate the stem cells into a pancreatic lineage in vitro. We observed that dBM-MSCs rapidly differentiated and matured into insulin-producing islet-like cell aggregates. Our study thus demonstrates that the diabetic hyperglycemic microenvironment is relevant for the differentiation and maturation of human BM-MSCs in vivo and in vitro.
Material and methods
Clinical characteristics of patients
After pre-informed consent, bone marrow samples were obtained from the sternum of diabetic patients, who underwent cardiac bypass surgery. Ninety-five samples from type 2 diabetes patients, aged 15 to 80 years, were used for bone marrow isolation. All patients were diagnosed according to ADA criteria and identified without major acute or chronic complications. The duration of diabetes ranged between 10 to 20 years. All procedures were approved by the ethical committee of the National Centre for Cell Science, an autonomous institute of the Department of Biotechnology, Government of India, India.
Isolation of human bone marrow cells
The bone marrow collected was mechanically disrupted to obtain a single cell suspension. The marrow was then diluted with α-MEM. Subsequently, diluted samples were overlaid with density gradient solution (Ficoll-Hypaque, 1.077 g/ml), and centrifuged for 30 minutes at 400 g. Following centrifugation, cells were removed from the plasma/Ficoll-Hypaque interface, and suspended in 5 ml of α-MEM (GIBCO) supplemented with 10% human umbilical cord blood serum (UCBS) [30], 100 U/ml penicillin, and 100 U/ml streptomycin (GIBCO). Cells were then plated at a density of 2 x 105/cm2 in 6 well culture plates (Corning, Fisher scientific), and incubated at 37°C in a humidified atmosphere containing 5% CO2. After 72 hours, the non-adherent cells were discarded and the adherent cells were cultured for approximately 10 days. Fresh medium was replaced twice a week and the cultures were maintained for 18-20 days. Upon reaching near confluence (90%), cells were detached with TPVG (0.25% trypsin, 1 mM/l EDTA) for 3-5 minutes at 37°C. After centrifugation, cells were re-suspended with fresh medium and replated at a ratio of 1:2. All chemicals were purchased from Sigma Aldrich, unless otherwise indicated.
Flow cytometry analysis of dBM-MSCs
BM-MSCs were trypsinized at different passages, fixed in 70% chilled ethanol, and then washed in chilled PBS (Ca/Mg-free) (GIBCO). These cells were then blocked using 4% NDS (v/v in Ca/Mg-free PBS) for 30 min on wet ice and incubated with fluorescent-tagged primary antibodies at 4°C for 30 min in the dark. Data was acquired on FACS Vantage (Becton Dickinson, Franklin Lakes, NJ). R-phycoerythrin-conjugated anti-human CD11b, anti-human CD14, anti-human CD29, mouse anti-human CD34, anti-mouse CD44, anti-mouse CD45, anti-human CD73, anti-human CD90, and anti-human CD105 were used at 1:100 dilution (all antibodies from BD Pharmingen, NJ, USA). Cells were also stained with PE-labeled isotype-matched immunoglobulins and used as negative controls.
Immunostaining and confocal microscopy
Guinea pig anti-insulin (Linco Research Inc, St. Charles, MO, USA), mouse anti-glucagon (Sigma, USA), mouse monoclonal anti-vimentin (Chemicon, Temecula, CA, USA), rabbit anti-human somatostatin (Chemicon Temecula, CA, USA), mouse anti-nestin (Chemicon, Temecla, CA, USA), mouse anti-smooth muscle actin (Sigma, USA), mouse anti-human CD31 (Chemicon, Temecula, CA, USA), rabbit anti-human Pdx1 (Millipore), and rabbit anti-human Glut2 (Chemicon, Temecula, CA, USA) were used at 1:100 dilution. Alexa-Fluor 488, 546, and 633 F(ab')2 secondary antibodies (Molecular Probes, OR, USA) were used at 1:100 dilution. Cells were also incubated with isotype-matched immunoglobulins and used as negative controls. Hoechst 33342 was used to visualize the nuclei. For immunostaining, cells from passage 5 to 7 or islet-like cell aggregates (ICAs) were fixed in 4% fresh paraformaldehyde, permeabilized using chilled 50% methanol or 0.1% triton, blocked in 4% NDS and incubated overnight with primary antibodies at 4°C. Cells were then washed with calcium-magnesium-containing PBS and incubated with secondary antibodies at 37°C for 1 hour. Cells were washed again and mounted in vectashield mountant containing Hoechst 33342. Confocal images were captured using a Zeiss LSM 510 meta laser scanning microscope using a 63 x 1.3 oil objective with optical slices of 1μ. Magnification, laser, and detector gains were identical across samples, and were set below saturation.
Multilineage differentiation
Cambrex bullet kit from Cambrex Bioscience Inc., Walkersville, MD, USA, was used as per the manufacturer's instructions to differentiate dBM-MSCs into adipogenic, osteogenic, and chondrogenic lineages. At the end of differentiation, cells were fixed using freshly prepared 4% PFA and stained with alizarin, safranin and Oil-Red-O for detection of osteogenesis, chondrogenesis, and adipogenesis respectively.
Preparation of pancreatic extract
After previous informed consent, second trimester human fetal pancreas (n = 3) were collected within 1 hour after medical termination of pregnancy, in accordance with the institutional guidelines for collection and use of human tissues. All procedures were approved by the institutional ethical committee. Fetal pancreases were snap frozen in liquid nitrogen for 2 minutes and homogenized in small volumes of 10X PBS, along with a cocktail of protease inhibitors and 0.1 mM phenylmethylsulfonyl fluoride (Sigma). After centrifugation (15,000 g for 15 minutes at 4°C), the supernatant (extract) was used fresh, or frozen, in liquid nitrogen and stored at -80oC [31]. The protein concentration of the extract was 30 mg/ml (Bradford).
Differentiation of dBM-MSCs into pancreatic lineages
Confluent monolayers of BM-MSCs were trypsinized, seeded on to untreated plastic petri plates with a density of 1.5 x 106 cells per 35 mm2 and exposed to 10 ng/mg of activin A and 10 µM sodium butyrate in serum free medium (DME-Ham's F12 media with 17.5 mM glucose, supplemented with 1.5% BSA and 1XITS (5 mg/l)). These mesenchymal cells migrated overnight to form islet-like cell aggregates (ICAs) within 12 hours of exposure to serum-free medium. After 24 hr, fresh medium was supplemented with 0.3 mM taurine and 1 nM of retinoic acid. On day three of differentiation, 1% pancreatic extract along with 1 μM trichostatin A was added to the medium. After 48 hr, fresh medium was supplemented with 1mM nicotinamide and 50 nM exendin. Clusters were harvested on day 7.
RNA isolation and quantitative real-time PCR
Cell pellets were homogenized and frozen in Trizol (Invitrogen, Carlsbad, CA). RNA was isolated according to the manufacturers' instructions, measured on ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE), and subjected to reverse transcription using a high capacity cDNA archival kit (Applied Biosystems, Foster City, CA). Quantitative real-time PCR (qRT-PCR) was performed using TaqMan based assay-on-demand (AoD) from Applied Biosystems. Target genes were detected with FAM-MGB probes while VIC-MGB 18S were used in duplex/single-plex on a 7500 FAST real time PCR system (Applied Biosystems, Foster City, CA, USA). PCR was performed in 5 μl total volume in 96-well plates using cDNA prepared from 100 ng equivalent total RNA. All qRT-PCR data was normalized to 18S rRNA, carried out in duplex/single-plex reaction to correct for any differences in RNA input in each well.
All values are represented as cycle threshold (Ct) values. Ct values are inversely proportional to the amount of target nucleic acid in the sample (i.e. the lower the Ct level the greater the amount of target nucleic acid in the sample). Samples with a Ct value of 39 and above have no target gene expression. In an ideal PCR reaction, the number of target molecules doubles in each PCR cycle. Therefore, a difference of 1 in the Ct value corresponds to a concentration difference of 2. For comparative purposes, fold difference over the detectable Ct value i.e. < 39, was calculated for each target gene, and was compared with the ICAs generated from the two protocols.
Statistical analysis
Values are expressed as mean ± SEM or median, and interquartile range, from at least three different experiments. Experimental groups were compared using Anova, or t test. Prism5, Graphpad Software, San Diego, was used for analysis.
Results
Characterization of diabetic BM-MSCs during in vitro expansion
Human bone marrow samples from donors were mechanically disrupted to yield single cell suspensions and then grown in adherent culture to generate human bone marrow-derived mesenchymal-like cells (BM-MSCs). Previously, we have shown that umbilical cord blood serum (UCBS) can be used for rapid and long term expansion of hBM-MSCs [30]. In the present study, all bone marrow samples (hBM-MSCs and dBM-MSCs) were cultured in a medium supplemented with 10% UCBS. One week after cultivation, adherent cells could be observed in 57% of the diabetic samples. We observed that adherent cells isolated from diabetic patients proliferated as a fairly homogenous population of mesenchymal-like cells expressing vimentin, nestin, smooth muscle actin, and fibronectin (Figure 1A). dBM-MSCs, in their exponential phase of growth (passage 5 to 7), were immunopositive for CD29 (>91.2 ± 1.73%), CD44 (>86.4 ± 5.76%), CD73 (>86.8 ± 2.9%), CD90 (>82 ± 9.59%), and CD105 (~37.6 ± 15.56%). In this phase, they also contained a subset of cells positive for CD11b (<0.68 ± 0.31%), CD14 (<1.43 ± 0.52%), CD34 (<17.4 ± 8.14%), and CD45 (<26.9 ± 12.92%) (Figure 1B). This data demonstrates that the human bone marrow-derived cells, irrespective of their disease status, represent a highly enriched population of mesenchymal stem-like cells similar to the hBM-MSCs obtained from normal donors [7, 8].
Figure 1. Characterization of dBM-MSCs.
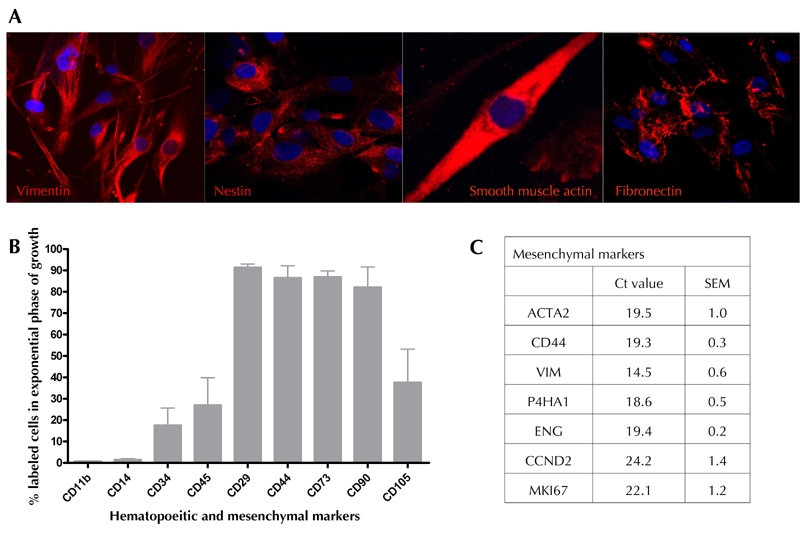
A: dBM-MSCs expressed vimentin, nestin, smooth muscle actin, and fibronectin. B: The expanding population of dBM-MSCs showed increased immunopositivity to mesenchymal (stained with CD29, CD44, CD73, CD90, and CD105) rather than hematopoietic (stained with CD11b, CD14, CD34, and CD45) markers. C: dBM-MSCs also showed abundant mesenchymal transcripts during expansion.
dBM-MSCs also show an abundance of mesenchymal gene transcripts such as ACTA2 (smooth muscle actin), CD44, Vim, P4HA1 (fibroblast surface marker), endoglin, and proliferation markers such as KI67 and CCND2 (Figure 1C). As observed in normal hBM-MSCs, the proliferation of dBM-MSCs was inversely related to the age of the patients [32, 33]. Younger dBM-MSCs (donor age: 15 to 30 yr) could proliferate up to 15 passages, while adult dBM-MSCs (donor age: 30 to 80 yr) could not be passaged for more than 3 times. Thus, dBM-MSCs from older donors had limited proliferative ability. We also observed that the proliferation was directly proportional to the duration of diabetes and that the proliferation ability of dBM-MSCs isolated from chronic and uncontrolled hyperglycemic patients was severely reduced (data not shown).
Multilineage differentiation
dBM-MSCs were exposed to conditions that induce differentiation into adipocytes, chondrocytes, and osteocytes. We observed that 40% of the cells showed uniform differentiation into adipocytes (Figure 2A). Upon exposure to osteogenic media, these cells showed an abundant production of extracellular matrix (ECM) and premature bone nodules (Figure 2B) around day 21 of differentiation. Cells at high density were exposed to chondrogenic media for approximately 30 days. The presence of proteoglycan-rich soft collagen ECM was evident by saffranin staining (Figure 2C).
Figure 2. Multilineage differentiation of dBM-MSCs.
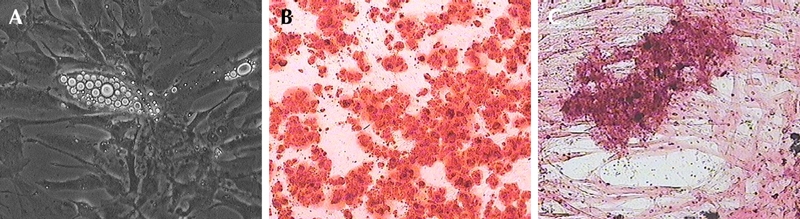
When exposed to lineage-specific media, dBM-MSCs differentiated into adipocytes (A), osteocytes (B), and chondrocytes (C).
Transcript analysis
To study the expanding population of dBM-MSCs, we characterized them on the basis of the abundance of endocrine pancreas-specific gene transcripts by using qRT-PCR. We observed that dBM-MSCs expressed epithelial transcripts (Figure 3A) such as INSR (Insulin receptor), and epithelial cell adhesion molecules such as NCAM-1, ICAM-5, PECAM-1, ITGAV, and E-cadherin (CDh1). dBM-MSCs also expressed few markers found on pancreatic cells such as PCSK1 (pro-hormone convertase, an enzyme required for correct processing of proinsulin in β-cells and pro-glucagon in α-cells) [34, 35], GATA6 (important regulator of endocrine pancreas development) [36], and surface receptors such as CXCR4 [37] and FGFR [38]. Islet-specific transcription factors were also expressed by these cells during the initial phase of expansion (Figure 3B).
Figure 3. Characterization of dBM-MSCs.
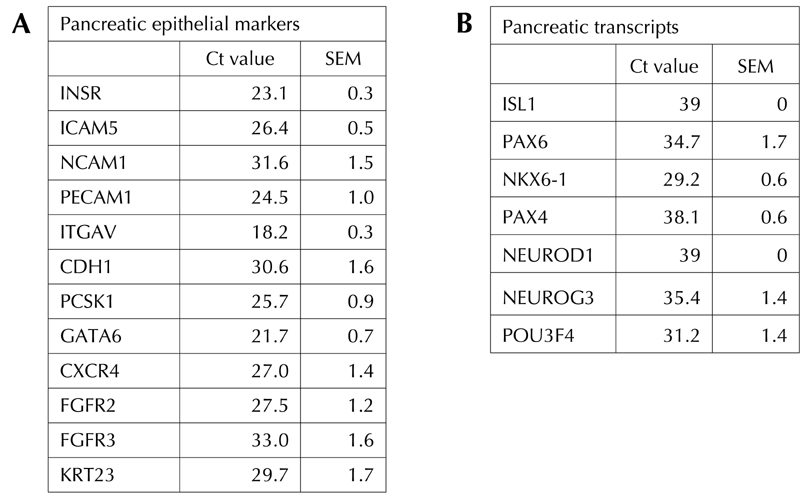
Few of the epithelial transcripts (A) such as INSR, ICAM5, NCAM1, PECAM1, ITGAV, CDH1, PCSK1, GATA6, CXCR4, FGFR2/3, and KRT23, along with pancreatic progenitor transcripts (B) such as PAX6, NKX6.1, NGN3, and POU3F4 are expressed in the undifferentiated adult hBM-MSCs, indicating their potential use as pancreatic progenitors.
POU3F4 (BRN4) is essential for the development of α-cells, whereas NGN3, NKX6.1, and PAX6 are transcription factors important for the development of insulin-producing β-cells. Freshly isolated dBM-MSCs were immunostained for the presence of pancreatic hormones. Surprisingly, we found that 6% (3/54) of the diabetic samples were immunopositive to insulin and C-peptide (Figure 4A, B, and E). These cells also coexpressed glucagon and somatostatin (Figure 4C and D). Real time RT-PCR studies further confirmed the low expression of pancreatic hormones such as proinsulin, glucagon, and somatostatin, along with ISL1 transcript, which was not detected in adult hBM-MSCs (Figure 4F). However, these cells could not be expanded for more than 3 passages. Hence, we were unable to study the differentiation and maturation of dBM-MSCs, which expressed proinsulin in an undifferentiated state. For differentiation studies, we focused on samples that could be obtained in large numbers.
Figure 4.
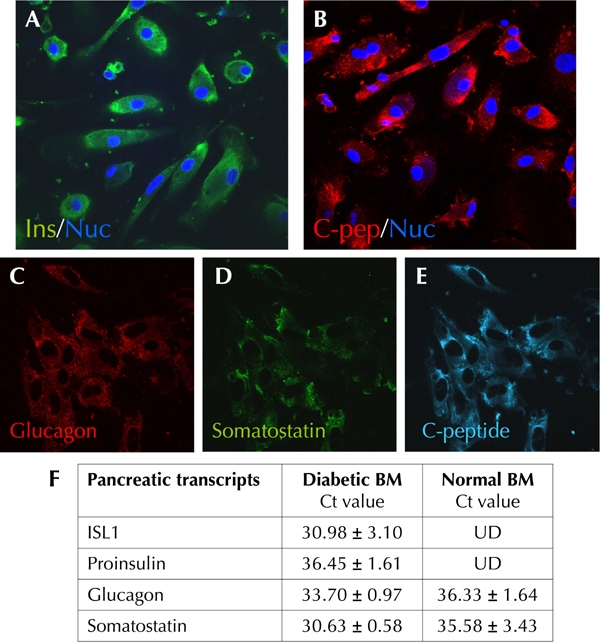
Undifferentiated dBM-MSCs show presence of insulin (A) and C-peptide (B, E) along with glucagon (C) and somatostatin (D). F: qRT-PCR studies compare normal bone marrow and diabetic bone marrow. Freshly isolated dBM-MSCs expresses proinsulin and pancreatic transcription factor ISL1 along with the other pancreatic hormones, which are also expressed in hBM-MSCs. UD: undetected.
In vitro differentiation of dBM-MSCs into pancreatic lineages
Figure 5A represents the pancreatic differentiation protocol. Upon exposure to serum free media, we observed that dBM-MSCs self-assembled to form clusters of cells called islet-like cell clusters (ICAs) (Figure 5A). As seen in Figure 6, these ICAs expressed insulin (Figure 6A) and C-peptide (Figure 6B) by day 7. We also observed that these ICAs expressed pancreatic and duodenal homeobox 1 (Pdx1) (Figure 6D), along with somatostatin (Figure 6C), glucagon (data not shown), glut2 (a β-cell-specific glucose transporter, Figure 6E), and endothelial marker CD31 (Figure 6F).
Figure 5.
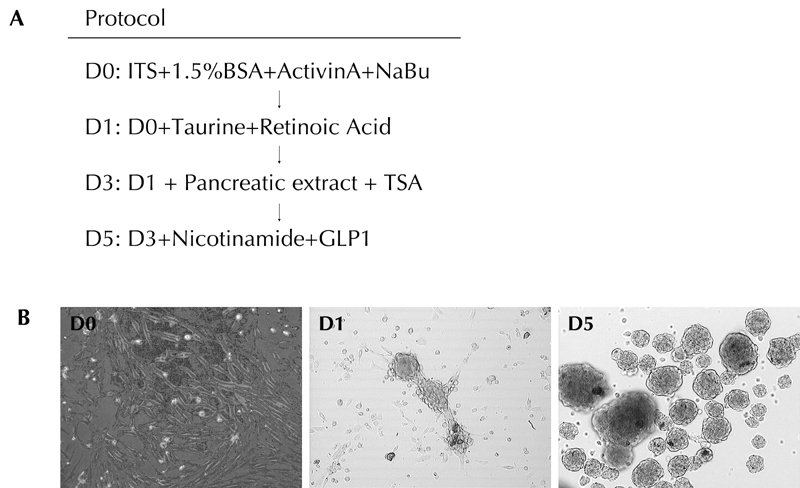
A: Protocol for differentiating dBM-MSCs into mature pancreatic hormone producing cells. B: dBM-MSCs migrate towards each other to form tight clusters called as Islet like cell aggregates (ICAs).
Figure 6. Characterization of ICAs.
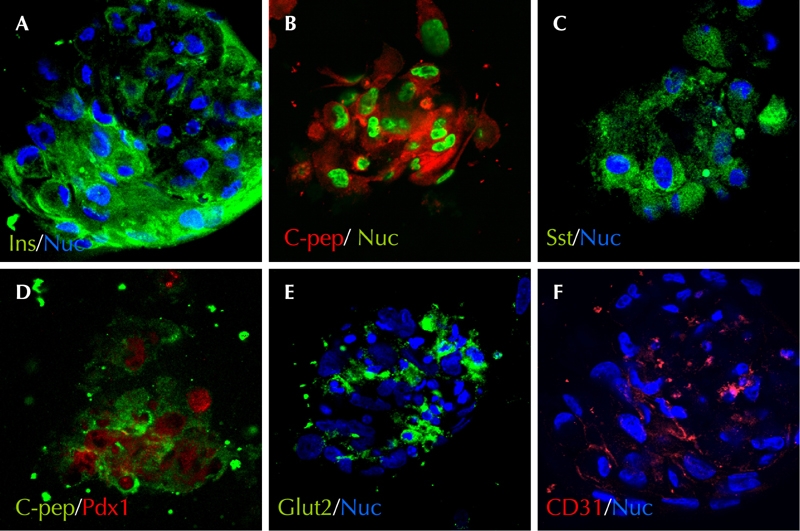
ICAs expressed insulin (A) and C-peptide (B, D). Somatostatin- (C) and Pdx1-expressing cells (D) were also present within the ICAs. Few cells from the ICAs also expressed Glut2 (E) and the endothelial marker CD31 (F).
Then, we compared the gene expression data of undifferentiated dBM-MSCs with that of ICAs. A significant increase in pancreas-specific transcripts was observed in the ICAs (Figure 7). The expression of PDX1, NGN3, and ISL1 in ICAs was 1000-fold higher than that observed in undifferentiated dBM-MSCs. In ICAs, an approximately 350-fold increase in the proinsulin transcript level was observed. There were also significant differences in the abundance of pro-glucagon and somatostatin transcripts in ICAs, as compared with undifferentiated dBM-MSCs (Figure 7). Thus, the qRT-PCR result indicates that dBM-MSCs transformed from a mesenchymal into a pancreatic epithelial lineage.
Figure 7. dBM-MSC-derived ICAs exhibit pancreatic transcription factors.
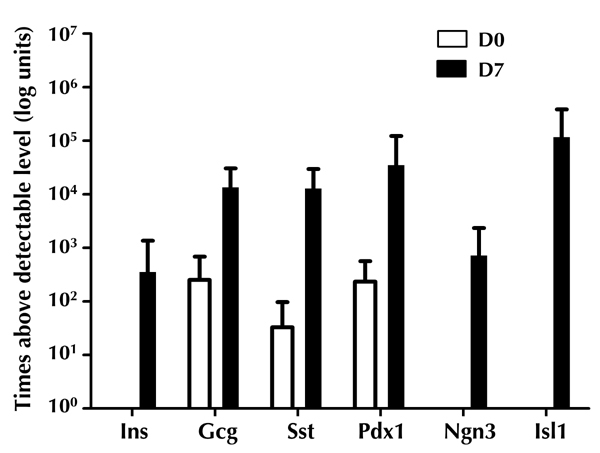
Abundance of proinsulin, proglucagon, somatostatin, PDX1, NGN3, and ISL1 transcripts were detectable in the ICAs.
Discussion and conclusions
In this study, we have investigated the characteristics of dBM-MSCs derived from diabetic patients. We successfully cultured 57% of diabetic samples. Many of the dBM-MSC samples derived from elderly chronic diabetic patients (>50 yr) were unable to expand and proliferate in vitro. dBM-MSCs derived from young adults (15 to 30 yr) displayed fibroblast-like morphology, and could be passaged up to 15 times. Flow cytometry studies showed that these cells express mesenchymal markers such as CD29, CD44, CD73, CD90, and CD105, along with a small subset of cells that were positive for hematopoietic markers such as CD11b, CD14, CD34, and CD45. These cells could also differentiate into adipocytes, chondrocytes, and osteocytes, indicating their unaltered multi-differentiation potential. We observed that undifferentiated dBM-MSCs, during the initial phase of growth, had low expression of islet-specific transcription factors (NKX6.1, PAX6, ISL1, BRN-4 (POU3F4), and GCG).
Kojima et al. made the surprising observation that hyperglycemia, with or without established diabetes, activated insulin gene transcription and proinsulin production in multiple extrapancreatic, extrathymic tissues [18]. Interestingly, we also observed that dBM-MSCs expressed C-peptide and insulin. A study by Oh et al. found that the incubation of isolated rat BM-MSCs in a high glucose medium induced insulin gene expression in about fifty percent of the cells within a week [19]. Glucose infusion in rats during a period of only 24 h increased the β-cell number by 50% [39]. However, unlike in β-cells, dBM-MSCs exclusively produced proinsulin and very little mature insulin, and did not significantly contribute to insulin production in vivo. Although high glucose concentration induces proinsulin transcription, it also stimulates the secretion of cytokines such as interleukin (IL)-1, which are known to cause β-cell apoptosis in vitro and in vivo [40, 41]. Kojima et al. observed that along with proinsulin, dBM-MSCs from diabetic rats also expressed TNF-alpha, a pro-inflammatory cytokine [42]. The authors hypothesized that these cells may mediate the ill effects of hyperglycemia, and may contribute to chronic diabetic complications such as diabetic neuropathy. In another study, Kume et al. reported that advanced glycation end-products (AGEs), which accumulate on long-lived proteins of various tissues in advanced age and diabetes mellitus, inhibit the differentiation potential of BM-MSCs in vitro [43].
Microenviromental niche in vivo governs cell and tissue homeostasis and plays a pivotal role in cell fate determination. Any disturbance in tissue homeostasis resulting from disease conditions affects the cells of the concerned tissue, and all the cells of the body. Ulrich et al. demonstrated differential growth patterns in the fibroblast cultures derived from skin biopsies of patients with rheumatoid arthritis, vitiligo, and schizophrenia. The patterns mirror the disease status in their proliferative potential [44]. The presence of insulin transcripts in the diabetic bone marrow that we observed in the present study probably reflects the disease status similar to those reported by Ulrich et al. Although the amount of proinsulin produced by the bone marrow cells exposed to hyperglycemia in vivo was extremely small, the appearance of proinsulin-producing cells outside the pancreas represents the body’s futile attempt to reverse hyperglycemia. In those samples that were unable to expand more than 3 passages we detected proinsulin transcripts in only 6%. Chronic exposure to hyperglycemia may be responsible for the reduced potential of these dBM-MSCs. Consequently, this characteristic expression of proinsulin in dBM-MSCs could be of therapeutic significance for identifying the state of diabetes in the patient. However, it precludes the use of dBM-MSCs for autologous stem cell therapy in diabetic individuals. These cells appear to be terminally differentiated, thus leading to a loss of stemness and failure of further propagation. In fact, these observations prompted us to investigate the influence of such microenvironmental milieu on dBM-MSC differentiation into islet-like aggregates.
Tissue extracts are known to mimic the tissue-specific microenvironment and to act as a natural inducer in promoting the differentiation of bone marrow stromal cells to the target lineage [45-47]. Based on this consideration, we used injured fetal pancreatic extract along with a high glucose containing medium in our differentiation protocol to imitate the in vivo hyperglycemic diabetic milieu. A significant increase in the pancreatic transcription factors within the ICAs and the expression of proinsulin transcript demonstrated that dBM-MSCs could be induced to differentiate into the pancreatic lineage. The presence of insulin as well as C-peptide indicated that these cells could synthesize, secrete, and process insulin in vitro. The exact mechanism by which the hyperglycemic and pancreatic cell extract-mediated reprogramming or differentiation occurs is not yet understood. However, our results suggest that analyzing the composition of the pancreatic extract and the diabetic status of the donors could indicate the relatively efficient and reproducible means of differentiating diabetic BM-MSCs into ICAs in vitro.
In conclusion, our study shows that the biological characteristics of dBM-MSCs derived from diabetic patients' bone marrow are similar to hBM-MSCs in phenotype, morphology, and multilineage differentiation potential. The present data also shows the impact of diabetic environment in vivo, as indicated by the presence of proinsulin and ISL1 transcripts in a few of the dBM-MSCs. Our data shows that MSCs derived from healthy donors are suitable for cell replacement therapy in diabetes, advocating allogeneic ex vivo expanded BM-MSCs as a source to trigger islet generation. The technique to demonstrate the effect of in vivo microenvironment on in vitro differentiation of BM-MSCs into the desired target organ may be applicable in other disease conditions.
Disclosures: The authors report no conflict of interests.
Acknowledgments
The authors thank Dr. Ashutosh Hardikar, Cardiothorasic Surgery Unit, DM Hospital, Pune and Dr. V. Kulkarni, Om laboratories, Pune, for their assistance in providing the marrow samples; and Dr. Satish Patki, Patki Hospital, Kolhapur for providing fetal pancreas. The authors wish to thank the Directors of NCCS and ARI for all the support. This project was supported through project grant to RRB, SMG and AAH from Department of Biotechnology, Government of India. SMP was supported by a senior research fellowship from the Council of Scientific and Industrial Research, New Delhi.
References
- 1.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 2.Li L, Xie T. Stem cell niche: structure and function. Annu Rev Cell Dev Biol. 2005;21:605–631. doi: 10.1146/annurev.cellbio.21.012704.131525. [DOI] [PubMed] [Google Scholar]
- 3.Gregory CA, Ylostalo J, Prockop DJ. Adult bone marrow stem/progenitor cells (MSCs) are preconditioned by microenvironmental "niches" in culture: a two-stage hypothesis for regulation of MSC fate. Sci STKE. 2005;2005(294):pe37. doi: 10.1126/stke.2942005pe37. [DOI] [PubMed] [Google Scholar]
- 4.Morrison SJ, Kimble J. Asymmetric and symmetric stem-cell divisions in development and cancer. Nature. 2006;441(7097):1068–1074. doi: 10.1038/nature04956. [DOI] [PubMed] [Google Scholar]
- 5.Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441(7097):1075–1079. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- 6.Li L, Neaves WB. Normal stem cells and cancer stem cells: the niche matters. Cancer Res. 2006;66(9):4553–4557. doi: 10.1158/0008-5472.CAN-05-3986. [DOI] [PubMed] [Google Scholar]
- 7.Flynn CM, Kaufman DS. Donor cell leukemia: insight into cancer stem cells and the stem cell niche. Blood. 2007;109(7):2688–2692. doi: 10.1182/blood-2006-07-021980. [DOI] [PubMed] [Google Scholar]
- 8.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mensenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 9.Moriscot C, de Fraipont F, Richard MJ, Marchand M, Savatier P, Bosco D, Favrot M, Benhamou PY. Human bone marrow mesenchymal stem cells can express insulin and key transcription factors of the endocrine pancreas developmental pathway upon genetic and/or microenvironmental manipulation in vitro. Stem Cells. 2005;23(4):594–603. doi: 10.1634/stemcells.2004-0123. [DOI] [PubMed] [Google Scholar]
- 10.Muller I, Lymperi S, Dazzi F. Mesenchymal stem cell therapy for degenerative inflammatory disorders. Curr Opin Organ Transplant. 2008;13(6):639–644. doi: 10.1097/MOT.0b013e328317a462. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Z, Wang X, Wang S. Isolation and characterization of mesenchymal stem cells derived from bone marrow of patients with Parkinson's disease. In Vitro Cell Dev Biol Anim. 2008;44(5-6):169–177. doi: 10.1007/s11626-008-9093-1. [DOI] [PubMed] [Google Scholar]
- 12.Ferrero I, Mazzini L, Rustichelli D, Gunetti M, Mareschi K, Testa L, Nasuelli N, Oggioni GD, Fagioli F. Bone marrow mesenchymal stem cells from healthy donors and sporadic amyotrophic lateral sclerosis patients. Cell Transplant. 2008;17(3):255–266. doi: 10.3727/096368908784153940. [DOI] [PubMed] [Google Scholar]
- 13.Zhao ZG, Liang Y, Li K, Li WM, Li QB, Chen ZC, Zou P. Phenotypic and functional comparison of mesenchymal stem cells derived from the bone marrow of normal adults and patients with hematologic malignant diseases. Stem Cells Dev. 2007;16(4):637–648. doi: 10.1089/scd.2007.0008. [DOI] [PubMed] [Google Scholar]
- 14.Drewa T, Joachimiak R, Kaznica A, Flisinski M, Brymora A, Manitius J. Bone marrow progenitors from animals with chronic renal failure lack capacity of in vitro proliferation. Transplant Proc. 2008;40(5):1668–1673. doi: 10.1016/j.transproceed.2008.03.141. [DOI] [PubMed] [Google Scholar]
- 15.Lopez-Villar O, Garcia JL, Sanchez-Guijo FM, Robledo C, Villaron EM, Hernandez-Campo P, Lopez-Holgado N, Diez-Campelo M, Barbado MV, Perez-Simon JA. et al. Both expanded and uncultured mesenchymal stem cells from MDS patients are genomically abnormal, showing a specific genetic profile for the 5q- syndrome. Leukemia. 2009;23(4):664–672. doi: 10.1038/leu.2008.361. [DOI] [PubMed] [Google Scholar]
- 16.Garayoa M, Garcia JL, Santamaria C, Garcia-Gomez A, Blanco JF, Pandiella A, Hernandez JM, Sanchez-Guijo FM, del Canizo MC, Gutierrez NC, San Miguel JF. Mesenchymal stem cells from multiple myeloma patients display distinct genomic profile as compared with those from normal donors. Leukemia. 2009;23(8):1515–1527. doi: 10.1038/leu.2009.65. [DOI] [PubMed] [Google Scholar]
- 17.Kastrinaki MC, Sidiropoulos P, Roche S, Ringe J, Lehmann S, Kritikos H, Vlahava VM, Delorme B, Eliopoulos GD, Jorgensen C. et al. Functional, molecular and proteomic characterisation of bone marrow mesenchymal stem cells in rheumatoid arthritis. Ann Rheum Dis. 2008;67(6):741–749. doi: 10.1136/ard.2007.076174. [DOI] [PubMed] [Google Scholar]
- 18.Banerjee M, Kumar A, Bhonde RR. Reversal of experimental diabetes by multiple bone marrow transplantation. Biochem Biophys Res Commun. 2005;328(1):318–325. doi: 10.1016/j.bbrc.2004.12.176. [DOI] [PubMed] [Google Scholar]
- 19.Kojima H, Fujimiya M, Matsumura K, Nakahara T, Hara M, Chan L. Extrapancreatic insulin-producing cells in multiple organs in diabetes. Proc Natl Acad Sci USA. 2004;101(8):2458–2463. doi: 10.1073/pnas.0308690100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oh SH, Muzzonigro TM, Bae SH, LaPlante JM, Hatch HM, Petersen BE. Adult bone marrow-derived cells trans-differentiating into insulin-producing cells for the treatment of type I diabetes. Lab Invest. 2004;84(5):607–617. doi: 10.1038/labinvest.3700074. [DOI] [PubMed] [Google Scholar]
- 21.Chang C, Niu D, Zhou H, Li F, Gong F. Mesenchymal stem cells contribute to insulin-producing cells upon microenvironmental manipulation in vitro. Transplant Proc. 2007;39(10):3363–3368. doi: 10.1016/j.transproceed.2007.09.026. [DOI] [PubMed] [Google Scholar]
- 22.Chang C, Niu D, Zhou H, Zhang Y, Li F, Gong F. Mesenchymal stroma cells improve hyperglycemia and insulin deficiency in the diabetic porcine pancreatic microenvironment. Cytotherapy. 2008;10(8):796–805. doi: 10.1080/14653240802461924. [DOI] [PubMed] [Google Scholar]
- 23.Chang C, Wang X, Niu D, Zhang Z, Zhao H, Gong F. Mesenchymal stem cells adopt beta-cell fate upon diabetic pancreatic microenvironment. Pancreas. 2009;38(3):275–281. doi: 10.1097/MPA.0b013e318191521c. [DOI] [PubMed] [Google Scholar]
- 24.Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276(5309):60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 25.Bonner-Weir S, Trent DF, Weir GC. Partial pancreatectomy in the rat and subsequent defect in glucose-induced insulin release. J Clin Invest. 1983;71(6):1544–1553. doi: 10.1172/JCI110910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonner-Weir S, Baxter LA, Schuppin GT, Smith FE. A second pathway for regeneration of adult exocrine and endocrine pancreas. A possible recapitulation of embryonic development. Diabetes. 1993;42(12):1715–1720. doi: 10.2337/diab.42.12.1715. [DOI] [PubMed] [Google Scholar]
- 27.Hardikar AA, Bhonde RR. Modulating experimental diabetes by treatment with cytosolic extract from the regenerating pancreas. Diabetes Res Clin Pract. 1999;46(3):203–211. doi: 10.1016/s0168-8227(99)00098-4. [DOI] [PubMed] [Google Scholar]
- 28.Hardikar AA, Karandikar MS, Bhonde RR. Effect of partial pancreatectomy on diabetic status in BALB/c mice. J Endocrinol. 1999;162(2):189–195. doi: 10.1677/joe.0.1620189. [DOI] [PubMed] [Google Scholar]
- 29.Choi KS, Shin JS, Lee JJ, Kim YS, Kim SB, Kim CW. In vitro trans-differentiation of rat mesenchymal cells into insulin-producing cells by rat pancreatic extract. Biochem Biophys Res Commun. 2005;330(4):1299–1305. doi: 10.1016/j.bbrc.2005.03.111. [DOI] [PubMed] [Google Scholar]
- 30.Xu YX, Chen L, Hou WK, Lin P, Sun L, Sun Y, Dong QY, Liu JB, Fu YL. Mesenchymal stem cells treated with rat pancreatic extract secrete cytokines that improve the glycometabolism of diabetic rats. Transplant Proc. 2009;41(5):1878–1884. doi: 10.1016/j.transproceed.2009.01.087. [DOI] [PubMed] [Google Scholar]
- 31.Phadnis SM, Joglekar MV, Venkateshan V, Ghaskadbi SM, Hardikar AA, Bhonde RR. Human umbilical cord blood serum promotes growth, proliferation, as well as differentiation of human bone marrow-derived progenitor cells. In Vitro Cell Dev Biol Anim. 2006;42(10):283–286. doi: 10.1290/0512087.1. [DOI] [PubMed] [Google Scholar]
- 32.Hakelien AM, Gaustad KG, Collas P. Transient alteration of cell fate using a nuclear and cytoplasmic extract of an insulinoma cell line. Biochem Biophys Res Commun. 2004;316(3):834–841. doi: 10.1016/j.bbrc.2004.02.127. [DOI] [PubMed] [Google Scholar]
- 33.D’Ippolito G, Diabira S, Howard GA, Menei P, Roos BA, Schiller PC. Marrow-isolated adult multilineage inducible (MIAMI) cells, a unique population of postnatal young and old human cells with extensive expansion and differentiation potential. J Cell Sci. 2004;117(Pt 14):2971–2981. doi: 10.1242/jcs.01103. [DOI] [PubMed] [Google Scholar]
- 34.Huang K, Zhou DH, Huang SL, Liang SH. Age-related biological characteristics of human bone marrow mesenchymal stem cells from different age donors. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2005;13(6):1049–1053. [PubMed] [Google Scholar]
- 35.Drucker DJ, Jin T, Asa SL, Young TA, Brubaker PL. Activation of proglucagon gene transcription by protein kinase-A in a novel mouse enteroendocrine cell line. Mol Endocrinol. 1994;8(12):1646–1655. doi: 10.1210/mend.8.12.7535893. [DOI] [PubMed] [Google Scholar]
- 36.Wideman RD, Covey SD, Webb GC, Drucker DJ, Kieffer TJ. A switch from prohormone convertase (PC)-2 to PC1/3 expression in transplanted alpha-cells is accompanied by differential processing of proglucagon and improved glucose homeostasis in mice. Diabetes. 2007;56(11):2744–2752. doi: 10.2337/db07-0563. [DOI] [PubMed] [Google Scholar]
- 37.Decker K, Goldman DC, Grasch CL, Sussel L. Gata6 is an important regulator of mouse pancreas development. Dev Biol. 2006;298(2):415–429. doi: 10.1016/j.ydbio.2006.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sordi V, Malosio ML, Marchesi F, Mercalli A, Melzi R, Giordano T, Belmonte N, Ferrari G, Leone BE, Bertuzzi F. et al. Bone marrow mesenchymal stem cells express a restricted set of functionally active chemokine receptors capable of promoting migration to pancreatic islets. Blood. 2005;106(2):419–427. doi: 10.1182/blood-2004-09-3507. [DOI] [PubMed] [Google Scholar]
- 39.Hardikar AA, Marcus-Samuels B, Geras-Raaka E, Raaka BM, Gershengorn MC. Human pancreatic precursor cells secrete FGF2 to stimulate clustering into hormone-expressing islet-like cell aggregates. Proc Natl Acad Sci USA. 2003;100(12):7117–7122. doi: 10.1073/pnas.1232230100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bernard C, Berthault MF, Saulnier C, Ktorza A. Neogenesis vs. apoptosis As main components of pancreatic beta cell ass changes in glucose-infused normal and mildly diabetic adult rats. FASEB J. 1999;13(10):1195–1205. doi: 10.1096/fasebj.13.10.1195. [DOI] [PubMed] [Google Scholar]
- 41.Donath MY, Gross DJ, Cerasi E, Kaiser N. Hyperglycemia-induced beta-cell apoptosis in pancreatic islets of Psammomys obesus during development of diabetes. Diabetes. 1999;48(4):738–744. doi: 10.2337/diabetes.48.4.738. [DOI] [PubMed] [Google Scholar]
- 42.Kaiser N, Leibowitz G, Nesher R. Glucotoxicity and beta-cell failure in type 2 diabetes mellitus. J Pediatr Endocrinol Metab. 2003;16(1):5–22. doi: 10.1515/jpem.2003.16.1.5. [DOI] [PubMed] [Google Scholar]
- 43.Terashima T, Kojima H, Fujimiya M, Matsumura K, Oi J, Hara M, Kashiwagi A, Kimura H, Yasuda H, Chan L. The fusion of bone-marrow-derived proinsulin-expressing cells with nerve cells underlies diabetic neuropathy. Proc Natl Acad Sci USA. 2005;102(35):12525–12530. doi: 10.1073/pnas.0505717102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kume S, Kato S, Yamagishi S, Inagaki Y, Ueda S, Arima N, Okawa T, Kojiro M, Nagata K. Advanced glycation end-products attenuate human mesenchymal stem cells and prevent cognate differentiation into adipose tissue, cartilage, and bone. J Bone Miner Res. 2005;20(9):1647–1658. doi: 10.1359/JBMR.050514. [DOI] [PubMed] [Google Scholar]
- 45.Ulrich-Merzenich G, Bhonde RR. Donor site, age and health affect fibroblast growth in culture. In Vitro Cell Dev Biol Anim. 1995;31(7):494–496. doi: 10.1007/BF02634025. [DOI] [PubMed] [Google Scholar]
- 46.Liu XN, Yin Q, Zhang H, Zhu SJ, Wei YJ, Hu SS. Tissue extracts from infarcted myocardium of rats in promoting the differentiation of bone marrow stromal cells into cardiomyocyte-like cells. Biomed Environ Sci. 2008;21(2):110–117. doi: 10.1016/S0895-3988(08)60015-X. [DOI] [PubMed] [Google Scholar]
- 47.Liu YX, Ji L, Yue W, Yan ZF, Wang J, Xi JF, Zhang R, Nan X, Bai CX, Chen L, Wang YF, Pei XT. Cells extract from fetal liver promotes the hematopoietic differentiation of human embryonic stem cells. Cloning Stem Cells. 2009;11(1):51–60. doi: 10.1089/clo.2008.0049. [DOI] [PubMed] [Google Scholar]
- 48.Li W, He H, Chen YT, Hayashida Y, Tseng SC. Reversal of myofibroblasts by amniotic membrane stromal extract. J Cell Physiol. 2008;215(3):657–664. doi: 10.1002/jcp.21345. [DOI] [PMC free article] [PubMed] [Google Scholar]


