Abstract
Inhibitors of the insulin-like growth factor-1 receptor (IGF-IR) have been widely studied for their ability to enhance the killing of a variety of malignant cells, but the role of IGF-I and its receptor in the differential protection of host and cancer cells against chemotherapy is unknown. We previously showed that starvation protects mice but not cancer cells against high dose chemotherapy (Differential Stress Resistance, DSR). Here we provide evidence for the role of IGF-I reduction in mediating the effect of starvation in DSR. A 72-hour fast reduced circulating IGF-I by 70% and increased the level of the IGF-I inhibitor IGFBP-1 by 11-fold in mice. LID mice, with a 70–80% reduction in circulating IGF-I levels, were protected against 3 out of 4 chemotherapy drugs tested. Restoration of IGF-I during fasting was sufficient to reverse its protective effect. 60% of melanoma-bearing LID mice treated with doxorubicin reached long-term survival whereas all control mice died of either metastases or chemo toxicity. Reduction of IGF-I/IGF-I signaling protected primary glia, but not glioma cells against cyclophosphamide and protected mouse embryonic fibroblasts (MEFs) against doxorubicin-induced DNA damage. Similarly, S. cerevisiae lacking homologues of IGF-I signaling proteins displayed protection against chemotherapy-dependent DNA damage, which was reversed by expression of an oncogene homolog. We conclude that reducing circulating IGF-I protects normal cells and mice against chemotherapy-dependent DNA damage by a mechanism that involves down-regulation of proto-oncoproteins.
Introduction
Most chemotherapy agents cause considerable damage to normal cells, leading to toxicity which is dose limiting and causes both short- and long-term side effects in patients. Although drug development has reduced these side effects with a succession of selective anti-tumor agents such as antibodies that target specific antigens on cancer or agents with a wider therapeutic index, toxicity continues to limit cancer treatment. Thus, interventions that reduce the undesired toxic side-effects could increase the efficacy of many chemotherapy drugs. Chemoprotectants such as amifostine, glutathione, mesna, and dexrazoxane have been investigated and shown to provide drug-dependent protection to specific tissues, but the use of these compounds has not been shown to increase disease-free or overall survival 1. Recently, we reported that short-term starvation (STS) differentially protects normal but not, or much less, malignant cells, leading to improved survival 2. Here we present evidence that reduced IGF-I is a major mediator of STS-dependent differential protection.
Biogerontologists have long known that calorie restriction and/or deficiencies in the pro-growth GH/IGF-I axis increase stress resistance and lifespan, and also share many physiological characteristics in various model organisms 3. These beneficial effects can be explained, in part, by the active diversion of energy utilization in starved or IGF-I deficient organisms. The finite energy source of an organism is finely balanced between growth and maintenance under normal conditions 4. However, under challenging conditions such as starvation, the energy is diverted from growth to maintenance, thereby enhancing protection and survival at the price of growth 5.
During starvation, several changes in the GH/IGF-I axis occur as a result of physiological adaptation to the new environment. Generally, growth hormone (GH) directly regulates the production of IGF-I, which is the major mediator of the growth effects of GH 6. In humans, IGF-I levels decrease dramatically in response to short-term starvation (36–120 hours) despite increased GH secretion 7, 8. Long-lived organisms that are deficient in IGF-I signaling have been shown to be resistant to multiple types of stress 9, 10. However, unlike normal cells, cancer cells are self-sufficient in growth signals and insensitive to growth inhibitory signals 11. Self-sufficiency in growth signals is often enabled by gain-of-function mutations in oncogenes (e.g. IGF-IR or its downstream Ras, Akt, mTor, etc) that result in constitutive activation of proliferation pathways independently or partially independently of external growth factor level. Notably, in normal cells, the RAS/RAF/MAPK and the PTEN/PI3K/AKT pathways can be down-regulated by CR or starvation 12, possibly mediated by reduced IGF-I. On the other hand, insensitivity to growth inhibitory signals is due to loss-of-function mutations in tumor-suppressor genes (e.g. Rb, p53, PTEN, etc), enabling cancer cells to disregard anti-proliferation signals 11, 13. Here we test the hypothesis that the reduction of circulating IGF-I and its signaling mediates the protection of normal cells and mice against chemotherapy toxicity, whereas oncogene-bearing cancer cells do not respond to reduced IGF-I.
Materials and Methods
Stress resistance against chemotherapy treatments in LID mice
LID mice of 75–100 weeks of age were used to model human cancer onset. Since liver is the major source of IGF-I production, mice with a conditional hepatic igf1 gene knockout have reduced circulating IGF-I levels by 80% 40. Because albumin is expressed in the liver after 10 days of birth, resulting in liver igf1 gene deletion, LID mice do not experience early death, growth retardation, or developmental defects like the igf1 gene knock-out (igf1−/−) mice 39. LID and its control mice were given 100 mg/kg etoposide intravenously. CP was given at 500 mg/kg. CP was dissolved in saline at 40 mg/ml and injected intraperitoneally. 5-Fluorouracil (5-FU, Sigma) was injected at 400 mg/kg intraperitoneally. Doxorubicin (DXR, Sigma) was prepared at 5 mg/ml in saline and injected intravenously first at 20 mg/kg and 22 days later at 28 mg/kg. All drugs have been selected from different categories. All mice were monitored daily for weight loss and signs of pain and stress. Mice determined terminally moribund were euthanized by CO2 narcosis and necropsy was performed. Experiments were performed in accordance with Institutional Animal Care and Use Committee (University of Southern California, Los Angeles, CA) and the National Institutes of Health guidelines.
Differential stress resistance against DXR in LID mice
In order to study differential stress resistance, mice were injected with highly metastatic melanoma cells. LID and its control mice of ages 75–100 weeks were used. B16Fluc melanoma cells were a generous gift of Dr. Noah Craft at UCLA. B16Fluc cells are derivatives of B16 cells but produce light by stable transfection of the Firefly luciferase gene driven by the CMV promoter 43. Prior to injection, cells were washed and resuspended in sterile saline. Each mouse received 2×105 cells in 100 μl saline, followed by another 100 μl of sterile saline to wash off remaining cells in the tails. 3 days after tumor inoculation, the first DXR (Bedford Laboratories) injections were given at 16 mg/kg. 2 weeks following the initial DXR administration, the second DXR injection was given at 12 mg/kg. Mice were observed daily for signs of stress or pain and body weight was recorded. Mice determined terminally moribund were sacrificed by CO2 narcosis and necropsy was performed. The heart was collected for further histological examination.
Results
Short-term starvation regulates components of the pro-growth GH/IGF-I axis
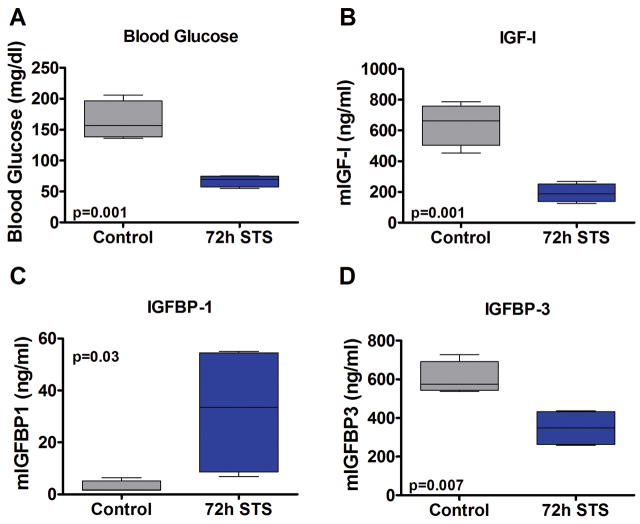
To investigate the role of the GH/IGF-I axis in the beneficial effects of short-term starvation (STS) on differential stress resistance (DSR), we started by measuring the level of circulating GH, IGF-I and its binding proteins IGFBP-1 and -3 in mice undergoing STS. CD-1 mice were fasted for 72 hours and blood was collected to measure glucose levels and plasma GH, IGF-I, and IGFBP-1 and -3 levels. After a 72-hour STS, mice had lost approximately 20% of body weight, glucose levels were reduced by 41%, GH levels were slightly increased, IGF-I levels decreased 70% (Fig. 1A,B; Fig. S1A,B). The bioavailability of IGF-I, which can activate IGF-I receptors (IGF-IR), is regulated by IGF binding proteins. In fasted mice, the level of IGFBP-1, which normally reduces IGF-I signaling, increased 11.4-fold (Fig. 1C). These results are in agreement with the reports showing that IGFBP-I increases in response to fasting in humans and rats 14–16, and also that its overexpression in mice effectively retards growth by sequestering IGF-I 17. Furthermore, the 72-hour fast decreased IGFBP-3 levels by 42% (Fig. 1D) in agreement with reports in short-term fasted humans and rats 16, 18. However, the mechanistic explanation for the decrease in IGFBP-3 is not clear.
Figure 1.
The effect of 72 hour fasting on glucose levels, IGF-I, and IGFBP-1/3. 30 week old CD-1 mice were fasted for 72 hours and sacrificed. Blood was collected via cardiac puncture under anesthesia, and blood glucose was measured immediately. Plasma IGF-1 and IGFBP-1/3 levels were measured by a mouse-specific in-house ELISA. All P values were calculated by Student’s t-test except for IGFBP-1 which was done by the Mann-Whitney U test.
To test if restoring the level of IGF-I during STS reverses the protection against chemotherapy toxicity, CD-1 mice underwent a 48-hour STS with IGF-I (200 μg/kg) administration every 12 hours. The level of injected IGF-I was determined from prior serum IGF-I measurements of ad lib fed mice. Following the STS/IGF-I treatments, mice were intravenously injected with 16 mg/kg doxorubicin (DXR), a widely used chemotherapy drug acting as an intercalating agent and topoisomerase II inhibitor 19. Indeed, the restoration of IGF-I during STS abolished the protective effect of STS on DXR toxicity, resulting in a 100% vs. 38% survival in the STS and STS/IGF-I group, respectively (Fig. S2).
Previously, we showed that primary glia but not glioma cell lines pre-incubated with low glucose (50 mg/dl compared to the normal 100 mg/dl) and low serum (1% fetal bovine serum with the consequent reduction of several growth factors including IGF-I) showed enhanced protection against the alkylating chemotherapy agent cyclophosphamide 2. The glucose levels of fasted mice were reduced to a similar level, along with a dramatic decrease in IGF-I levels (Fig. 1A,B). Therefore, the reduction of IGF-I, a potent growth factor, may mediate part of the effect of fasting on DSR.
Reduced IGF-I signaling protects primary glia but not glioma cells against high-dose cyclophosphamide
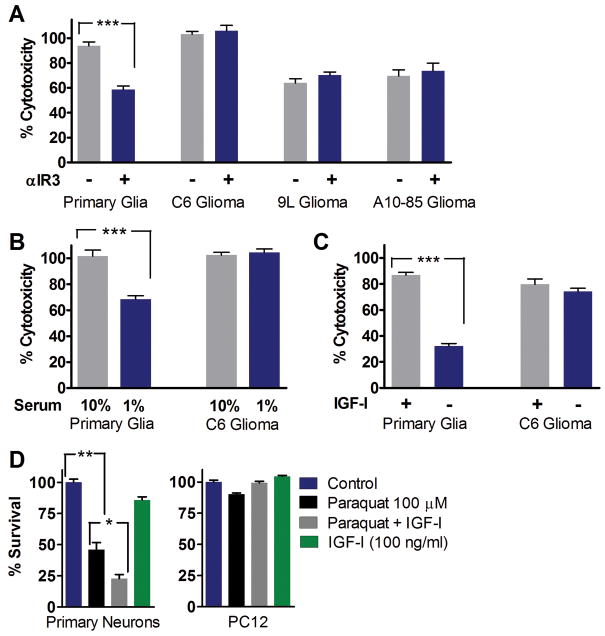
IGF-I-like signaling pathways are implicated in regulating life span and stress resistance in organisms ranging from the simple yeast to worms, flies, and mice 3, 20–22. To test the role of IGF-I signaling in DSR against chemotherapeutic drugs in vitro, we incubated normal and the equivalent cancer cell lines with either an IGF-I receptor (IGF-IR) blocking antibody, low serum concentrations, or excess IGF-I prior to treatment with cyclophosphamide (CP), a commonly used chemo drug based on its DNA alkylating properties 23. Primary mixed rat glia (astrocytes + 5–10% microglia) and 3 different rat glioma cell lines (C6, A10–85 and 9L) were tested. All cells were grown to confluence to minimize differences in proliferation rate. Pre-incubation with an antagonistic IGF-IR antibody (αIR3) protected primary glia but not the three glioma cell lines against CP toxicity (Fig. 2A). Reduction of serum level from the standard 10% to 1%, with consequent reduction of growth factors including IGF-I, decreased the toxicity of 15 mg/ml CP to primary glia but not to C6 glioma cells (Fig. 2B). On the other hand, pre-incubation with 100ng/ml IGF-I (in the low normal range for adult human serum) 24 caused a 3-fold increase in the toxicity of CP to primary mixed glia but did not increase the toxicity of CP to C6 glioma cells (Fig. 2C). Similar results were obtained with primary neurons and neuron-like pheochromocytoma cells (PC12) treated with a combination of IGF-I and the oxidative stress agent paraquat (Fig. 2D). These results are consistent with our previous studies on fasting and DSR 2 and support the hypothesis that down-regulation of IGF-I signaling can protect normal but not cancer cells against cytotoxic agents.
Figure 2.
in vitro DSR to CP treatments by reducing IGF-I. Primary rat glial cells and rat glioma cell lines (C6, 9L, and A10–85) cell lines were tested. (A) Cells were pre-incubated in DMEM/F12 with 1% serum and neutralizing anti-IGF-IR monoclonal antibody alpha-IR3 (1 ug/ml) for 24 hours (15 mg/ml; n=12). (B) Cells were pre-incubated in medium with either 1% (STS) or 10% FBS for 24 hours (15 mg/ml; n=12). (C) Cells were pre-incubated in medium with 1% serum with or without rhIGF-I (100ng/ml) for 48 hours (12 mg/ml; n=21). (D) The effect of IGF-I on DSR against oxidative stress. Chemotherapy drugs such as etoposide, cyclophosphamide, 5-fluorouracil, and DXR have been shown to increase reactive oxygen species (ROS) and cause oxidative stress. Primary neurons and PC12 pheochromocytoma cells were pre-treated with IGF-I (100ng/ml) for 30 minutes, followed by paraquat treatments for 24 hours. Cytotoxicity (LDH assay) was determined following treatment. * P <0.05, ** P <0.005, *** P<0.0001 by Student’s t test.
Effect of IGF-IR deletion or overexpression on stress resistance in mouse embryonic fibroblast cells
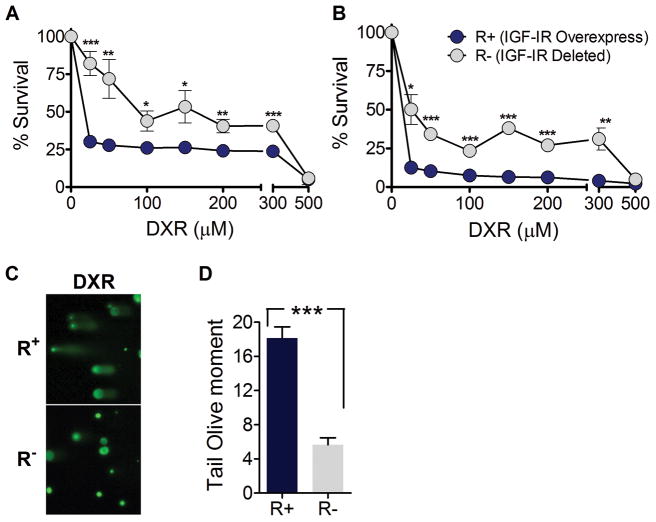
To begin to investigate the mechanism responsible for differential stress resistance, we treated mouse embryonic fibroblasts (MEF) bearing an igf1r deletion (R− cells) or overexpressing IGF-IR (R+ cells) with DXR 25. All cells were grown to confluence to minimize the difference in proliferation rate and were treated with DXR for 24 or 48 hours. After a 24 hour DXR treatment, R− cells showed greater survival compared to R+ cells. The effect was most pronounced at 25μM where more than 80% of R− cells were viable, whereas only 30% of R+ cells were alive (Fig. 3A, P <0.0005). Similar results were observed when cells were treated for 48 hours, with 50% vs. 12% survival rate for R− and R+ cells, respectively, at 25μM (Fig. 3B, P <0.02).
Figure 3.
R+ and R− cells were grown to confluence and treated with DXR (0–500μM) in DMEM/F12 supplemented with 10% FBS for (A) 24 hours or (B) 48 hours. Viability was determined by the relative degree of MTT reduction compared to untreated; mean ± SD. * P <0.05, ** P <0.01, *** P <0.001 by Student’s t test; R+ vs. R− cells at same DXR concentration. (C) Comet assay. Cells overexpressing IGF-IR or with IGF-IR deficiency (R+ and R−) were treated with 50μM DXR for 1 hour. Significant DNA damage was observed in the DXR treated R+ cells, whereas R− cells showed enhanced protection against DXR induced DNA damage. (D) Tail olive moment analysis of the comet assay. *** P <0.001 by Student’s t test; R+ DXR vs. R− DXR. Similar results were obtained from two independent experiments. Representative experiment is shown.
To further investigate how deficiency in IGF-I signaling protects against chemotoxicity we measured DNA damage using the comet assay. DXR induced DNA damage was significantly higher in R+ cells compared to R− cells, with more than a 3-fold difference as assessed by the comet assay (Fig. 3C,D, P <0.001). These results support our hypothesis that reduced IGF-I signaling protects normal cells by reducing chemotherapy-dependent DNA damage 26. Notably, R− cells have been shown to be resistant against transformation by the SV40 large T-antigen, which is remarkable considering that fibroblasts frequently transform in culture spontaneously 27.
The role of yeast homologs of downstream effectors of the IGF-IR in DSR
To investigate the mechanisms by which down-regulation of the IGF-IR signaling protects against chemotoxicity and its effect on DNA damage, we turned to the simple model system S. cerevisiae. The rationale for utilizing yeast is based on the role of Ras2 and Sch9, homologs of the mammalian Ras and Akt/S6K, respectively, in modulating cellular defense against oxidative stress, DNA alkylation, and thermal stress demonstrated in our previous studies 2, 28, 29, and on the central signaling role of homologs of SCH9 and RAS2 downstream of IGF-IR (Fig. S3A). We tested the effect of the deletion of RAS2 and SCH9 on the resistance against DXR. To further investigate DSR, we also studied cells transformed with a gene expressing a constitutively active RAS2 (RAS2Val19) that models human oncogenic Ras mutations. The deletion of SCH9 (sch9Δ) or both SCH9 and RAS2 (sch9Δ ras2Δ) provided remarkable protection against DXR compared to its wild-type (WT) strains (Fig. S3B). However, analogously to what we observed in mammalian cells, the expression of the oncogene-like RAS2Val19 reversed the protection provided by RAS2 and SCH9 deficiency. Following 48 hours of DXR treatment, 50% of WT and RAS2val19 expressing cells survived, whereas 70% of sch9Δ and more than 90% of sch9Δ ras2Δ survived (Fig. S3B). The protection was more pronounced after 72 hours of DXR treatment where sch9Δ ras2Δ and sch9Δ were highly protected (88% and 70% survival respectively) but the protection was reversed by the expression of RAS2val19 (sch9Δ RAS2val19; 27% survival). To study the molecular mechanisms of differential resistance to DXR, we monitored DNA mutation frequency, measured as mutations in the CAN1 gene (Canr colonies/106 cells) 30. DXR treatments increased mutation frequency in all strains. In agreement with the survival analysis, sch9Δ and sch9Δ ras2Δ exhibited the lowest mutation frequency, whereas RAS2val19 expression increased mutation frequency (Fig. S3C). The expression of RAS2val19 in sch9Δ (sch9Δ RAS2val19) completely reversed the protection provided by the Sch9 deficiency resulting in a 3-fold increase in mutation frequency (Fig. S3B,C). These data suggest that lowered Ras2 and Sch9 signaling has a beneficial effect that could be explained, at least in part, due to the enhanced protection against DNA damage in the cell, which can be reversed by the expression of oncogenes.
Octreotide sensitizes NXS2 neuroblastoma cells but does not protect mice against high-dose etoposide
Since reduction of IGF-I provided differential chemotherapy protection in mammalian cell culture, we tested if pharmacological manipulation of the GH/IGF-I axis could induce DSR in vivo. The somatostatin analogue octreotide is used in clinics to reduce GH secretion and IGF-I production primarily in acromegaly patients. Also, octreotide was selected because somatostatin increases in response to fasting 31. Interestingly, octreotide and other somatostatin analogs have been shown to have therapeutic effects in a number of cancers 32 through two distinct effects: direct actions on tumors mediated by somatostatin receptors 33, 34, and indirect effects through inhibition of growth hormone release and the lowering of IGF-I 33–35. In a previous report, we showed that short-term starvation (STS) provides DSR against high-dose etoposide 2, a widely used chemotherapy drug that inhibits topoisomerase II 36. Here we tested if inhibiting the GH/IGF-I axis with octreotide could increase the protection against etoposide. We selected a particularly aggressive tumor line (NXS2) that models neuroblastoma (NB) 37. Intravenous injection of NXS2 cells results in a consistent formation of metastasis in multiple organs including the liver, kidneys, adrenal gland, and ovaries 37. A single injection of high-dose etoposide (80 mg/kg) extended the lifespan of tumor-bearing mice, which otherwise would have succumbed to metastasis within 40 days. In our previous study, STS caused a remarkable reduction in acute chemotoxicity-related deaths, but also provided partial protection to the cancer cells 2. Our present results indicate that octreotide is not sufficient to protect the animals against chemotherapy but its combination with STS sensitizes the NXS2 cancer cells to etoposide (Fig. S4A,B; Fig. S4C, Gr. 4 vs. Gr.7, P <0.01). However, octreotide had a minimal effect on lowering IGF-I levels in mice (Fig. S4D), which could explain its inability to protect the animal. It is possible that homeostatic mechanisms counteract the effect of somatostatin and lead to tachyphylaxis to octreotide treatment, thus failing to reduce IGF-I levels significantly.
To test if octreotide exerted its sensitizing effect on NXS2 cells directly or indirectly, we treated NXS2 cells with octreotide and etoposide in vitro (Fig. S4E). Octreotide did not alter the toxicity of etoposide to NXS2 cells in cell culture, suggesting the sensitizing effect of octreotide in mice is indirect. Together, this implies that octreotide alone does not provide starvation-like host protection, but may reverse the partial protection provided by STS to cancer cells by sensitizing them. Further studies are necessary to investigate the possibility that octreotide may sensitize other tumors against chemotherapy.
Enhanced stress resistance in LID mice against high-dose chemotherapy
Mice with genetic disruptions in the IGF-IR or its downstream effectors are more resistant to oxidative stress 9, 38. To determine whether reducing IGF-I signaling protects from chemotoxicity, we tested a transgenic mouse model with a conditional liver igf1 gene deletion (LID)39, resulting in a post-natal 70–80% reduction of circulating IGF-I 40, which is similar to that of a 72-hour fasted mice (Fig. 1B). The LID mice provides a model for investigating the mechanistic relationship between IGF-I and fasting in chemotherapy resistance 41. A single administration of high-dose etoposide led to 50% vs. 88% survival rate respectively in the LID and control mice (Fig. 4A; Fig. S5A). Based on our in vitro results, we tested CP in LID mice. LID mice treated with 500 mg/kg CP showed significantly higher resistance, with 70% vs. 30% survival rate for LID and control mice respectively (Fig. 4B). Furthermore, while LID mice only lost an average of 10% of their weight, control mice lost 20% (Fig. S5B). The surviving LID mice also did not show signs of toxicity (Fig. S6). To determine the range of protection by reduced IGF-I, we tested two additional drugs, 5-fluorouracil (5-FU) and doxorubicin (DXR), which represent different classes of chemotherapy drugs. 5-FU is an anti-metabolite 42. Survival after a treatment with high-dose 5-FU was improved, with a 55% vs. 10% survival rate in LID and controls respectively, although the difference was not statistically significant (Fig. 4C). Similar but more pronounced effects were observed with DXR. Unlike etoposide and other drugs that can cause irreversible damage to the tail vein of rodents after a single high-dose injection, DXR can be injected for up to 2–3 cycles (data not shown). In order to test the effect of multiple cycles of chemotherapy, we challenged LID mice with 2 cycles of high-dose DXR. The first DXR injection (20 mg/kg) did not result in any toxicity related deaths, but led to considerable weight loss in all mice (Fig. 4D; Fig. S5C). Weight loss was more evident in LID mice during the first 5 days following DXR injection, but unlike controls who continued to lose weight and showed signs of toxicity, LID mice regained their weight during the following 3 weeks. The second DXR injection (28 mg/kg) caused a considerable amount of DXR-related deaths in the control (25% survival) but not in the LID mice (100% survival) (Fig. 4D).
Figure 4.
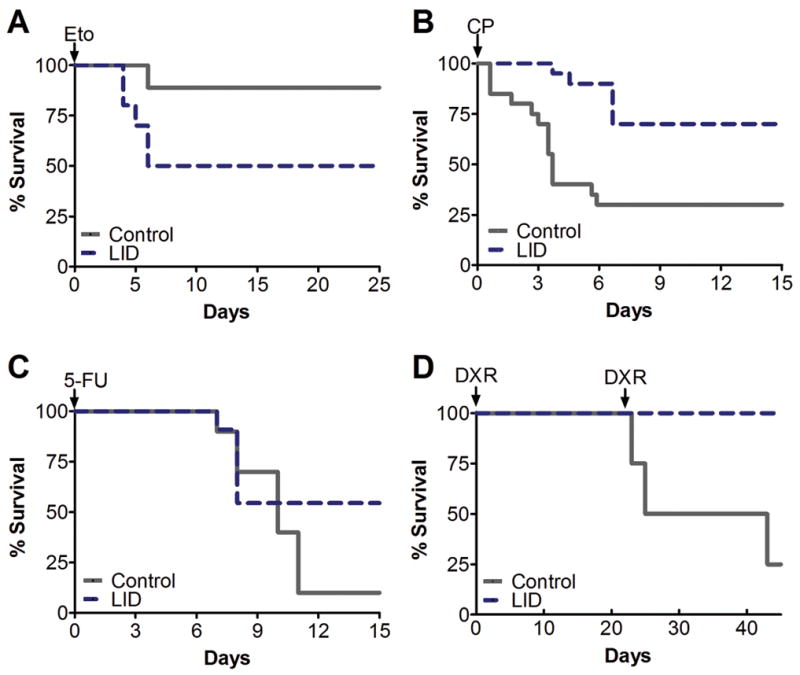
Stress resistance testing in LID mice with various high-dose chemotherapeutic drugs. LID and control mice received (A) a single injection of 100 mg/kg etoposide (n=10/LID, n=9/control, P=0.064), (B) a single injection of 500 mg/kg CP (n=20/group, P=0.001), (C) a single injection of 400 mg/kg 5-fluorouracil (n=11/LID, n=10/control, P=0.148), (D) two injections of doxorubicin (DXR). The first injection of 20 mg/kg was given on day 0, and the second injection of 28 mg/kg was given on day 22 (n=5/LID, n=4/control, P=0.022). Toxicity evaluated by percent survival is shown. P values by Peto’s log rank test.
Differential stress resistance in melanoma bearing LID mice against high-dose doxorubicin
Next, we tested DSR in vivo by monitoring LID mice inoculated with a highly aggressive melanoma cell line (B16Fluc) that metastasizes primarily to the lungs 43 and treating them with DXR. B16Fluc is a luminescent derivative of the B16 mouse melanoma cell line. Therefore tumor progression and regression can be visualized and quantified in vivo using bioluminescence imaging technology (BLI) 43. LID and its control mice were intravenously injected with B16Fluc (2×105 cells/mouse) melanoma cells and treated with 2 cycles of high-dose DXR (Fig. 5A). Although IGF-I plays a major role in tumor growth and metastasis 44, both LID and its control mice started to succumb to metastasis as early as 25 days following cancer inoculation. The 2 cycles of high-dose DXR extended survival time by delaying metastasis in all mice (Fig. 5C). 43% of control mice died with signs of DXR-induced cardiac myopathy, whereas none of the LID mice died from DXR toxicity (Fig. 5D; Fig. S7A). In addition, LID mice showed a slight advantage in weight maintenance (Fig. S8). 90 days after cancer inoculation, all control mice that received chemotherapy had died from either cancer metastases or DXR toxicity, but 60% of LID mice that received 2 cycles of high-dose DXR treatment were cancer-free with no apparent toxic side-effects (Fig. 5B–C; Fig. S7). All the LID mice deaths were caused by cancer metastases. The progression of cancer between control and LID mice was similar after DXR treatments (Fig. S7B), suggesting that the reduction of circulating IGF-I protects the host but not cancer cells against high dose chemotherapy.
Figure 5.
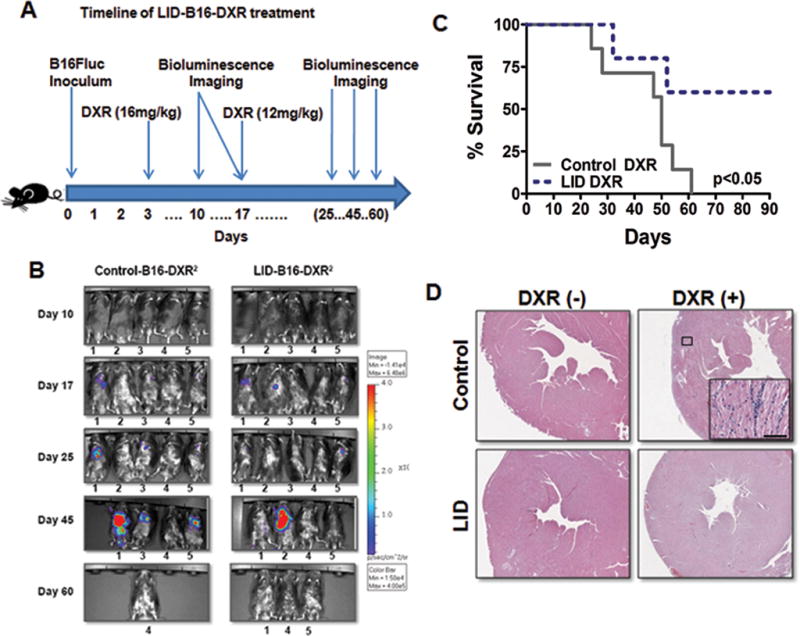
Differential stress resistance (DSR) against 2 cycles of high-dose DXR in melanoma bearing LID mice. (A) Timeline of experimental procedures (n=4/LID-B16, n=5/LID-B16-DXR, n=8/Control-B16, n=7/Control-B16-DXR). (B) Bioluminesence imaging of B16Fluc melanoma bearing LID mice and control mice treated with 2 cycles of high-dose DXR. Five mice were randomly selected and followed throughout the experiment to monitor tumor progression or regression. (C) Survival rate comparison between B16Fluc melanoma bearing LID and control mice treated with 2 cycles of high-dose DXR (P <0.05). (D) DXR induced cardiomyopathy in control and LID mice. Heart failure is a major outcome of acute DXR toxicity. Histological slides of the heart from DXR treated control mice showed loss of myofibrils and infiltration of immune cells, whereas DXR dependent cardiac myopathy was not observed in LID mice. Hematoxylin and eosin staining (H&E). Representative slide shown. Bar, 100μm.
Discussion
In a previous report, we described a STS-based DSR method to protect the host but not cancer cells against high-dose chemotherapy. The basis for this appears to be the existence of a non-dividing state, which normal cells enter in response to starvation for the purpose of investing the remaining energy resources in cellular protection against various insults. Here we show that a major reduction in circulating IGF-I can protect the host but not cancer cells against chemotherapy. Low levels of IGF-I can reduce intracellular mitogenic signaling pathways, including those regulated by Ras and Akt, two of the major pathways downstream of the IGF-IR. We believe that the reduction of mitogenic stimuli may protect normal cells in part by inducing cell cycle arrest 45–47 and in part by shifting the energy towards repair by mechanisms regulated by proteins including Akt, Ras/ERK, FOXO, SirT1, SODs, and DNA repair genes 10, 26, 48, thereby entering a highly protected ‘maintenance mode’ 2, 46. In yeast, we have previously shown that protection can be increased in non-dividing cells by up to 1,000-fold, suggesting that a major component of the protective mechanisms is independent of the switch from a dividing to a non-dividing state, at least in this simple organism 2. This is also in agreement with the effect of IGF-IR overexpression in sensitizing fibroblasts grown to confluence to doxorubicin (Fig. 3). On the other hand, cancer cells are self sufficient in growth signals, less or not responsive to physiological anti-growth signals, and in many cases do not undergo cell cycle arrest due to check point dysregulation 11, 26, 47. In fact, it has been shown that pre-treatment with non-toxic doses of cell cycle arresting drugs (e.g. DXR) or growth factor inhibitors (inhibitors of MEK or EGF receptor) protect normal cells but not cancer cells against chemotherapy 49–51.
In support of our hypothesis, our yeast experiments show that the deletion of the homologs of RAS and/or SCH9 (AKT/S6K) promotes protection against DXR, but the expression of the oncogenic RAS2Val19 reverses this cellular and DNA protection independently of cell division. These results raise the possibility that oncogenic mutations that activate pathways such as Ras, AKT or PKA may reverse the protective effect of reduced IGF-I signaling in malignant cells, thus allowing differential protection of host and various cancers. Notably, inhibition of the pathway downstream of oncogenic mutations could have either a positive or negative effect on the protection of cancer cells. Preclinical studies show that IGF-IR targeting strategies can be effective in the treatment of multiple myelomas, prostate, breast and colon cancer in addition to other cancers 35, 52. The antitumor effect seen with such agents is thought to be dependent on apoptosis resulting from IGF-IR inactivation 52. However, it must be noted that IGF-IR blockade could also trigger apoptosis in normal cells, and may not protect against high dose chemotherapy by interfering with the growth/recovery of certain types of cells (e.g. bone marrow cells). As observed with our LID mice, reduced IGF-I, unlike IGF-IR blockade, does not cause cancer cell death but can selectively enhance the resistance of normal cells against chemotoxicity and may sensitize cancer cells to chemotherapy. This is in agreement with the normal development of prostatic carcinoma in the LID-TRAMP model 40. Based on our results from etoposide treated LID mice, strategies that reduce circulating IGF-I may also increase the toxicity of certain chemotherapy drugs. Therefore, the compatibility between each drug and IGF-I reduction/blockade therapy should be carefully tested in pre-clinical studies before being considered as a candidate. Although it appears to be central, IGF-I may represent simply one of a number of growth factors that can activate Ras, Akt etc in normal cells and promote cell death in cancer cells and therefore only one of the factors that can be down-regulated to provide differential stress resistance 47.
In summary, our studies in mice indicate that a major reduction in circulating IGF-I and in intracellular IGF-I signalling enhances resistance of the host, but not cancer cells against chemotherapy, thus providing the foundation for a method to augment cancer treatment without the need to fast. However, the combination of fasting and IGF-I reduction could result in an even more pronounced effect.
Supplementary Material
Acknowledgments
We thank T. Chen of USC for providing glioma cell lines, R. Baserga of Thomas Jefferson University for providing R+ and R− cells, N. Craft of UCLA for providing B16Fluc cells, and D. LeRoith of Mt. Sinai School of Medicine for providing LID and its control mice. We also thank L. Dubeau of USC for histology expertise. Lizzia Raffaghello is a recipient of a Fondazione Italiana per la Lotta al Neuroblastoma fellowship and a MFAG (My First AIRC Grant). This study was also funded in part by NIH/NIA AG20642, AG025135, Ted Bakewell (The Bakewell Foundation), the V Foundation for Cancer Research, and a USC Norris Cancer Center pilot grant to VDL.
References
- 1.Links M, Lewis C. Chemoprotectants: a review of their clinical pharmacology and therapeutic efficacy. Drugs. 1999;57:293–308. doi: 10.2165/00003495-199957030-00003. [DOI] [PubMed] [Google Scholar]
- 2.Raffaghello L, Lee C, Safdie FM, et al. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc Natl Acad Sci U S A. 2008;105:8215–20. doi: 10.1073/pnas.0708100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Longo VD, Finch CE. Evolutionary Medicine: from Dwarf Model Systems to Healthy Centenarians. Science. 2003;299:1342–6. doi: 10.1126/science.1077991. [DOI] [PubMed] [Google Scholar]
- 4.Kirkwood TB, Shanley DP. Food restriction, evolution and ageing. Mech Ageing Dev. 2005;126:1011–6. doi: 10.1016/j.mad.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 5.Shanley DP, Kirkwood TB. Calorie restriction and aging: a life-history analysis. Evolution. 2000;54:740–50. doi: 10.1111/j.0014-3820.2000.tb00076.x. [DOI] [PubMed] [Google Scholar]
- 6.Bartke A. Minireview: role of the growth hormone/insulin-like growth factor system in mammalian aging. Endocrinology. 2005;146:3718–23. doi: 10.1210/en.2005-0411. [DOI] [PubMed] [Google Scholar]
- 7.Thissen JP, Underwood LE, Ketelslegers JM. Regulation of insulin-like growth factor-I in starvation and injury. Nutr Rev. 1999;57:167–76. doi: 10.1111/j.1753-4887.1999.tb06939.x. [DOI] [PubMed] [Google Scholar]
- 8.Merimee TJ, Zapf J, Froesch ER. Insulin-like growth factors in the fed and fasted states. J Clin Endocrinol Metab. 1982;55:999–1002. doi: 10.1210/jcem-55-5-999. [DOI] [PubMed] [Google Scholar]
- 9.Holzenberger M, Dupont J, Ducos B, et al. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–7. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- 10.Murakami S. Stress resistance in long-lived mouse models. Exp Gerontol. 2006;41:1014–9. doi: 10.1016/j.exger.2006.06.061. [DOI] [PubMed] [Google Scholar]
- 11.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 12.Xie L, Jiang Y, Ouyang P, et al. Effects of dietary calorie restriction or exercise on the PI3K and Ras signaling pathways in the skin of mice. J Biol Chem. 2007;282:28025–35. doi: 10.1074/jbc.M604857200. [DOI] [PubMed] [Google Scholar]
- 13.Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789–99. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 14.Cotterill AM, Holly JM, Wass JA. The regulation of insulin-like growth factor binding protein (IGFBP)-1 during prolonged fasting. Clin Endocrinol (Oxf) 1993;39:357–62. doi: 10.1111/j.1365-2265.1993.tb02377.x. [DOI] [PubMed] [Google Scholar]
- 15.Katz LE, Satin-Smith MS, Collett-Solberg P, Baker L, Stanley CA, Cohen P. Dual regulation of insulin-like growth factor binding protein-1 levels by insulin and cortisol during fasting. J Clin Endocrinol Metab. 1998;83:4426–30. doi: 10.1210/jcem.83.12.5347. [DOI] [PubMed] [Google Scholar]
- 16.Frystyk J, Delhanty PJ, Skjaerbaek C, Baxter RC. Changes in the circulating IGF system during short-term fasting and refeeding in rats. Am J Physiol. 1999;277:E245–52. doi: 10.1152/ajpendo.1999.277.2.E245. [DOI] [PubMed] [Google Scholar]
- 17.Murphy LJ. Overexpression of insulin-like growth factor binding protein-1 in transgenic mice. Pediatr Nephrol. 2000;14:567–71. doi: 10.1007/s004670000347. [DOI] [PubMed] [Google Scholar]
- 18.Norrelund H, Frystyk J, Jorgensen JO, et al. The effect of growth hormone on the insulin-like growth factor system during fasting. J Clin Endocrinol Metab. 2003;88:3292–8. doi: 10.1210/jc.2002-021983. [DOI] [PubMed] [Google Scholar]
- 19.Zeman SM, Phillips DR, Crothers DM. Characterization of covalent adriamycin-DNA adducts. Proc Natl Acad Sci U S A. 1998;95:11561–5. doi: 10.1073/pnas.95.20.11561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown-Borg HM, Rakoczy SG, Romanick MA, Kennedy MA. Effects of growth hormone and insulin-like growth factor-1 on hepatocyte antioxidative enzymes. Exp Biol Med. 2002;227:94–104. doi: 10.1177/153537020222700203. [DOI] [PubMed] [Google Scholar]
- 21.Suh Y, Atzmon G, Cho MO, et al. Functionally significant insulin-like growth factor I receptor mutations in centenarians. Proc Natl Acad Sci U S A. 2008;105:3438–42. doi: 10.1073/pnas.0705467105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yan L, Vatner DE, O’Connor JP, et al. Type 5 adenylyl cyclase disruption increases longevity and protects against stress. Cell. 2007;130:247–58. doi: 10.1016/j.cell.2007.05.038. [DOI] [PubMed] [Google Scholar]
- 23.de Jonge ME, Huitema AD, Rodenhuis S, Beijnen JH. Clinical pharmacokinetics of cyclophosphamide. Clin Pharmacokinet. 2005;44:1135–64. doi: 10.2165/00003088-200544110-00003. [DOI] [PubMed] [Google Scholar]
- 24.Manetta J, Brun JF, Maimoun L, Callis A, Prefaut C, Mercier J. Effect of training on the GH/IGF-I axis during exercise in middle-aged men: relationship to glucose homeostasis. Am J Physiol Endocrinol Metab. 2002;283:E929–36. doi: 10.1152/ajpendo.00539.2001. [DOI] [PubMed] [Google Scholar]
- 25.Drakas R, Tu X, Baserga R. Control of cell size through phosphorylation of upstream binding factor 1 by nuclear phosphatidylinositol 3-kinase. Proc Natl Acad Sci U S A. 2004;101:9272–6. doi: 10.1073/pnas.0403328101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Longo VD, Lieber MR, Vijg J. Turning anti-ageing genes against cancer. Nat Rev Mol Cell Biol. 2008;9:903–10. doi: 10.1038/nrm2526. [DOI] [PubMed] [Google Scholar]
- 27.Baserga R. The IGF-I receptor in cancer research. Exp Cell Res. 1999;253:1–6. doi: 10.1006/excr.1999.4667. [DOI] [PubMed] [Google Scholar]
- 28.Wei M, Fabrizio P, Hu J, et al. Life span extension by calorie restriction depends on Rim15 and transcription factors downstream of Ras/PKA, Tor, and Sch9. PLoS Genet. 2008;4:e13. doi: 10.1371/journal.pgen.0040013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Madia F, Gattazzo C, Wei M, et al. Longevity mutation in SCH9 prevents recombination errors and premature genomic instability in a Werner/Bloom model system. J Cell Biol. 2008;180:67–81. doi: 10.1083/jcb.200707154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Madia F, Gattazzo C, Fabrizio P, Longo VD. A simple model system for age-dependent DNA damage and cancer. Mech Ageing Dev. 2007;128:45–9. doi: 10.1016/j.mad.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ishikawa M, Mizobuchi M, Takahashi H, Bando H, Saito S. Somatostatin release as measured by in vivo microdialysis: circadian variation and effect of prolonged food deprivation. Brain Res. 1997;749:226–31. doi: 10.1016/S0006-8993(96)01163-8. [DOI] [PubMed] [Google Scholar]
- 32.Hejna M, Schmidinger M, Raderer M. The clinical role of somatostatin analogues as antineoplastic agents: much ado about nothing? Ann Oncol. 2002;13:653–68. doi: 10.1093/annonc/mdf142. [DOI] [PubMed] [Google Scholar]
- 33.Susini C, Buscail L. Rationale for the use of somatostatin analogs as antitumor agents. Ann Oncol. 2006;17:1733–42. doi: 10.1093/annonc/mdl105. [DOI] [PubMed] [Google Scholar]
- 34.Zalatnai A, Schally AV. Treatment of N-nitrosobis(2-oxopropyl)amine-induced pancreatic cancer in Syrian golden hamsters with D-Trp-6-LH-RH and somatostatin analogue RC-160 microcapsules. Cancer Res. 1989;49:1810–5. [PubMed] [Google Scholar]
- 35.Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4:505–18. doi: 10.1038/nrc1387. [DOI] [PubMed] [Google Scholar]
- 36.Hande KR. Etoposide: four decades of development of a topoisomerase II inhibitor. Eur J Cancer. 1998;34:1514–21. doi: 10.1016/s0959-8049(98)00228-7. [DOI] [PubMed] [Google Scholar]
- 37.Lode HN, Xiang R, Varki NM, Dolman CS, Gillies SD, Reisfeld RA. Targeted interleukin-2 therapy for spontaneous neuroblastoma metastases to bone marrow. J Natl Cancer Inst. 1997;89:1586–94. doi: 10.1093/jnci/89.21.1586. [DOI] [PubMed] [Google Scholar]
- 38.Migliaccio E, Giorgio M, Mele S, et al. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999;402:309–13. doi: 10.1038/46311. [DOI] [PubMed] [Google Scholar]
- 39.Yakar S, Liu JL, Stannard B, et al. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci U S A. 1999;96:7324–9. doi: 10.1073/pnas.96.13.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anzo M, Cobb LJ, Hwang DL, et al. Targeted deletion of hepatic Igf1 in TRAMP mice leads to dramatic alterations in the circulating insulin-like growth factor axis but does not reduce tumor progression. Cancer Res. 2008;68:3342–9. doi: 10.1158/0008-5472.CAN-07-3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patel AC, Nunez NP, Perkins SN, Barrett JC, Hursting SD. Effects of energy balance on cancer in genetically altered mice. J Nutr. 2004;134:3394S–8S. doi: 10.1093/jn/134.12.3394S. [DOI] [PubMed] [Google Scholar]
- 42.Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330–8. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 43.Craft N, Bruhn KW, Nguyen BD, et al. Bioluminescent imaging of melanoma in live mice. J Invest Dermatol. 2005;125:159–65. doi: 10.1111/j.0022-202X.2005.23759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Samani AA, Yakar S, LeRoith D, Brodt P. The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr Rev. 2007;28:20–47. doi: 10.1210/er.2006-0001. [DOI] [PubMed] [Google Scholar]
- 45.Keyomarsi K, Pardee AB. Selective protection of normal proliferating cells against the toxic effects of chemotherapeutic agents. Prog Cell Cycle Res. 2003;5:527–32. [PubMed] [Google Scholar]
- 46.Blagosklonny MV, Pardee AB. Exploiting cancer cell cycling for selective protection of normal cells. Cancer Res. 2001;61:4301–5. [PubMed] [Google Scholar]
- 47.Blagosklonny MV, Darzynkiewicz Z. Cyclotherapy: protection of normal cells and unshielding of cancer cells. Cell Cycle. 2002;1:375–82. doi: 10.4161/cc.1.6.259. [DOI] [PubMed] [Google Scholar]
- 48.Li Y, Xu W, McBurney MW, Longo VD. SirT1 Inhibition Reduces IGF-I/IRS-2/Ras/ERK1/2 Signaling and Protects Neurons. Cell Metab. 2008;8:38–48. doi: 10.1016/j.cmet.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blagosklonny MV, Bishop PC, Robey R, Fojo T, Bates SE. Loss of cell cycle control allows selective microtubule-active drug-induced Bcl-2 phosphorylation and cytotoxicity in autonomous cancer cells. Cancer Res. 2000;60:3425–8. [PubMed] [Google Scholar]
- 50.Blagosklonny MV, Robey R, Bates S, Fojo T. Pretreatment with DNA-damaging agents permits selective killing of checkpoint-deficient cells by microtubule-active drugs. J Clin Invest. 2000;105:533–9. doi: 10.1172/JCI8625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Demidenko ZN, Halicka D, Kunicki J, McCubrey JA, Darzynkiewicz Z, Blagosklonny MV. Selective killing of adriamycin-resistant (G2 checkpoint-deficient and MRP1-expressing) cancer cells by docetaxel. Cancer Res. 2005;65:4401–7. doi: 10.1158/0008-5472.CAN-04-4428. [DOI] [PubMed] [Google Scholar]
- 52.Tao Y, Pinzi V, Bourhis J, Deutsch E. Mechanisms of disease: signaling of the insulin-like growth factor 1 receptor pathway--therapeutic perspectives in cancer. Nat Clin Pract Oncol. 2007;4:591–602. doi: 10.1038/ncponc0934. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.