Abstract
The main cytokine induced by the interaction of oral epithelial cells with C. glabrata is granulocyte monocyte colony-stimulating factor (GM-CSF); however, the mechanisms regulating this response are unknown. Based on previously published information on the interactions of C. albicans with oral epithelial cells, we hypothesized that interaction with viable C. glabrata triggers GM-CSF synthesis via NF-κB activation. We found that C. glabrata-induced GM-CSF synthesis was adhesion-dependent, enhanced by endocytosis, and required fungal viability. NF-κB activation was noted during interaction of epithelial cells with C. glabrata, and pre-treatment with an NF-κB inhibitor partly inhibited GM-CSF synthesis. Blocking TLR4 with anti-TLR4 antibody did not inhibit GM-CSF production. In contrast, an anti-CDw17 antibody triggered significant inhibition of NF-κB activation and GM-CSF synthesis. β-glucans did not stimulate GM-CSF synthesis, suggesting that the CDw17/NF-κB/GM-CSF pathway may be β-glucan-independent. This study provides new insights into the mechanism of GM-CSF induction by C. glabrata.
Keywords: C. glabrata, oral epithelial cell, GM-CSF, TLR4, CDw17
Introduction
Candidiasis is the most common oral fungal infection in humans (Muzyka, 2005). C. glabrata has emerged as the second or third most frequently isolated Candida species in the oral flora of persons with diabetes mellitus, advanced cancer, and HIV infection (Masia Canuto et al., 2000; Davies et al., 2002; Kadir et al., 2002). In addition to maintaining an intact invasion barrier, oral epithelial cells (OECs) can regulate the inflammatory host response to Candida by releasing a wide array of chemotactic and priming molecules for innate immune effector cells (Dongari-Bagtzoglou and Kashleva, 2003; Dongari-Bagtzoglou et al., 2005).
We have shown that C. glabrata is a potent inducer of granulocyte monocyte colony-stimulating factor (GM-CSF) in human oral keratinocytes (Li and Dongari-Bagtzoglou, 2007; Li et al., 2007), and that strains differ in eliciting GM-CSF responses, with certain oroesophageal candidiasis isolates possessing higher GM-CSF induction capacity than oral commensal isolates (Li and Dongari-Bagtzoglou, 2007). By enhancing the proliferation, activation, as well as fungicidal activity of immuno-effector cells such as neutrophils and monocytes, GM-CSF may play a major role in local control of Candida and the prevention of invasive infection, as suggested in clinical reports (Nicolatou-Galitis et al., 2001). Given the potential importance of this cytokine in the host defense against Candida infection, a better understanding of the specific mechanisms involved in GM-CSF induction in response to C. glabrata is needed. The current study was therefore undertaken to investigate the mechanisms of C. glabrata-induced GM-CSF expression in OECs.
Materials & Methods
Organisms
C. glabrata strains GDH2269 and 94-11 were obtained from ATCC. Two pathogenic esophageal candidiasis isolates, MRL2302 and MRL7525, were kindly provided by Dr. M. Ghannoum (Case Western Reserve University, Cleveland, OH, USA). Stationary-phase C. glabrata yeast cells were prepared by growth for 18 hrs at room temperature in yeast extract, peptone, and dextrose (Difco Laboratories, Detroit, MI, USA), supplemented with 2% glucose. All of the strains used in this study had similar growth rates in Keratinocyte Serum-free culture media (KSFM, Invitrogen, Carlsbad, CA, USA), as determined by direct cell counting of yeast cells. Non-viable C. glabrata were prepared by fixation in 4% formaldehyde-PBS for 30 min and overnight washing in PBS.
Cell Cultures
OKF6/TERT-2 cells represent normal oral mucosal epithelium immortalized by forced expression of telomerase via retroviral transduction (Dickson et al., 2000). The SCC15 oral carcinoma line was obtained from ATCC. OECs were maintained in KSFM, supplemented with 0.4 mM CaCl2, 0.1 ng/mL bovine pituitary extract, and antibiotics. Leukocytic cell lines HL-60 (ATCC) and THP-1 (ATCC) were maintained in RPMI 1640 supplemented with 10% fetal bovine serum. HEK293 cells stably transfected with TLR4 were a generous gift from Dr. Douglas Golenbock (University of Massachusetts) and were cultured in DMEM containing 10% FBS.
Experimental System
OKF6/TERT-2 cells were seeded at or near confluence in 6-, 12-, or 24-well polystyrene plates (Corning Incorporated, Corning, NY, USA) and incubated overnight in complete KSFM at 37°C in 5% CO2 atmosphere. The following day, media were discarded, and cells were challenged with suspensions of stationary-phase organisms at various fungal cell-to-host cell ratios. Supernatants and cell or nuclear extracts were collected and stored at −80°C until they were assayed. Cytokines were quantified by ELISA and/or Q-RT-PCR (see Appendix).
In adhesion experiments, OKF6/TERT-2 cells were co-cultured with C. glabrata for 4 hrs, and the attached yeast cells were quantified by a modification of the 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazoliumhydroxide (XTT) assay (see Appendix).
In receptor blocking experiments, cells were pre-incubated for 30 min with 20 µg/mL anti-TLR4 mAb (IgG2a, eBioscience, San Diego, CA, USA), or 20 µg/mL anti-CDw17 mAb (IgMκ, Ancell, Bayport, MN, USA) and then challenged with C. glabrata for up to 36 hrs. To block endocytosis, we pre-treated OKF6/TERT-2 cells with cytochalasin D (100 ng/mL) for 30 min (Neal et al., 2006), followed by challenge with C. glabrata in the presence of cytochalasin D for up to 20 hrs.
To examine the effect of β-glucan on GM-CSF production, we stimulated OKF6/TERT-2 cells with curdlan, zymosan, or laminarin (all from Sigma, St. Louis, MO, USA) at concentrations ranging from 1 to 100 µg/mL for 24 hrs, or with PGG-glucan (Biothera, Eagan, MN, USA), at 100-200 µg/mL, for 36 hrs. The effect of PGG-glucan was also tested in cells stimulated by C. glabrata or IL-1α.
Transmission Electron Microscopy
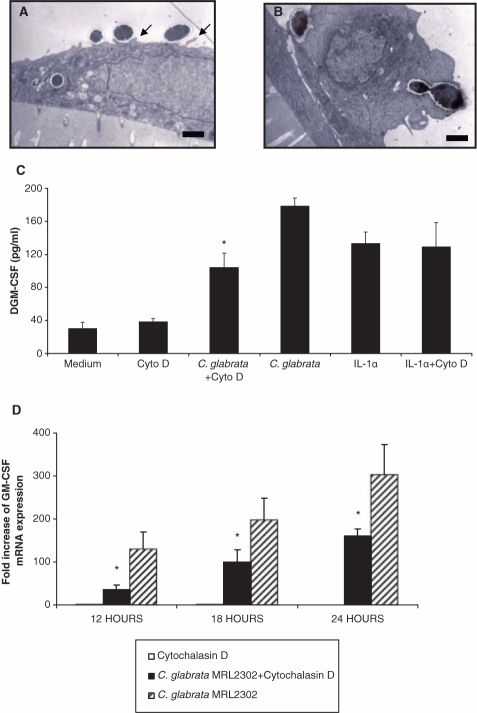
OKF6/TERT-2 and SCC15 cells (5 x 104/well) were co-cultured with viable or non-viable C. glabrata cells (1.5 x 104/well) for up to 12 hrs and processed for TEM as described elsewhere (Villar et al., 2007). Sections were collected on grids, stained with 2% uranyl acetate and lead citrate, and examined and photographed by transmission electron microscopy (Philips CM10; Philips, Eindhoven, the Netherlands).
TLR4 and CDw17 Detection
Formalin-fixed OKF6/TERT-2 cells were stained with 20 µg/mL anti-TLR4 mAb (IgG2a, eBioscience HTA125) with the use of an avidin-biotin-peroxidase kit (Vector Laboratories, Burlingame, CA, USA). Cells stained with isotype control antibody (IgG2a) were used as a negative control. Expression of TLR4 was confirmed by RT-PCR (Appendix).
CDw17 was detected by flow cytometry with HL-60 cells as positive control (Iwabuchi and Nagaoka, 2002). Briefly, cells were dislodged with a Hanks’ salt-based, enzyme-free cell dissociation buffer (Sigma-Aldrich). After Fc receptor was blocked with IgG from human serum (Sigma-Aldrich), cells were stained with anti-CDw17 mAb or control mouse IgMκ on ice for 30 min, followed by Cy3-conjugated affiniPure F(ab′)2 fragment goat anti-mouse IgM (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) for an additional 30 min. Cells were analyzed with a FACSCalibur cytometer (BD Biosciences, Palo Alto, CA, USA).
NF-κB Activation Assays
To detect NF-κB activation, we co-cultured OKF6/TERT-2 cells with C. glabrata MRL2302 at 0.1:1, 1:1, 10:1, and 100:1 yeast-to-epithelial-cell ratios, for 5 min-18 hrs. In certain experiments, we also added IL-1 receptor antagonist (IL-1ra, 1 µg/mL; R&D Systems, Minneapolis, MN, USA) to rule out a secondary role of IL-1 in NF-κB activation. To inhibit NF-κB activation, we pre-treated cells with 15 µM pyrrolidine dithiocarbamate (PDTC, Sigma) for 1 hr (Evans et al., 2005), prior to the addition of C. glabrata. At this concentration, PDTC did not affect the growth or binding of C. glabrata to epithelial cells (not shown). Nuclear protein extracts were obtained from cell cultures by means of a Nuclear Extract Kit (Active Motif, Carlsbad, CA, USA). P65 and P50 DNA binding activity was assessed by the TransAM™ NF-κB Kit (Active Motif).
Statistical Analysis
The statistical significance of the differences between experimental and control groups was determined by two-tailed t test, assuming equal variances.
Results
C. glabrata-induced GM-CSF Responses in Oral Epithelial Cells Were Dependent on Host Cell-Candida Contact and Fungal Viability
Previous dose response studies showed that a maximal GM-CSF response is detected after prolonged incubation at a 0.1:1 C. glabrata-to-epithelial-cell ratio (Li and Dongari-Bagtzoglou, 2007; Li et al., 2007), and we obtained similar data with strain MRL2302 in this study (Fig. 1A). When fungal and epithelial cells were separated by well inserts, GM-CSF secretion was completely inhibited (Fig. 1A). Increasing the fungal challenge (1000:1 yeast-to-epithelial-cell ratio) in the presence of well inserts did not result in GM-CSF induction (Fig. 1A). These results showed a requirement of fungal-to-epithelial-cell contact in GM-CSF induction.
Figure 1.
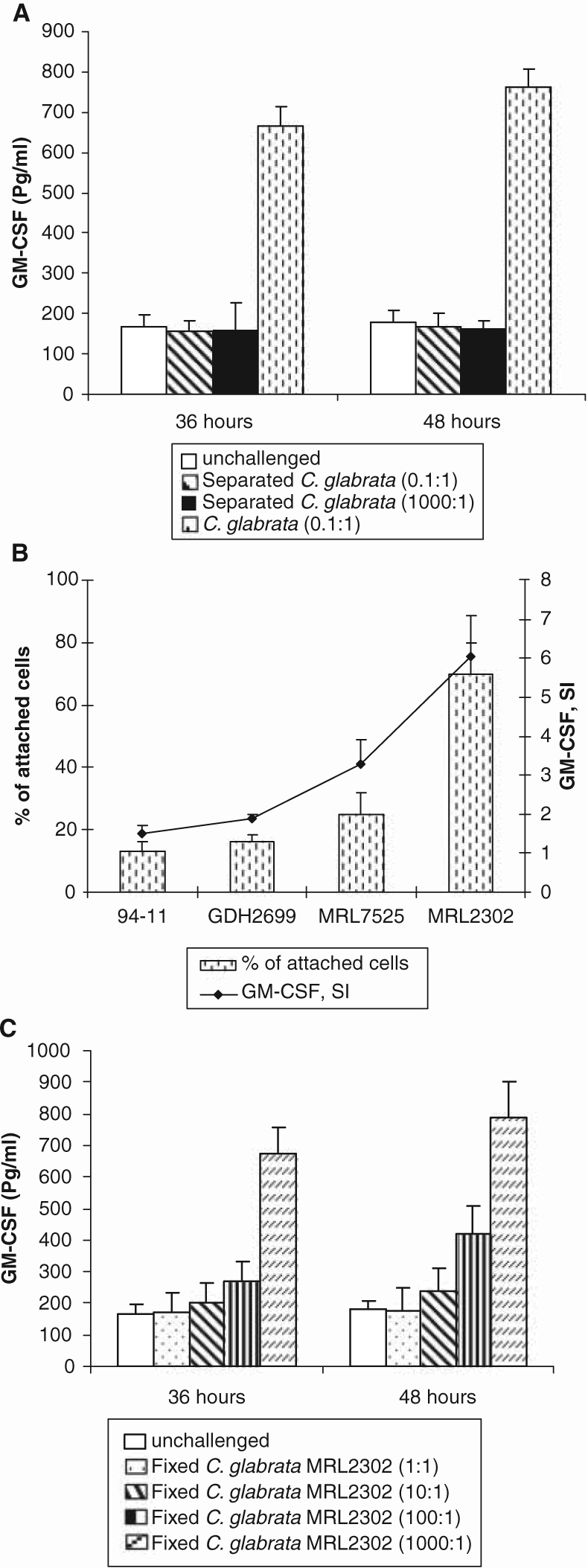
Effects of fungal-epithelial cell contact, yeast viability, and adhesion on GM-CSF responses by OKF6/TERT-2 cells. (A) Epithelial cells were challenged with C. glabrata MRL2302 in the presence (no contact) or absence (contact) of a separating well insert for up to 48 hrs, and GM-CSF release was quantified in culture medium. (B) OKF6/TERT-2 cells were co-cultured with C. glabrata (strains 94-11, GDH2269, MRL2302, and MRL7525) at a 1:5 epithelial-to-yeast-cell ratio for 4 hrs, and adhesion was quantified by XTT assay. Culture supernatants were tested for the presence of GM-CSF. (C) Epithelial cells were challenged with fixed C. glabrata MRL2302 at various epithelial-to-yeast-cell ratios for up to 48 hrs, and GM-CSF release was quantified in culture medium. Replicate samples from at least two independent experiments were analyzed. Error bars indicate one standard deviation of the mean.
Variable GM-CSF-inducing potentials among C. glabrata strains have been noted in our previous studies (Li and Dongari-Bagtzoglou, 2007). Because the types and quantities of surface adhesins differ in genetically distinct C. glabrata strains (deGroot et al., 2008), we next examined whether the GM-CSF induction potential was commensurate with the adhesion potential of these strains. Adhesion of C. glabrata strains to OECs was time-dependent, with maximum levels obtained after 4 hrs of co-culture (not shown). GM-CSF stimulation was commensurate with each strain’s ability to adhere to OECs, with strain MRL2302 having the greatest adhesion potential and triggering the highest GM-CSF stimulation, followed by strains MRL7525, GDH2269, and 94-11 (Fig. 1B).
To determine whether fungal viability is required for GM-CSF induction, we used fixed C. glabrata MRL2302 yeast cells in cell interaction assays. Extremely high (1000:1) doses of fixed yeast cells were needed to induce GM-CSF levels comparable with those induced by low (0.1:1) doses of viable yeasts, indicating that C. glabrata viability is important in GM-CSF induction (Fig. 1C).
C. glabrata-induced GM-CSF Responses in Oral Epithelial Cells Were Enhanced by Endocytosis
It has been reported that C. glabrata can be detected within the cytoplasm of murine vaginal epithelial cells, raising the possibility that endocytosis of this organism by epithelial cells leads to internalization (Fidel et al., 1999). Using two OEC lines, we observed the intracytoplasmic presence of C. glabrata yeast cells as early as 4 hrs after co-culture, with extension of membrane-bound pseudopods around yeast cells (Figs. 2A, 2B). Pre-treatment of OKF6/TERT-2 cells with endocytosis inhibitor cytochalasin D prevented internalization (not shown) and triggered a significant, but incomplete, inhibition of GM-CSF gene expression (Figs. 2C, 2D). Cytochalasin D treatment did not affect IL-1α-triggered GM-CSF induction, suggesting that this treatment did not result in a non-specific adverse effect on cell metabolism (Fig. 2C). Analysis of these data, taken together, suggested an active involvement of actin polymerization in the uptake of C. glabrata by OECs and confirmed a role for endocytosis in C. glabrata-induced GM-CSF release.
Figure 2.
Endocytosis of C. glabrata by OECs and effect of cytochalasin D on GM-CSF responses. Epithelial cells OKF6/TERT-2 (A) and SCC15 (B) were co-cultured with C. glabrata MRL2302 at a 1:5 epithelial-to-yeast-cell ratio for 4 hrs. The intracellular presence of yeast cells was observed by TEM. Bar = 1 µm. (C,D) OKF6/TERT-2 cells were pre-treated with cytochalasin D and challenged with C. glabrata MRL2302 (0.1:1 fungal-to-epithelial-cell ratio) for up to 24 hrs. IL-1α-stimulated GM-CSF induction was used as a negative control for the effects of cytochalasin D. Supernatants were analyzed by ELISA (C), and cell lysates were analyzed by real-time RT-PCR (D). Mean values were obtained by analysis of three individual experiments, with each condition set up in duplicate, and error bars indicate one standard deviation of the mean. * P < 0.05 for a comparison with the C. glabrata MRL2302-stimulated group.
CDw17, but not TLR4, was Involved in the GM-CSF Response to C. glabrata
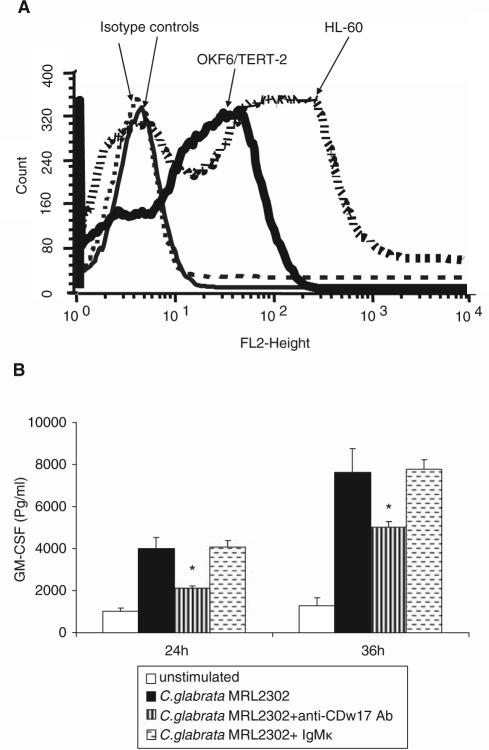
Although OKF6/TERT-2 cells constitutively express TLR4 (Appendix Figs. 1A, 1B), a neutralizing anti-TLR4 mAb did not inhibit GM-CSF production (Appendix Fig. 1C), suggesting that the GM-CSF response to C. glabrata was not mediated by TLR4.
CDw17 has been suggested to be an adhesion receptor for several fungal organisms, including Candida (Jimenez-Lucho et al., 1990). Moreover, interaction of Pneumocystis carinii cell wall β-glucan with CDw17 has been reported to induce release of pro-inflammatory cytokines from alveolar epithelial cells (Hahn et al., 2003). Therefore, we hypothesized that C. glabrata-induced GM-CSF response was mediated by β-glucan-CDw17 interaction. Expression of CDw17 was detected in both OKF6/TERT-2 and HL-60 cells, with slightly higher expression levels in the latter (Fig. 3A). Next, an anti-CDw17 mAb was used to block surface interaction with OECs. Pre-treatment of OKF6/TERT-2 cells with anti-CDw17 significantly suppressed GM-CSF production (Fig. 3B), indicating that this molecule partly regulates secretion of GM-CSF from OECs. However, challenging OECs with increasing concentrations of zymosan, PGG-glucan, curdlan, or laminarin (Appendix Fig. 2A), or seeding OECs on curdlan- or laminarin-coated plates (not shown), did not induce a GM-CSF response. Furthermore, PGG-glucan inhibited both C. glabrata- and IL-1α-induced GM-CSF synthesis (Appendix Fig. 2B). Analysis of these data, taken together, suggests that β-glucan is not the moiety on the yeast cell triggering GM-CSF induction.
Figure 3.
Involvement of CDw17 in the GM-CSF responses of OECs to C. glabrata. (A) Expression of CDw17 in OKF6/TERT-2 cells and HL-60 cells was assessed by flow cytometry. Results are representative of 3 experiments. (B) OKF6/TERT-2 cells were challenged with C. glabrata MRL2302 (0.1:1 yeast-to-epithelial-cell ratio) for up to 36 hrs, in the presence of anti-CDw17 or isotype control Ab IgMκ. * P < 0.05 for a comparison with C. glabrata-challenged cells in the absence of Ab. Error bars indicate one standard deviation of the mean.
Activation of NF-κB was Involved in C. glabrata-induced GM-CSF Production
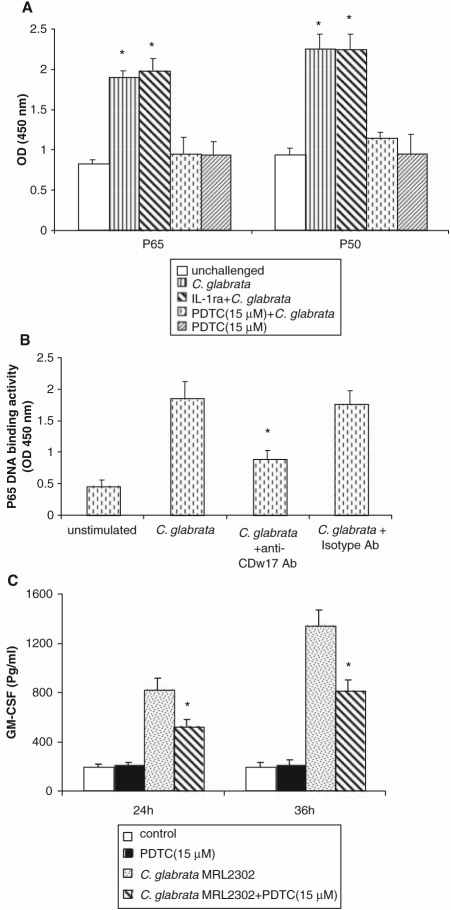
Since NF-κB activation is involved in pro-inflammatory cytokine synthesis of keratinocytes in response to fungal pathogens (Pivarcsi et al., 2003), we investigated its role in GM-CSF stimulation. P65 and p50 DNA binding activity in challenged OKF6/TERT-2 cells increased significantly after 2 hrs of co-culture (Fig. 4A) and continued to be significantly elevated even after 12 hrs of challenge (not shown). NF-κB activation was inhibited by PDTC, but not by IL-1ra (Fig. 4A), excluding a role of IL-1 in NF-κB activation. Pre-treatment of OKF6/TERT-2 with anti-CDw17 significantly inhibited activation of NF-κB in C. glabrata-challenged OECs (Fig. 4B). The NF-κB inhibitor PDTC also triggered a significant reduction in GM-CSF release (Fig. 4C). Analysis of these data, taken together, suggests that the GM-CSF response to C. glabrata is mediated, at least partly, by a CDw17-NF-κB signaling pathway.
Figure 4.
NF-κB activation in C. glabrata-challenged OECs. (A) OKF6/TERT-2 cells were pre-treated with PDTC (15 µM) or IL-1ra (1µg/mL) for 1 hr, followed by C. glabrata challenge (100:1 yeast-to-epithelial-cell ratio) for 2 hrs. * P < 0.05 for a comparison with unchallenged control. (B) Pre-treatment with anti-CDw17 Ab (20 µg/mL) partially inhibited NF-κB activation. *P < 0.05 for a comparison with C. glabrata-challenged cells in the absence of antibody. (C) OKF6/TERT-2 cells were pre-treated with PDTC (15 µM) for 1 hr prior to fungal challenge, and supernatants were analyzed by ELISA for GM-CSF. *P < 0.05 for a comparison with C. glabrata-challenged cells without PDTC treatment. Error bars indicate one standard deviation of the mean.
Discussion
Oral Candida infections are characterized by intense and persistent intra-epithelial inflammation (Eversole et al., 1997). Previous work from our lab has shown that OECs respond to C. glabrata infection by secreting substantial amounts of the phagocyte priming and growth enhancing cytokine GM-CSF (Li and Dongari-Bagtzoglou, 2007; Li et al., 2007). To our knowledge, this is the first study showing that the C. glabrata-induced GM-CSF response in OECs: (a) requires direct physical contact and fungal viability; (b) is enhanced by endocytosis; and (c) is at least partly regulated by a CDw17-NF-κB signaling pathway.
The involvement of TLR4 in Candida-induced inflammatory cytokine production has been controversial (Netea et al., 2006). It has been reported that activation of TLR4 signaling pathways was required for Candida-induced inflammatory cytokine expression by human monocytes (Van der Graaf et al., 2005). However, TLR4 was also reported as dispensable for the production of inflammatory cytokines in host cells in response to Candida albicans in vivo (Murciano et al., 2006) and in vitro (Villamón et al., 2004). In OECs, the expression of TLRs, especially TLR4, remains controversial (Asai et al., 2001; Kusumoto et al., 2004). Although this study demonstrated expression of TLR4 in the oral epithelial cell line OKF6/TERT-2, analysis of our data indicated that TLR4 was not involved in C. glabrata-induced GM-CSF production.
We next examined the role of CDw17 in C. glabrata-induced GM-CSF response in OECs. CDw17 was reported to act as receptor for bacteria (Krivan et al., 1989), viruses (Markwell et al., 1981), and fungi, including C. albicans (Jimenez-Lucho et al., 1990). Expression of CDw17 was detected in human buccal and bronchial epithelial cells (Baker et al., 1990). In this study, we detected surface expression of CDw17 in the oral epithelial cell line OKF6/TERT-2 for the first time. Blocking of CDw17 resulted in a significant, but partial, inhibition of NF-κB activity and GM-CSF response, suggesting the existence of additional surface receptor(s) on OECs which may interact with C. glabrata to stimulate GM-CSF expression. Although β-glucan-CDw17 interaction has been reported to mediate cytokine synthesis in epithelial cells (Hahn et al., 2003; Evans et al., 2005), β-glucans failed to activate GM-CSF in our system, and thus we conclude that the CDw17/NF-κB/GM-CSF pathway may be β-glucan-independent. It is possible that binding and signaling are mediated by an alternative C. glabrata lectin-type ligand (Fantini et al., 2006) from the Epa family of adhesins, which has the potential to bind to a core structural motif shared by CDw17 and other membrane glycosphingolipids (Cormack et al., 1999). However, given the relatively late activation of NF-κB in epithelial cells after contact with C. glabrata, there is also a distinct possibility that NF-κB activation is not the result of immediate recognition of the fungus, but rather a more indirect effect of downstream mediators other than IL-1. To our knowledge, this is the first report of CDw17 receptor involvement in generating a pro-inflammatory response to C. glabrata infection, suggesting its potential role in host innate immune defense against fungal infections.
In summary, we demonstrated that C. glabrata-induced GM- CSF responses in OECs were contact-dependent, and enhanced by fungal viability and endocytosis. We further demonstrated that the surface receptor CDw17, but not TLR4, participated in GM-CSF gene expression, via NF-κB activation. This is the first report describing an inflammatory signaling pathway triggered by C. glabrata in OECs.
Supplementary Material
Footnotes
This work was supported by NIH/NIDCR grant RO1 DE13986.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Asai Y, Ohyama Y, Gen K, Ogawa T. (2001). Bacterial fimbriae and their peptides activate human gingival epithelial cells through Toll-like receptor 2. Infect Immun 69:7387-7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker N, Hansson GC, Leffler H, Riise G, Svanborg-Edén C. (1990). Glycosphingolipid receptors for Pseudomonas aeruginosa. Infect Immun 58:2361-2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cormack BP, Ghori N, Falkow S. (1999). An adhesin of the yeast pathogen Candida glabrata mediating adherence to human epithelial cells. Science 285:578-582. [DOI] [PubMed] [Google Scholar]
- Davies AN, Brailsford S, Broadley K, Beighton D. (2002). Oral yeast carriage in patients with advanced cancer. Oral Microbiol Immunol 17:79-84. [DOI] [PubMed] [Google Scholar]
- deGroot PW, Kraneveld EA, Yin QY, Dekker HL, Gross U, Crielaard W, et al. (2008). The cell wall of the human pathogen Candida glabrata: differential incorporation of novel adhesin-like wall proteins. Eukaryot Cell 7:1951-1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson MA, Hahn WC, Ino Y, Ronfard V, Wu JY, Weinberg RA, et al. (2000). Human keratinocytes that express hTERT and also bypass a p16 INK4a- enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol Cell Biol 20:1436-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dongari-Bagtzoglou A, Kashleva H. (2003). Candida albicans triggers interleukin-8 secretion by oral epithelial cells. Microb Pathog 34:169-177. [DOI] [PubMed] [Google Scholar]
- Dongari-Bagtzoglou A, Villar CC, Kashleva H. (2005). Candida albicans-infected oral epithelial cells augment the anti-fungal activity of human neutrophils in vitro. Med Mycol 43:545-549. [DOI] [PubMed] [Google Scholar]
- Evans SE, Hahn PY, McCann F, Kottom TJ, Pavlović ZV, Limper AH. (2005). Pneumocystis cell wall beta-glucans stimulate alveolar epithelial cell chemokine generation through nuclear factor-kappaB-dependent mechanisms. Am J Respir Cell Mol Biol 32:490-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eversole LR, Reichart PA, Ficarra G, Schmidt-Westhausen A, Romagnoli P, Pimpinelli N. (1997). Oral keratinocyte immune responses in HIV-associated candidiasis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 84:372-380. [DOI] [PubMed] [Google Scholar]
- Fantini J, Garmy N, Yahi N. (2006). Prediction of glycolipid-binding domains from the amino acid sequence of lipid raft-associated proteins: application to HpaA, a protein involved in the adhesion of Helicobacter pylori to gastrointestinal cells. Biochemistry 45:10957-10962. [DOI] [PubMed] [Google Scholar]
- Fidel PL, Jr, Vazquez JA, Sobel JD. (1999). Candida glabrata: review of epidemiology, pathogenesis, and clinical disease with comparison to C. albicans. Clin Microbiol 12:80-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn PY, Evans SE, Kottom TJ, Standing JE, Pagano RE, Limper AH. (2003). Pneumocystis carinii cell wall beta-glucan induces release of macrophage inflammatory protein-2 from alveolar epithelial cells via a lactosylceramide-mediated mechanism. J Biol Chem 278:2043-2050. [DOI] [PubMed] [Google Scholar]
- Iwabuchi K, Nagaoka I. (2002). Lactosylceramide-enriched glycosphingolipid signaling domain mediates superoxide generation from human neutrophils. Blood 100:1454-1464. [PubMed] [Google Scholar]
- Jimenez-Lucho V, Ginsburg V, Krivan HC. (1990). Cryptococcus neoformans, Candida albicans, and other fungi bind specifically to the glycosphingolipid lactosylceramide (Gal beta 1-4Glc beta 1-1Cer), a possible adhesion receptor for yeasts. Infect Immun 58:2085-2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadir T, Pisiriciler R, Akyuz S, Yarat A, Emekli N, Ipbuker A. (2002). Mycological and cytological examination of oral candidal carriage in diabetic patients and non-diabetic control subjects: thorough analysis of local aetiologic and systemic factors. J Oral Rehabil 29:452-457. [DOI] [PubMed] [Google Scholar]
- Krivan HC, Olson LD, Barile MF, Ginsburg V, Roberts DD. (1989). Adhesion of Mycoplasma pneumoniae to sulfated glycolipids and inhibition by dextran sulfate. J Biol Chem 264:9283-9288. [PubMed] [Google Scholar]
- Kusumoto Y, Hirano H, Saitoh K, Yamada S, Takedachi M, Nozaki T, et al. (2004). Human gingival epithelial cells produce chemotactic factors interleukin-8 and monocyte chemoattractant protein-1 after stimulation with Porphyromonas gingivalis via Toll-like receptor 2. J Periodontol 75:370-379. [DOI] [PubMed] [Google Scholar]
- Li L, Dongari-Bagtzoglou A. (2007). Oral epithelium-Candida glabrata interactions in vitro. Oral Microbiol Immunol 22:182-187. [DOI] [PubMed] [Google Scholar]
- Li L, Kashleva H, Dongari-Bagtzoglou A. (2007). Cytotoxic and cytokine-inducing properties of Candida glabrata in single and mixed oral infection models. Microb Pathog 42:138-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell MA, Svennerholm L, Paulson JC. (1981). Specific gangliosides function as host cell receptors for Sendai virus. Proc Natl Acad Sci USA 78:5406-5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masia Canuto M, Gutierrez Rodero F, Ortiz de la Tabla Ducasse V, Hernandez Aguado I, Martin Gonzalez C, Sanchez Sevillano A, et al. (2000). Determinants for the development of oropharyngeal colonization or infection by fluconazole-resistant Candida strains in HIV-infected patients. Eur J Clin Microbiol Infect Dis 19:593-601. [DOI] [PubMed] [Google Scholar]
- Murciano C, Villamon E, Gozalbo D, Roig P, O’Connor JE, Gil ML. (2006). Toll-like receptor 4 defective mice carrying point or null mutations do not show increased susceptibility to Candida albicans in a model of hematogenously disseminated infection. Med Mycol 44: 149-157. [DOI] [PubMed] [Google Scholar]
- Muzyka BC. (2005). Oral fungal infections. Dent Clin North Am 49:49-65. [DOI] [PubMed] [Google Scholar]
- Neal MD, Leaphart C, Levy R, Prince J, Billiar T, Watkins S, et al. (2006). Enterocyte TLR4 mediates phagocytosis and translocation of bacteria across the intestinal barrier. J Immunol 176:3070-3079. [DOI] [PubMed] [Google Scholar]
- Netea MG, van der Meer JW, Kullberg BJ. (2006). Both TLR2 and TLR4 are involved in the recognition of Candida albicans. Reply to “TLR2, but not TLR4, triggers cytokine production by murine cells in response to Candida albicans yeasts and hyphae” by Gil and Gozalbo (Microbes Infect 8:2821-2822). Microbes Infect 8:2823-2824. [DOI] [PubMed] [Google Scholar]
- Nicolatou-Galitis O, Dardoufas K, Markoulatos P, Sotiropoulou-Lontou A, Kyprianou K, Kolitsi G, et al. (2001). Oral pseudomembranous candidiasis, herpes simplex virus-1 infection, and oral mucositis in head and neck cancer patients receiving radiotherapy and granulocyte-macrophage colony-stimulating factor (GM-CSF) mouthwash. J Oral Pathol Med 30:471-480. [DOI] [PubMed] [Google Scholar]
- Pivarcsi A, Bodai L, Réthi B, Kenderessy-Szabó A, Koreck A, Széll M, et al. (2003). Expression and function of Toll-like receptors 2 and 4 in human keratinocytes. Int Immunol 15:721-730. [DOI] [PubMed] [Google Scholar]
- Van der Graaf CA, Netea MG, Verschueren I, van der Meer JW, Kullberg BJ. (2005). Differential cytokine production and Toll-like receptor signaling pathways by Candida albicans blastoconidia and hyphae. Infect Immun 73:7458-7464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villamón E, Gozalbo D, Roig P, O’Connor JE, Fradelizi D, Gil ML. (2004). Toll-like receptor 2 is essential in murine defenses against Candida albicans infections. Microbes Infect 6:1-7. [DOI] [PubMed] [Google Scholar]
- Villar CC, Kashleva H, Nobile CJ, Mitchell AP, Dongari-Bagtzoglou A. (2007). Mucosal tissue invasion by Candida albicans is associated with E-cadherin degradation, mediated by transcription factor Rim101p and protease Sap5p. Infect Immun 75:2126-2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.